Aptamer Efficacies for In Vitro and In Vivo Modulation of αC-Conotoxin PrXA Pharmacology
Abstract
:1. Introduction
2. Results
2.1. αC-Conotoxin PrXA Lethality and Earlier Data on Aptamer Neutralization
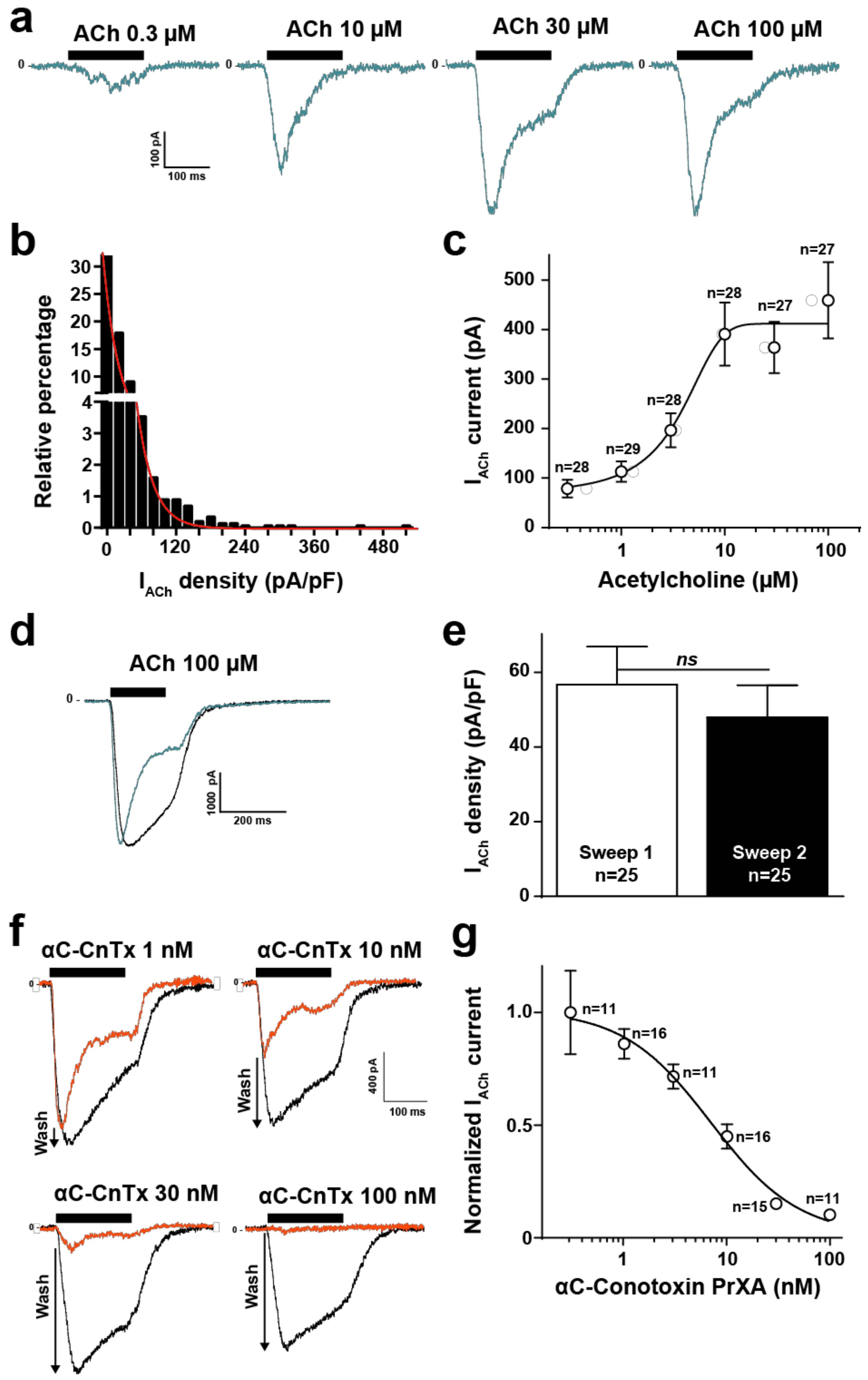
2.2. High Throughput Evaluation of ACh and αC-Conotoxin PrXA Effects on the TE671 Cell Line
2.3. Evaluation of Aptamer Efficacies in Neutralizing αC-Conotoxin PrXA-Mediated Inhibition of ACh Response
2.4. Mice Intoxication Symptoms Induced by αC-Conotoxin PrXA
2.5. Combined or Separated Injection of Aptamers Neutralize Toxin-Induced Abdominal Myoclonus
2.6. Combined or Separated Injection of Aptamers Neutralize Toxin-Induced Lethality
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. Toxin and Aptamers Preparation
4.4. Aptamer Potency to Neutralize Abdominal Myoclonus
4.5. Aptamer Potency to Neutralize Toxin-Induced Lethality
4.6. Automated Patch-Clamp Recordings
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rojas, G.; Jimenez, J.M.; Gutierrez, J.M. Caprylic acid fractionation of hyperimmune horse plasma: Description of a simple procedure for antivenom production. Toxicon 1994, 32, 351–363. [Google Scholar] [CrossRef]
- Hawgood, B.J. Doctor Albert Calmette 1863–1933: Founder of antivenomous serotherapy and of antituberculous BCG vaccination. Toxicon 1999, 37, 1241–1258. [Google Scholar] [CrossRef]
- Wade, L. Drug industry. For Mexican antivenom maker, U.S. market is a snake pit. Science 2014, 343, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Leon, G.; Burnouf, T. Antivenoms for the treatment of snakebite envenomings: The road ahead. Biol. J. Int. Assoc. Biol. Stand. 2011, 39, 129–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Froude, J.W.; Stiles, B.; Pelat, T.; Thullier, P. Antibodies for biodefense. mAbs 2011, 3, 517–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stechmann, B.; Bai, S.K.; Gobbo, E.; Lopez, R.; Merer, G.; Pinchard, S.; Panigai, L.; Tenza, D.; Raposo, G.; Beaumelle, B.; et al. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell 2010, 141, 231–242. [Google Scholar] [CrossRef] [PubMed]
- El-Aziz, T.M.A.; Ravelet, C.; Molgo, J.; Fiore, E.; Pale, S.; Amar, M.; Al-Khoury, S.; Dejeu, J.; Fadl, M.; Ronjat, M.; et al. Efficient functional neutralization of lethal peptide toxins in vivo by oligonucleotides. Sci. Rep. 2017, 7, 7202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesselberth, J.R.; Miller, D.; Robertus, J.; Ellington, A.D. In vitro selection of RNA molecules that inhibit the activity of ricin A-chain. J. Biol. Chem. 2000, 275, 4937–4942. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Dutertre, S.; Vetter, I.; Christie, M.J. Conus venom peptide pharmacology. Pharmacol. Rev. 2012, 64, 259–298. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, E.C.; Olivera, B.M.; Teichert, R.W. AlphaC-conotoxin PrXA: A new family of nicotinic acetylcholine receptor antagonists. Biochemistry 2007, 46, 8717–8724. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.R.; Darling, J.; Pilkington, G.J.; Lantos, P.L.; Reeves, B.R.; Cooper, C.S. Characterization of the human cell line TE671. Carcinogenesis 1989, 10, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, R.; Luther, M.; Lindstrom, J. The human medulloblastoma cell line TE671 expresses a muscle-like acetylcholine receptor. Cloning of the alpha-subunit cDNA. FEBS Lett. 1988, 226, 235–240. [Google Scholar] [CrossRef]
- Shao, Z.; Mellor, I.R.; Brierley, M.J.; Harris, J.; Usherwood, P.N. Potentiation and inhibition of nicotinic acetylcholine receptors by spermine in the TE671 human muscle cell line. J. Pharmacol. Exp. Ther. 1998, 286, 1269–1276. [Google Scholar] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, S.C.; Awazu, K.; Fujimaki, M.; Shimizu, K.; Mizutani, W.; Tsukagoshi, K. Surface functionalization chemistries on highly sensitive silica-based sensor chips. Analyst 2012, 137, 3520–3527. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the αC-conotoxin PrXA are available from the authors. |

| Treatments | Concentration (µg/g b.w.) | Sex | D/T | Mortality Latency (min) | Toxic Symptoms |
|---|---|---|---|---|---|
| Control | – | Male Female | 0/3 0/3 | – – – – | None |
| αC-conotoxin PrXA | 0.005 | Male Female | 3/3 0/3 | >23, <25 | Hypoactivity, asthenia, tremors, loss of the righting reflex, myoclonus, exophthalmos |
| 0.008 | Male Female | 3/3 0/3 | >23, <24 | Hypoactivity, asthenia, tremors, loss of the righting reflex, myoclonus, exophthalmos | |
| 0.01 | Male Female | 2/3 1/3 | >10, <12 >12, <12 | Hypoactivity, asthenia, tremors, loss of the righting reflex, myoclonus, exophthalmos | |
| 0.05 | Male Female | 3/3 2/3 | >1, <3 >1, <2 | Hypoactivity, asthenia, tremors, loss of the righting reflex, myoclonus, exophthalmos | |
| 0.1 | Male Female | 3/3 2/3 | >1, <3 >1, <2 | Hypoactivity, asthenia, tremors, loss of the righting reflex, myoclonus, exophthalmos, salivation and syncope | |
| 0.5 | Male Female | 3/3 3/3 | >1, <2 >1, <2 | Hypoactivity, salivation, tachypnea, tremors, loss of the righting reflex, myoclonus, exophthalmos, salivation and syncope | |
| 1 | Male Female | 3/33/3 | >1, <2 >1, <2 | Hypoactivity, salivation, tachypnea, tremors, loss of the righting reflex, myoclonus, exophthalmos, salivation and syncope | |
| 1.5 | Male Female | 3/3 3/3 | >1, <2 >1, <2 | Salivation, tachypnea, tremors, loss of the righting reflex, myoclonus, exophthalmos, salivation and syncope |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taiwe, G.S.; Montnach, J.; Nicolas, S.; De Waard, S.; Fiore, E.; Peyrin, E.; El-Aziz, T.M.A.; Amar, M.; Molgó, J.; Ronjat, M.; et al. Aptamer Efficacies for In Vitro and In Vivo Modulation of αC-Conotoxin PrXA Pharmacology. Molecules 2019, 24, 229. https://doi.org/10.3390/molecules24020229
Taiwe GS, Montnach J, Nicolas S, De Waard S, Fiore E, Peyrin E, El-Aziz TMA, Amar M, Molgó J, Ronjat M, et al. Aptamer Efficacies for In Vitro and In Vivo Modulation of αC-Conotoxin PrXA Pharmacology. Molecules. 2019; 24(2):229. https://doi.org/10.3390/molecules24020229
Chicago/Turabian StyleTaiwe, Germain Sotoing, Jérôme Montnach, Sébastien Nicolas, Stéphan De Waard, Emmanuelle Fiore, Eric Peyrin, Tarek Mohamed Abd El-Aziz, Muriel Amar, Jordi Molgó, Michel Ronjat, and et al. 2019. "Aptamer Efficacies for In Vitro and In Vivo Modulation of αC-Conotoxin PrXA Pharmacology" Molecules 24, no. 2: 229. https://doi.org/10.3390/molecules24020229
APA StyleTaiwe, G. S., Montnach, J., Nicolas, S., De Waard, S., Fiore, E., Peyrin, E., El-Aziz, T. M. A., Amar, M., Molgó, J., Ronjat, M., Servent, D., Ravelet, C., & De Waard, M. (2019). Aptamer Efficacies for In Vitro and In Vivo Modulation of αC-Conotoxin PrXA Pharmacology. Molecules, 24(2), 229. https://doi.org/10.3390/molecules24020229









