Effects of High-Intensity Ultrasound Pretreatment on Structure, Properties, and Enzymolysis of Soy Protein Isolate
Abstract
:1. Introduction
2. Results and Discussion
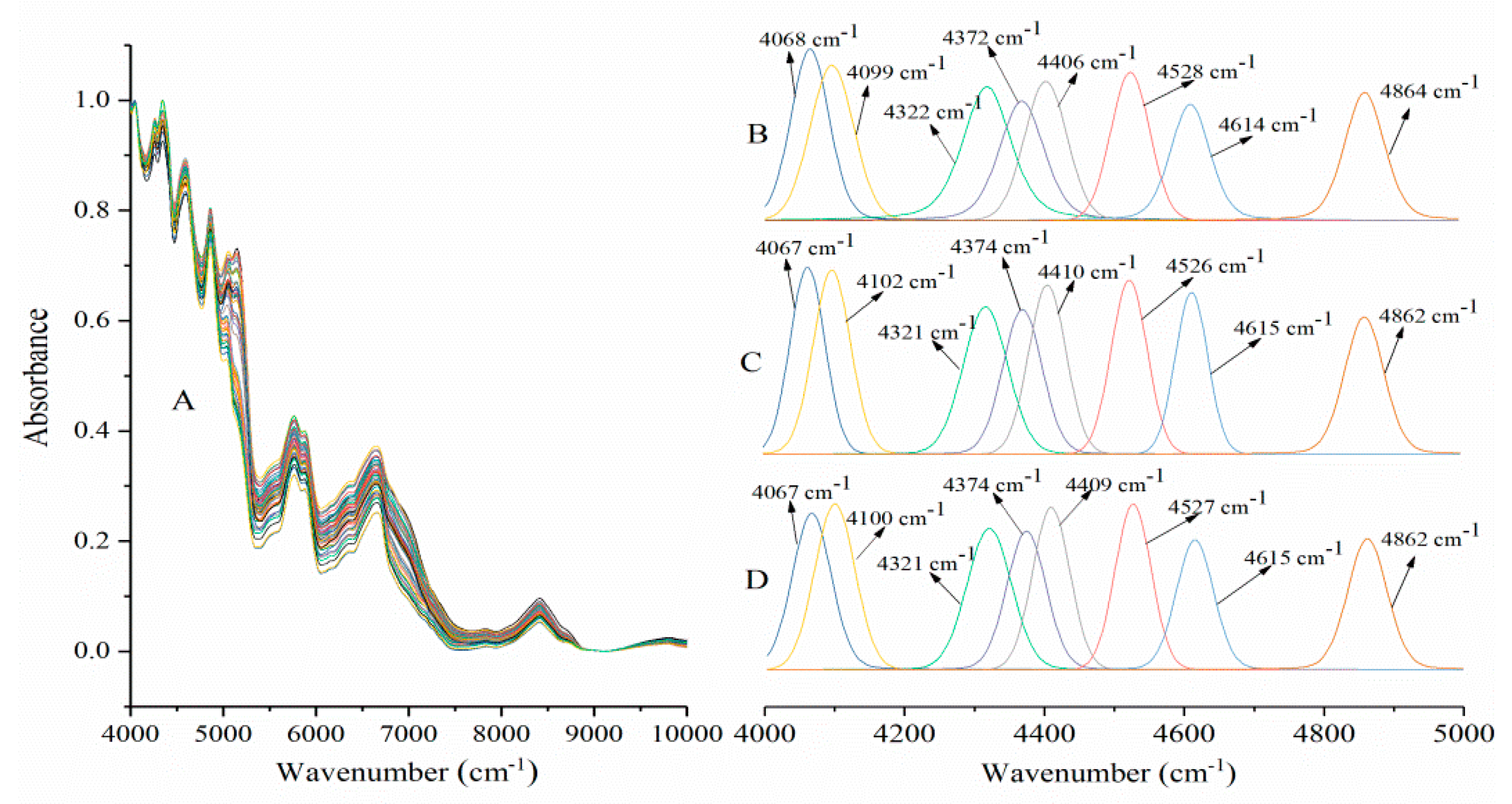
2.1. Near-Infrared Spectroscopy Analysis
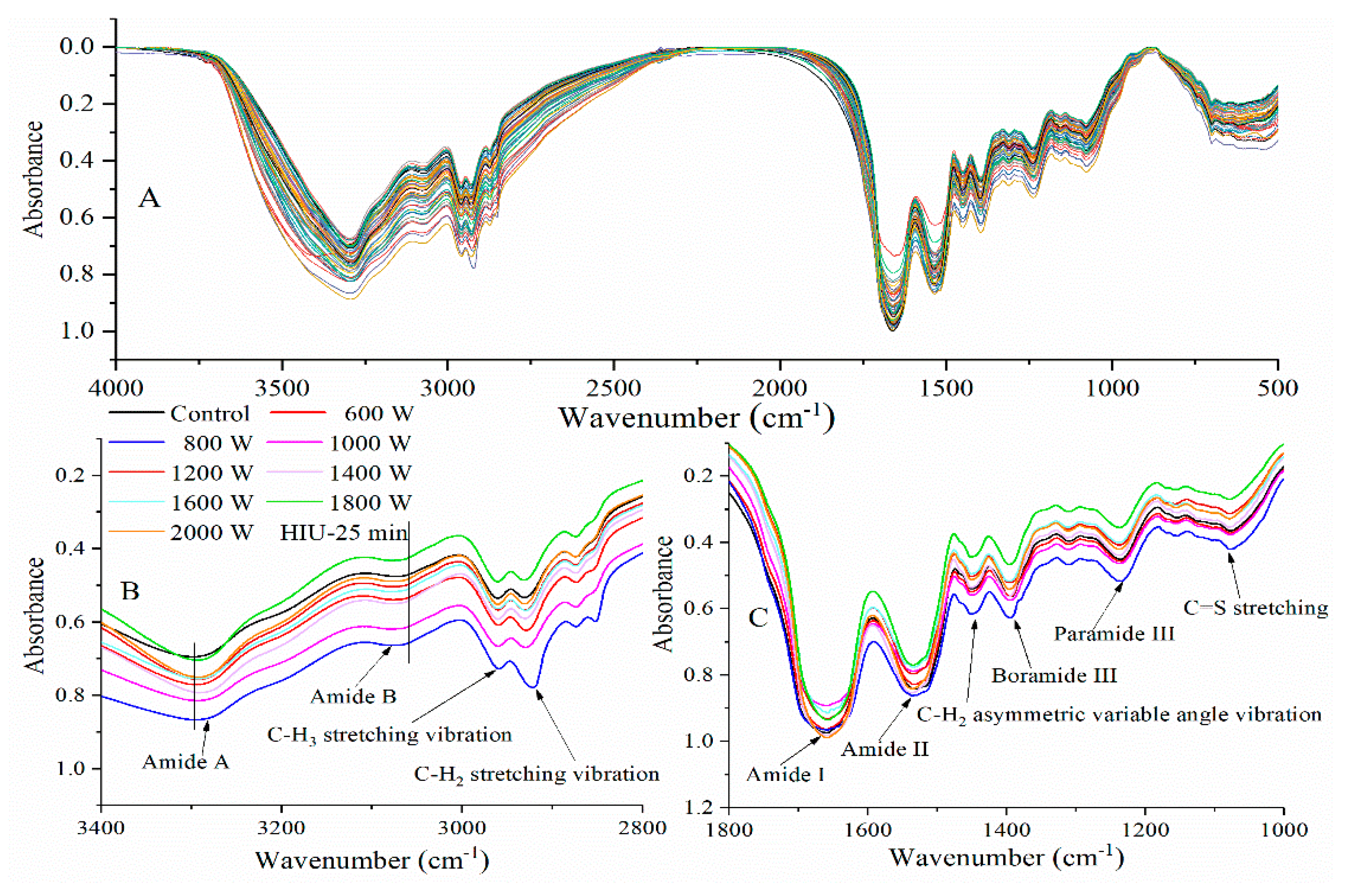
2.2. Fourier-Transform Infrared Spectroscopy Analysis
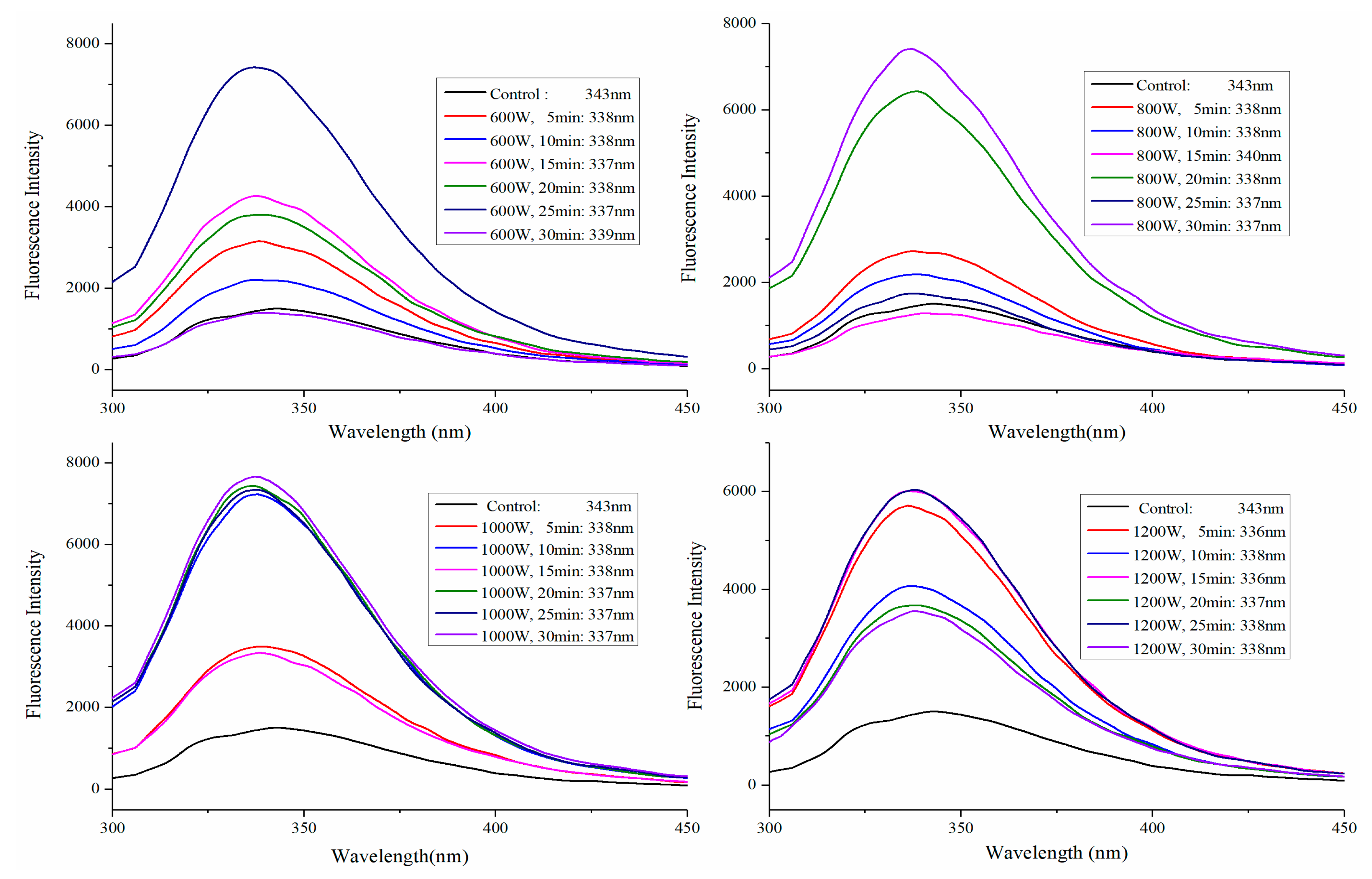
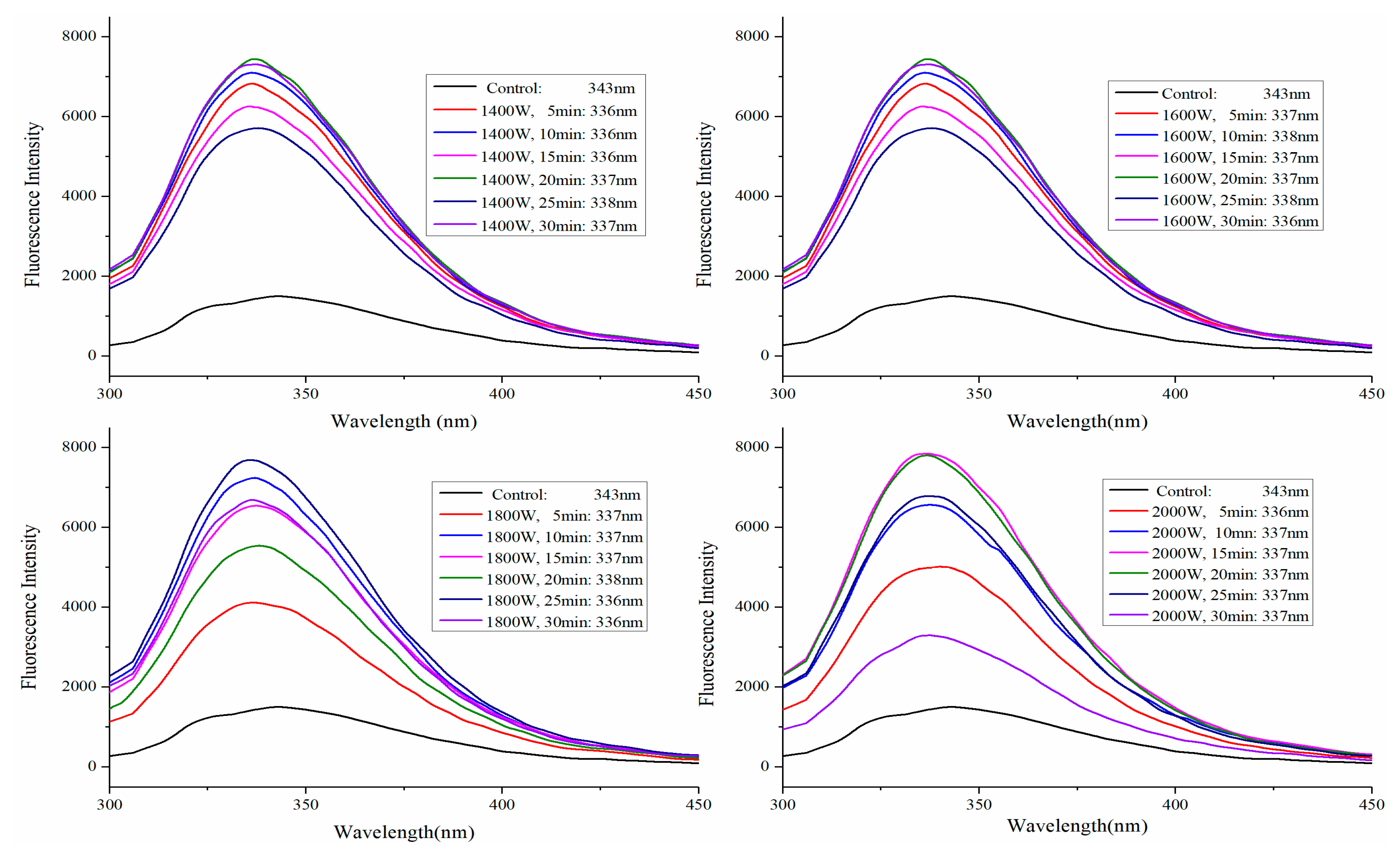
2.3. Fluorescence Spectra Analysis
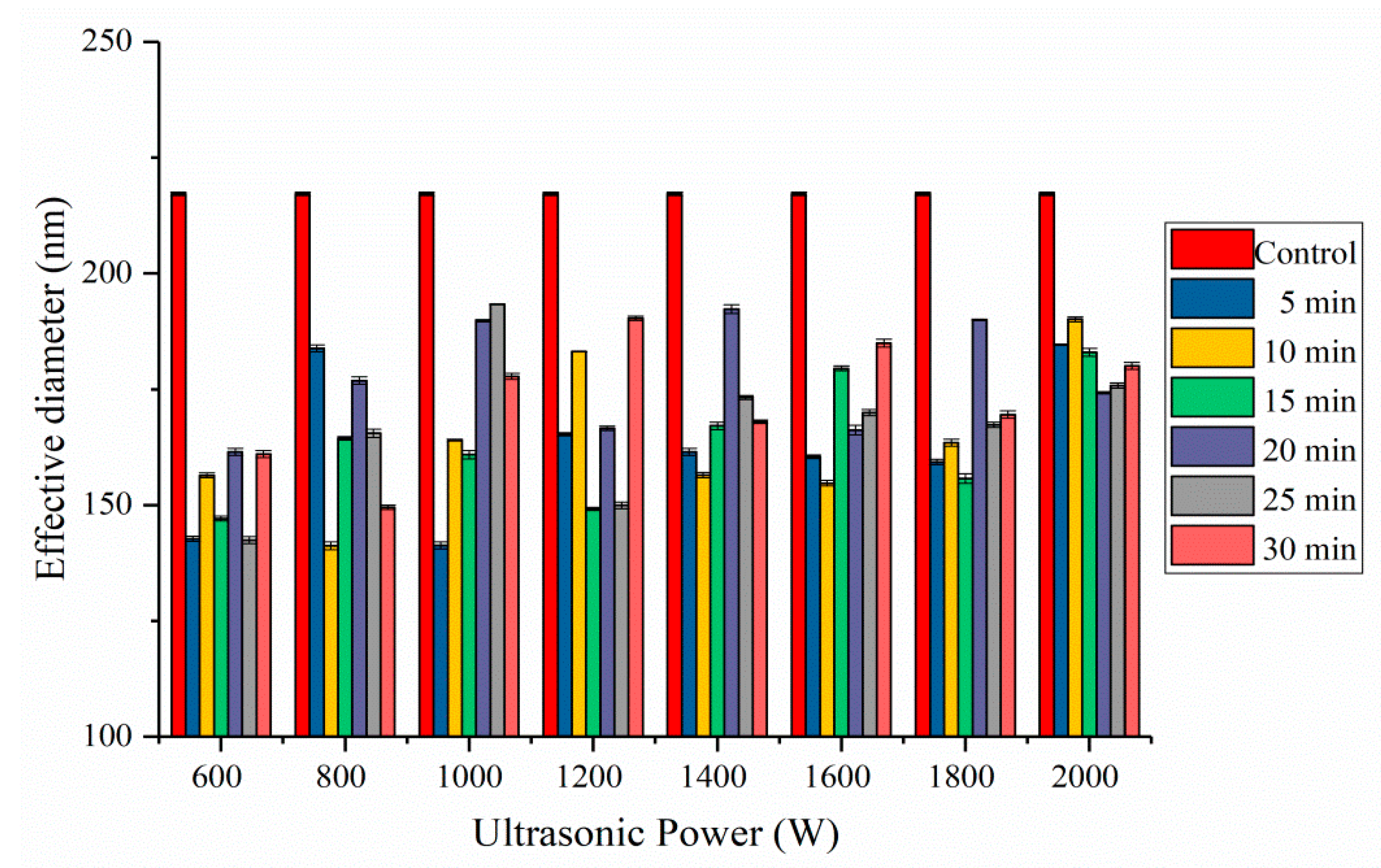
2.4. Effective Diameter Analysis
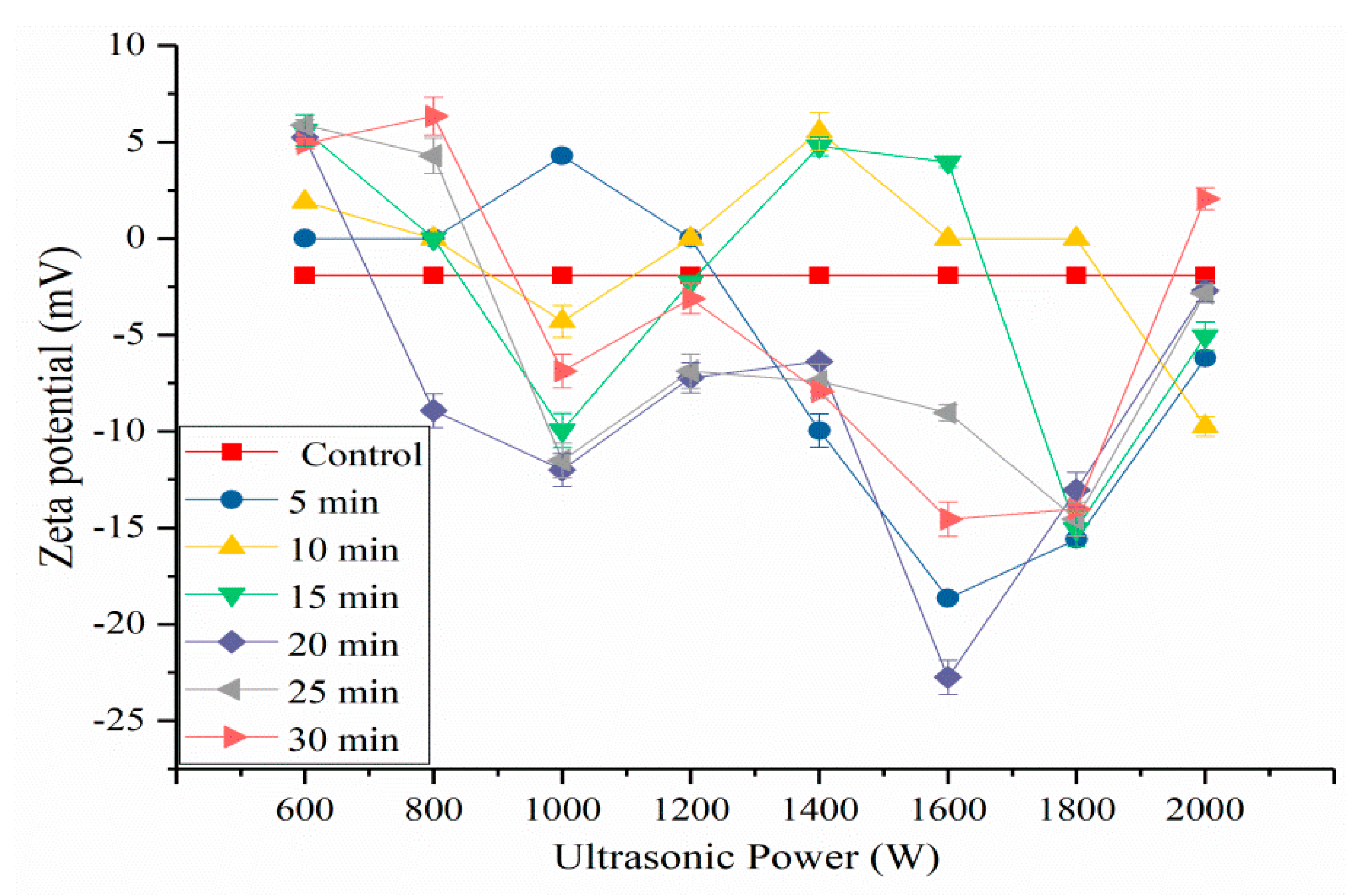
2.5. Effects of HIU Treatment on ζ-Potential of SPI
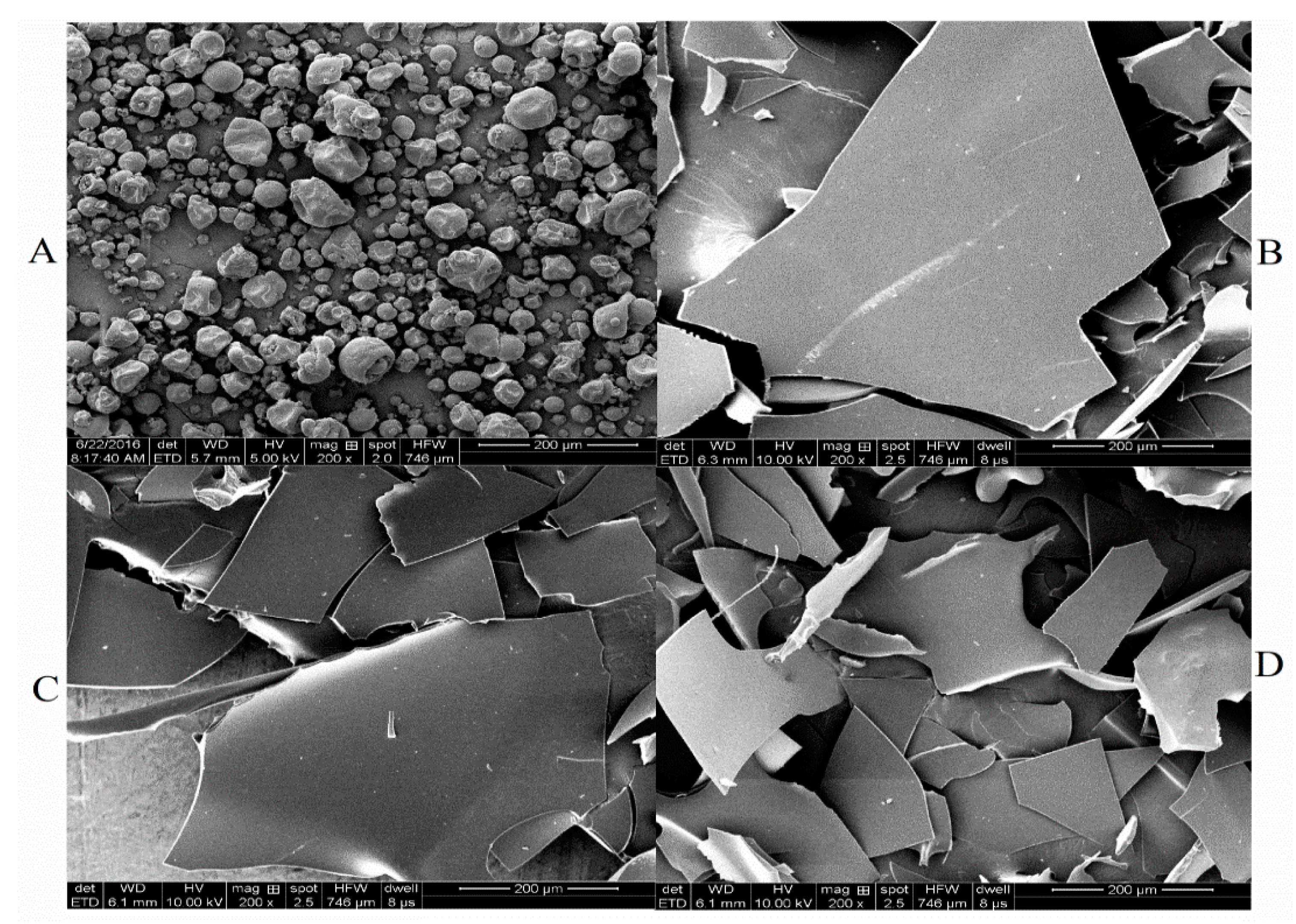
2.6. Scanning Electron Microscopy
2.7. Light Scattering Analysis
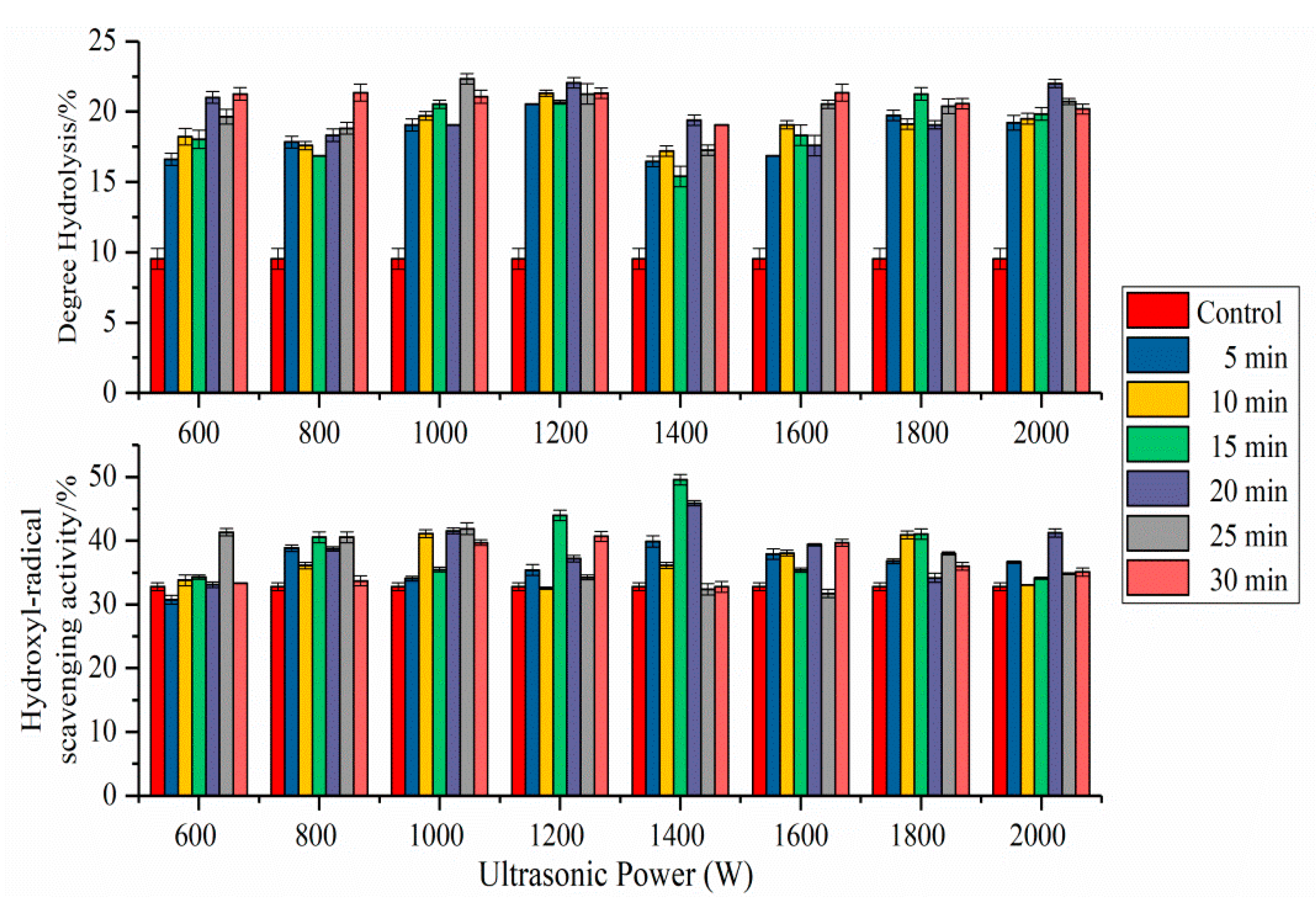
2.8. Antioxidant Activity of SPI Hydrolysates
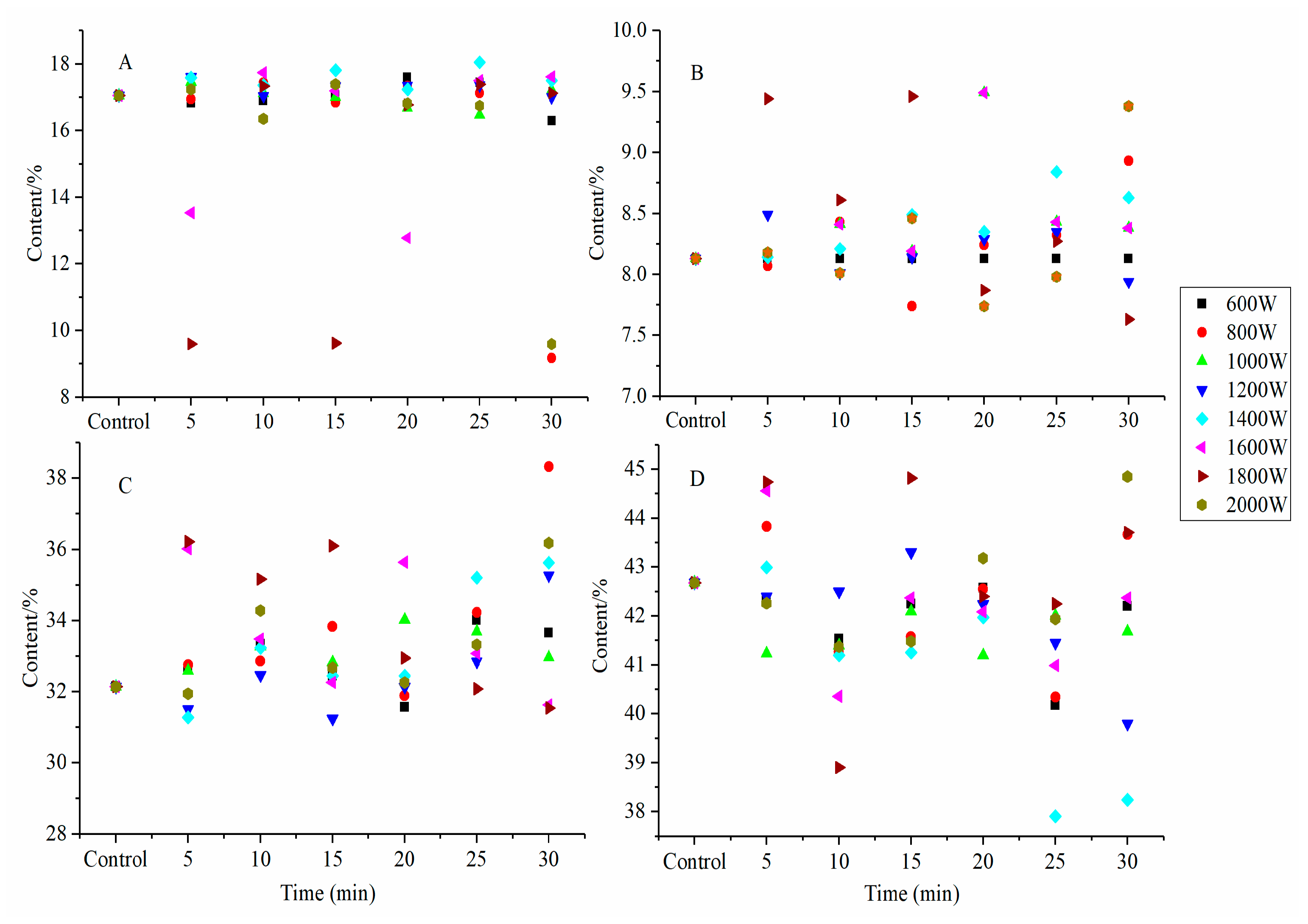
2.9. Degree of Hydrolysis of SPI Hydrolysates
3. Materials and Methods
3.1. Materials
3.2. High-Intensity Ultrasound Treatment of SPI
3.3. Near-Infrared Spectroscopy
3.4. Fourier-Transformed Infrared Spectroscopy
3.5. Fluorescence Measurements
3.6. Particle Size Determination
3.7. Zeta (ζ) Potential Measurements
3.8. Scanning Electron Microscopy
3.9. Light Scattering
3.10. Preparation of SPI Hydrolysates
3.11. Degree of Hydrolysis
3.12. Determination of Hydroxyl-Radical Scavenging Capacity
3.13. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, B.; Ren, J.; Zhao, M.; Luo, D.; Gu, L. Effects of limited enzymatic hydrolysis with pepsin and on the functional properties of soybean protein isolate. LWT Food Sci. Technol. 2012, 46, 453–459. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Melachouris, N. Functional properties of proteins in foods: A survey. Crit. Rev. Food Sci. Nutr. 1976, 7, 219–280. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, L.; Wei, D.; Li, Y.; Sui, X.; Wang, Z.; Li, D. Effect of Secondary Structure determined by FTIR Spectra on Surface Hydrophobicity of Soybean Protein Isolate. Procedia Eng. 2018, 15, 4819–4827. [Google Scholar] [CrossRef]
- Jia, J.; Ma, H.; Zhao, W.; Wang, Z.; Tian, W.; Luo, L.; He, R. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010, 119, 336–342. [Google Scholar] [CrossRef]
- Wang, B.; Meng, T.; Ma, H.; Zhang, Y.; Li, Y.; Jin, J.; Ye, X. Mechanism study of dual-frequency ultrasound assisted enzymolysis on rapeseed protein by immobilized Alcalase. Ultrason. Sonochem. 2016, 32, 307–313. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo Furtado, G.; Mantovani, R.A.; Consoli, L.; Hubinger, M.D.; da Cunha, R.L. Structural and emulsifying properties of sodium caseinate and lactoferrin influenced by ultrasound process. Food Hydrocoll. 2017, 63, 178–188. [Google Scholar] [CrossRef]
- Tatar, F.; Tunç, M.T.; Kahyaoglu, T. Turkish Tombul hazelnut (Corylus avellana L.) protein concentrates: Functional and rheological properties. J. Food Sci. Technol. 2015, 52, 1024–1031. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Lee, J.; Kentish, S.; Grieser, F. Bubbles in an acoustic field: An overview. Ultrason. Sonochemistry 2007, 14, 470–475. [Google Scholar] [CrossRef]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Li-Chan, E.C.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pa, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Handa, C.; Xu, J. Effects of ultrasound pre-treatment on the structure of β-conglycinin and glycinin and the antioxidant activity of their hydrolysates. Food Chem. 2017, 218, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, H.; Wang, B.; Qu, W.; Li, Y.; He, R.; Wali, A. Effects of ultrasound pretreatment on the enzymolysis and structural characterization of wheat gluten. Food Biophys. 2015, 10, 385–395. [Google Scholar] [CrossRef]
- Hu, H.; Cheung, I.W.; Pan, S.; Li-Chan, E.C. Effect of high intensity ultrasound on physicochemical and functional properties of aggregated soybean β-conglycinin and glycinin. Food Hydrocoll. 2015, 45, 102–110. [Google Scholar] [CrossRef]
- Izutsu, K.; Fujimaki, Y.; Kuwabara, A.; Hiyama, Y.; Yomota, C.; Aoyagi, N. Near-infrared analysis of protein secondary structure in aqueous solutions and freeze-dried solids. J. Pharm. Sci. 2006, 95, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Coello, J.; Iturriaga, H.; Maspoch, S.; de la Pezuela, C. Near-infrared spectroscopy in the pharmaceutical industry. Analyst 1998, 123, 135R–150R. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Devaux, M.F.; Mouhous, N.; Dufour, E. Monitoring the secondary structure of proteins by near-infrared spectroscopy. Appl. Spectrosc. 1999, 53, 226–232. [Google Scholar] [CrossRef]
- Bai, S.; Nayar, R.; Carpenter, J.F.; Manning, M.C. Noninvasive determination of protein conformation in the solid state using near infrared (NIR) spectroscopy. J. Pharm. Sci. 2005, 94, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Workman, J.; Weyer, J.L. Practical Guide to Interpretive Near-Infrared Spectroscopy, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 72–82. [Google Scholar]
- Jin, J.; Ma, H.; Wang, K.; Yagoub, A.; Owusu, J.; Qu, W.; He, R.; Zhou, C.; Ye, X. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015, 24, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Sonoyama, M. Second derivative near infrared studies on the structural characterisation of proteins. J. Near Infrared Spectrosc. 1998, 6, 253–257. [Google Scholar] [CrossRef]
- Weng, S.; Xu, Y.Z. Fourier Transform Infrared Spectroscopy, 3rd ed.; Chemistry Industry Press: Beijing, China, 2016; pp. 490–507. [Google Scholar]
- Zhou, M.; Liu, J.; Zhou, Y.; Huang, X.; Liu, F.; Pan, S.; Hu, H. Effect of high intensity ultrasound on physicochemical and functional properties of soybean glycinin at different ionic strengths. Innov. Food Sci. Emerg. Technol. 2016, 34, 205–213. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.; Yu, X.; Yagoub, A.; Zhang, Y.; Ma, H.; Gao, X.; Yarley, P. Heat and/or ultrasound pretreatments motivated enzymolysis of corn gluten meal: Hydrolysis kinetics and protein structure. LWT Food Sci. Technol. 2016, 77, 488–496. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Et Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ding, X.; Dai, C.; Ma, H. Changes in the structure and dissociation of soybean protein isolate induced by ultrasound-assisted acid pretreatment. Food Chem. 2017, 232, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Saini, C.S. Rheological and structural properties of protein isolates extracted from dephenolized sunflower meal: Effect of high intensity ultrasound. Food Hydrocoll. 2018, 81, 229–241. [Google Scholar] [CrossRef]
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of Acid Soluble Collagen and Pepsin Soluble Collagen from Scale of Spotted Golden Goatfish (Parupeneus heptacanthus). Food Chem. 2011, 129, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Decker, E.A.; McClements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties. Ultrason. Sonochem. 2017, 39, 511–519. [Google Scholar] [CrossRef]
- Pallares, I.; Vendrell, J.; Aviles, F.; Ventura, S. Amyloid fibril formation by a partially structured intermediate state of a-chymotrypsin. J. Mol. Biol. 2004, 342, 321–331. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: Effect on surface properties, foaming ability and structural changes. Ultrason. Sonochem. 2018, 44, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Wooster, T.; Ashokkumar, M.; Balachandran, S.; Mawson, R.; Simons, L. The use of ultrasonics for nanoemulsion preparation. Innov. Food Sci. Emerg. Technol. 2008, 9, 170–175. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, Y.; Han, J.; Chen, Q.; Kong, B. Structure-modification by moderate oxidation in hydroxyl radical-generating systems promote the emulsifying properties of soy protein isolate. Food Struct. 2015, 6, 21–28. [Google Scholar] [CrossRef]
- Bouzid, H.; Rabiller-Baudry, M.; Paugam, L.; Rousseau, F.; Derriche, Z.; Bettahar, N.E. Impact of zeta potential and size of caseins as precursors of fouling deposit on limiting and critical fluxes in spiral ultrafiltration of modified skim milks. J. Membr. Sci. 2008, 314, 67–75. [Google Scholar] [CrossRef]
- Song, X.; Zhou, C.; Fu, F.; Chen, Z.; Wu, Q. Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Ind. Crop. Prod. 2013, 43, 538–544. [Google Scholar] [CrossRef]
- Resendiz-Vazquez, J.A.; Ulloa, J.A.; Urías-Silvas, J.E.; Bautista-Rosales, P.U.; Ramírez-Ramírez, J.C.; Rosas-Ulloa, P.; González-Torres, L. Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate. Ultrason. Sonochem. 2017, 37, 436–444. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, D.F.; Li, X.Y.; Dong, H.Z. High-Pressure Homogenization Pretreatment before Enzymolysis of Soy Protein Isolate: The Effect of Pressure Level on Aggregation and Structural Conformations of the Protein. Molecules 2018, 23, 1775. [Google Scholar] [CrossRef]
- Tang, C.; Wang, X.; Yang, X.; Li, L. Formation of soluble aggregates from insoluble commercial soy protein isolate by means of ultrasonic treatment and their gelling properties. J. Food Eng. 2009, 92, 432–437. [Google Scholar] [CrossRef]
- Burchard, W. Light scattering techniques. In Physical Techniques for the Study of Food Biopolymers; Ross-Murphy, S.B., Ed.; Springer: Boston, MA, USA, 1994; pp. 151–213. [Google Scholar]
- Li, W.; Steve, W.; Wang, C.; Rickey, Y. Studies of aggregation behaviours of cereal β-glucans in dilute aqueous solutions by light scattering: Part, I., Structure effects. Food Hydrocoll. 2011, 25, 189–195. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Nakamura, A.; Burchard, W.; Ross Hallettd, F. Molecular characfterisation of soybean polysaccharides: An approach by size exclusion chromatography, dynamic and static light scattering methods. Carbohydr. Res. 2005, 340, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, S. Light Scattering from Polymer Solutions and Nanoparticle Dispersions Series; Springer: Boston, MA, USA, 2007. [Google Scholar]
- Wu, J.; Zhang, K.; Zhou, H.; Tang, S. Study on the preparation of soybean protein antioxidant peptides by ultrasonic assisted enzymatic method. Sci. Technol. Food Ind. 2012, 33, 212–219. [Google Scholar]
- Wang, Z.; Tian, W.; Zhao, X.; Zheng, J.; Cui, S. Effect of high hydrostatic pressure treatment on enzymolysis characteristics of thermodenatured rice protein. Food Sci. 2012, 33, 11–15. [Google Scholar]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzene sulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q. Hyroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Entry | Mw (g/mol) | Rg (nm) | Rh (nm) | A2 | ρ | Structure |
|---|---|---|---|---|---|---|
| Control | 2.89 ± 0.17 × 106 | 96 ± 5.4 | 122.97 | −3.28 ± 0.6 × 10−4 | 0.78 | Hard-sphere |
| 600 W-5 min | 2.57 ± 0.39 × 108 | 100 ± 18 | 95.99 | 8 ± 3.8 × 10−7 | 1.04 | Hollow-sphere |
| 600 W-15 min | 8.2 ± 0.33 × 107 | 93 ± 4.8 | 89.86 | 4.31 ± 0.8 × 10−6 | 1.03 | Hollow-sphere |
| 600 W-30 min | 3.11 ± 0.2 × 108 | 96.1 ± 7.4 | 90.63 | 4.23 ± 0.38 × 10−6 | 1.06 | Hollow-sphere |
| 800 W-5 min | 5.44 ± 0.22 × 107 | 97.5 ± 4.7 | 123.56 | 4.7 ± 1.3 × 10−7 | 0.79 | Hard-sphere |
| 800 W-15 min | 1.41 ± 0.26 × 107 | 166 ± 20 | 121.53 | 1.3 ± 0.81 × 10−5 | 1.37 | GCM (θ-solvent) |
| 800 W-30 min | 3.2 ± 1.2 × 107 | 224 ± 48 | 123.77 | 3.6 ± 1.3 × 10−5 | 1.81 | GCM (good-solvent) |
| 1000 W-5 min | 1.05 ± 0.17 × 107 | 95 ± 18 | 94.36 | 8.5 ± 1.1 × 10−7 | 1.01 | Hollow-sphere |
| 1000 W-15 min | 1.90 ± 0.32 × 107 | 157 ± 20 | 123.76 | 9.46 ± 0.8 × 10−7 | 1.27 | GCM (θ-solvent) |
| 1000 W-30 min | 2.59 ± 0.51 × 107 | 198 ± 24 | 137.34 | 9 ± 10 × 10−6 | 1.44 | GCM (θ-solvent) |
| 1200 W-5 min | 1.12 ± 0.54 × 107 | 300 ± 79 | 142.8 | 7 ± 4.1 × 10−5 | 2.1 | GCP (good-solvent) |
| 1200 W-15 min | 1.76 ± 0.27 × 107 | 178 ± 18 | 119.49 | 1.7 ± 1.3 × 10−5 | 1.49 | GCM (θ-solvent) |
| 1200 W-30 min | 2.01 ± 0.53 × 107 | 214 ± 33 | 138.21 | 1.31 ± 0.85 × 10−5 | 1.55 | GCM (θ-solvent) |
| 1400 W-5 min | 1.51 ± 0.16 × 107 | 136 ± 10 | 115.42 | 1.5 ± 0.98 × 10−5 | 1.18 | Hollow-sphere |
| 1400 W-15 min | 2.49 ± 0.26 × 107 | 159 ± 11 | 124.75 | 1.1 ± 0.43 × 10−5 | 1.27 | GCM (θ-solvent) |
| 1400 W-30 min | 5.7 ± 1.5 × 107 | 213 ± 32 | 129.22 | 2.51 ± 0.26 × 10−5 | 1.65 | GCP (θ-solvent) |
| 1600 W-5 min | 6.9 ± 1.8 × 106 | 169 ± 28 | 128.97 | 1.3 ± 5 × 10−5 | 1.31 | GCM (θ-solvent) |
| 1600 W-15 min | 2.3 ± 1 × 107 | 224 ± 58 | 137.19 | 3.8 ± 1.6 × 10−5 | 1.63 | GCP (θ-solvent) |
| 1600 W-30 min | 2.52 ± 0.44 × 107 | 200 ± 21 | 133.82 | 1.41 ± 0.58 × 10−5 | 1.49 | GCM (θ-solvent) |
| 1800 W-5 min | 7.8 ± 1.2 × 106 | 148 ± 16 | 125.61 | 8 ± 120 × 10−7 | 1.18 | Hollow-sphere |
| 1800 W-15 min | 1.62 ± 0.31 × 107 | 209 ± 24 | 130.15 | 3.2 ± 1.4 × 10−5 | 1.61 | GCP (θ-solvent) |
| 1800 W-30 min | 3.32 ± 0.95 × 107 | 223 ± 37 | 129.97 | 2.29 ± 0.65 × 10−5 | 1.72 | GCP (θ-solvent) |
| 2000 W-5 min | 3.32 ± 0.57 × 106 | 120 ± 17 | 129.56 | −5.6 ± 3 × 10−5 | 0.93 | Hollow-sphere |
| 2000 W-15 min | 7 ± 2.2 × 106 | 173 ± 36 | 137.78 | −1.1 ± 1.8 × 10−5 | 1.26 | GCM (θ-solvent) |
| 2000 W-30 min | 2.67 ± 0.74 × 107 | 227 ± 36 | 138.9 | 1.5 ± 0.65 × 10−5 | 1.63 | GCP (θ-solvent) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Liu, X.; Ding, X.; Dong, H.; Wang, W. Effects of High-Intensity Ultrasound Pretreatment on Structure, Properties, and Enzymolysis of Soy Protein Isolate. Molecules 2019, 24, 3637. https://doi.org/10.3390/molecules24203637
Zhao F, Liu X, Ding X, Dong H, Wang W. Effects of High-Intensity Ultrasound Pretreatment on Structure, Properties, and Enzymolysis of Soy Protein Isolate. Molecules. 2019; 24(20):3637. https://doi.org/10.3390/molecules24203637
Chicago/Turabian StyleZhao, Fei, Xuemei Liu, Xiuzhen Ding, Haizhou Dong, and Wentao Wang. 2019. "Effects of High-Intensity Ultrasound Pretreatment on Structure, Properties, and Enzymolysis of Soy Protein Isolate" Molecules 24, no. 20: 3637. https://doi.org/10.3390/molecules24203637
APA StyleZhao, F., Liu, X., Ding, X., Dong, H., & Wang, W. (2019). Effects of High-Intensity Ultrasound Pretreatment on Structure, Properties, and Enzymolysis of Soy Protein Isolate. Molecules, 24(20), 3637. https://doi.org/10.3390/molecules24203637





