Protective Effects of Astragaloside IV against LPS-Induced Endometritis in Mice through Inhibiting Activation of the NF-κB, p38 and JNK Signaling Pathways
Abstract
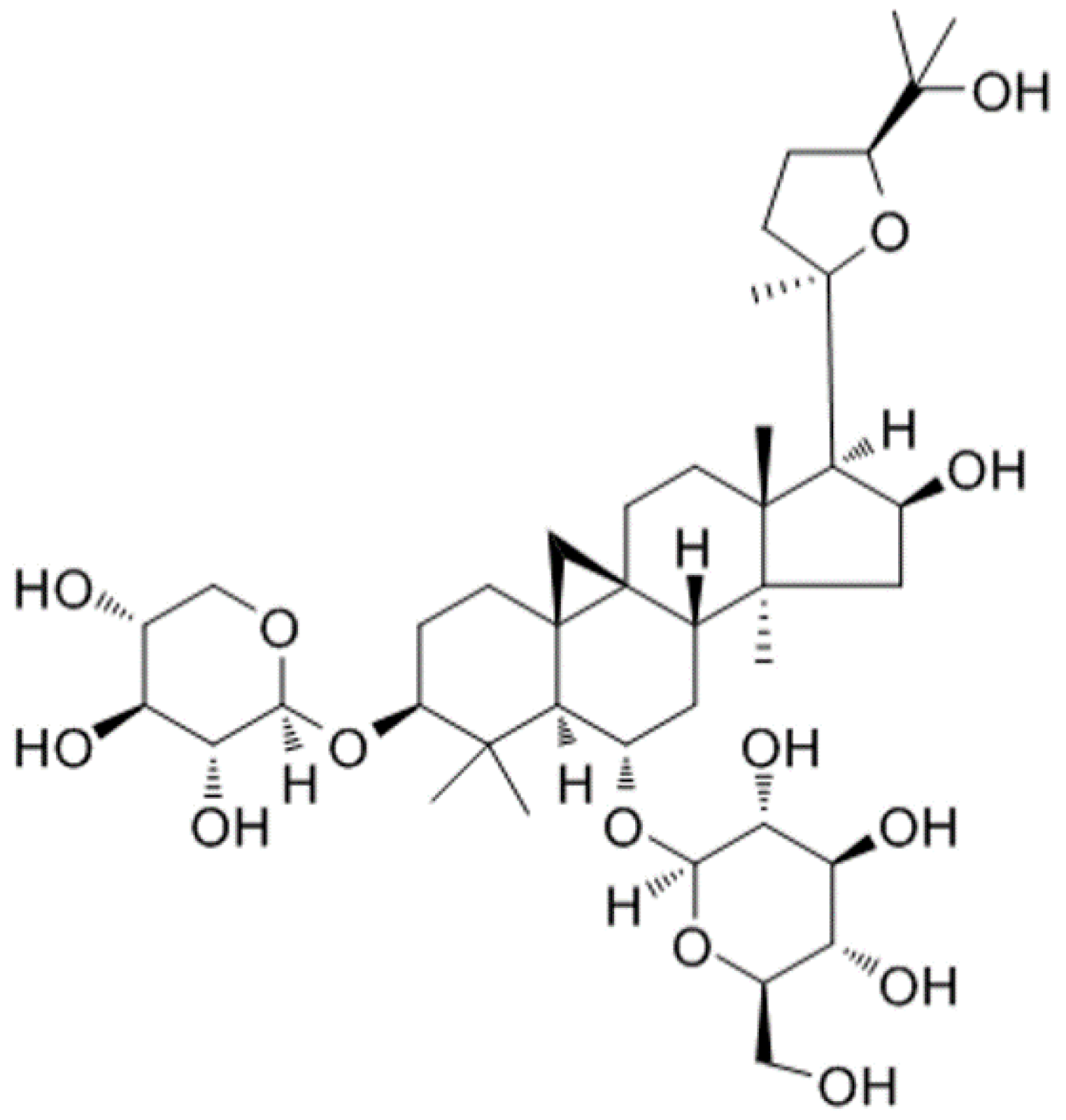
1. Introduction
2. Results
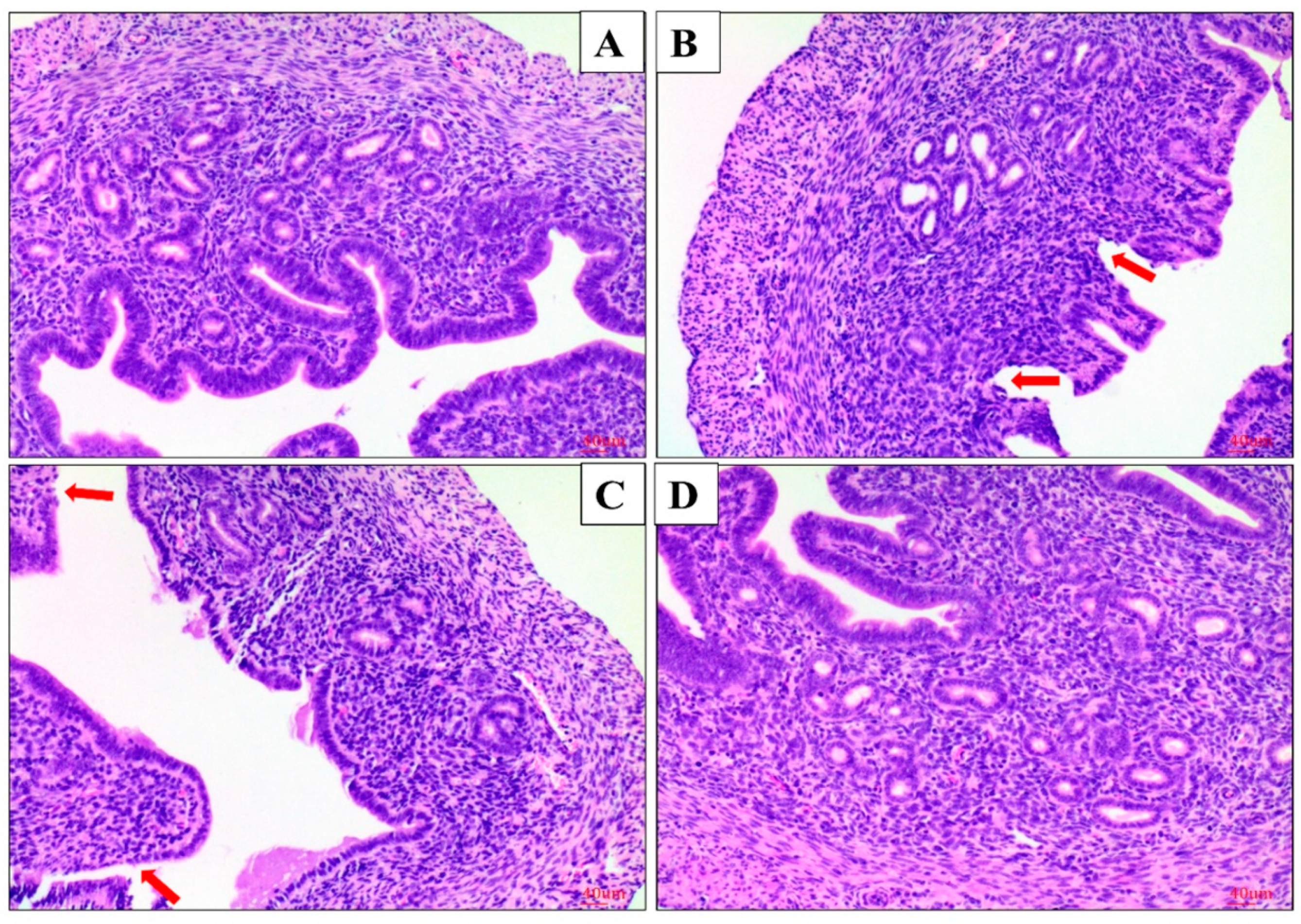
2.1. Effect of AS IV on LPS-Induced Histopathological Changes of Uterus
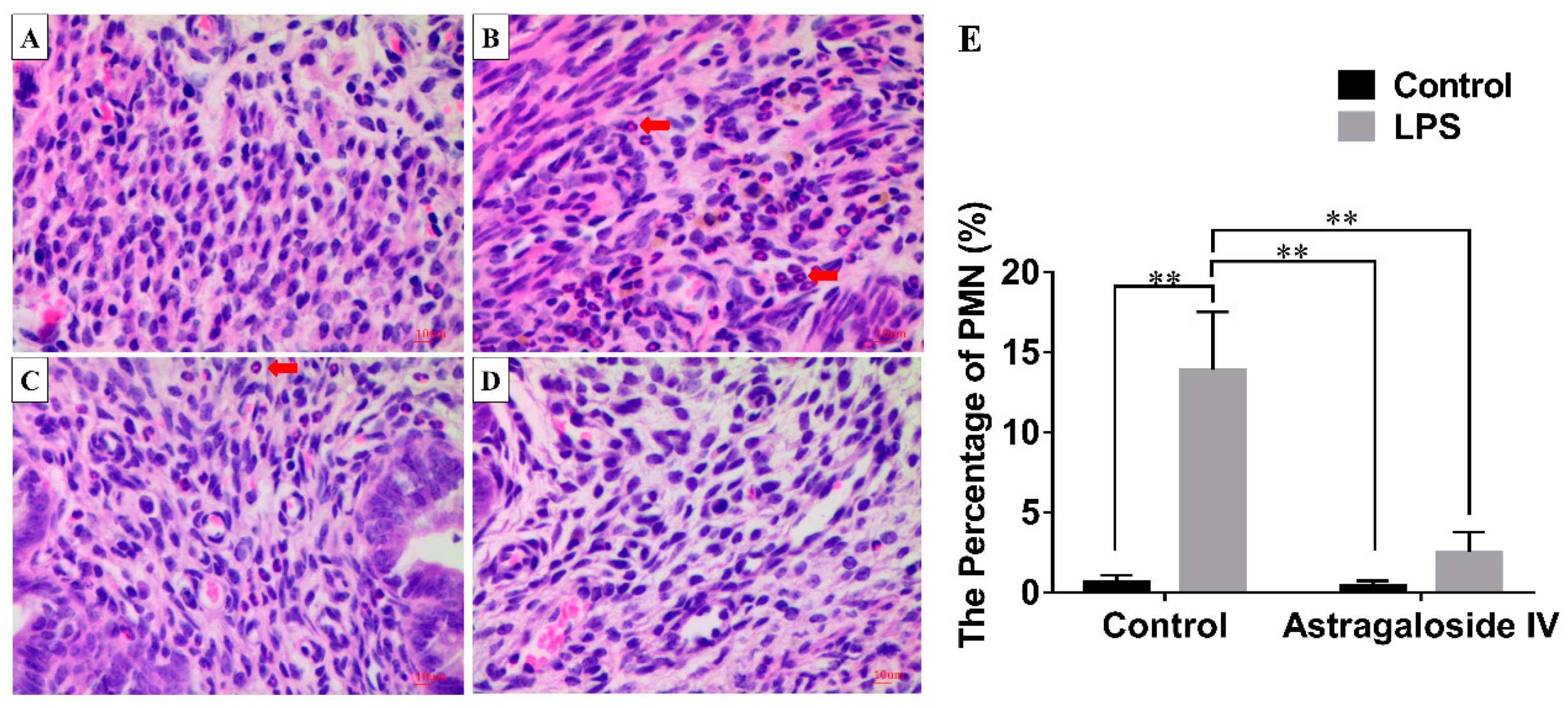
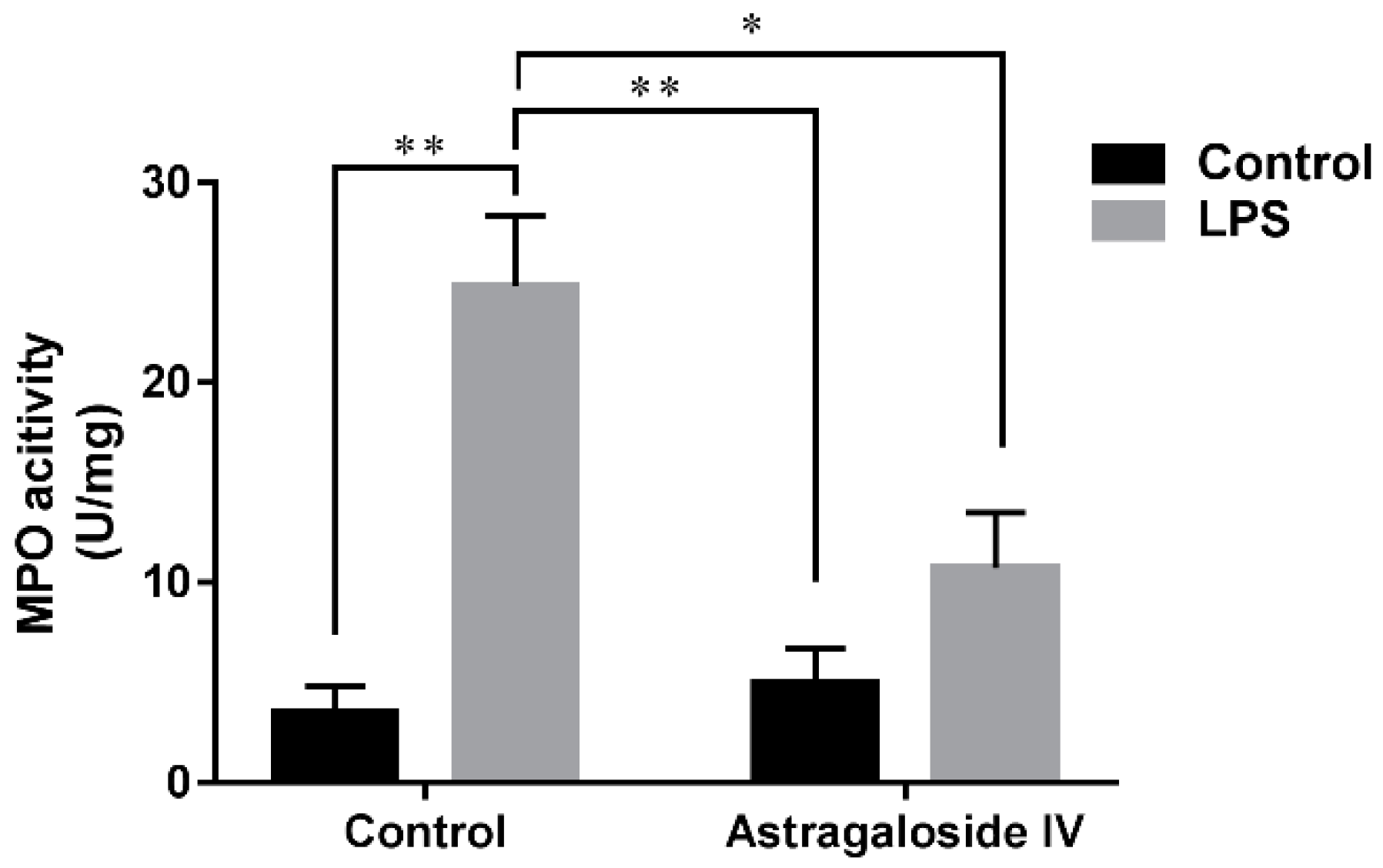
2.2. Effect of AS IV on Myeloperoxidase (MPO) Activity in LPS-Induced Uterus
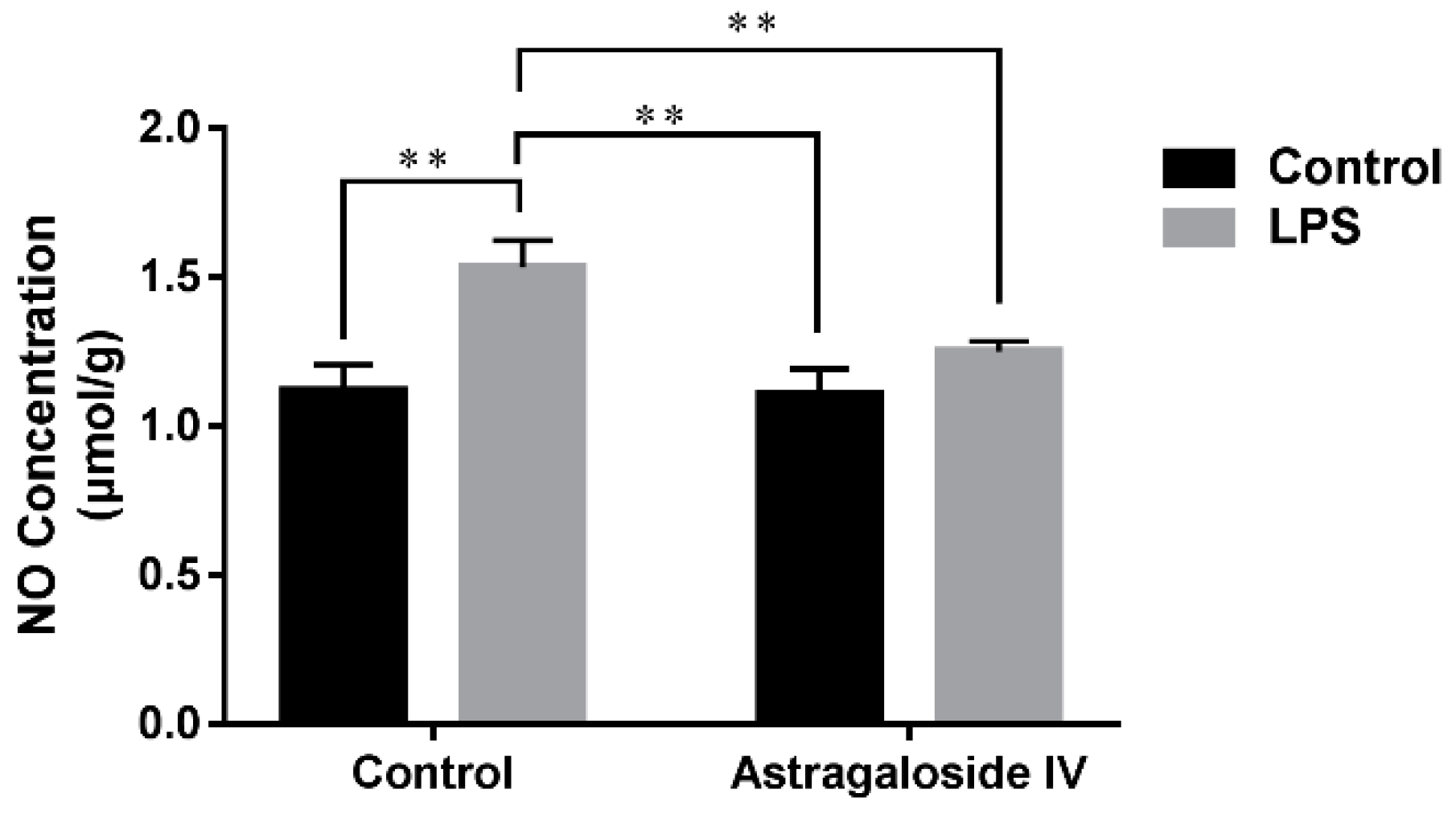
2.3. Effect of AS IV on Concentration of NO in LPS-Induced Uteri
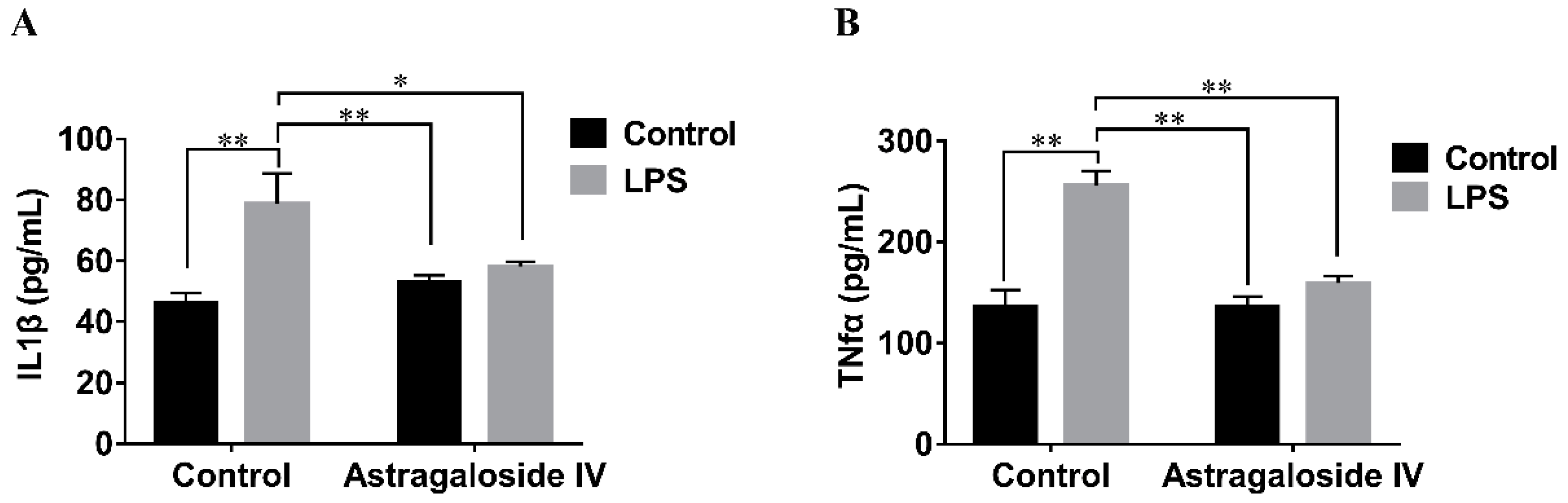
2.4. Effect of AS IV on LPS-Induced IL-1β and TNF-α Production in Mouse Uteri
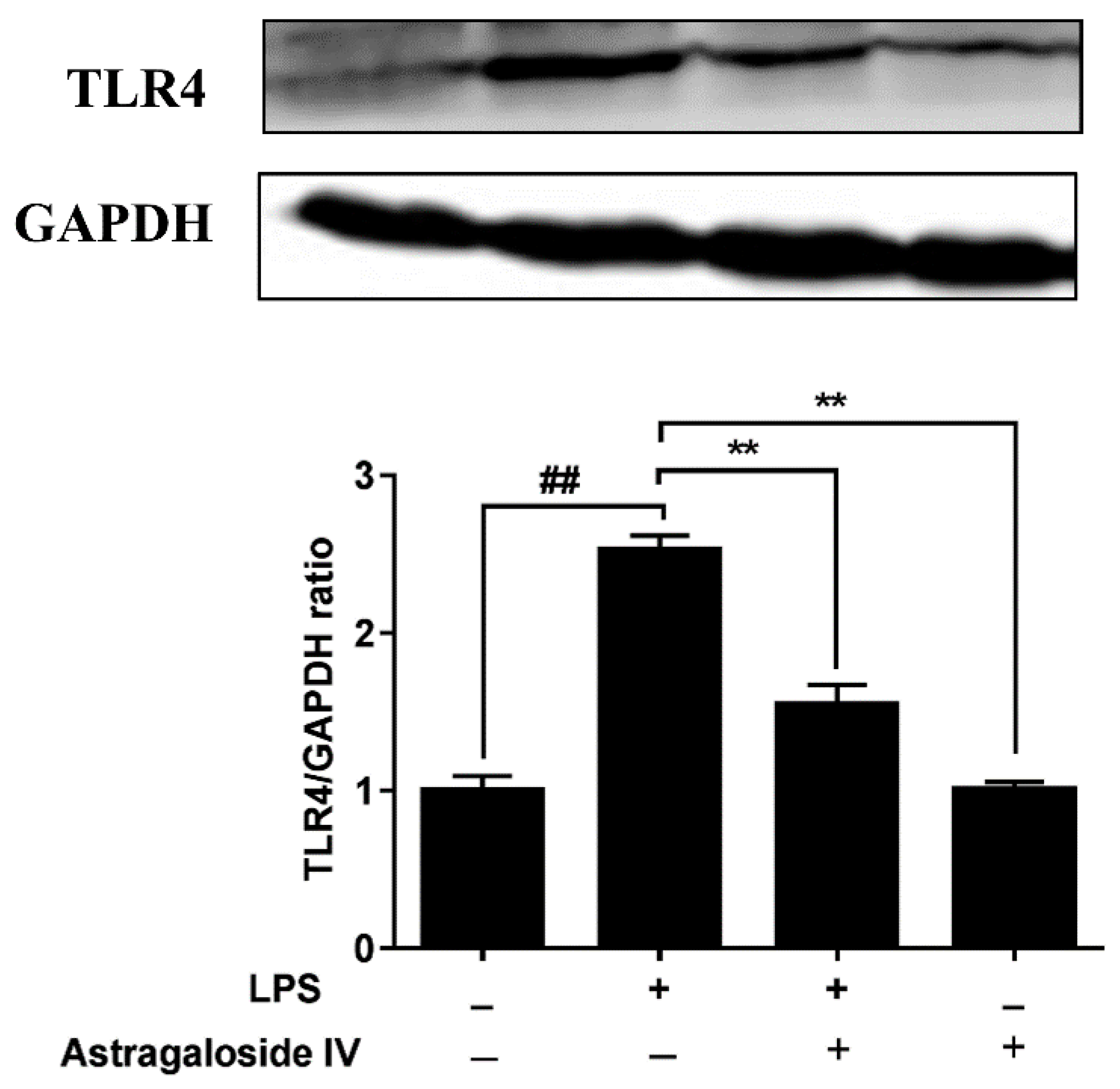
2.5. AS IV on LPS-Induced Activation of TLR4 and the NF-κB Signal Pathway
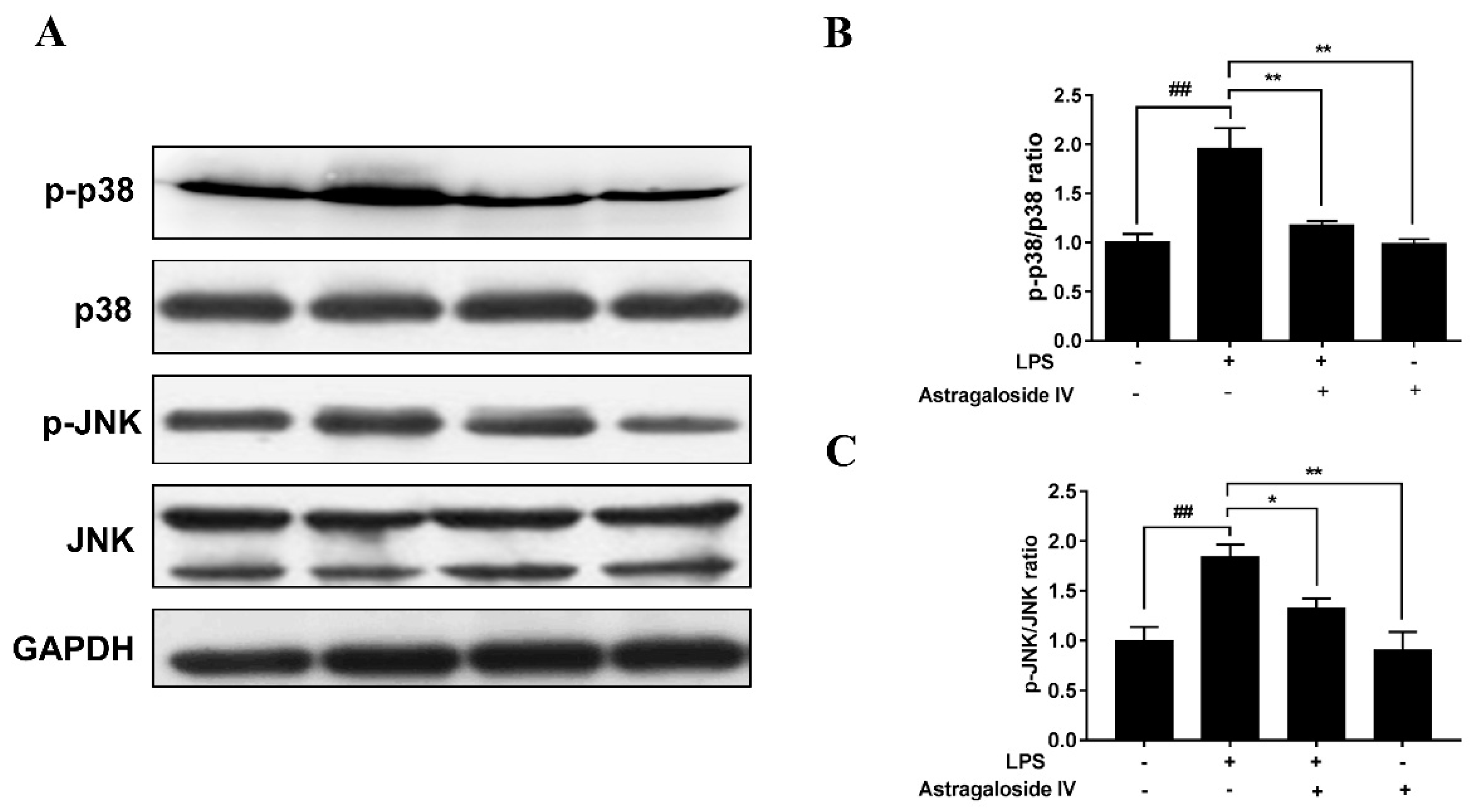
2.6. AS IV on LPS-Induced Activation of p38 and JNK Signal Pathways
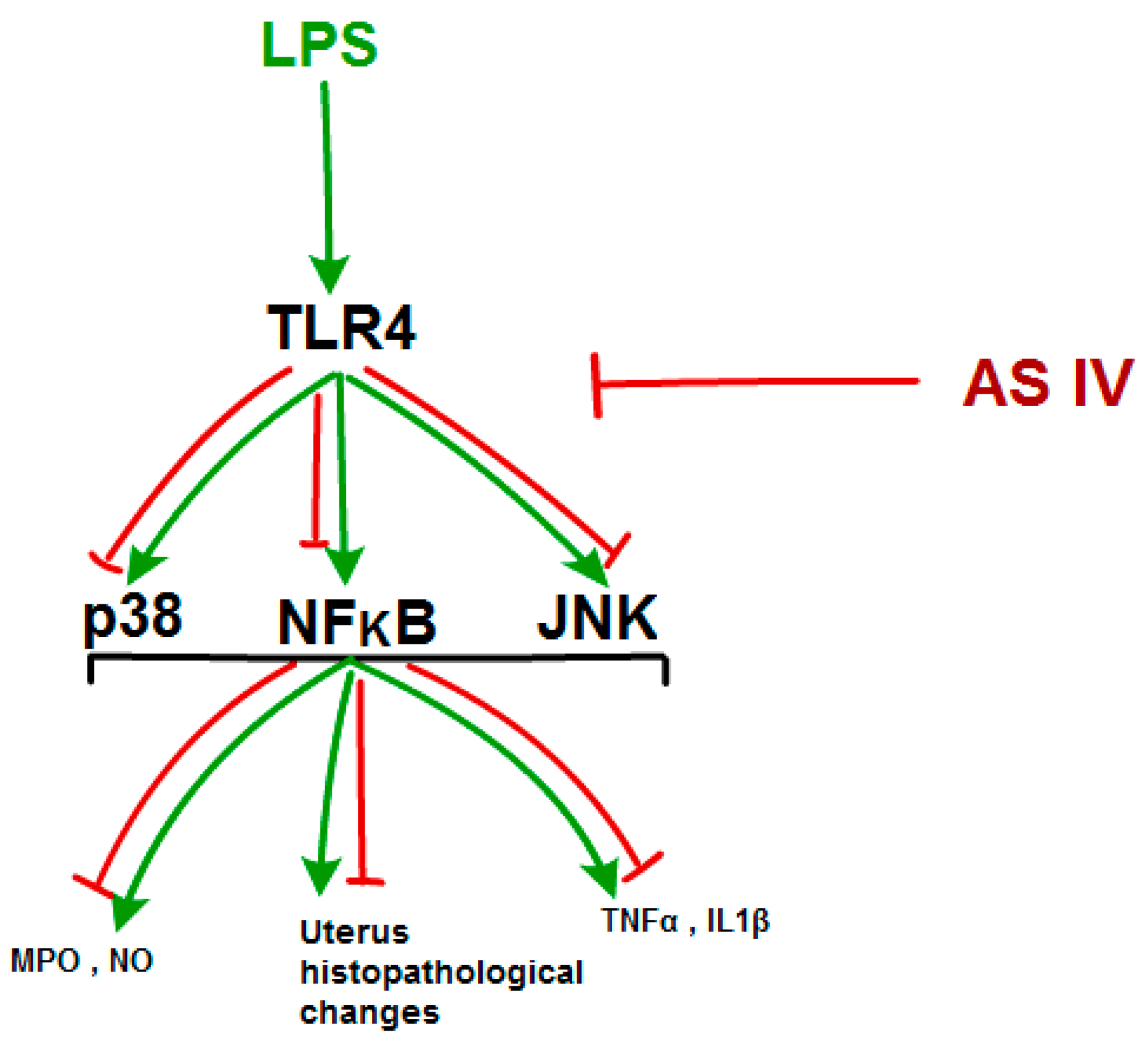
3. Discussion
4. Materials and Methods
4.1. Chemical and Animal Experiments
4.2. Histological Assay
4.3. MPO Assay
4.4. NO Assay
4.5. Enzyme-Linked Immunosorbent Assays
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AS IV | Astragaloside IV |
| MPO | Myeloperoxidase |
| LPS | Lipopolysaccharide |
References
- Gautam, G.; Nakao, T.; Yusuf, M.; Koike, K. Prevalence of endometritis during the postpartum period and its impact on subsequent reproductive performance in two Japanese dairy herds. Anim. Reprod. Sci. 2009, 116, 175–187. [Google Scholar] [CrossRef]
- Lewis, G.S. Uterine health and disorders. J. Dairy Sci. 1997, 80, 984–994. [Google Scholar] [CrossRef]
- Doerrler, W.T. Lipid trafficking to the outer membrane of Gram-negative bacteria. Mol. Microbiol. 2006, 60, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Karkhanis, Y.D.; Zeltner, J.Y.; Jackson, J.J.; Carlo, D.J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal. Biochem. 1978, 85, 595–601. [Google Scholar] [CrossRef]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- Lv, X.; Fu, K.; Li, W.; Wang, Y.; Wang, J.; Li, H.; Tian, W.; Cao, R. TIIA attenuates LPS-induced mouse endometritis by suppressing the NF-kappaB signaling pathway. Can. J. Physiol. Pharmacol. 2015, 93, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Hajibemani, A.; Mirzaei, A.; Rowshan Ghasrodashti, A.; Memarzadeh, M.R. The effect of Zataria multiflora extract on the clinical endometritis and reproductive indices in lactating Holstein dairy cows. Vet. Res. Forum Int. Q. J. 2016, 7, 309–315. [Google Scholar]
- Kalayou, S.; Haileselassie, M.; Gebre-Egziabher, G.; Tiku’e, T.; Sahle, S.; Taddele, H.; Ghezu, M. In-vitro antimicrobial activity screening of some ethnoveterinary medicinal plants traditionally used against mastitis, wound and gastrointestinal tract complication in Tigray Region, Ethiopia. Asian Pac. J. Trop. Biomed. 2012, 2, 516–522. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Xie, D.; Chen, Y.X.; Zhang, H.Y.; Xia, Z.X. Protective effect of Gui Qi mixture on the progression of diabetic nephropathy in rats. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2006, 114, 563–568. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Zhao, J. Preparation and suppressive effect of astragalus polysaccharide in glomerulonephritis rats. Int. Immunopharmacol. 2007, 7, 23–28. [Google Scholar] [CrossRef]
- Cho, W.C.; Leung, K.N. In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J. Ethnopharmacol. 2007, 113, 132–141. [Google Scholar] [CrossRef]
- Zhang, W.J.; Hufnagl, P.; Binder, B.R.; Wojta, J. Antiinflammatory activity of astragaloside IV is mediated by inhibition of NF-kappaB activation and adhesion molecule expression. Thromb. Haemost. 2003, 90, 904–914. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, Y.; Wu, H.; Zhu, X.; Zheng, X.; Jiang, S.; Zhuo, H.; Shen, J.; Li, L.; Qiu, J. Protective effects of Astragalus saponin I on early stage of diabetic nephropathy in rats. J. Pharmacol. Sci. 2004, 95, 256–266. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, C.; Wang, X.H.; Gao, P.J.; Zhu, D.L.; Chen, H.; Liu, R.H.; Li, H.L. Astragaloside IV dilates aortic vessels from normal and spontaneously hypertensive rats through endothelium-dependent and endothelium-independent ways. Planta Medica 2006, 72, 621–626. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Y.; Zhang, X. Astragaloside IV Alleviates Lipopolysaccharide-Induced Acute Kidney Injury Through Down-Regulating Cytokines, CCR5 and p-ERK, and Elevating Anti-Oxidative Ability. Med. Sci. Monit. Int. Med J. Exp. Clin. Res. 2017, 23, 1413–1420. [Google Scholar] [CrossRef]
- Kasius, J.C.; Fatemi, H.M.; Bourgain, C.; Sie-Go, D.M.; Eijkemans, R.J.; Fauser, B.C.; Devroey, P.; Broekmans, F.J. The impact of chronic endometritis on reproductive outcome. Fertil. Steril. 2011, 96, 1451–1456. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Huang, H.; Gao, W.; Zhuang, C.; Li, B.; Zhou, P.; Kong, D. Anti-hepatitis B virus activities of astragaloside IV isolated from radix Astragali. Biol. Pharm. Bull. 2009, 32, 132–135. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Sun, S.; Shen, J.; Qiu, J.; Yin, X.; Yin, H.; Jiang, S. Inhibitory effects of astragaloside IV on diabetic peripheral neuropathy in rats. Can. J. Physiol. Pharmacol. 2006, 84, 579–587. [Google Scholar] [CrossRef]
- Li, Z.P.; Cao, Q. Effects of astragaloside IV on myocardial calcium transport and cardiac function in ischemic rats. Acta Pharmacol. Sin. 2002, 23, 898–904. [Google Scholar]
- Reumaux, D.; de Boer, M.; Meijer, A.B.; Duthilleul, P.; Roos, D. Expression of myeloperoxidase (MPO) by neutrophils is necessary for their activation by anti-neutrophil cytoplasm autoantibodies (ANCA) against MPO. J. Leukoc. Biol. 2003, 73, 841–849. [Google Scholar] [CrossRef]
- Gunnett, C.A.; Lund, D.D.; McDowell, A.K.; Faraci, F.M.; Heistad, D.D. Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arter. Thromb. Vasc. Biol. 2005, 25, 1617–1622. [Google Scholar] [CrossRef]
- Neurath, M.F.; Becker, C.; Barbulescu, K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut 1998, 43, 856–860. [Google Scholar] [CrossRef]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef]
- Herlaar, E.; Brown, Z. p38 MAPK signalling cascades in inflammatory disease. Mol. Med. Today 1999, 5, 439–447. [Google Scholar] [CrossRef]
- Ip, Y.T.; Davis, R.J. Signal transduction by the c-Jun N-terminal kinase (JNK)—From inflammation to development. Curr. Opin. Cell Biol. 1998, 10, 205–219. [Google Scholar] [CrossRef]
- Sheldon, I.M. Metabolic stress and endometritis in dairy cattle. Vet. Rec. 2018, 183, 124–125. [Google Scholar] [CrossRef]
- Jiang, P.Y.; Zhu, X.J.; Zhang, Y.N.; Zhou, F.F.; Yang, X.F. Protective effects of apigenin on LPS-induced endometritis via activating Nrf2 signaling pathway. Microb. Pathog. 2018, 123, 139–143. [Google Scholar] [CrossRef]
- Matteo, M.; Cicinelli, E.; Greco, P.; Massenzio, F.; Baldini, D.; Falagario, T.; Rosenberg, P.; Castellana, L.; Specchia, G.; Liso, A. Abnormal pattern of lymphocyte subpopulations in the endometrium of infertile women with chronic endometritis. Am. J. Reprod. Immunol. 2009, 61, 322–329. [Google Scholar] [CrossRef]
- Brodzki, P.; Kostro, K.; Brodzki, A.; Wawron, W.; Marczuk, J.; Kurek, L. Inflammatory cytokines and acute-phase proteins concentrations in the peripheral blood and uterus of cows that developed endometritis during early postpartum. Theriogenology 2015, 84, 11–18. [Google Scholar] [CrossRef]
- Giebelen, I.A.; van Westerloo, D.J.; LaRosa, G.J.; de Vos, A.F.; van der Poll, T. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock 2007, 28, 700–703. [Google Scholar]
- Gitter, A.H.; Bendfeldt, K.; Schulzke, J.D.; Fromm, M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000, 14, 1749–1753. [Google Scholar]
- Weinstein, J.A.; Taylor, J.M. Interleukin-1 and the acute-phase response: Induction of mouse liver serum amyloid A mRNA by murine recombinant interleukin-1. J. Trauma 1987, 27, 1227–1232. [Google Scholar] [CrossRef]
- Gnainsky, Y.; Granot, I.; Aldo, P.B.; Barash, A.; Or, Y.; Schechtman, E.; Mor, G.; Dekel, N. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil. Steril. 2010, 94, 2030–2036. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Wang, J.; Guo, J.; Li, Y.; Cao, Y.; Zhang, N.; Fu, Y. Melatonin inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in lipopolysaccharide-induced endometritis in mice. Int. Immunopharmacol. 2018, 64, 101–109. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Chen, S.; Deng, L.; Chen, L.; Huang, Y.; Tian, M.; Li, C.; Zhou, X. Protective Effects of Astragaloside IV against LPS-Induced Endometritis in Mice through Inhibiting Activation of the NF-κB, p38 and JNK Signaling Pathways. Molecules 2019, 24, 373. https://doi.org/10.3390/molecules24020373
Wang F, Chen S, Deng L, Chen L, Huang Y, Tian M, Li C, Zhou X. Protective Effects of Astragaloside IV against LPS-Induced Endometritis in Mice through Inhibiting Activation of the NF-κB, p38 and JNK Signaling Pathways. Molecules. 2019; 24(2):373. https://doi.org/10.3390/molecules24020373
Chicago/Turabian StyleWang, Fengge, Shuxiong Chen, Liang Deng, Lu Chen, Yuwen Huang, Meng Tian, Chunjin Li, and Xu Zhou. 2019. "Protective Effects of Astragaloside IV against LPS-Induced Endometritis in Mice through Inhibiting Activation of the NF-κB, p38 and JNK Signaling Pathways" Molecules 24, no. 2: 373. https://doi.org/10.3390/molecules24020373
APA StyleWang, F., Chen, S., Deng, L., Chen, L., Huang, Y., Tian, M., Li, C., & Zhou, X. (2019). Protective Effects of Astragaloside IV against LPS-Induced Endometritis in Mice through Inhibiting Activation of the NF-κB, p38 and JNK Signaling Pathways. Molecules, 24(2), 373. https://doi.org/10.3390/molecules24020373





