Anion-Binding-Induced Electrochemical Signal Transduction in Ferrocenylimidazolium: Combined Electrochemical Experimental and Theoretical Investigation
Abstract
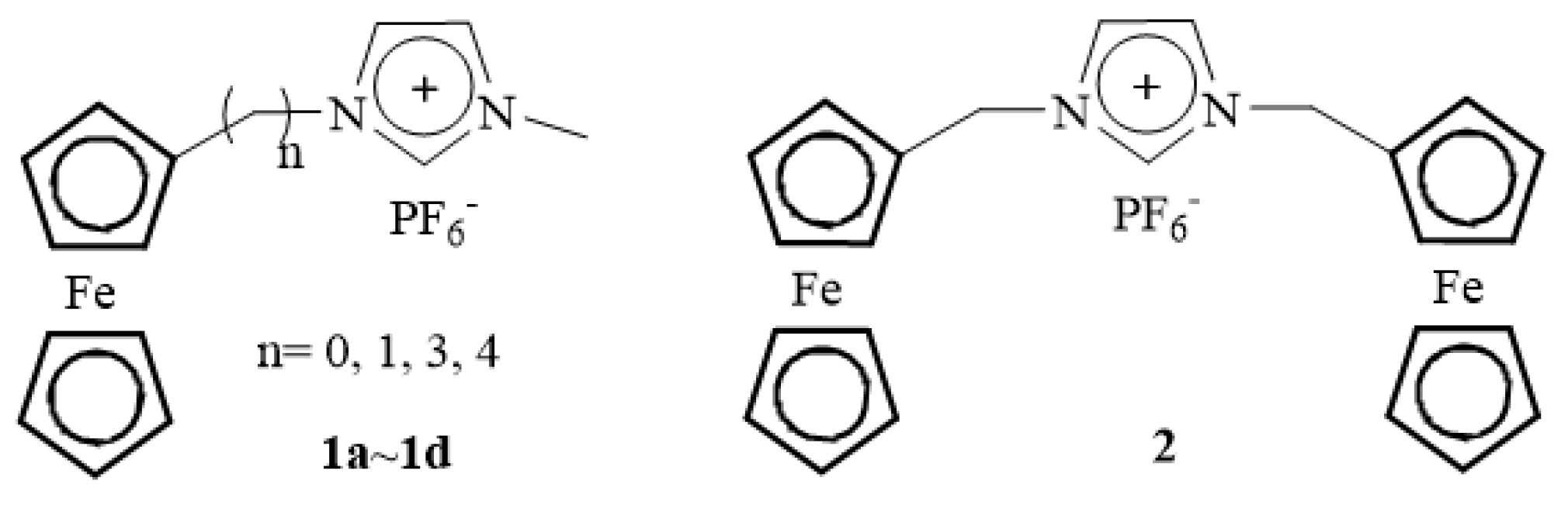
1. Introduction
2. Results
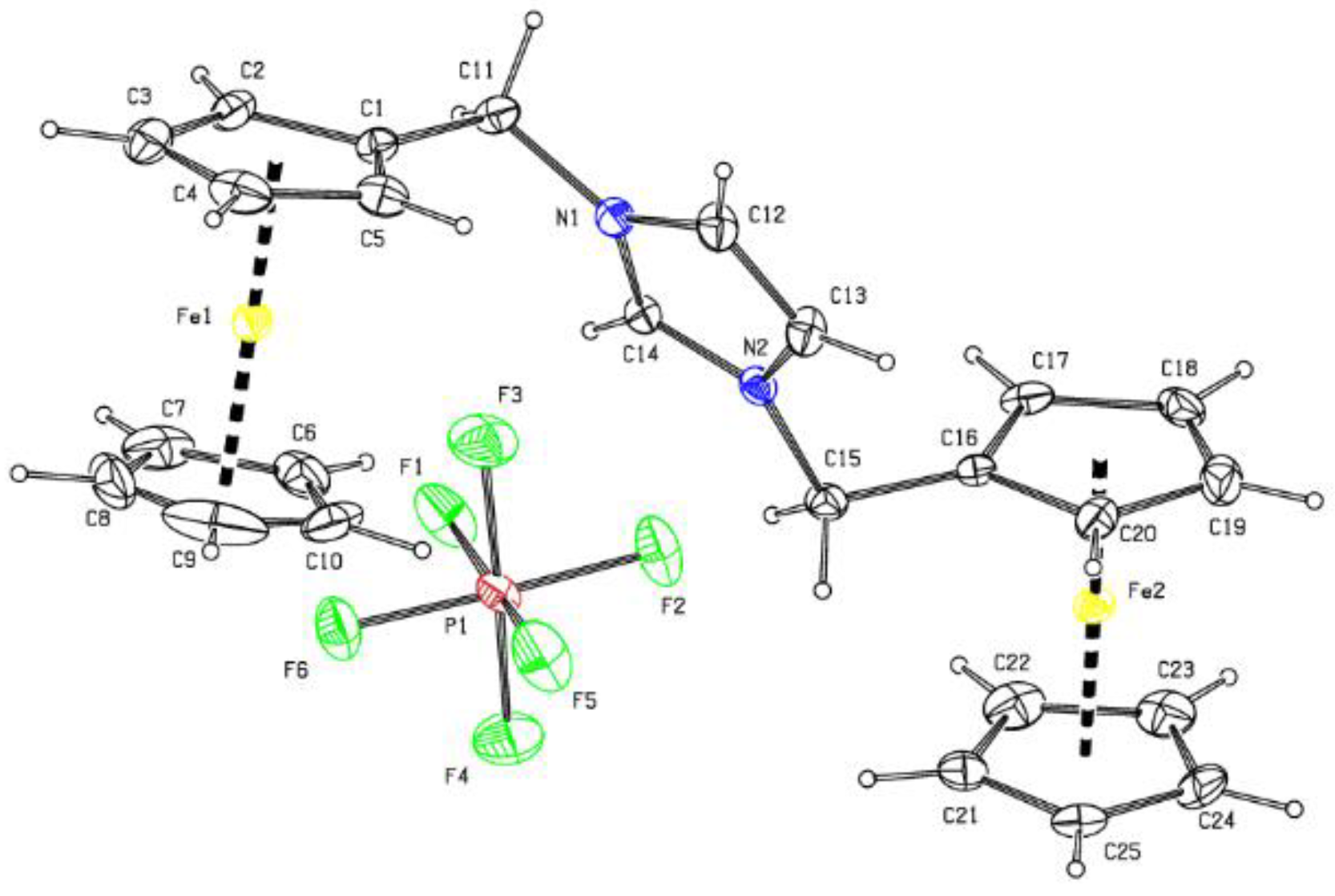
2.1. Crystal Structures
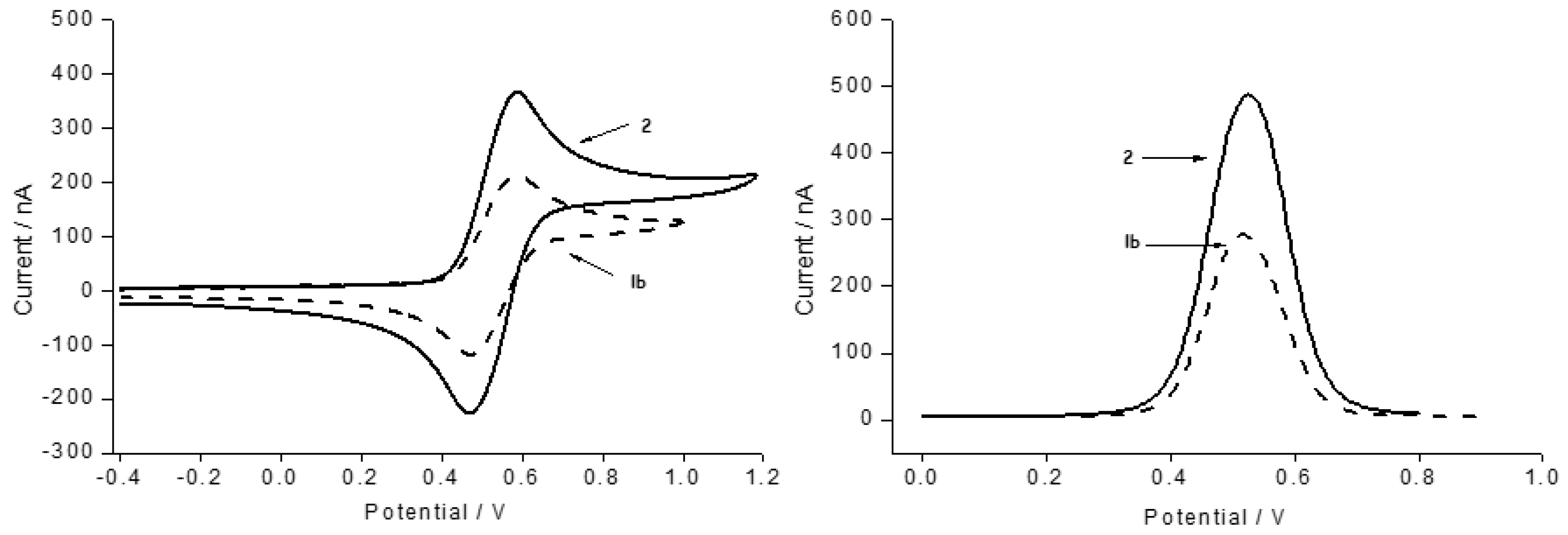
2.2. Electrochemical Behavior of the Ligand 2
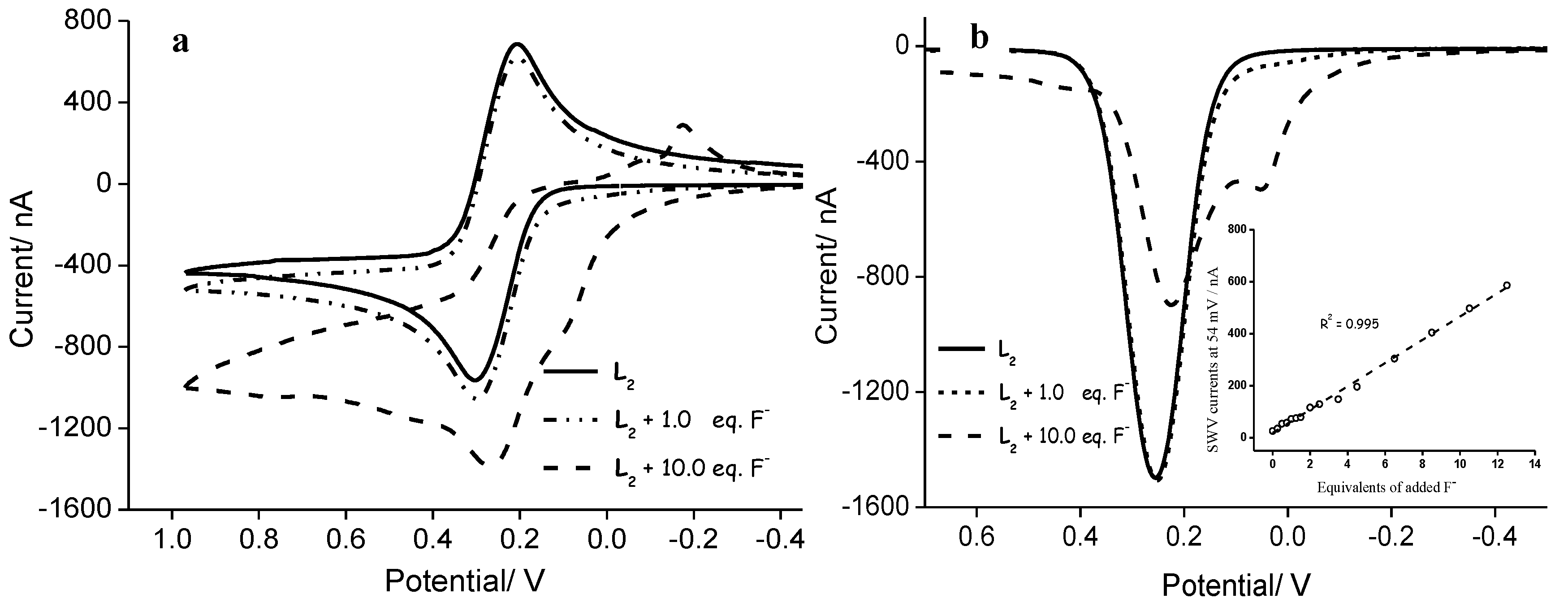
2.3. Ligand 2 Electrochemical Recognition of Anions
3. Discussion
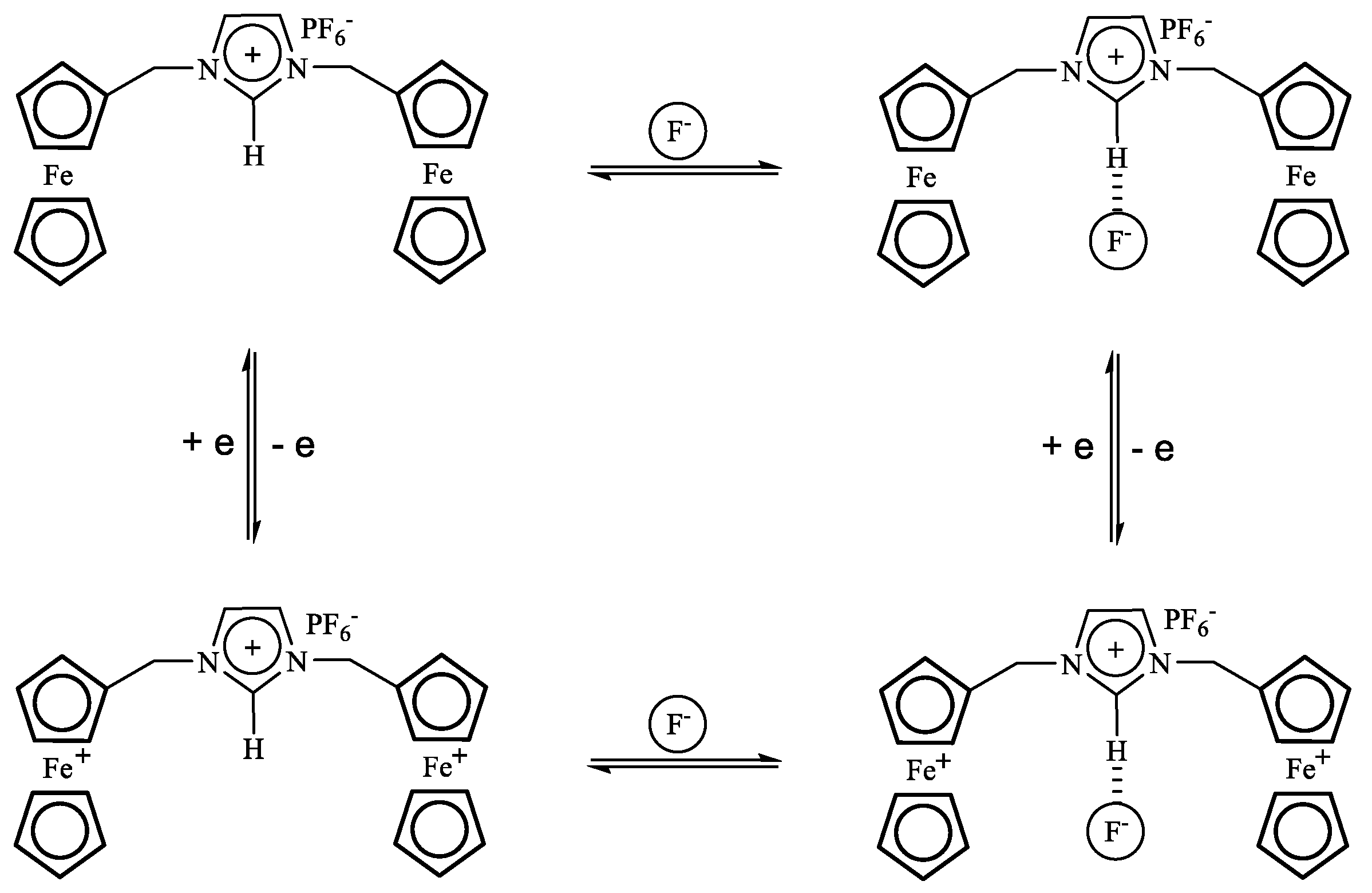
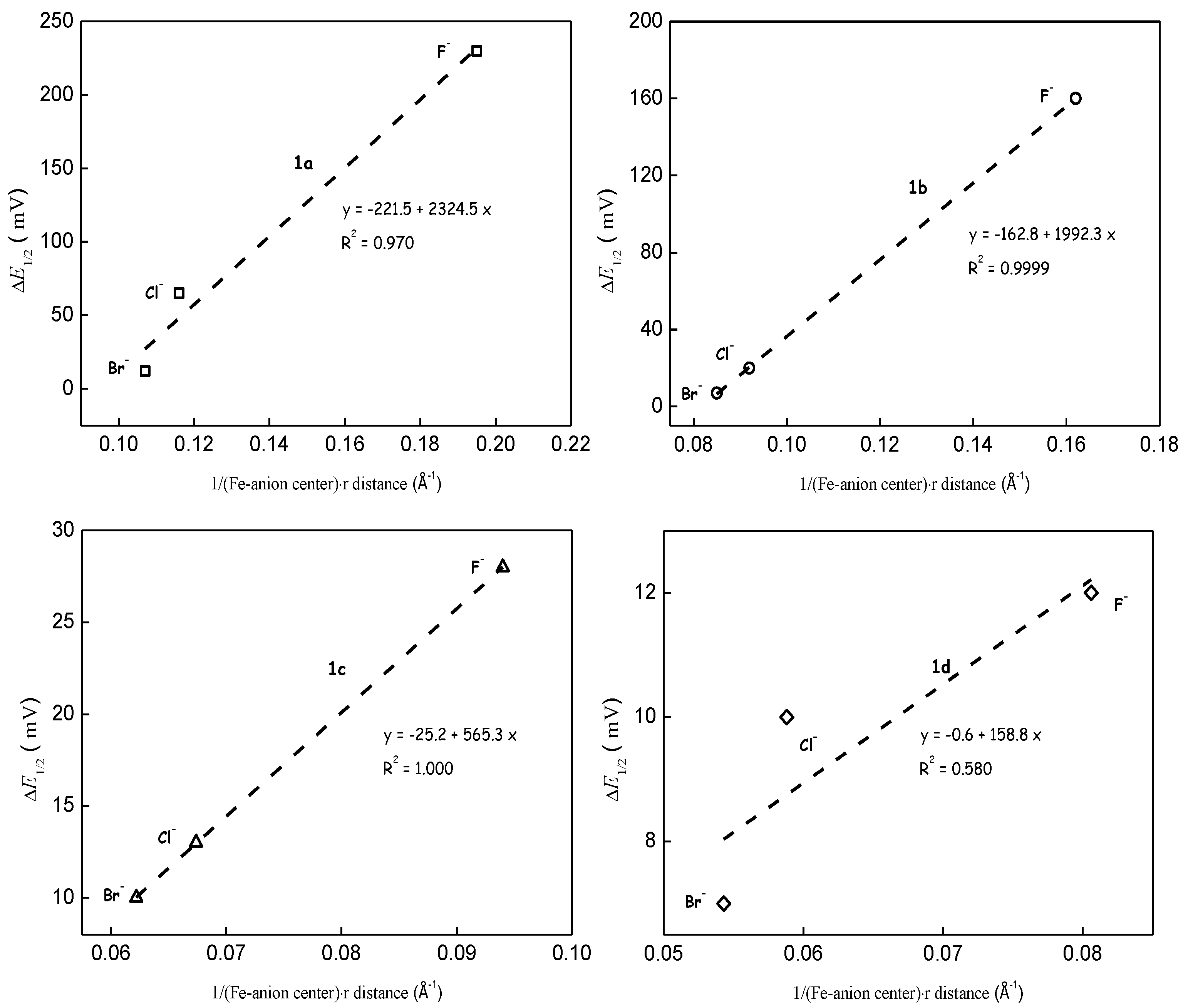
3.1. Coupled Electron and Inter- or Intramolecular Anion Transfer Processes
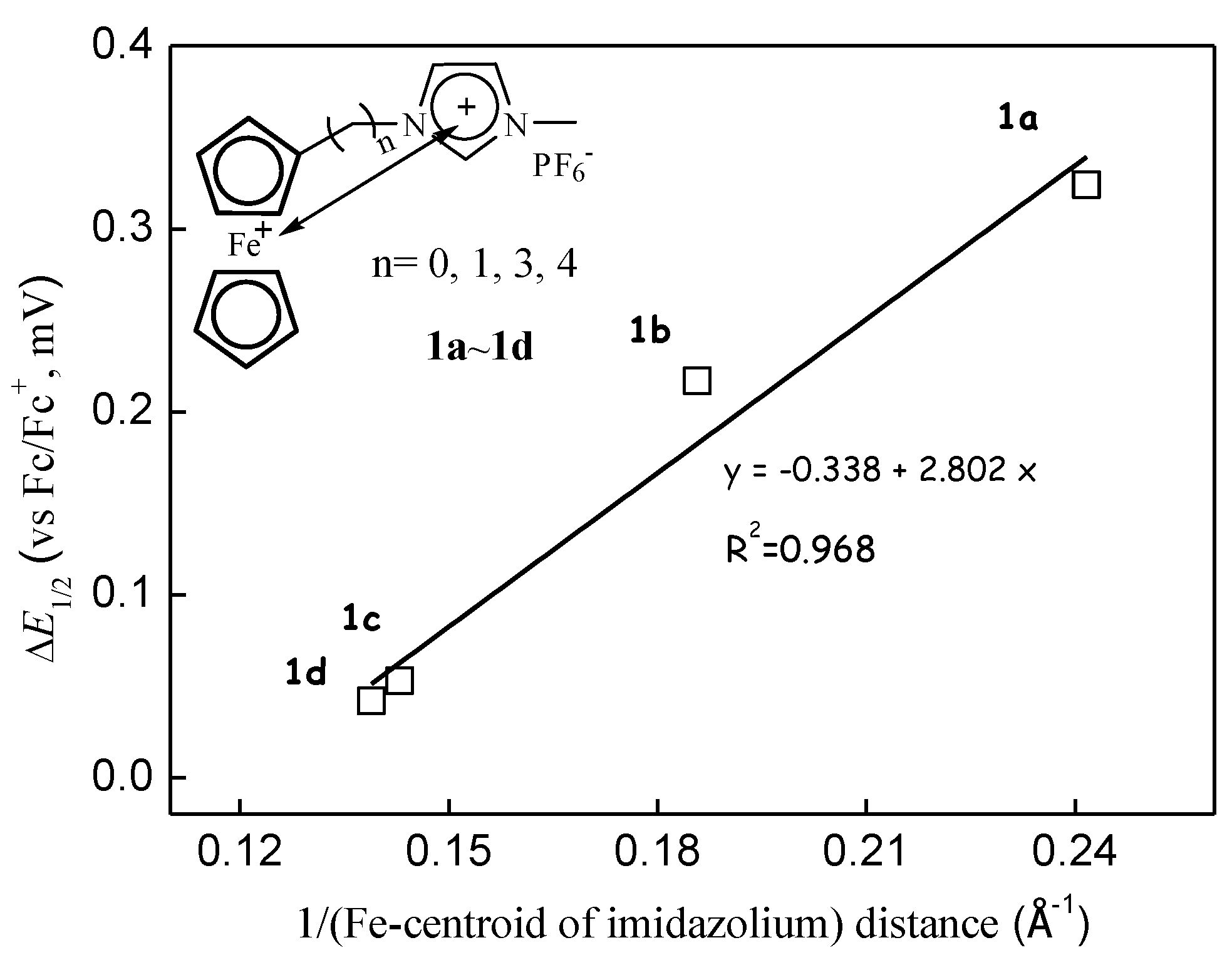
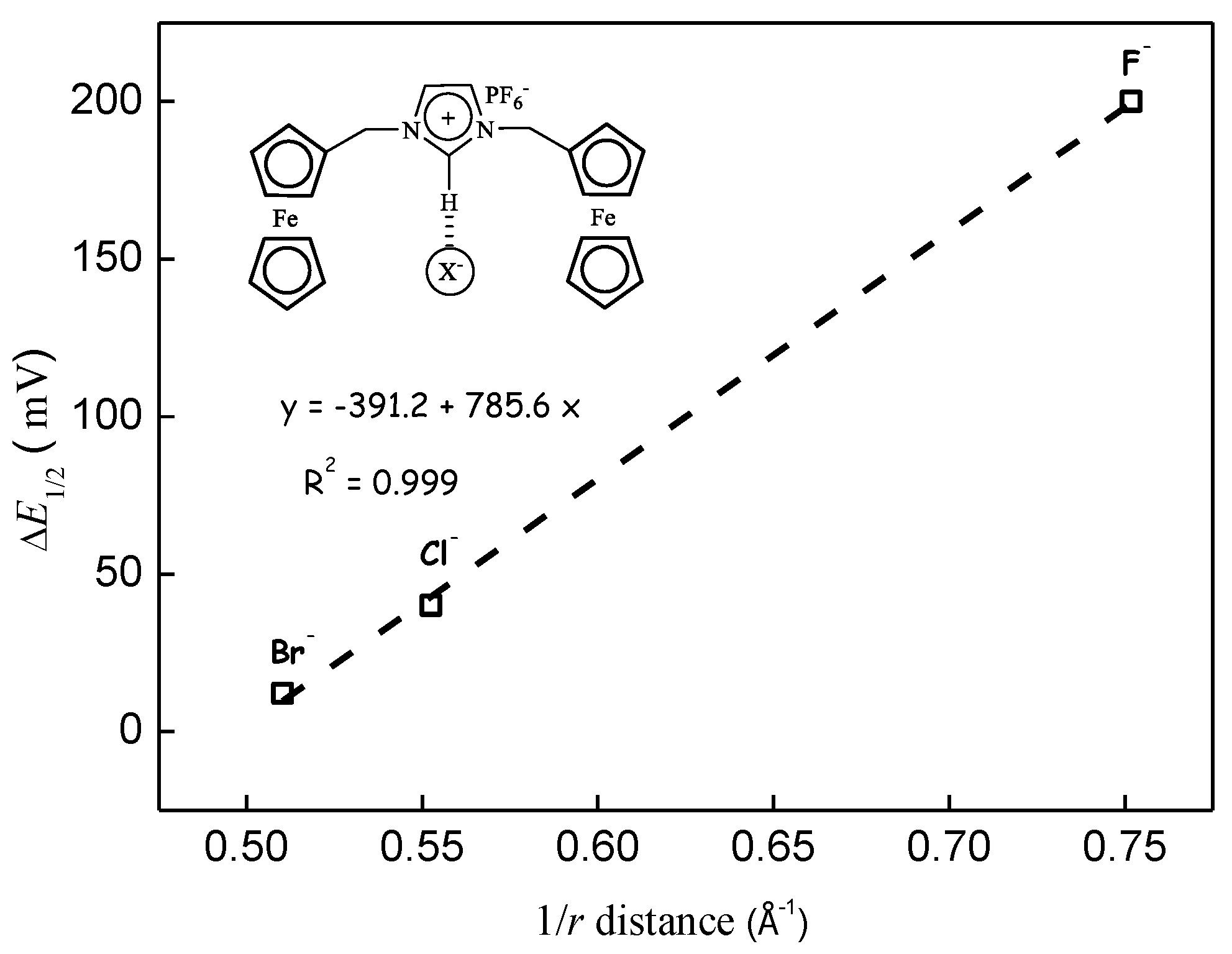
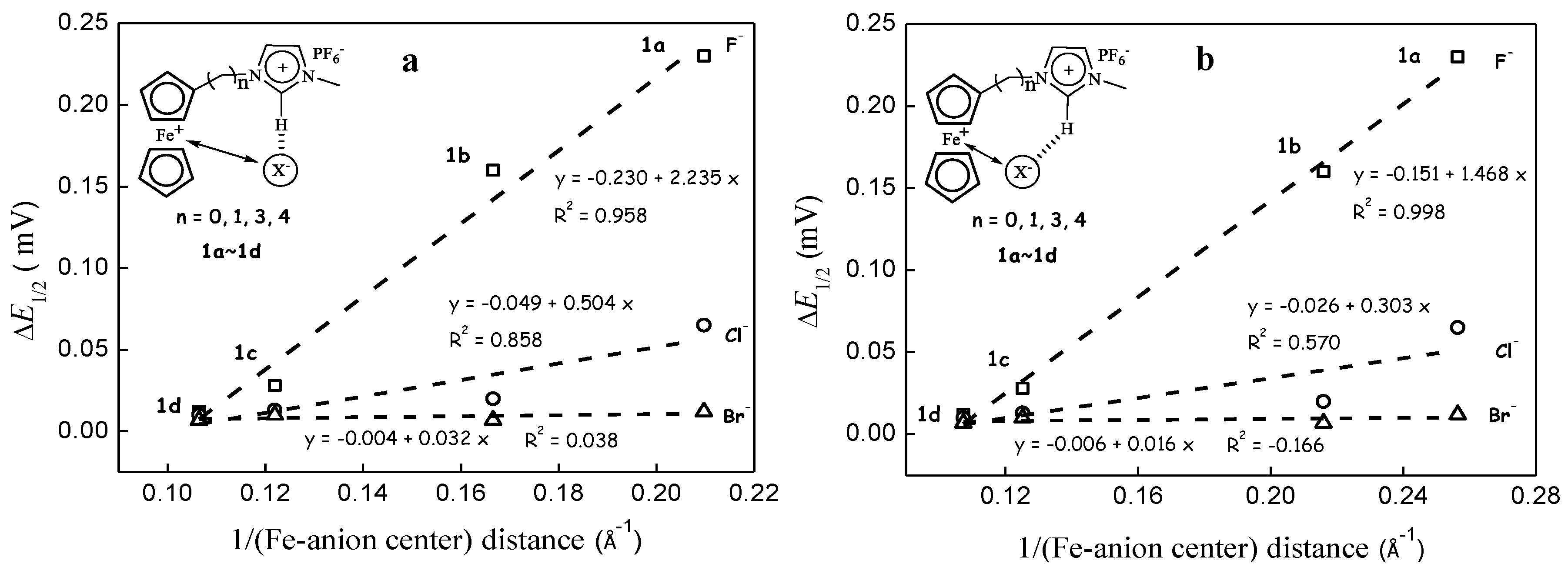
3.2. Through-Space Intramolecular Electrostatics
4. Materials and Methods
4.1. Instrumentation
4.2. Chemicals
4.3. Crystallography
4.4. Computational Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Nussinov, R. Close-range electrostatic interactions in proteins. Chembiochem 2002, 3, 604–617. [Google Scholar] [CrossRef]
- Coleman, J.A.; Gouaux, E. Structural basis for recognition of diverse antidepressants by the human serotonin transporter. Nat. Struct. Mol. Biol. 2018, 25, 170–175. [Google Scholar] [CrossRef]
- Purohit, A.; England, J.K.; Douma, L.G.; Tondnevis, F.; Bloom, L.B.; Levitus, M. Electrostatic Interactions at the Dimer Interface Stabilize the E. coli β Sliding Clamp. Biophys. J. 2017, 113, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.S.; Ziganshina, A.Y.; Lee, J.W.; Ko, Y.H.; Kang, J.K.; Lee, C.; Kim, K. A [2]pseudorotaxane-based molecular machine: Reversible formation of a molecular loop driven by electrochemical and photochemical stimuli. Angew. Chem. Int. Ed. 2003, 42, 4097–4100. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed; Garland Science: New York, NY, USA, 2001; pp. 2–10. [Google Scholar]
- Jeon, W.S.; Kim, E.; Ko, Y.H.; Hwang, I.H.; Lee, J.W.; Kim, S.Y.; Kim, H.J.; Kim, K. Molecular loop lock: A redox-driven molecular machine based on a host-stabilized charge-transfer complex. Angew. Chem. Int. Ed. 2005, 44, 87–91. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A.; Chen, G.Z. Electrochemical molecular recognition: Pathways between complexation and signalling. J. Chem. Soc. Dalton Trans. 1999, 1897–1909. [Google Scholar] [CrossRef]
- Caltagirone, C.; Gale, P.A. Anion receptor chemistry: Highlights from 2007. Chem. Soc. Rev. 2009, 38, 520–563. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.; Zapata, F.; Caballero, A. Anion Recognition Strategies Based on Combined Noncovalent Interactions. Chem. Rev. 2017, 117, 9907–9972. [Google Scholar] [CrossRef]
- Bdjic, J.D.; Balzani, V.; Credi, A.; Silvi, S.; Stoddart, J.F. A Molecular Elevator. Science 2004, 303, 1845–1849. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Zink, J.I.; Skelton, J.M.; Bayer, M.J.; Liu, C.; Livshits, E.; Baer, R.; Neuhauser, D. Electrical or photocontrol of the rotary motion of a metallacarborane. Science 2004, 303, 1849–1851. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A.; Chen, G.Z. Mechanisms of electrochemical recognition of cations, anions and neutral guest species by redox-active receptor molecules. Coord. Chem. Rev. 1999, 185–186, 3–36. [Google Scholar] [CrossRef]
- Saleem, M.; Yu, H.; Wang, L.; Zain ul, A.; Khalid, H.; Akram, M.; Abbasi, N.M.; Chen, Y. Study on synthesis of ferrocene-based boronic acid derivatives and their saccharides sensing properties. J. Electroanal. Chem. 2016, 763, 71–78. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, Y.; Rui, Q.; Yao, C. Rhodamine-Ferrocene Conjugate Chemosensor for Selectively Sensing Copper(II) with Multisignals: Chromaticity, Fluorescence, and Electrochemistry and Its Application in Living Cell Imaging. Organometallics 2015, 34, 2962–2970. [Google Scholar] [CrossRef]
- Caballero, A.; White, N.G.; Beer, P.D. A ferrocene imidazolium-based macrocycle as an electrochemical chemosensor for halide anions. CrystEngComm 2014, 16, 3694–3698. [Google Scholar] [CrossRef]
- Beer, P.D.; Chen, Z.; Ogden, M.I. Voltammetric and NMR studies of a bis(ferrocenecarboxamide)-substituted diaza 18-crown-6 receptor that simultaneously complexes and electrochemically recognises both cations and anions. J. Chem. Soc. Faraday Trans. 1995, 91, 295–302. [Google Scholar] [CrossRef]
- Chen, Z.; Graydon, A.R.; Beer, P.D. Electrochemical response to anions in acetonitrile by neutral molecular receptors containing ferrocene, amide and amine moieties. J. Chem. Soc., Faraday Trans. 1996, 92, 97–102. [Google Scholar] [CrossRef]
- Goel, A.; Brennan, N.; Brady, N.; Kenny, P.T.M. Electrochemical recognition of anions by 1,1′-N,N′ ferrocenoylbisamino acid esters. Biosens. Bioelectron. 2007, 22, 2047–2050. [Google Scholar] [CrossRef]
- Reynes, O.; Bucher, C.; Moutet, J.C.; Royal, G.; Saint-Aman, E. Electrochemical sensing of dihydrogen phosphate and adenosine-5′-triphosphate anions by self-assembled monolayers of (ferrocenylmethyl)trialkylammonium cations on gold electrodes. Inorg. Chim. Acta 2008, 361, 1784–1788. [Google Scholar] [CrossRef]
- Reynes, O.; Maillard, F.; Moutet, J.C.; Royal, G.; Saint-Aman, E.; Stanciu, G.; Dutasta, J.P.; Gosse, I.; Mulatier, J.C. Complexation and electrochemical sensing of anions by amide-substituted ferrocenyl ligands. J. Organomet. Chem. 2001, 637, 356–363. [Google Scholar] [CrossRef]
- Gale, P.A.; Quesada, R. Anion coordination and anion-templated assembly: Highlights from 2002 to 2004. Coord. Chem. Rev. 2006, 250, 3219–3244. [Google Scholar] [CrossRef]
- Gale, P.A.; Garcia-Garrido, S.E.; Garric, J. Anion receptors based on organic frameworks: Highlights from 2005 and 2006. Coord. Chem. Rev. 2008, 37, 151–190. [Google Scholar] [CrossRef] [PubMed]
- Mullen, K.M.; Beer, P.D. Sulfate anion templation of macrocycles, capsules, interpenetrated and interlocked structures. Coord. Chem. Rev. 2009, 38, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, A.; Aller, E.; Molina, P. Iminophosphorane-based synthesis of multinuclear ferrocenyl urea, thiourea and guanidine derivatives and exploration of their anion sensing properties. Tetrahedron 2009, 65, 1397–1401. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, S.K.; Singh, N.J.; Kim, K.S. Imidazolium receptors for the recognition of anions. Coord. Chem. Rev. 2006, 35, 355–360. [Google Scholar]
- Reynes, O.; Bucher, C.; Moutet, J.C.; Royal, G.; Saint-Aman, E.; Ungureanu, E.M. Electrochemical sensing of anions by redox-active receptors built on the ferrocenyl cyclam framework. J. Electroanal. Chem. 2005, 580, 291–299. [Google Scholar] [CrossRef]
- Reynes, O.; Royal, G.; Chainet, E.; Moutet, J.C.; Saint-Aman, E. Poly(ferrocenylalkylammonium): A molecular electrode material for dihydrogenphosphate sensing. Electroanalysis 2003, 15, 65–69. [Google Scholar] [CrossRef]
- Bucher, C.; Devillers, C.H.; Moutet, J.C.; Royal, G.; Saint-Aman, E. Ferrocene-appended porphyrins: Syntheses and properties. Coord. Chem. Rev. 2009, 253, 21–36. [Google Scholar] [CrossRef]
- Thomas, J.L.; Howarth, J.; Kennedy, A.M. Electrochemical anion recognition by novel ferrocenyl imidazole systems. Molecules 2002, 7, 861–866. [Google Scholar] [CrossRef]
- Xu, Z.; Kim, S.K.; Yoon, J. Revisit to imidazolium receptors for the recognition of anions: Highlighted research during 2006–2009. Chem. Soc. Rev. 2010, 39, 1457–1466. [Google Scholar] [CrossRef]
- Xu, Z.; Singh, N.J.; Kim, S.K.; Spring, D.R.; Kim, K.S.; Yoon, J. Induction-Driven Stabilization of the Anion–π Interaction in Electron-Rich Aromatics as the Key to Fluoride Inclusion in Imidazolium-Cage Receptors. Chem. Eur. J. 2011, 17, 1163–1170. [Google Scholar] [CrossRef]
- Xu, Z.; Kim, S.K.; Han, S.J.; Lee, C.; Kociok-Kohn, G.; James, T.D.; Yoon, J. Ratiometric Fluorescence Sensing of Fluoride Ions by an Asymmetric Bidentate Receptor Containing a Boronic Acid and Imidazolium Group. Eur. J. Org. Chem. 2009, 2009, 3058–3065. [Google Scholar] [CrossRef]
- Kong, D.D.; Weng, T.Q.; He, W.X.; Liu, B.; Jin, S.; Hao, X.; Liu, S.H. Synthesis, characterization, and electrochemical properties of ferrocenylimidazolium. J. Organomet. Chem. 2013, 727, 19–27. [Google Scholar] [CrossRef]
- Su, Z.-M.; Yan, X.-Q.; Liang, C.-L.; Lin, C.-X.; Xie, L.-L.; Yuan, Y.-F. Highly selective detection of fluoride based on 2,2-diferrocenylpropane benzimidazolium borate-ester salt. Tetrahedron Lett. 2016, 57, 1250–1255. [Google Scholar] [CrossRef]
- Manivannan, R.; Elango, K.P. Spectral and electrochemical studies on anion recognition by ferrocene based imidazoles possessing different electron acceptor moieties. J. Organomet. Chem. 2015, 799–800, 99–107. [Google Scholar] [CrossRef]
- Zhuo, J.-B.; Lin, C.-X.; Wan, Q.; Xie, L.-L.; Yuan, Y.-F. Anion receptors of N-ferrocenylmethylene-substituted bis-imidazolium salts linked by xylene spacers. J. Organomet. Chem. 2015, 791, 289–297. [Google Scholar] [CrossRef]
- Zhuo, J.-B.; Zhu, X.-X.; Lin, C.-X.; Bai, S.; Xie, L.-L.; Yuan, Y.-F. Design, synthesis and anion recognition of ferrocene-based benzimidazolium receptors. J. Organomet. Chem. 2014, 770, 85–93. [Google Scholar] [CrossRef]
- Gilday, L.C.; White, N.G.; Beer, P.D. Triazole- and triazolium-containing porphyrin-cages for optical anion sensing. Dalton Trans. 2012, 23, 7092–7097. [Google Scholar] [CrossRef] [PubMed]
- Picot, S.C.; Mullaney, B.R.; Beer, P.D. Ion-Pair Recognition by a Heteroditopic Triazole-Containing Receptor. Chem. Eur. J. 2012, 18, 6230–6237. [Google Scholar] [CrossRef]
- Niu, H.T.; Yin, Z.M.; Su, D.D.; Niu, D.; Ao, Y.B.; He, J.Q.; Cheng, J.P. Ferrocene-based imidazolium receptors for anions. Tetrahedron 2008, 64, 6300–6306. [Google Scholar] [CrossRef]
- Niu, H.T.; Yin, Z.M.; Su, D.D.; Niu, D.; He, J.Q.; Cheng, J.P. Imidazolium-based macrocycles as multisignaling chemosensors for anions. Dalton Trans. 2008, 28, 3694–3700. [Google Scholar] [CrossRef]
- Thomas, J.L.; Howarth, J.; Hanlon, K.; McGuirk, D. Ferrocenyl imidazolium salts as a new class of anion receptors with C-H center dot center dot center dot X- hydrogen bonding. Tetrahedron Lett. 2000, 41, 413–416. [Google Scholar] [CrossRef]
- Jin, S.; Wang, D.H.; Jin, X.B.; Chen, G.Z. Intramolecular electrostatics: Coulomb’s law at sub-nanometers. Chemphyschem 2004, 5, 1623–1629. [Google Scholar] [CrossRef]
- Jin, S.; Jin, X.B.; Wang, D.H.; Cheng, G.Z.; Peng, L.; Chen, G.Z. Voltammetric studies of through-space and through-bond electrostatic interactions in alkyl linked ferrocene and benzoaza-15-crown-5 receptor molecules in acetonitrile. J. Phys. Chem. B 2005, 109, 10658–10667. [Google Scholar] [CrossRef] [PubMed]
- Camire, N.; Mueller-Westerhoff, U.T.; Geiger, W.E. Improved electrochemistry of multi-ferrocenyl compounds: Investigation of biferrocene, terferrocene, bis(fulvalene)diiron and diferrocenylethane in dichloromethane using [NBu4][B(C6F5)4] as supporting electrolyte. J. Organomet. Chem. 2001, 637, 823–826. [Google Scholar] [CrossRef]
- Wu, X.H.; Weng, T.Q.; Jin, S.; Liang, J.H.; Guo, R.; Yu, G.A.; Liu, S.H. Synthesis, characterization, and substituent effects of mononuclear ruthenium complexes [RuCl(CO)(PMe3)3 (CH=CH-C6H4-R-p)] (R = H, CH3, OCH3, NO2, NH2, NMe2). J. Organomet. Chem. 2009, 694, 1877–1883. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods Fundamentals and Applications, 2nd ed; John Wiley & Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Gao, Y.; Twamley, B.; Shreeve, J.M. The first (ferrocenylmethyl)imidazollum and (ferrocenylmethyl)triazolium room temperature ionic liquids. Inorg. Chem. 2004, 43, 3406–3412. [Google Scholar] [CrossRef]
- Coleman, K.S.; Turberville, S.; Pascu, S.I.; Green, M.L.H. Synthesis of a new bidentate ferrocenyl N-heterocyclic carbene ligand precursor and the palladium (II) complex trans-[PdCl2(C^fc^C)], where (C^fc^C) = 1,1′-di-tert-butyl-3,3′(1,1′-dimethyleneferrocenyl)-diimidazol-2-ylidene. J. Organomet. Chem. 2005, 690, 653–658. [Google Scholar] [CrossRef]
- Ozcubukcu, S.; Schmitt, E.; Leifert, A.; Bolm, C. A general and efficient synthesis of nitrogen-substituted ferrocenes. Synthesis 2007, 3, 389–392. [Google Scholar] [CrossRef]
- Howarth, J.; James, P.; Ryan, R. Sodium borohydride reduction of aldehydes and ketones in the recyclable ionic liquid [BMIM]PF6. Synth. Commun. 2001, 31, 2935–2938. [Google Scholar] [CrossRef]
- Seo, H.; Kim, B.Y.; Lee, J.H.; Park, H.J.; Son, S.U.; Chung, Y.K. Synthesis of chiral ferrocenyl imidazolium salts and their rhodium(I) and iridium(I) complexes. Organometallics 2003, 22, 4783–4791. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-2013, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 2013. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a. Conductor Solvent Model. J. Phys. Chem. A. 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a–1d and 2 are available or not from the authors. |

| Cation | 1a | 1b | 1c | 1d | 2 |
|---|---|---|---|---|---|
| E1/2 (vs. Ag/Ag+, mV) | 413 | 256 | 72 | 56 | 262 |
| E1/2 (vs. Fc/Fc+, mV) | 324 | 167 | −17 | −33 | 173 |
| ΔEp (mV) | 74 | 72 | 66 | 74 | 70 |
| ipa/ipc | 1.00 | 1.07 | 0.99 | 0.98 | 0.99 |
| Compound | ΔE1/2 (mV) | |||
|---|---|---|---|---|
| F− | Cl− | Br− | HSO4− | |
| 1a | 230 | 65 | 12 | 108 |
| 1b | 160 | 21 | 7 | 24 |
| 1c | 28 | 13 | 10 | 24 |
| 1d | 12 | 10 | 7 | 12 |
| 2 | 200 | 40 | 13 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, T.-Q.; Huang, Y.-F.; Xue, L.-S.; Cheng, J.; Jin, S.; Liu, S.-H.; Wu, D.-Y.; Chen, G.Z. Anion-Binding-Induced Electrochemical Signal Transduction in Ferrocenylimidazolium: Combined Electrochemical Experimental and Theoretical Investigation. Molecules 2019, 24, 238. https://doi.org/10.3390/molecules24020238
Weng T-Q, Huang Y-F, Xue L-S, Cheng J, Jin S, Liu S-H, Wu D-Y, Chen GZ. Anion-Binding-Induced Electrochemical Signal Transduction in Ferrocenylimidazolium: Combined Electrochemical Experimental and Theoretical Investigation. Molecules. 2019; 24(2):238. https://doi.org/10.3390/molecules24020238
Chicago/Turabian StyleWeng, Tan-Qing, Yi-Fan Huang, Lou-Sha Xue, Jie Cheng, Shan Jin, Sheng-Hua Liu, De-Yin Wu, and George Z. Chen. 2019. "Anion-Binding-Induced Electrochemical Signal Transduction in Ferrocenylimidazolium: Combined Electrochemical Experimental and Theoretical Investigation" Molecules 24, no. 2: 238. https://doi.org/10.3390/molecules24020238
APA StyleWeng, T.-Q., Huang, Y.-F., Xue, L.-S., Cheng, J., Jin, S., Liu, S.-H., Wu, D.-Y., & Chen, G. Z. (2019). Anion-Binding-Induced Electrochemical Signal Transduction in Ferrocenylimidazolium: Combined Electrochemical Experimental and Theoretical Investigation. Molecules, 24(2), 238. https://doi.org/10.3390/molecules24020238







