MicroRNAs as a Novel Tool in the Diagnosis of Liver Lipid Dysregulation and Fatty Liver Disease
Abstract
1. Introduction
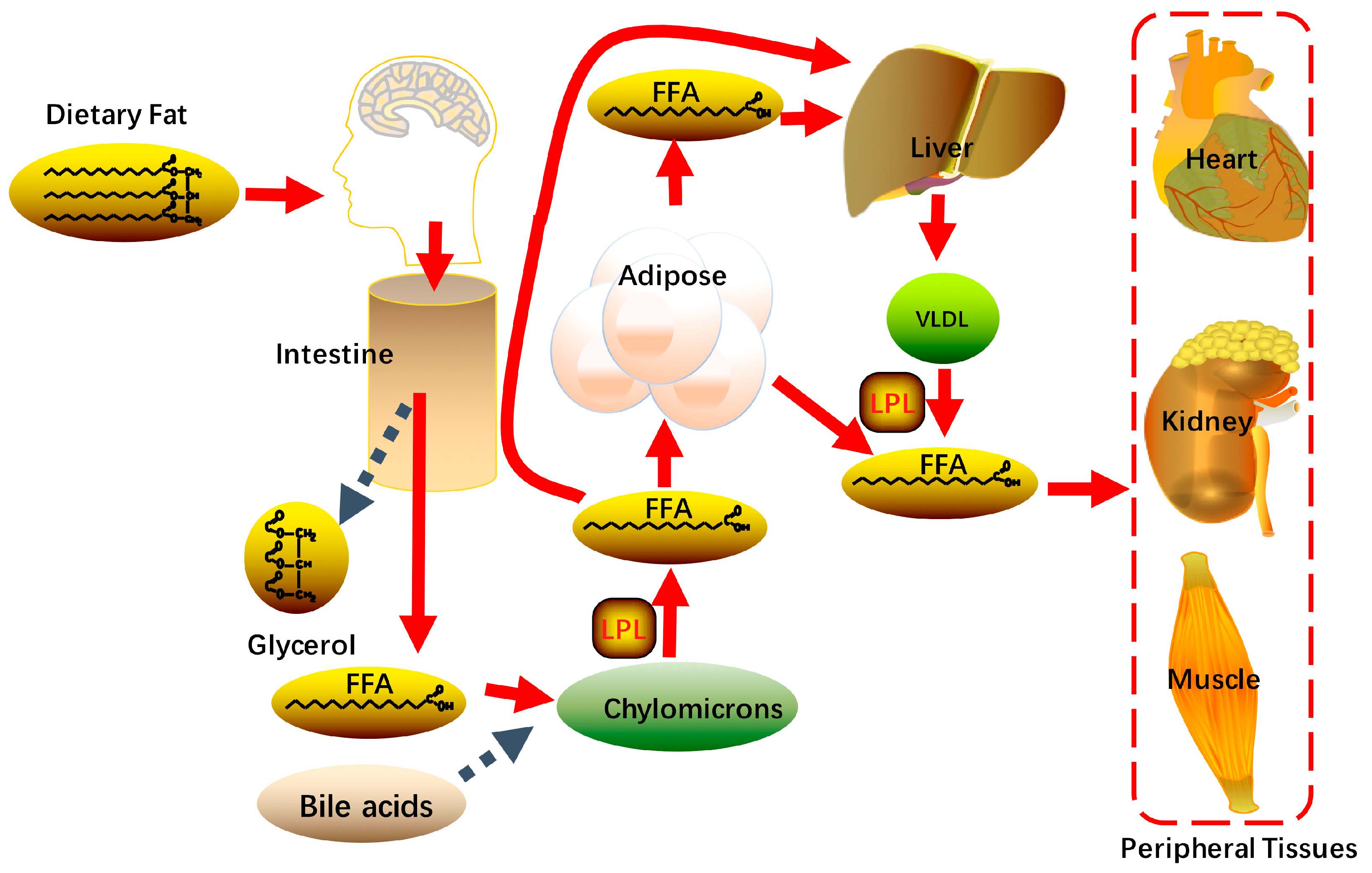
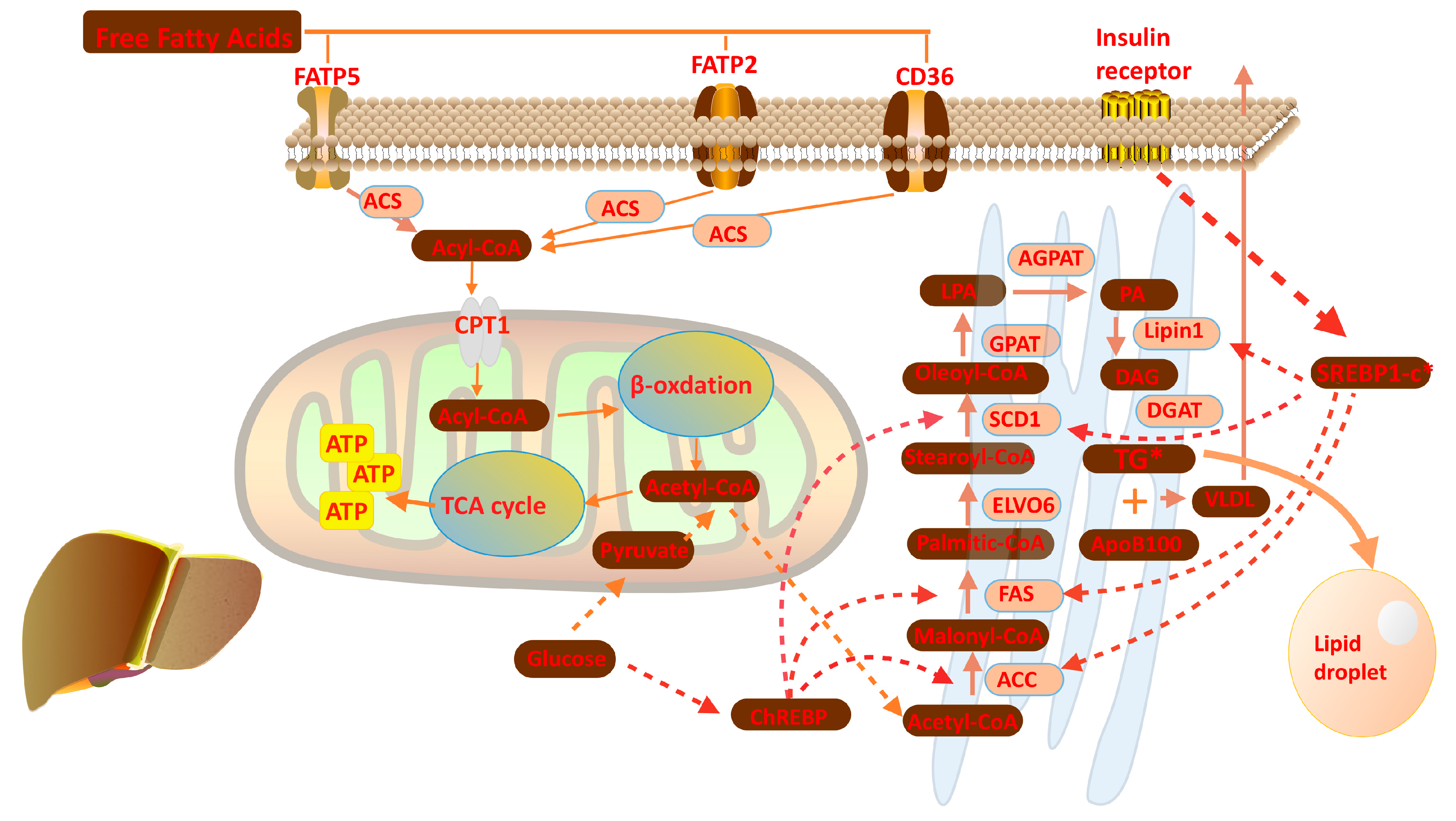
2. Overview of Liver Lipid Metabolism
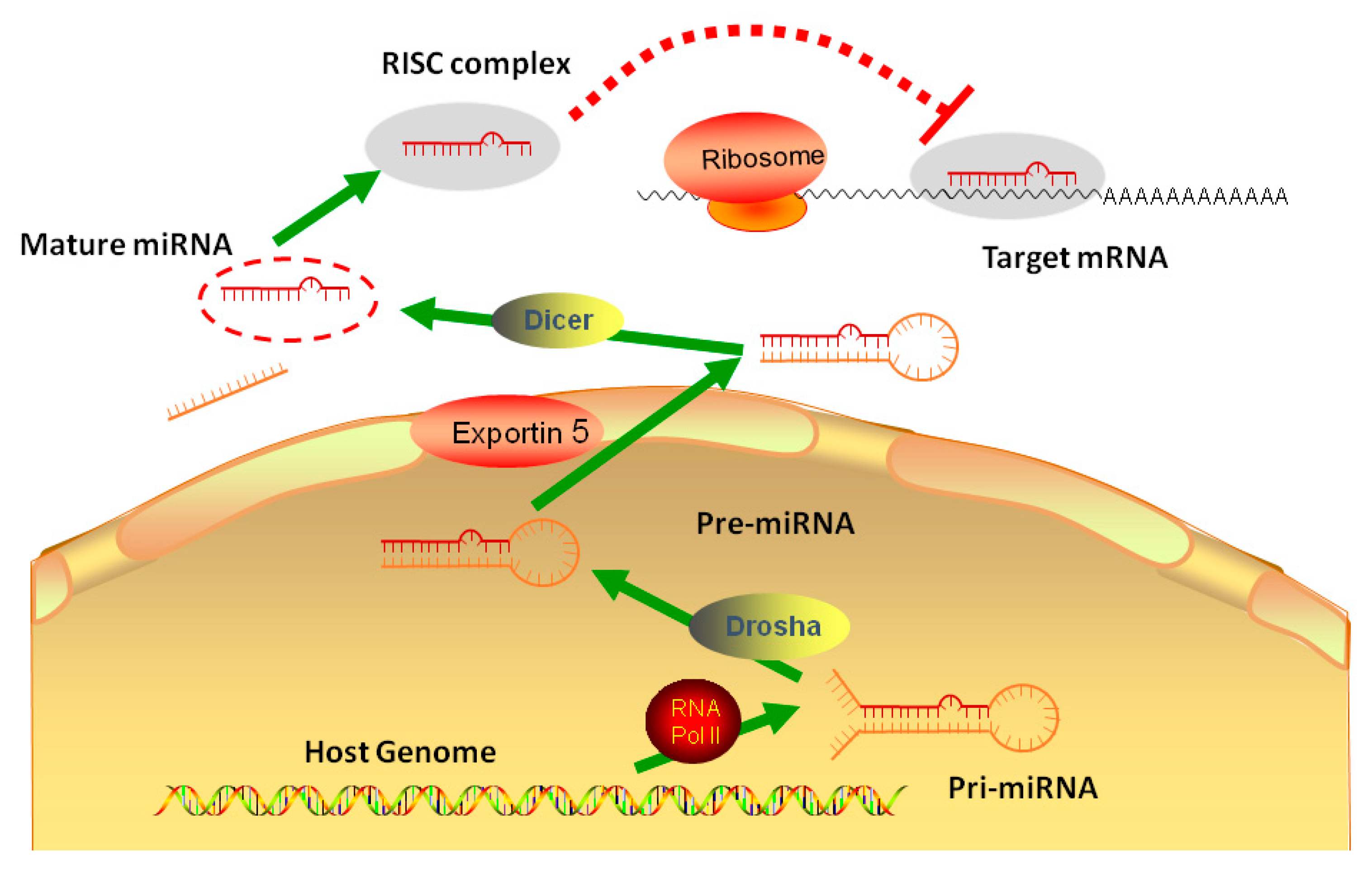
3. miRNAs in Lipid Metabolism
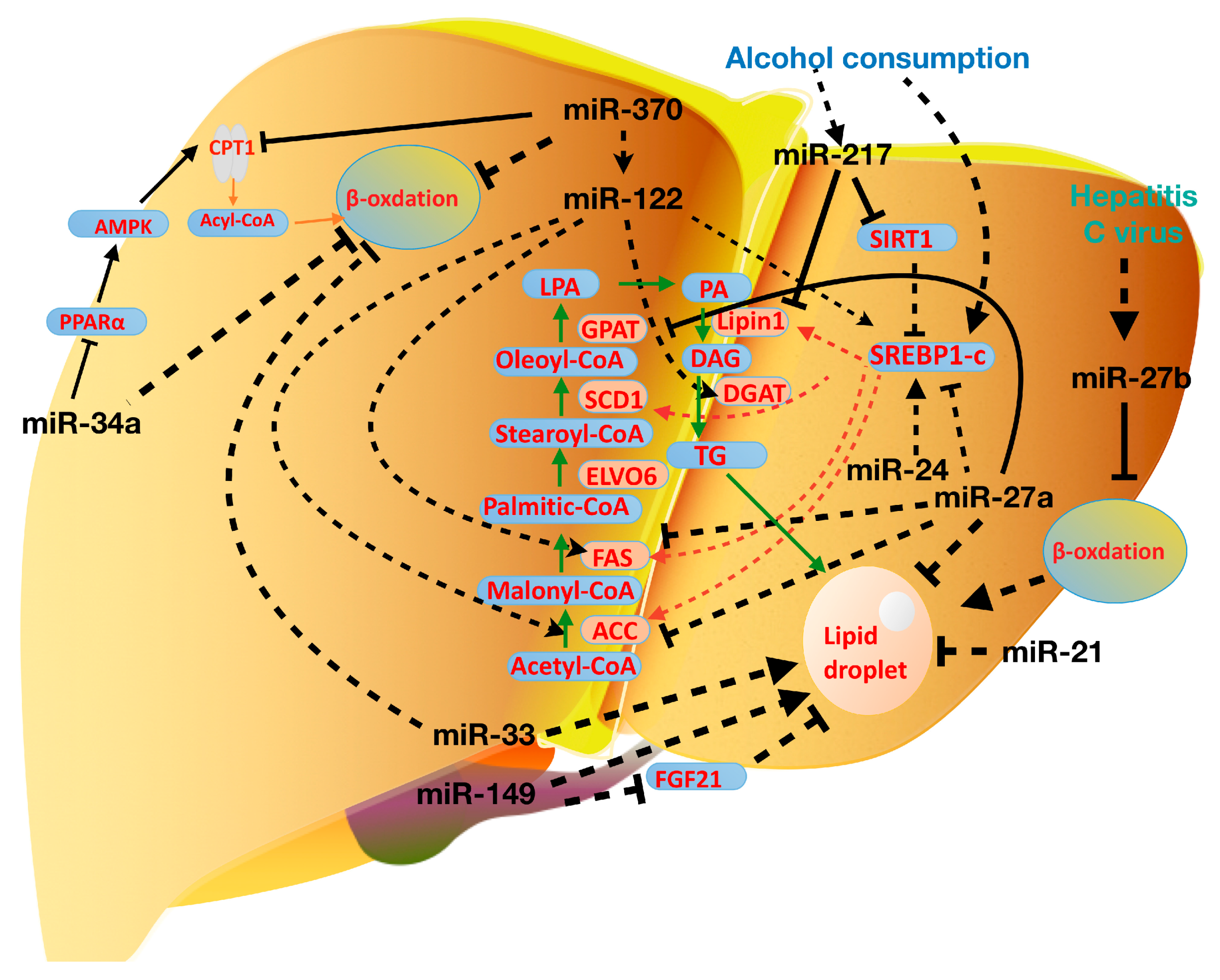
4. miRNAs in Nonalcoholic Fatty Liver Disease
5. miRNAs in Alcohol and Virus-Induced Fatty Liver
6. Novel Diagnostic Tools and Treatments for Fatty Liver Diseases
7. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Diehl-Jones, W.L.; Askin, D.F. The neonatal liver, Part 1: Embryology, anatomy, and physiology. Neonatal Netw. 2002, 21, 5–12. [Google Scholar] [CrossRef]
- Abdel-Misih, S.R.; Bloomston, M. Liver anatomy. Surg. Clin. N. Am. 2010, 90, 643–653. [Google Scholar] [CrossRef]
- Peng, J.; Yu, J.; Xu, H.; Kang, C.; Shaul, P.W.; Guan, Y.; Zhang, X.; Su, W. Enhanced Liver Regeneration after Partial Hepatectomy in Sterol Regulatory Element-Binding Protein (SREBP)-1c-Null Mice is Associated with Increased Hepatocellular Cholesterol Availability. Cell Physiol. Biochem. 2018, 47, 784–799. [Google Scholar] [CrossRef]
- Ai, R.; Laragione, T.; Hammaker, D.; Boyle, D.L.; Wildberg, A.; Maeshima, K.; Palescandolo, E.; Krishna, V.; Pocalyko, D.; Whitaker, J.W.; et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat. Commun. 2018, 9, 1921. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Rottiers, V.; Najafi-Shoushtari, S.H.; Kristo, F.; Gurumurthy, S.; Zhong, L.; Li, Y.; Cohen, D.E.; Gerszten, R.E.; Bardeesy, N.; Mostoslavsky, R.; et al. MicroRNAs in metabolism and metabolic diseases. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 225–233. [Google Scholar] [CrossRef]
- Saunders, M.A.; Liang, H.; Li, W.H. Human polymorphism at microRNAs and microRNA target sites. Proc. Natl. Acad. Sci. USA 2007, 104, 3300–3305. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Le Bloc’h, J.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef]
- Spector, A.A. Fatty acid binding to plasma albumin. J. Lipid Res. 1975, 16, 165–179. [Google Scholar] [PubMed]
- Ashbrook, J.D.; Spector, A.A.; Santos, E.C.; Fletcher, J.E. Long chain fatty acid binding to human plasma albumin. J. Biol. Chem. 1975, 250, 2333–2338. [Google Scholar] [PubMed]
- Ashbrook, J.D.; Spectro, A.A.; Fletcher, J.E. Medium chain fatty acid binding to human plasma albumin. J. Biol. Chem. 1972, 247, 7038–7042. [Google Scholar]
- Krammer, J.; Digel, M.; Ehehalt, F.; Stremmel, W.; Fullekrug, J.; Ehehalt, R. Overexpression of CD36 and acyl-CoA synthetases FATP2, FATP4 and ACSL1 increases fatty acid uptake in human hepatoma cells. Int. J. Med. Sci. 2011, 8, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Bian, Y.; Yuan, S.; Chen, K.; Sheng, Y.; Fu, T.; Wei, L.; Pei, Y.; Sun, H.J.P. Identification of 4-aminoquinoline core for the design of new cholinesterase inhibitors. PeerJ. 2016, 4. [Google Scholar] [CrossRef]
- Hajri, T.; Han, X.X.; Bonen, A.; Abumrad, N.A. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J. Clin. Investig. 2002, 109, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Pei, K.; Cai, H.; Tu, S.; Zhang, Z.; Cheng, X.; Qiao, F.; Fan, K.; Qin, K.; Liu, X.; et al. Bioactivity evaluation-based ultra high-performance liquid chromatography coupled with electrospray ionization tandem quadrupole-time-of-flight mass spectrometry and novel distinction of multi-subchemome compatibility recognition strategy with Astragali Radix-Fructus Corni herb-pair as a case study. J. Pharm. Biomed. Anal. 2016, 129, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Smith, A.J.; Bernlohr, D.A. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J. Biol. Chem. 2003, 278, 43008–43013. [Google Scholar] [CrossRef]
- Richards, M.R.; Harp, J.D.; Ory, D.S.; Schaffer, J.E. Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J. Lipid Res. 2006, 47, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Cases, S.; Smith, S.J.; Zheng, Y.W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collins, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl CoA: Diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Q.; Hong, L.; Chen, K.; Yang, L.; Guo, T.; Ke, S.; Yan, C.; Cheng, G.; Lee, R.J. Lipid Nanoparticles Loaded with an Antisense Oligonucleotide Gapmer Against Bcl-2 for Treatment of Lung Cancer. Pharm. Res. 2017, 34, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Munday, M.R. Regulation of mammalian acetyl-CoA carboxylase. Biochem. Soc. Trans. 2002, 30, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Munday, M.R.; Haystead, T.A.; Holland, R.; Carling, D.A.; Hardie, D.G. The role of phosphorylation/dephosphorylation of acetyl-CoA carboxylase in the regulation of mammalian fatty acid biosynthesis. Biochem. Soc. Trans. 1986, 14, 559–562. [Google Scholar] [CrossRef]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef]
- Fan, S.; Huang, K.; Ai, R.; Wang, M.; Wang, W.J.G. Predicting CpG methylation levels by integrating Infinium HumanMethylation450 BeadChip array data. Genomics 2016, 107, 132–137. [Google Scholar] [CrossRef]
- Fan, S.; Chi, W. Methods for genome-wide DNA methylation analysis in human cancer. Brief Funct. Genom. 2016, 15, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Foufelle, F.; Ferre, P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: A role for the transcription factor sterol regulatory element binding protein-1c. Biochem. J. 2002, 366, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Sun, Y.; Chen, K.; Sun, H.; Wei, W. Amphiphilic dendritic nanomicelle-mediated co-delivery of 5-fluorouracil and doxorubicin for enhanced therapeutic efficacy. J. Drug Target. 2016, 25, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Li, C.; Ai, R.; Firestein, G.S.; Wang, W. Computationally expanding Infinium HumanMethylation450 BeadChip array data to reveal distinct DNA methylation patterns of rheumatoid arthritis. Bioinformatics 2016, 32, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Hersch, S.J.; Wang, M.; Zou, S.B.; Moon, K.M.; Foster, L.J.; Ibba, M.; Navarre, W.W. Divergent protein motifs direct elongation factor P-mediated translational regulation in Salmonella enterica and Escherichia coli. MBio 2013, 4, e00180-13. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Hernandez, V.A.; Hu, K. Functional interaction of the two-pore domain potassium channel TASK-1 and caveolin-3. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Kang, C. Ion channels, protein kinase C and caveolae in cardioprotection. Ph.D. Thesis, Ohio State University, Columbus, OH, USA, 2015. [Google Scholar]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Gondret, F.; Ferre, P.; Dugail, I. ADD-1/SREBP-1 is a major determinant of tissue differential lipogenic capacity in mammalian and avian species. J. Lipid Res. 2001, 42, 106–113. [Google Scholar] [PubMed]
- Kang, C.; Qin, J.; Osei, W.; Hu, K. Age-dependent Mitochondrial Targeting Of Protein Kinase C Epsilon In Cardioprotection. FASEB J. 2017. [Google Scholar] [CrossRef]
- Willy, P.J.; Mangelsdorf, D.J. Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR. Genes Dev. 1997, 11, 289–298. [Google Scholar] [CrossRef]
- Kang, C.; Sun, Y.; Zhu, J.; Li, W.; Zhang, A.; Kuang, T.; Xie, J.; Yang, Z. Delivery of Nanoparticles for Treatment of Brain Tumor. Curr. Drug Metab. 2016, 17, 745–754. [Google Scholar] [CrossRef]
- Kang, C.; Qin, J.; Osei, W.; Hu, K. Regulation of protein kinase C-epsilon and its age-dependence. Biochem. Biophys. Res. Commun. 2017, 482, 1201–1206. [Google Scholar] [CrossRef]
- Dentin, R.; Denechaud, P.D.; Benhamed, F.; Girard, J.; Postic, C. Hepatic gene regulation by glucose and polyunsaturated fatty acids: A role for ChREBP. J. Nutr. 2006, 136, 1145–1149. [Google Scholar] [CrossRef]
- Kang, C.N.A.; Hu, K. Role of caveolin-3 in adenosine-induced increase in mitochondrial PKCε. FASEB J. 2013. [Google Scholar] [CrossRef]
- Adeli, K.; Taghibiglou, C.; Van Iderstine, S.C.; Lewis, G.F. Mechanisms of hepatic very low-density lipoprotein overproduction in insulin resistance. Trends Cardiovasc. Med. 2001, 11, 170–176. [Google Scholar] [CrossRef]
- Taghibiglou, C.; Carpentier, A.; Van Iderstine, S.C.; Chen, B.; Rudy, D.; Aiton, A.; Lewis, G.F.; Adeli, K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J. Biol. Chem. 2000, 275, 8416–8425. [Google Scholar]
- Lei, S.; Chen, K.; Yuan, S.; Huang, W.; Wei, L.; Qian, Z. Biochemistry. Crocetin Inhibits Lipopolysaccharide-Induced Inflammatory Response in Human Umbilical Vein Endothelial Cells. Cell. Physiol. Biochem. 2016, 40, 443–452. [Google Scholar]
- White, D.A.; Bennett, A.J.; Billett, M.A.; Salter, A.M. The assembly of triacylglycerol-rich lipoproteins: An essential role for the microsomal triacylglycerol transfer protein. Br. J. Nutr. 1998, 80, 219–229. [Google Scholar] [PubMed]
- Li, Q.; Yang, H.; Mo, J.; Chen, Y.; Wu, Y.; Kang, C.; Sun, Y.; Sun, H. Identification by shape-based virtual screening and evaluation of new tyrosinase inhibitors. PeerJ. 2018, 6. [Google Scholar] [CrossRef]
- Esau, C.; Kang, X.L.; Peralta, E.; Hanson, E.; Marcusson, E.G.; Ravichandran, L.V.; Sun, Y.Q.; Koo, S.; Perera, R.J.; Jain, R.; et al. MicroRNA-143 regulates adipocyte differentiation. J. Biol. Chem. 2004, 279, 52361–52365. [Google Scholar] [CrossRef]
- Qiao, H.; Dong, F.; Jing, C.; Yuan, S.; Chen, K.; Di, L.; Li, J.; Chen, Z.; Chen, J.; Gao, Y. Orally delivered polycurcumin responsive to bacterial reduction for targeted therapy of inflammatory bowel disease. Drug Deliv. 2017, 24, 233–242. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Y.; Kang, C.; Zhu, H. Pegylated drug delivery systems: From design to biomedical applications. Nano LIFE 2016, 6, 1642002. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C.; Liu, F.; Song, L. Delivery of Antipsychotics with Nanoparticles. Drug Dev. Res. 2016, 77, 393–399. [Google Scholar] [CrossRef]
- Qiao, H.; Zhu, Z.; Fang, D.; Sun, Y.; Kang, C.; Di, L.; Zhang, L.; Gao, Y. Redox-triggered mitoxantrone prodrug micelles for overcoming multidrug-resistant breast cancer. J. Drug Target. 2017, 26, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Zhang, H.; Liang, X.; Xiang, Y.; Yu, C.; Zen, K.; Li, Y.; Zhang, C.Y. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status. J. Lipid Res. 2009, 50, 1756–1765. [Google Scholar] [CrossRef]
- Tome, M.; Lopez-Romero, P.; Albo, C.; Sepulveda, J.C.; Fernandez-Gutierrez, B.; Dopazo, A.; Bernad, A.; Gonzalez, M.A. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ. 2011, 18, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kang, C.; Liu, F.; Zhou, Y.; Luo, L.; Qiao, H. RGD Peptide-Based Target Drug Delivery of Doxorubicin Nanomedicine. Drug Dev. Res. 2017, 78, 283–291. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C.; Yao, Z.; Liu, F.; Zhou, Y. Peptide-Based Ligand for Active Delivery of Liposomal Doxorubicin. Nano Life 2016, 6. [Google Scholar] [CrossRef]
- Shirasaki, T.; Honda, M.; Shimakami, T.; Horii, R.; Yamashita, T.; Sakai, Y.; Sakai, A.; Okada, H.; Watanabe, R.; Murakami, S.; et al. MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J. Virol. 2013, 87, 5270–5286. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.P.; George, M.; Kalyanasundaram, A.; Chen, K.; Periasamy, M.; Hu, K.; Lacombe, V.A. GLUT12 functions as a basal and insulin-independent glucose transporter in the heart. Biochim. Biophys. Acta. 2013, 1832, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Dou, S.; Xiong, M.; Sun, T.; Nano, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano. 2011, 5, 3679–3692. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, N.Y.; Yu, H.T.; Sun, Y.; Kang, C.; Huang, Q.B.; Sun, H.P.; Wang, X.L.; Li, N.G. Discovery of novel inhibitors disrupting HIF-1α/von Hippel–Lindau interaction through shape-based screening and cascade docking. PeerJ. 2016, 4. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Elmen, J.; Lindow, M.; Schutz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjarn, M.; Hansen, H.F.; Berger, U.; et al. LNA-mediated microRNA silencing in non-human primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xie, J.; Zhu, J.; Kang, C.; Chiang, C.; Wang, X.; Wang, X.; Kuang, T.; Chen, F.; Chen, Z.; et al. Functional exosome-mimic for delivery of siRNA to cancer: In vitro and in vivo evaluation. J. Control. Release 2016, 243, 160–171. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Drosatos, K.; Hiyama, Y.; Goldberg, I.J.; Zannis, V.I. MicroRNA-370 controls the expression of microRNA-122 and Cpt1alpha and affects lipid metabolism. J. Lipid Res. 2010, 51, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.Y.; Hsiao, J.K.; Wang, Y.P.; Lan, C.H.; Wu, H.C. Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer. Biomaterials 2016, 99, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Feinberg, M.W. MicroRNA-management of lipoprotein homeostasis. Circ. Res. 2014, 115, 2–6. [Google Scholar] [CrossRef]
- Najafi-Shoushtari, S.H.; Kristo, F.; Li, Y.; Shioda, T.; Cohen, D.E.; Gerszten, R.E.; Naar, A.M. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010, 328, 1566–1569. [Google Scholar] [CrossRef]
- Brown, M.S.; Ye, J.; Goldstein, J.L. HDL miR-ed down by SREBP introns. Science 2010, 328, 1495–1496. [Google Scholar] [CrossRef]
- Yung, B.C.; Li, J.; Zhang, M.; Cheng, X.; Li, H.; Yung, E.M.; Kang, C.; Cosby, L.E.; Liu, Y.; Teng, L.; et al. Lipid nanoparticles composed of quaternary amine-tertiary amine cationic lipid combination (QTsome) for therapeutic delivery of antimiR-21 for lung cancer. Mol. Pharm. 2016, 13, 653–662. [Google Scholar] [CrossRef]
- Rayner, K.J.; Esau, C.C.; Hussain, F.N.; McDaniel, A.L.; Marshall, S.M.; van Gils, J.M.; Ray, T.D.; Sheedy, F.J.; Goedeke, L.; Liu, X.; et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 2011, 478, 404–407. [Google Scholar] [CrossRef]
- Davalos, A.; Goedeke, L.; Smibert, P.; Ramirez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K.; Suresh, U.; Pastor-Pareja, J.C.; et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef]
- Vickers, K.C.; Shoucri, B.M.; Levin, M.G.; Wu, H.; Pearson, D.S.; Osei-Hwedieh, D.; Collins, F.S.; Remaley, A.T.; Sethupathy, P. MicroRNA-27b Is a Regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology 2013, 57, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.; William-Olsson, L.; Ahnmark, A.; Ekroos, K.; Hallberg, C.; Sjogren, H.P.; Becker, B.; Svensson, L.; Clapham, J.C.; Oscarsson, J.; et al. Liver-directed overexpression of mitochondrial glycerol-3-phosphate acyltransferase results in hepatic steatosis, increased triacylglycerol secretion and reduced fatty acid oxidation. FASEB J. 2006, 20, 434–443. [Google Scholar] [CrossRef]
- van Ree, J.H.; van den Broek, W.J.; Dahlmans, V.E.; Groot, P.H.; Vidgeon-Hart, M.; Frants, R.R.; Wieringa, B.; Havekes, L.M.; Hofker, M.H. Diet-induced hypercholesterolemia and atherosclerosis in heterozygous apolipoprotein E-deficient mice. Atherosclerosis 1994, 111, 25–37. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C. Self-Assembly of Peptides into Hydrogel. J. Org. Inorg. Chem. 2016, 2. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Arrese, M.; Zamora-Valdes, D.; Uribe, M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007, 27, 423–433. [Google Scholar] [CrossRef]
- Ekstedt, M.; Franzen, L.E.; Mathiesen, U.L.; Kechagias, S. Low clinical relevance of the nonalcoholic fatty liver disease activity score (NAS) in predicting fibrosis progression. Scand. J. Gastroenterol. 2012, 47, 108–115. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015, 149, 389–397. [Google Scholar] [CrossRef]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Design 2010, 16, 1941–1951. [Google Scholar] [CrossRef]
- Youssef, W.; McCullough, A.J. Diabetes mellitus, obesity, and hepatic steatosis. Semin. Gastrointest. Dis. 2002, 13, 17–30. [Google Scholar]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ye, Y.F.; Chen, S.H.; Yu, C.H.; Liu, J.; Li, Y.M. MicroRNA expression pattern in different stages of nonalcoholic fatty liver disease. Dig. Liver Dis. 2009, 41, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Chung, C.H.; Ha, T. High fat diet induced downregulation of microRNA-467b increased lipoprotein lipase in hepatic steatosis. Biochem. Biophys. Res. Commun. 2011, 414, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Zhu, L.; Gupta, N.; Chang, Y.; Fang, F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol. Endocrinol. 2007, 21, 2785–2794. [Google Scholar] [CrossRef]
- Xu, J.; Wu, C.; Che, X.; Wang, L.; Yu, D.; Zhang, T.; Huang, L.; Li, H.; Tan, W.; Wang, C.; et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol. Carcinog. 2011, 50, 136–142. [Google Scholar] [CrossRef]
- Hoekstra, M.; van der Sluis, R.J.; Kuiper, J.; Van Berkel, T.J.C. Nonalcoholic fatty liver disease is associated with an altered hepatocyte microRNA profile in LDL receptor knockout mice. J. Nutr. Biochem. 2012, 23, 622–628. [Google Scholar] [CrossRef]
- Cermelli, S.; Ruggieri, A.; Marrero, J.A.; Ioannou, G.N.; Beretta, L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS ONE 2011, 6, e23937. [Google Scholar] [CrossRef]
- Lee, J.; Padhye, A.; Sharma, A.; Song, G.; Miao, J.; Mo, Y.Y.; Wang, L.; Kemper, J.K. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J. Biol. Chem. 2010, 285, 12604–12611. [Google Scholar] [CrossRef] [PubMed]
- Xiuyun, H.; Shanqin, X.; Maitland-Toolan, K.A.; Kaori, S.; Bingbing, J.; Yasuo, I.; Fan, L.; Kenneth, W.; Michel, W.; Verbeuren, T.J. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar]
- Ding, J.; Li, M.; Wan, X.; Jin, X.; Chen, S.; Yu, C.; Li, Y. Effect of miR-34a in regulating steatosis by targeting PPARalpha expression in nonalcoholic fatty liver disease. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Peters, J.M.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J. Biol. Chem. 1998, 273, 5678–5684. [Google Scholar] [CrossRef]
- Nakajima, T.; Tanaka, N.; Kanbe, H.; Hara, A.; Kamijo, Y.; Zhang, X.; Gonzalez, F.J.; Aoyama, T. Bezafibrate at clinically relevant doses decreases serum/liver triglycerides via down-regulation of sterol regulatory element-binding protein-1c in mice: A novel peroxisome proliferator-activated receptor alpha-independent mechanism. Mol. Pharmacol. 2009, 75, 782–792. [Google Scholar] [CrossRef]
- Cheung, O.; Puri, P.; Eicken, C.; Contos, M.J.; Mirshahi, F.; Maher, J.W.; Kellum, J.M.; Min, H.; Luketic, V.A.; Sanyal, A.J. Nonalcoholic steatohepatitis is associated with altered hepatic micro RNA expression. Hepatology 2008, 48, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Wu, H.; Xiao, H.; Chen, X.; Willenbring, H.; Steer, C.J.; Song, G. Inhibition of microRNA-24 expression in liver prevents hepatic lipid accumulation and hyperlipidemia. Hepatology 2014, 60, 554–564. [Google Scholar] [CrossRef]
- Hornby, R.J.; Starkey Lewis, P.; Dear, J.; Goldring, C.; Park, B.K. MicroRNAs as potential circulating biomarkers of drug-induced liver injury: Key current and future issues for translation to humans. Expert Rev. Clin. Pharmacol. 2014, 7, 349–362. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, S.; Marzolf, B.; Troisch, P.; Brightman, A.; Hu, Z.; Hood, L.E.; Galas, D.J. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc. Natl. Acad. Sci. USA 2009, 106, 4402–4407. [Google Scholar] [CrossRef]
- Yamada, H.; Suzuki, K.; Ichino, N.; Ando, Y.; Sawada, A.; Osakabe, K.; Sugimoto, K.; Ohashi, K.; Teradaira, R.; Inoue, T. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin. Chim. Acta 2013, 424, 99–103. [Google Scholar] [CrossRef]
- Sun, C.; Huang, F.; Liu, X.; Xiao, X.; Yang, M.; Hu, G.; Liu, H.; Liao, L. miR-21 regulates triglyceride and cholesterol metabolism in non-alcoholic fatty liver disease by targeting HMGCR. Int. J. Mol. Med. 2015, 35, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lv, D.; Zhao, Y.; Chen, X.; Song, M.; Liu, J.; Bei, Y.; Wang, F.; Yang, W.; Yang, C. miR-149 controls non-alcoholic fatty liver by targeting FGF-21. J. Cell. Mol. Med. 2016, 20, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Bei, Y.; Liu, J.; Dimitrova-Shumkovska, J.; Kuang, D.; Zhou, Q.; Li, J.; Yang, Y.; Xiang, Y.; Wang, F.; et al. miR-212 downregulation contributes to the protective effect of exercise against non-alcoholic fatty liver via targeting FGF-21. J. Cell. Mol. Med. 2016, 20, 204–216. [Google Scholar] [CrossRef]
- Murata, Y.; Konishi, M.; Itoh, N. FGF21 as an Endocrine Regulator in Lipid Metabolism: From Molecular Evolution to Physiology and Pathophysiology. J. Nutr. Metabol. 2011, 2011. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, J.; Dai, Y.; Zhou, N.; Ji, C.; Li, X. MiR-146b attenuates high-fat diet-induced non-alcoholic steatohepatitis in mice. J. Gastroenterol. Hepatol. 2015, 30, 933–943. [Google Scholar] [CrossRef]
- Miller, A.M.; Gilchrist, D.S.; Nijjar, J.; Araldi, E.; Ramirez, C.M.; Lavery, C.A.; Fernándezhernando, C.; Mcinnes, I.B.; Kurowskastolarska, M. MiR-155 has a protective role in the development of non-alcoholic hepatosteatosis in mice. PLoS ONE 2013, 8, e72324. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lv, G.C.; Sheng, J.; Yang, Y.D. Effect of miRNA-10b in regulating cellular steatosis level by targeting PPAR-alpha expression, a novel mechanism for the pathogenesis of NAFLD. J. Gastroenterol. Hepatol. 2010, 25, 156–163. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Costinean, S.; Croce, C.M. MicroRNAs, the immune system and rheumatic disease. Nat. Clin. Pract. Rheum. 2008, 4, 534–541. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Starlard-Davenport, A.; Tryndyak, V.P.; Han, T.; Ross, S.A.; Rusyn, I.; Beland, F.A. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Lab. Investig. 2010, 90, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Csak, T.; Bala, S.; Lippai, D.; Kodys, K.; Catalano, D.; Iracheta-Vellve, A.; Szabo, G. MicroRNA-155 deficiency attenuates liver steatosis and fibrosis without reducing inflammation in a mouse model of steatohepatitis. PLoS ONE 2015, 10, e0129251. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Cimino, A.; Costinean, S.; Dumitru, C.D.; Adair, B.; Fabbri, M.; Alder, H.; Liu, C.G.; Calin, G.A.; et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 2007, 179, 5082–5089. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, N.; Wang, Z.; Ai, D.M.; Cao, Z.Y.; Pan, H.P. Decreased MiR-155 level in the peripheral blood of non-alcoholic fatty liver disease patients may serve as a biomarker and may influence LXR activity. Cell. Physiol. Biochem. 2016, 39, 2239–2248. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. New pathway of ethanol metabolism in the liver. Gastroenterology 1970, 59, 930–937. [Google Scholar] [PubMed]
- Lieber, C.S. Hepatic, metabolic and toxic effects of ethanol: 1991 update. Alcohol. Clin. Exp. Res. 1991, 15, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, L.; Song, Z.; Lambert, J.C.; McClain, C.J.; Kang, Y.J. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am. J. Pathol. 2003, 163, 1137–1146. [Google Scholar] [CrossRef]
- O’Shea, R.S.; Dasarathy, S.; McCullough, A.J. Alcoholic liver disease. Hepatology 2010, 51, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Marcos, M.; Kodys, K.; Csak, T.; Catalano, D.; Mandrekar, P.; Szabo, G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J. Biol. Chem. 2011, 286, 1436–1444. [Google Scholar] [CrossRef]
- Bala, S.; Szabo, G. MicroRNA Signature in Alcoholic Liver Disease. Int. J. Hepatol. 2012, 2012. [Google Scholar] [CrossRef]
- Donohue, T.M., Jr. Alcohol-induced steatosis in liver cells. World J. Gastroenterol. 2007, 13, 4974–4978. [Google Scholar]
- Ponugoti, B.; Kim, D.H.; Xiao, Z.; Smith, Z.; Miao, J.; Zang, M.; Wu, S.Y.; Chiang, C.M.; Veenstra, T.D.; Kemper, J.K. SIRT1 Deacetylates and Inhibits SREBP-1C Activity in Regulation of Hepatic Lipid Metabolism. J. Biol. Chem. 2010, 285, 33959–33970. [Google Scholar] [CrossRef]
- You, M.; Jogasuria, A.; Taylor, C.; Wu, J. Sirtuin 1 signaling and alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 88–100. [Google Scholar]
- Liang, X.M.; Hu, M.; Rogers, C.Q.; Shen, Z.; You, M. Role of SIRT1-FoxO1 signaling in dietary saturated fat-dependent upregulation of liver adiponectin receptor 2 in ethanol-administered mice. Antioxid. Redox Signal. 2011, 15, 425–435. [Google Scholar] [CrossRef]
- Yin, H.; Hu, M.; Zhang, R.; Shen, Z.; Flatow, L.; You, M. MicroRNA-217 promotes ethanol-induced fat accumulation in hepatocytes by down-regulating SIRT1. J. Biol. Chem. 2012, 287, 9817–9826. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Wang, R.; Tuo, H.; Guo, Y.; Yi, L.; Wang, D.; Wang, J. MicroRNA-217 promotes angiogenesis of human cytomegalovirus-infected endothelial cells through downregulation of SIRT1 and FOXO3A. PLoS ONE 2013, 8, e83620. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Liang, X.; Rogers, C.Q.; Rideout, D.; You, M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G364–G374. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.E.; Finck, B.N. Dual function lipin proteins and glycerolipid metabolism. Trends Endocrin. Met. 2011, 22, 226–233. [Google Scholar] [CrossRef]
- Hu, M.; Wang, F.M.; Li, X.; Rogers, C.Q.; Liang, X.M.; Finck, B.N.; Mitra, M.S.; Zhang, R.; Mitchell, D.A.; You, M. Regulation of hepatic lipin-1 by ethanol: Role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice. Hepatology 2012, 55, 437–446. [Google Scholar] [CrossRef]
- Khalil, M.B.; Sundaram, M.; Zhang, H.Y.; Links, P.H.; Raven, J.F.; Manmontri, B.; Sariahmetoglu, M.; Tran, K.; Reue, K.; Brindley, D.N.; et al. The level and compartmentalization of phosphatidate phosphatase-1 (lipin-1) control the assembly and secretion of hepatic VLDL. J. Lipid Res. 2009, 50, 47–58. [Google Scholar] [CrossRef]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef]
- Lavanchy, D. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 2011, 17, 107–115. [Google Scholar] [CrossRef]
- Naoki, T.; Kyoji, M.; Kendo, K.; Kazuhiko, K.; Gonzalez, F.J.; Toshifumi, A. PPARalpha activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J. Clin. Investig. 2008, 118, 683–694. [Google Scholar]
- Ferguson, D.; Zhang, J.; Davis, M.A.; Helsley, R.N.; Vedin, L.L.; Lee, R.G.; Crooke, R.M.; Graham, M.J.; Parini, P.; Brown, J.M. The Lipid Droplet Associated Protein Perilipin 3 facilitates hepatitis C Virus-Driven Hepatic Steatosis. J. Lipid Res. 2016. [Google Scholar] [CrossRef]
- Sarnow, P.; Jopling, C.L.; Norman, K.L.; Schutz, S.; Wehner, K.A. MicroRNAs: Expression, avoidance and subversion by vertebrate viruses. Nat. Rev. Microbiol. 2006, 4, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, R.; Chen, R.; Lyn, R.K.; Jones, D.M.; O’Hara, S.; Rouleau, Y.; Cheng, J.; Srinivasan, P.; Nasheri, N.; Russell, R.S.; et al. Hepatitis C Virus Induced Up-Regulation of MicroRNA-27: A Novel Mechanism for Hepatic Steatosis. Hepatology 2014, 59, 98–108. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Liu, S.A.; Zhang, J.Q.; Ju, W.; Quan, M.; Feng, S.H.; Dong, J.L.; Gao, P.; Cheng, J. MicroRNA-185-5p mediates regulation of SREBP2 expression by hepatitis C virus core protein. World J. Gastroenterol. 2015, 21, 4517–4525. [Google Scholar] [CrossRef]
- Liu, Z.C.; Wang, Y.P.; Borlak, J.; Tong, W.D. Mechanistically linked serum miRNAs distinguish between drug induced and fatty liver disease of different grades. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ge, G.; Pan, T.; Wen, D.; Gan, J. A pilot study of serum microRNAs panel as potential biomarkers for diagnosis of nonalcoholic fatty liver disease. PLoS ONE 2014, 9, e105192. [Google Scholar] [CrossRef]
- Pirola, C.J.; Gianotti, T.F.; Castano, G.O.; Sookoian, S. Circulating MicroRNA-122 signature in nonalcoholic fatty liver disease and cardiovascular disease: A new endocrine system in metabolic syndrome. Hepatology 2013, 57, 2545–2547. [Google Scholar] [CrossRef]
- Miyaaki, H.; Ichikawa, T.; Kamo, Y.; Taura, N.; Honda, T.; Shibata, H.; Milazzo, M.; Fornari, F.; Gramantieri, L.; Bolondi, L.; et al. Significance of serum and hepatic microRNA-122 levels in patients with non-alcoholic fatty liver disease. Liver Int. 2014, 34, e302–e307. [Google Scholar] [CrossRef]
- Bala, S.; Petrasek, J.; Mundkur, S.; Catalano, D.; Levin, I.; Ward, J.; Alao, H.; Kodys, K.; Szabo, G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 2012, 56, 1946–1957. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Saha, B.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J. Transl. Med. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, J.K.; Gerhard, G.S. Circulating microRNAs in nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Venugopal, S.K. Circulating microRNAs: Possible role as non-invasive diagnostic biomarkers in liver disease. Clin. Res. Hepatol. Gastroenterol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, N.; Steer, B.M.; Ingram, A.J.; Gupta, M.; Al-Omran, M.; et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 2012, 126. [Google Scholar] [CrossRef]
- Uzumcu, A.; Tekguc, M.; Sevli, S.; Bayrak, O.F.; Ozen, M. The role of microRNA (miRNA)s as a novel tool in cancer diagnosis and therapy. Int. Un. Biochem. Mol. Biol. Life 2009, 61, 298. [Google Scholar]
- Xu, S.; Kang, C.; Chen, M.; Zhou, P.; He, G.; Cui, Y.; Yang, D.; Wu, Y. Dynamic expression of AQP4 in early stage of ischemia/reperfusion rats and cerebral edema. Chin. Pharmacol. Bull. 2016, 32, 1433–1441. [Google Scholar]
- Sun, Y.; Kang, C.; Zhang, A.; Liu, F.; Hu, J.; Zhong, X.; Xie, J. Co-delivery of dual-drugs with nanoparticle to overcome multidrug resistance. Eur. J. Biomed. Res. 2016, 2, 12–18. [Google Scholar] [CrossRef]
- Jing, X.; Yang, Z.; Zhou, C.; Jing, Z.; Lee, R.J.; Teng, L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol. Adv. 2016, 34, 343–353. [Google Scholar]
- Yang, Z.; Yu, B.; Zhu, J.; Huang, X.; Xie, J.; Xu, S.; Yang, X.; Wang, X.; Yung, B.C.; Lee, L.J. A microfluidic method to synthesize transferrin-lipid nanoparticles loaded with siRNA LOR-1284 for therapy of acute myeloid leukemia. Nanoscale 2014, 6, 9742–9751. [Google Scholar] [CrossRef]
- Kang, C.; Sun, Y.; Wang, M.; Cheng, X. Nanosized camptothecin conjugates for single and combined drug delivery. Eur. J. Biomed. Res. 2016, 2, 8–14. [Google Scholar] [CrossRef]
- Kang, C.; Hu, K. Modulation of the two-pore domain potassium channel TASK-1 by caveolin-3. FASEB J. 2015. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, X.; Zhang, X.; Liu, B.; Huang, L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for Cancer Therapy. FASEB J. 2010, 18, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.B.; Zhu, L.Y.; Wang, Y.G.; Li, F.; Zhang, X.Y.; Dai, W.J. Systemic transcriptome analysis of hepatocellular carcinoma. Tumour Biol. 2016, 37, 13323–13331. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Sun, Y.; Kang, C.; Wan, G. The theory of dielectrophoresis and its applications on medical and materials research. Eur. J. BioMed. Res. 2017, 2, 7–11. [Google Scholar] [CrossRef]
- Yao, Z.; Sun, Y.; Kang, C. Structure and self-assembly of multicolored naphthalene diimides semiconductor. Nano Life 2016, 6, 6. [Google Scholar] [CrossRef]
- Yan, G.; Du, Q.; Wei, X.; Miozzi, J.; Kang, C.; Wang, J.; Han, X.; Pan, J.; Xie, H.; Chen, J.; et al. Application of Real-Time Cell Electronic Analysis System in Modern Pharmaceutical Evaluation and Analysis. Molecules 2018, 23, 3280. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Sun, Y.; Kang, C. Controlling amphiphilic functional block copolymers’ self-assembly: From structure to size. Glob. J. Nanomed. 2016. [Google Scholar] [CrossRef]
| miRNA | Species | Disease-Association | Expression | Target Genes | Findings | Pathologic (+) or Protective (−) | Reference |
|---|---|---|---|---|---|---|---|
| miR-122 | Human, mouse | NAFLD | ↑ (circulation) ↓ | HMGCR, MTTP, HMGCS1PGC1-α | Upregulate the expression of SREBP1-c, DGAT2, FAS and ACC1 | + | [37,112,113] |
| miR-370 | Human | NAFLD | ↑ | SREBP-1c, DGAT2 | Upregulate the expression of genes involved in lipogenesis Upregulate the expression of miR-122; | + | [39] |
| miR-29c | Human | NAFLD | ↑ | HMGR, Sirt1 | Regulate insulin resistance and lipid metabolism | − | [84] |
| miR-216 | Mouse | NAFLD | ↓ | FAS, SREBP-1c | Regulate the lipid synthesis | / | [61] |
| miR-302a | Mouse | NAFLD | ↓ | ELOVL; ABCA1 | Regulate hepatic lipid accumulation | / | [61] |
| miR-34a | Mouse, Human | NAFLD | ↑ | PPARα, SIRT1 | Decrease FA β-oxidation | − | [68,75,84] |
| miR-24 | Human, Mouse | NAFLD | ↑ | Insig1 | Downregulate Insig1 expression; Promote SREBP-1 processing | − | [72] |
| miR-21 | Mouse | NAFLD | ↓ | HMGCR | Regulate liver TG and cholesterol metabolism | + | [76] |
| miR-149 | Mouse | NAFLD | ↑ | FGF21 | Regulate lipogenesis in HepG2 cells | − | [77] |
| miR-10b | Human | NAFLD | ↑ | PPARα | Overexpression of miR-10b increases the triglyceride levels in hepatocytes | − | [82] |
| miR-155 | Mouse, Human | NAFLD; Alcoholic fatty liver | ↓ | LXRα | Regulate LXRα/SREBP-1c signaling and influence liver lipid accumulation. | +/− | [117,118,119,124,125] |
| miR-467 | Mouse | NAFLD | ↓ | LPL | Regulate lipid metabolism through target LPL | + | [86] |
| miR-217 | Human | Alcoholic fatty liver | ↑ | SIRT1; Lipin1 | Promote ethanol induced -fat accumulation in hepatocytes | +/− | [133] |
| miR-27 | Human | DyslipidemiaVirus hepatitis | ↑ | PPARα; ANGPTL3 | Promote triglyceride accumulation in hepatocytes and inhibit hepatitis C virus replication dyslipidemia animal model | +/− | [60,81,136] |
| miR-185-5p | Human | Virus hepatitis | ↓ | SREBP2 | Regulate cholesterol homeostasis in liver | − | [137] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Peng, J.; Luan, Z.; Zheng, F.; Su, W. MicroRNAs as a Novel Tool in the Diagnosis of Liver Lipid Dysregulation and Fatty Liver Disease. Molecules 2019, 24, 230. https://doi.org/10.3390/molecules24020230
Yu J, Peng J, Luan Z, Zheng F, Su W. MicroRNAs as a Novel Tool in the Diagnosis of Liver Lipid Dysregulation and Fatty Liver Disease. Molecules. 2019; 24(2):230. https://doi.org/10.3390/molecules24020230
Chicago/Turabian StyleYu, Jingwei, Jun Peng, Zhilin Luan, Feng Zheng, and Wen Su. 2019. "MicroRNAs as a Novel Tool in the Diagnosis of Liver Lipid Dysregulation and Fatty Liver Disease" Molecules 24, no. 2: 230. https://doi.org/10.3390/molecules24020230
APA StyleYu, J., Peng, J., Luan, Z., Zheng, F., & Su, W. (2019). MicroRNAs as a Novel Tool in the Diagnosis of Liver Lipid Dysregulation and Fatty Liver Disease. Molecules, 24(2), 230. https://doi.org/10.3390/molecules24020230




