Anti-Platelet Properties of Phenolic Extracts from the Leaves and Twigs of Elaeagnus rhamnoides (L.) A. Nelson
Abstract
1. Introduction
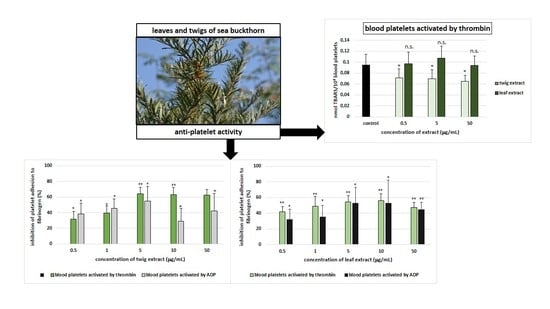
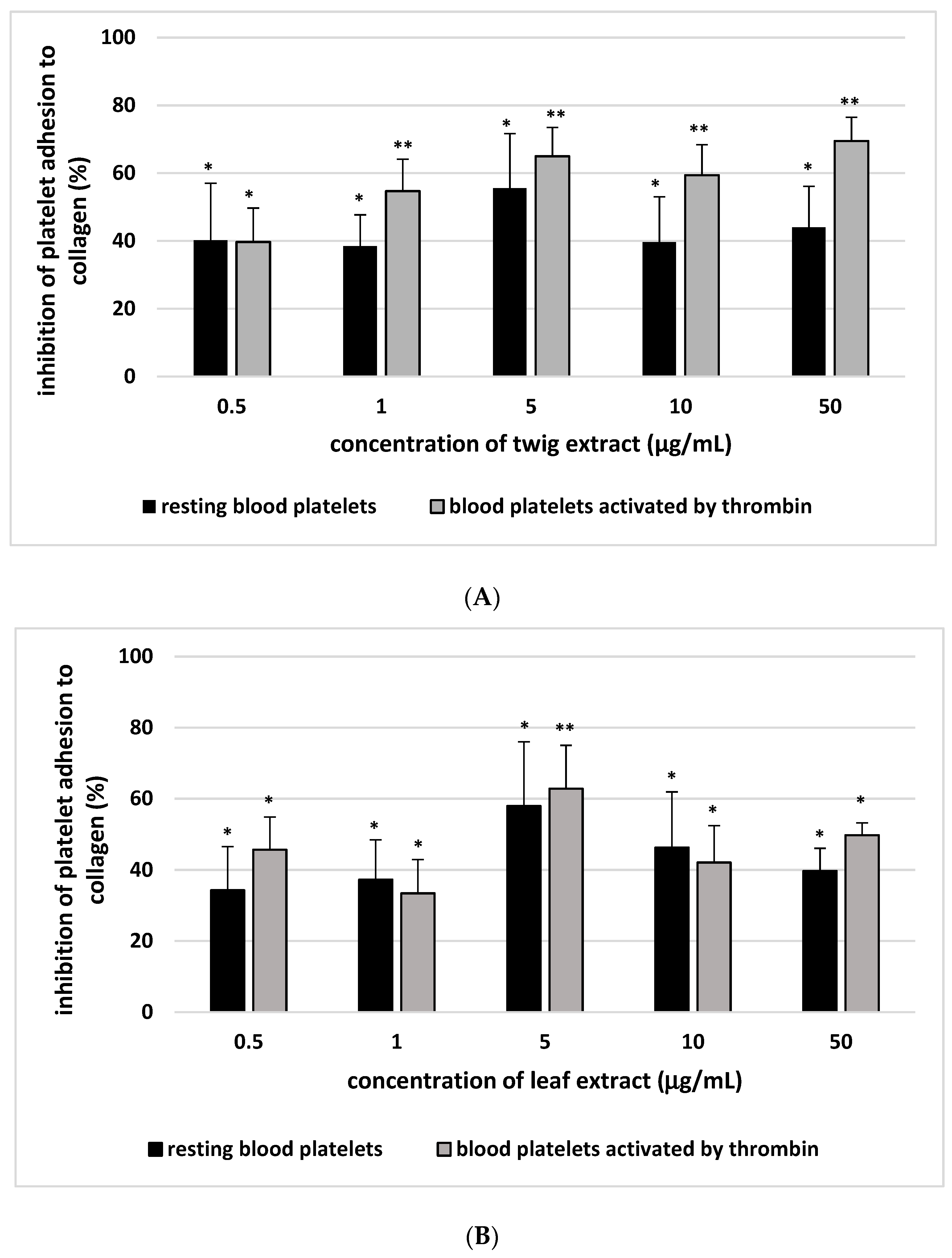
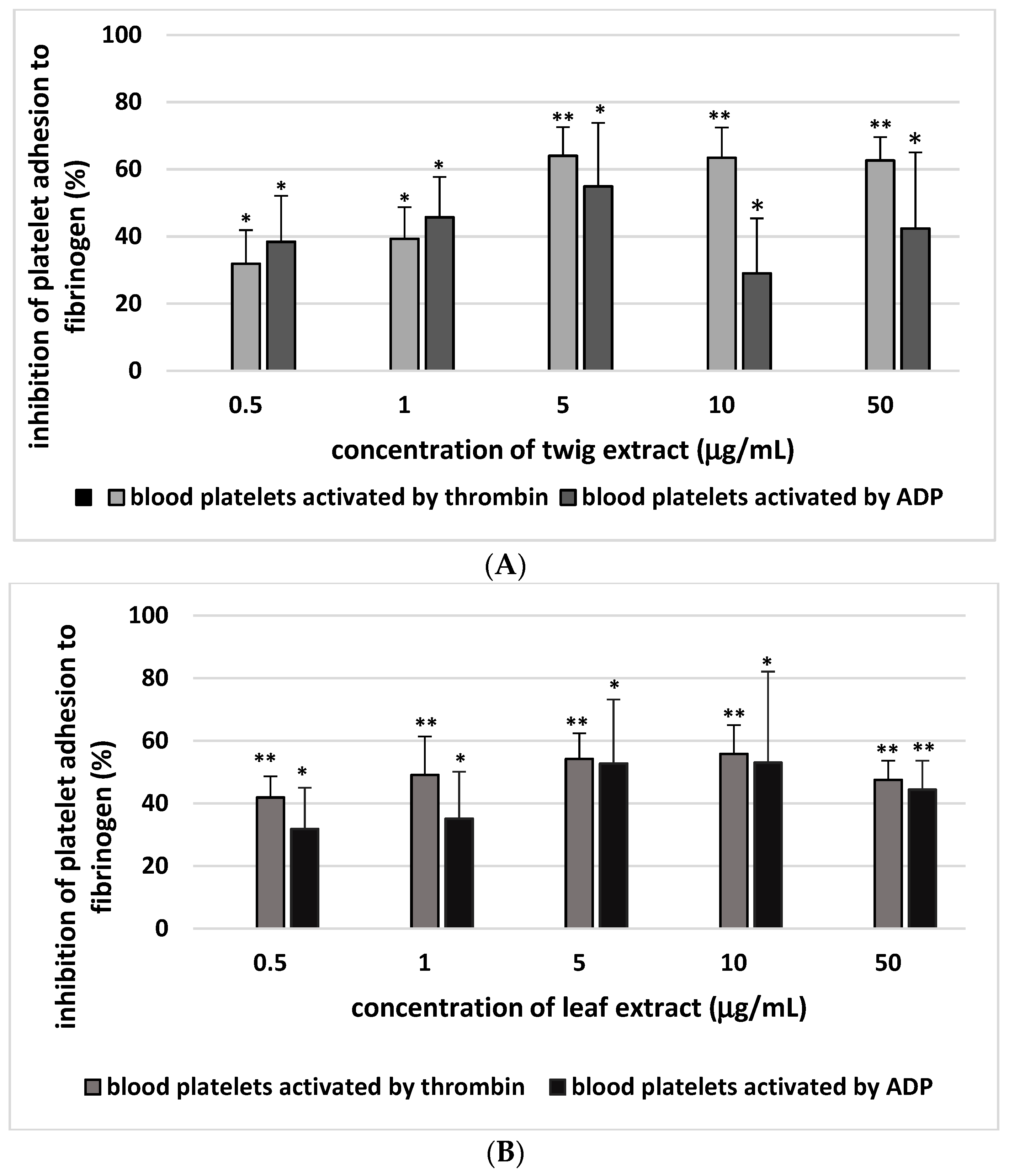
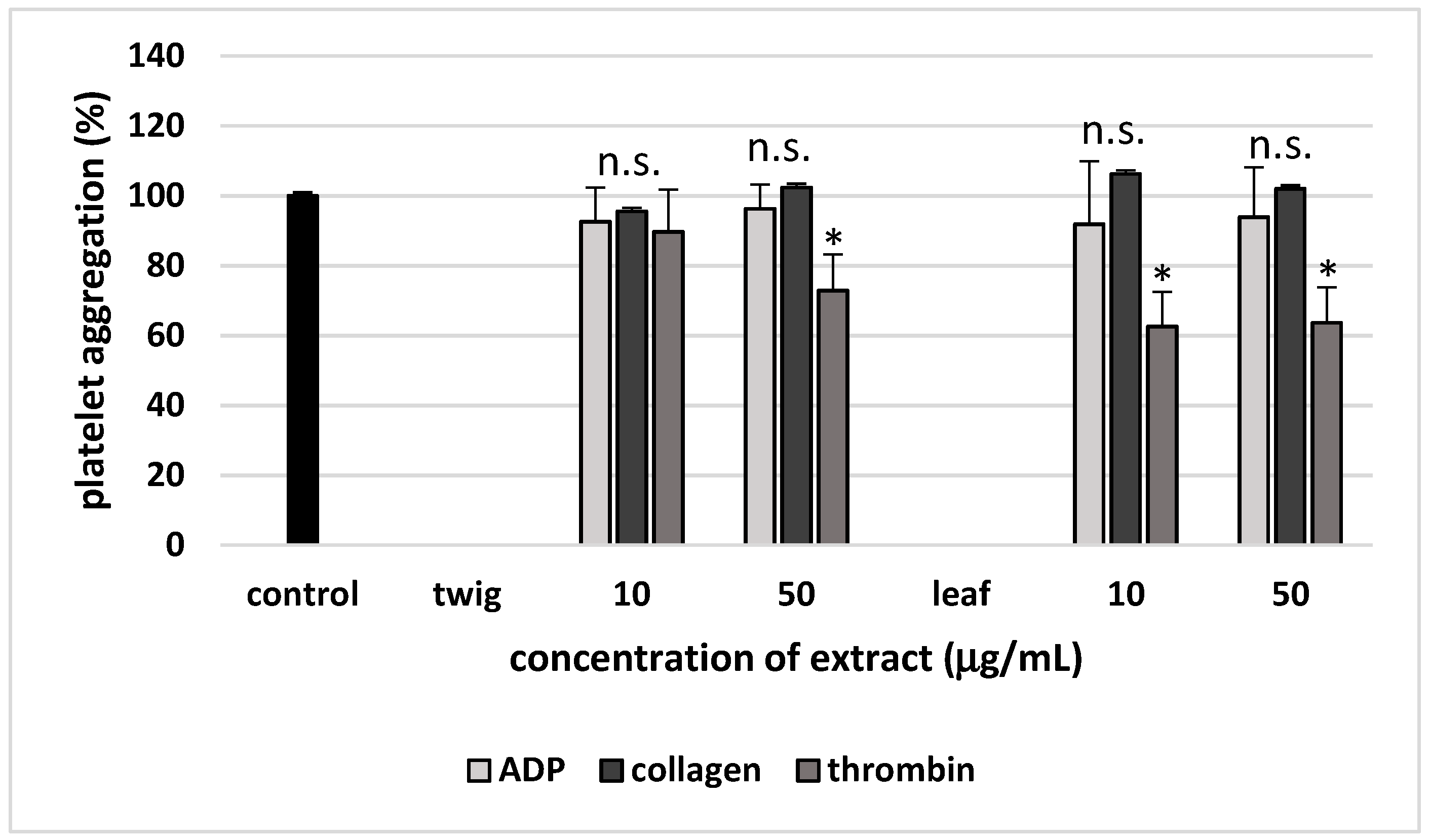
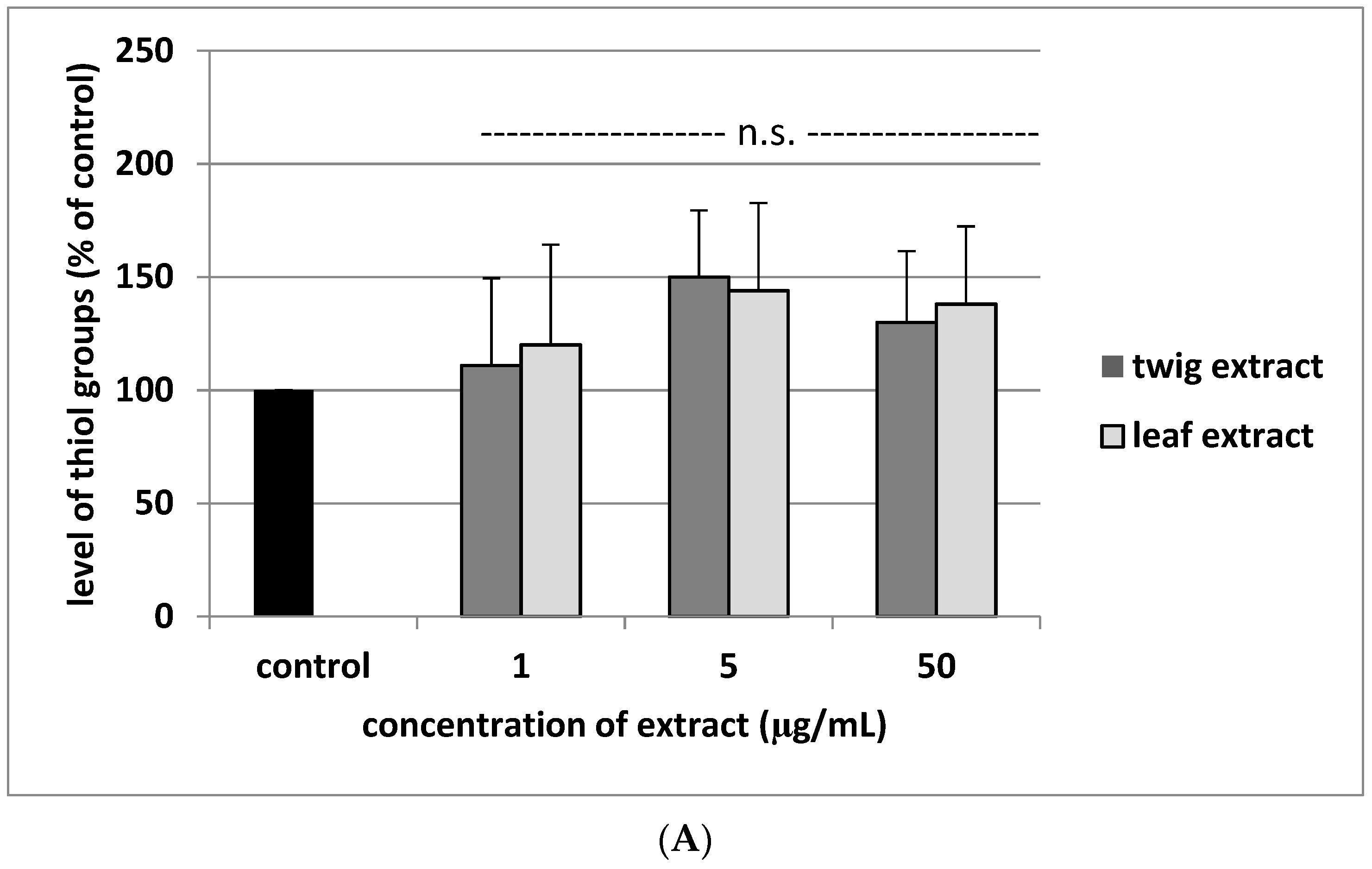
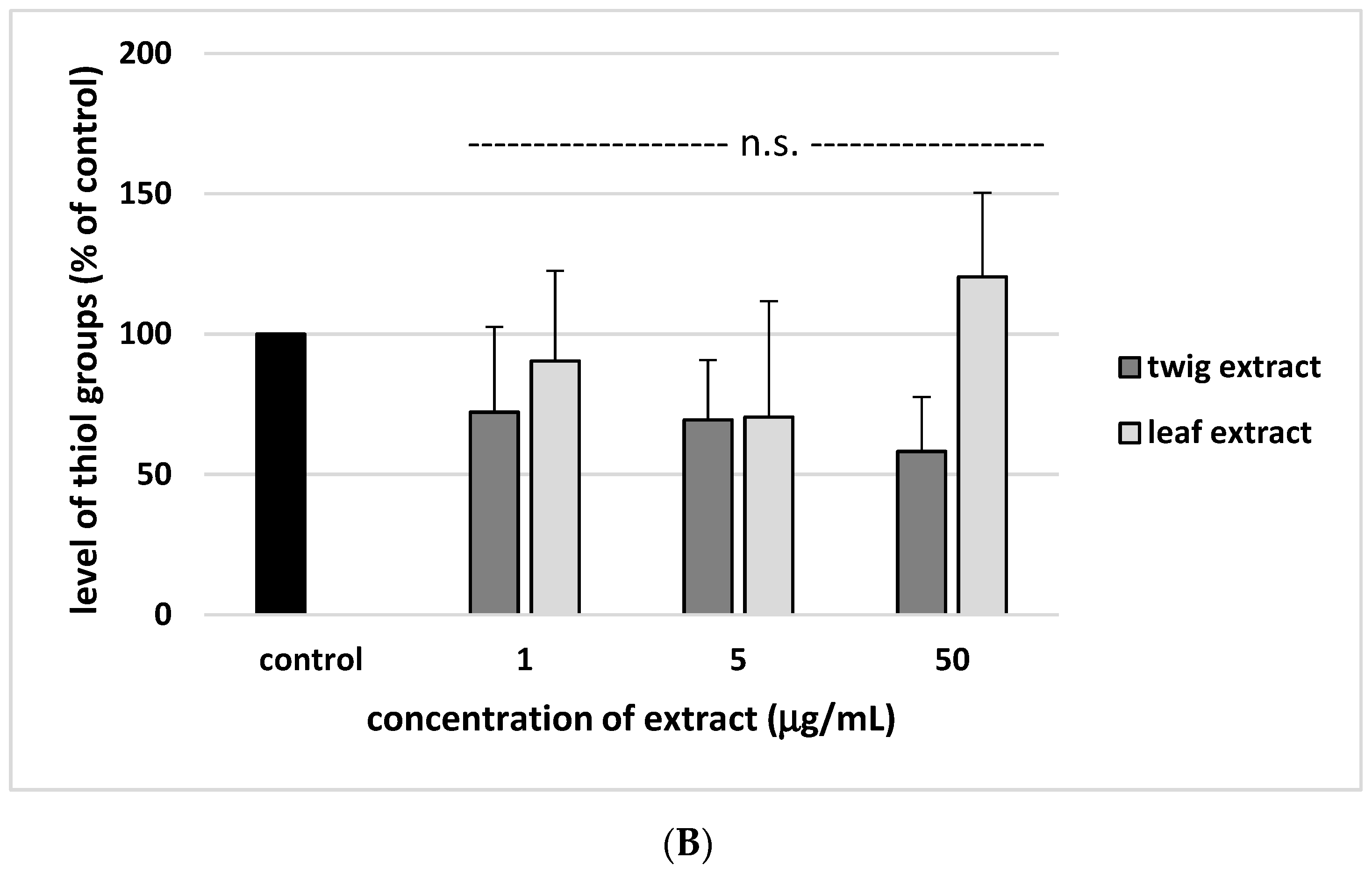
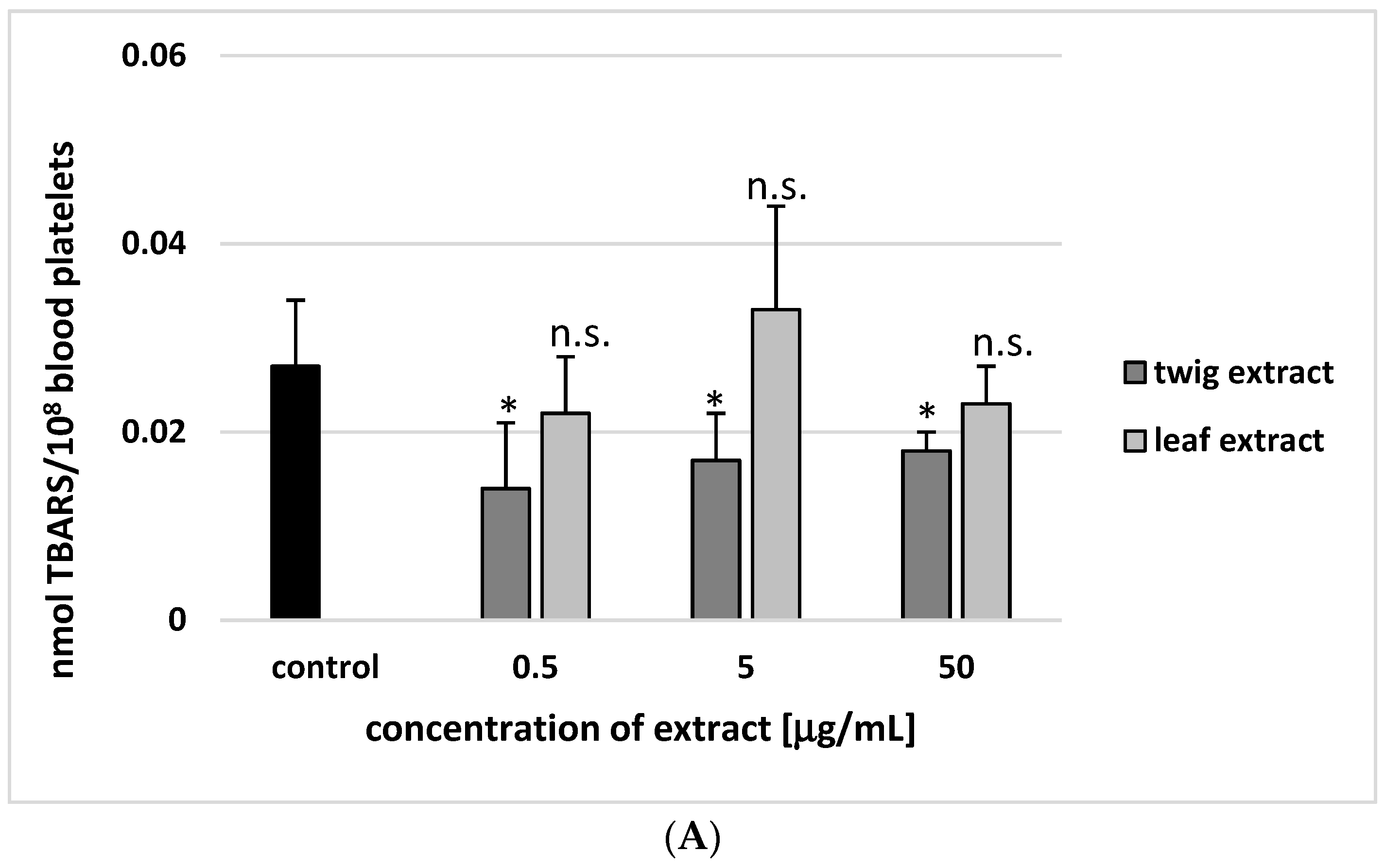
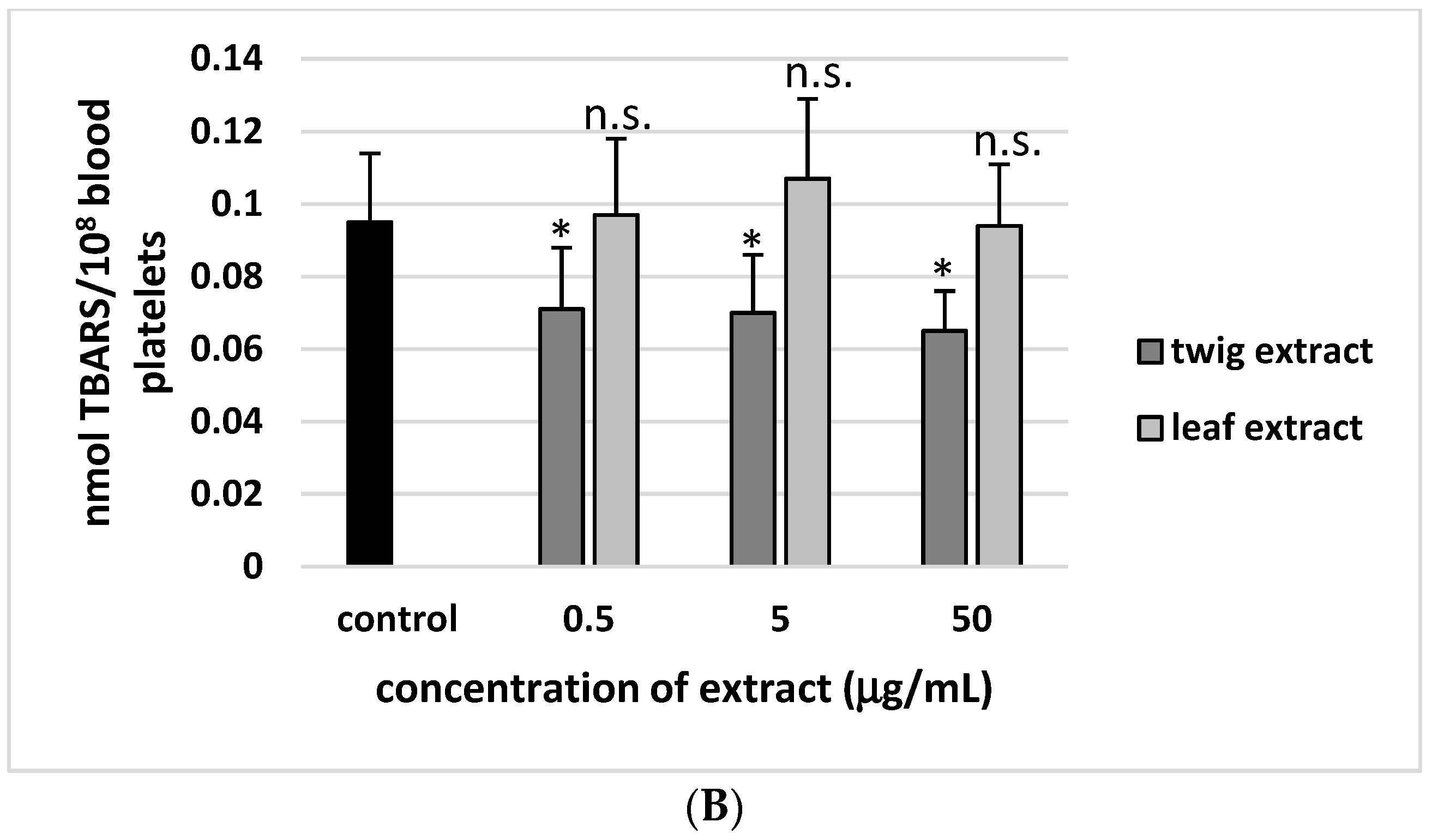
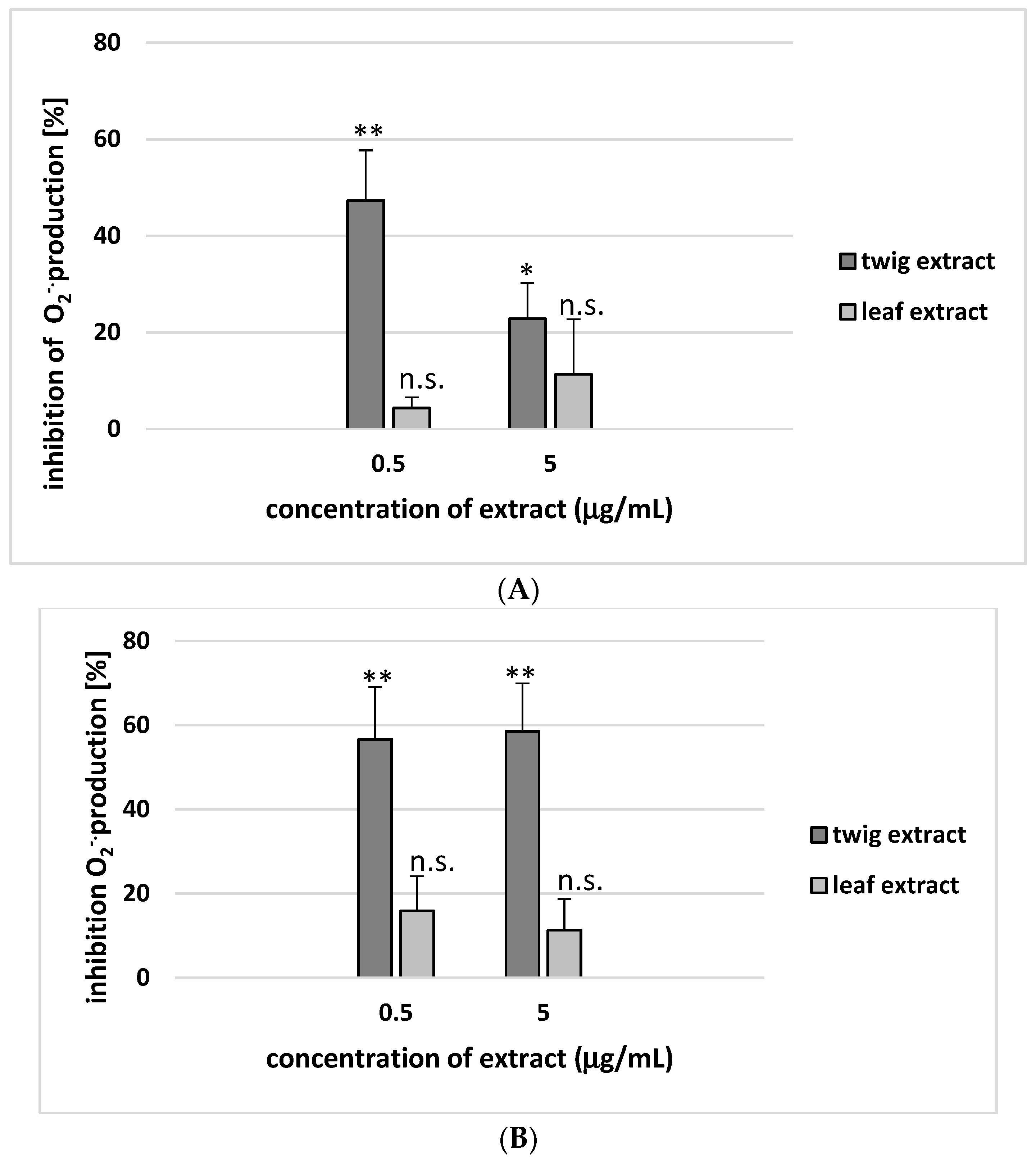
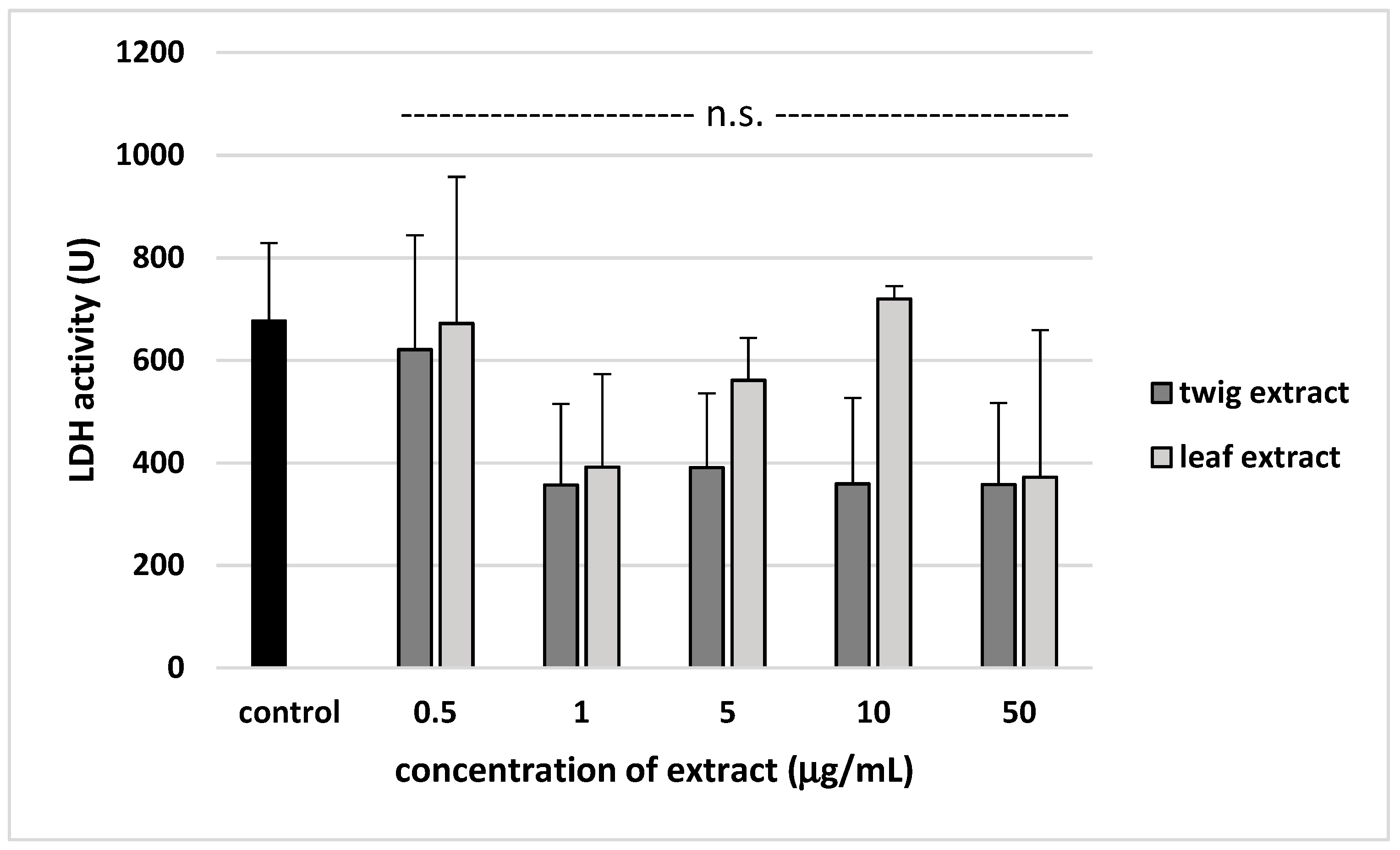
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Chemical Characteristics of Extracts from Sea Buckthorn Twigs and Leaves
4.4. Blood Platelet Isolation
4.5. Platelet Adhesion
4.6. Platelet Aggregation
4.7. Glutathione and Thiol Group Measurement
4.8. Lipid Peroxidation Measurement
4.9. Superoxide Anion Measurement
4.10. Lactate Dehydrogenase (LDH) Activity Measurement
4.11. Data Analysis
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
Abbreviations
References
- Blockmans, D.; Deckmyn, H.; Vermylen, J. Platelet activation. Blood Rev. 1995, 9, 143–156. [Google Scholar] [CrossRef]
- Stakos, D.A.; Tziakas, D.N.; Stellos, K. Mechanisms of platelet activation in acute coronary syndromes. Curr. Vasc. Pharmacol. 2012, 10, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Allender, S.; Foster, C.; Hutchinsons, L.; Arambepola, C. Quantification of urbanization in relation to chronic diseases in developing countries: A systematic review. J. Urban Health. 2008, 85, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, I.I.; Tillmann, T.; Banerjee, A. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study. Lancet 2013, 385, 117–171. [Google Scholar]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Olas, B. The multifunctionality of berries toward blood platelet and the role of berry phenolics in cardiovascular disorders. Platelets. 2017, 28, 540–549. [Google Scholar] [CrossRef]
- Olas, B. Berry phenolic antioxidants–implications for human health? Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Olas, B. Dietary supplements with anti-platelet activity: A solution for everyone? Adv. Nutr. 2018, 9, 51–57. [Google Scholar] [CrossRef]
- Eccleston, C.; Baoru, Y.; Tahvonen, R.; Kallio, H.; Rimbach, G.H.; Minihane, A.M. Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans. J. Nutr. Biochem. 2002, 13, 346–354. [Google Scholar] [CrossRef]
- Basu, M.; Prasad, R.; Jayamurthy, P.; Pal, K.; Arumughan, C.; Sawhney, R.C. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine 2007, 14, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhao, J.; Zhang, W.; Zhuang, X.; Wang, J.; Xu, R.; Xu, Z.; Qu, W. Antihypertensive effect of total flavones extracted from seed residues of Hippophae rhamnoides L. in sucrose-fed rats. J. Ethnopharmacol. 2008, 117, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Negi, B.; Kaur, R.; Dey, G. Protective effects of a novel sea buckthorn wine on oxidative stress and hipercholesterolemia. Food Funct. 2013, 4, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sayegh, M.; Miglio, C.; Ray, S. Potential cardiovascular implications of sea buckthorn berry consumption in humans. Int. J. Food Sci. Nutr. 2014, 65, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Kontek, B.; Szczęsna, M.; Grabarczyk, Ł.; Stochmal, A.; Żuchowski, J. Inhibition of blood platelet adhesion by the phenolics’ rich fraction of Hippophae rhamnoides L. fruits. J. Physiol. Pharmacol. 2017, 2, 23–29. [Google Scholar]
- Skalski, B.; Kontek, B.; Olas, B.; Żuchowski, J.; Stochmal, A. Phenolic fraction and non-polar fraction from sea buckthorn leaves and twigs: Chemical profile and biological activity. Future Med. Chem. 2018, 10, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Wachowicz, B.; Nowak, P.; Kędzierska, M.; Tomczak, A.; Stochmal, A.; Oleszek, W.; Jeziorski, A.; Piekarski, J. Studies on antioxidant properties of polyphenol- rich extract from berries of Aronia melanocarpa on blood platelets. J. Phys. Pharm. 2008, 59, 823–835. [Google Scholar]
- Olas, B.; Wachowicz, B.; Tomczak, A.; Erler, J.; Stochmal, A.; Oleszek, W. Comparative anti-platelet and antioxidant properties of polyphenol-rich extracts from: Berries of Aronia melanocarpa, seeds of grape and bark of Yucca schidigera in vitro. Platelets. 2008, 19, 70–77. [Google Scholar] [CrossRef]
- Kędzierska, M.; Olas, B.; Wachowicz, B.; Stochmal, A.; Oleszek, W.; Jeziorski, A.; Piekarski, J.; Głowacki, R. An extract from berries of Aronia melanocarpa modulates the generation of superoxide anion radicals in blood platelets from breast cancer patients. Planta Med. 2009, 75, 1405–1409. [Google Scholar] [CrossRef]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Kontek, B.; Malinowska, P.; Żuchowski, J.; Stochmal, A. Hippophae rhamnoides L. fruits reduce the oxidative stress in human blood platelets and plasma. Oxid. Med. Cell Longev. 2016, 2016, 4692486. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.K.; Kumar, R.; Mandotra, S.K.; Meena, R.N.; Siddiqui, M.S.; Sawhney, R.C.; Gupta, A. Safety and wound healing efficacy of sea buckthorn (Hippophae rhamnoides L.) seed oil in experimental rats. Food Chem. Toxicol. 2009, 47, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, D.T.; Yogendra Kumar, M.S.; Verma, S.K.; Singh, V.K.; Som Nath Singh. Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 2011, 49, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Mann, K.; Chinchubose; Das, D.K.; Sinha, M.; Kesh, S.B.; Das, U.; Dey, R.S.; Banerji, A.; Dey, S. Sea buckthorn (Hippophae rhamnoides L.) leaf extract ameliorates the gamma radiation mediated DNA damage and hepatic alterations. Indian J. Exp. Biol. 2014, 52, 952–964. [Google Scholar]
- Sadowska, B.; Budzynska, A.; Stochmal, A.; Zuchowski, J.; Rozalska, B. Novel properties of Hippophae rhamnoides L. twig and leaf extracts–anti-virulence action and synergy with antifungals studied in vitro on Candida spp. model. Microb. Pathog. 2017, 107, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liimatainen, J.; Allanne, A.-L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berriesand leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Różalska, B.; Sadowska, B.; Żuchowski, J.; Więckowska-Szakiel, M.; Budzyńska, A.; Wójcik, U.; Stochmal, A. Phenolic and nonpolar fractions of Elaeagnus rhamnoides (L.) Nelson extracts as virulence modulators—In vitro study on bacteria, fungi, and epithelial cells. Molecules 2018, 23, 1498. [Google Scholar] [CrossRef]
- Essex, D.W.; Li, N. Redox control of platelet aggregation. Biochemistry 2003, 42, 129–136. [Google Scholar] [CrossRef]
- Karolczak, K.; Olas, B.; Kołodziejczyk, J. The role of thiols in blood platelet activation. Adv. Cell Biol. 2009, 1, 101–120. [Google Scholar]
- Hirano, I.; Mori, H.; Kato, T.; Haga, M. Safety examination of some edible plants. Part 2. J. Environ. Pathol. Toxicol. 1978, 1, 71–74. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remsey, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remsey, C. Bioavailability and bioafficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, R.; Pal, K.; Banerjee, P.K.; Sawhney, R.C. A preclinical study of the effects of sea buckthorn (Hippophae rhamnoides L.) leaf extract on cutaneous wound healing in albino rats. Int. J. Low. Extrem. Wounds. 2005, 4, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Koo, S.I.; Song, W.O.; Chun, O.K. Food matrix affecting anthocynanin bioavailability: Review. Curr. Med. Chem. 2011, 18, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Toromanovic, J.; Kovac-Besovic, E.; Sapcainin, A.; Tahirovic, I.; Rimpapa, Z.; Kroyer, G.; Sofic, E. Urinary hippuric acid after ingestion of edible fruits. Bosn. J. Basic Med. Sci. 2008, 8, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Stanley, R.; Singh, I. The ex vivo antiplatelet activation potential of fruit phenolic metabolite hippuric acid. Food Funct. 2015, 6, 2679–2683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chong, M.F.F.; Macdonald, R.; Lovegrove, J.A. Fruit polyphenols and CDV risk: A review of human intervention studies. B. J. Nutr. 2010, 104, S28–S39. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.F.; Monteiro, V.V.S.; de Souza Gomes, R.; do Carmo, M.M.; da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Broncel, M.; Markowicz, M.; Chalubinski, M.; Wojdan, K.; Mikiciuk-Olasik, E. Short-term supplementation with Aronia melanocarpa extract improves platelet aggregation, clotting, and fibrinolytsis in patients with metabolic syndrome. Eur. J. Nutr. 2012, 51, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Markowicz-Piasecka, M.; Broncel, M.; Mikiciuk-Olasik, E. Extract of Aronia melanocarpa-medified hemostasis: In vitro studies. Eur. J. Nutr. 2014, 53, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Chen, S.X.; Kini, R.M.; Xu, H.X. Effects of tannins from Geum japonicum on the catalytic activity of thrombin and factor Xa of blood coagulation cascade. J. Nat. Prod. 1998, 61, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F.; Schubert, D.; Schwartz, S.A. Amino acid sequence studies on artiodactyl fibrinopeptides I Dromedary camel, mule deer, and cape buffalo. Arch. Biochem. Biophys. 1967, 118, 456–467. [Google Scholar] [CrossRef]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Pop, R.M.; Socaciu, C.; Pintea, A.; Buzoianu, A.D.; Sanders, M.G.; Gruppen, H.; Vincken, J.P. UHPLC/PDA–ESI/MS analysis of the main berry and leaf flavonol glycosides from different Carpathian Hippophaë rhamnoides L. varieties. Phytochem. Anal. 2013, 24, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.G.; Wen, X.F.; Li, Y.H.; Matsuzaki, K.; Kitanaka, S. Inhibitory effects of the constituents of Hippophae rhamnoides on 3T3-L1 cell differentiation and nitric oxide production in RAW264. 7 cells. Chem. Pharm. Bull. 2013, 61, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.W.; Zhu, S.; Kazuma, K.; Wei, S.L.; Yoshimatsu, K.; Komatsu, K. Molecular ion index assisted comprehensive profiling of B-type oligomeric proanthocyanidins in rhubarb by high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 3555–3570. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak, B.; Kesy, A.; Michalec, L. Microplate leader—A convenient tool in studies of blood coagulation. Thromb. Res. 1997, 87, 95–103. [Google Scholar] [CrossRef]
- Bellavite, P.; Andrioli, G.; Guzzo, P.; Arigliano, P.; Chirumbolo, S.; Manzato, F.; Santonastaso, C. A colorimetric method for the measurement of platelet adhesion in microtiter plates. Anal. Biochem. 1994, 216, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Born, G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962, 194, 927–928. [Google Scholar] [CrossRef]
- Ando, Y.; Steiner, M. Sulphydryl and disulphide groups of platelet membranes: Determination of disulphide groups. Biochim. Biophys. Acta 1973, 311, 26–37. [Google Scholar] [CrossRef]
- Ando, Y.; Steiner, M. Sulphydryl and disulphide groups of platelet membranes: Determination of sulphydryl groups. Biochim. Biophys. Acta 1973, 311, 38–44. [Google Scholar] [CrossRef]
- Wachowicz, B. Adenine nucleotides in thrombocytes of birds. Cell Biochem. Funct. 1984, 2, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Druga Twarz Tlenu; Naukowe PWN: Warszawa, Poland, 1995; pp. 310–315. [Google Scholar]
- Olas, B.; Żbikowska, H.M.; Wachowicz, B.; Krajewski, T.; Buczynski, A.; Magnuszewska, A. Inhibitory effect on resveratrol on free radical generation in blood platelets. Acta Biochim. Pol. 1999, 46, 991–996. [Google Scholar]
- Wroblewski, F.; Ladue, J.S. Lactic dehydrogenase activity in blood. Proc. Soc. Exp. Biol. Med. 1955, 90, 210–213. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the Elaeagnus rhamnoides (L.) A. Nelson extracts are available from the authors. |
| Inhibition of Resting Blood Platelet Adhesion to Collagen (%) | Inhibition of Thrombin-Activated Platelets to Collagen (%) | Inhibition of Thrombin- Activated Platelets to Fibrinogen (%) | Inhibition of ADP-Activated Platelets to Fibrinogen (%) | |
|---|---|---|---|---|
| Sea buckthorn twig extract (a) | 39.6 ± 13.4 (p > 0.05, a vs. b, c) | 59.4 ± 9.0 (p < 0.05, a vs. b, c) | 63.4 ± 5.3 (p < 0.05, a vs. b, c) | 29.0 ± 16.4 (p > 0.05, a vs. b, c) |
| Sea buckthorn leaf extract (b) | 46.3 ± 15.6 (p > 0.05, b vs. c) | 42.1 ± 10.3 (p > 0.05, b vs. c) | 55.8 ± 9.2 (p < 0.05, b vs. c) | 53.0 ± 29.1 (p > 0.05, b vs. c) |
| Aronia berry extract (c) | 24.5 ± 11.4 | 34.9 ± 12.9 | 32.1 ± 17.4 | 30.7 ± 15.9 |
| No. | Compounds (Tentative Identification) | tR (min) | λ max (nm) | [M − H]− | Fragment Ions (+) (m/z) | Concentration (µg/mg) |
|---|---|---|---|---|---|---|
| [M + H]+ | ||||||
| 1 | strictinin/isomer | 13.92 | 220, 270 | 633 | 153, 277, 303, 447 | 6.6 ± 0.3 a |
| 635 | ||||||
| 2 | strictinin/isomer | 14.12 | 220, 270 | 633 | 153, 277, 303, 447 | 7.4 ± 0.3 a |
| 635 | ||||||
| 3 | stachyurin/isomer | 14.61 | 222, 270 | 935 | 153, 277, 345, 617 | 14.2 ± 0.5 a |
| 937 | ||||||
| 4 | casuarinin/isomer | 15.13 | 227, 270 | 935 | 153, 255, 345, 617 | 24.5 ± 0.5 a |
| 937 | ||||||
| 5 | casuarinin/isomer | 15.34 | 230, 270 | 935 | 153, 255, 345, 617 | 39.4 ± 0.5 a |
| 937 | ||||||
| 6 | hippophaenin B/isomer | 17.17 | 224, 270 | 1103 | 153, 345, 471, 617 | 8.8 ± 0.2 a |
| 1105 | ||||||
| 7 | hippophaenin B/isomer | 17.30 | 224, 270 | 1103 | 153, 345, 471, 617 | 19.6 ± 1.0 a |
| 1105 | ||||||
| 8 | ellagic acid-Hex | 19.30 | 253, 261 | 463 | 153, 303 | 7.4 ± 0.2 a |
| 465 | ||||||
| 9 | casuarictin/isomer | 21.56 | 224, 270sh | 935 | 153, 277, 303, 447, 785 | 21.7 ± 0.2 a |
| 937 | ||||||
| 10 | Q-Hex-dHex | 23.31 | 255, 352 | 609 | 303, 449 | 5.9 ± 0.0 b |
| 611 | ||||||
| 11 | ellagic acid-Pen | 24.02 | 253, 361 | 433 | 303 | 8.0 ± 0.2 a |
| 435 | ||||||
| 12 | ellagic acid | 24.37 | 253, 366 | 301 | 14.0 ± 0.3 a | |
| 303 | ||||||
| 13 | I-3-O-Hex-7-O-dHex | 26.31 | 255, 350 | 623 | 317, 463 | 8.4 ± 0.0 b |
| 625 | ||||||
| 14 | I-3-O-Glc-7-O-Rha | 27.33 | 255, 352 | 623 | 317, 463 | 15.9 ± 0.1 b |
| 625 | ||||||
| 15 | K-Hex-pCouA | 43.85 | 266, 314 | 593 | 147, 287 | 5.4 ± 0.1 b |
| 595 |
| No. | Compounds (Tentative Identification) | tR (min) | λ max (nm) | [M − H]− | Fragment Ions (+) (m/z) | Concentration (µg/mg) |
|---|---|---|---|---|---|---|
| [M + H]+ | ||||||
| 1 | gallocatechin-catechin | 5.38 | 270, 300sh | 593 | 259, 305, 465 | 9.2 ± 0.4 b |
| 595 | ||||||
| 2 | gallocatechin-catechin | 5.85 | 270, 300sh | 593 | 259, 305, 465 | 8.0 ± 0.4 b |
| 595 | ||||||
| 3 | dimeric proanthocyanidin | 9.71 | 200, 279 | 577 | 289 | 58.4 ± 0.7 b |
| 579 | ||||||
| 4 | catechin | 10.86 | 200, 278 | 289 | 139 | 76.1 ± 0.8 b |
| 291 | ||||||
| 5 | trimeric proanthocyanidin | 11.37 | 200, 279 | 865 | 139, 289, 579 | 23.3 ± 0.6 b |
| 867 | ||||||
| 6 | tetrameric proanthocyanidin | 14.90 | 200, 278 | 1153 | 289, 577, 865 | 12.3 ± 0.2 b |
| 1155 | ||||||
| 7 | trimeric proanthocyanidin | 16.14 | 200, 278 | 865 | 289, 577 | 13.8 ± 0.3 b |
| 867 | ||||||
| 8 | dimeric proanthocyanidin | 16.77 | 200, 278 | 577 | 291 | 7.4 ± 0.2 b |
| 579 | ||||||
| 9 | tetrameric proanthocyanidin | 19.23 | 200, 279 | 1153 | 289, 577, 865 | 11.8 ± 0.2 b |
| 1155 | ||||||
| 10 | ellagic acid | 24.46 | 253, 366 | 301 | 7.5 ± 0.1 a | |
| 303 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalski, B.; Kontek, B.; Rolnik, A.; Olas, B.; Stochmal, A.; Żuchowski, J. Anti-Platelet Properties of Phenolic Extracts from the Leaves and Twigs of Elaeagnus rhamnoides (L.) A. Nelson. Molecules 2019, 24, 3620. https://doi.org/10.3390/molecules24193620
Skalski B, Kontek B, Rolnik A, Olas B, Stochmal A, Żuchowski J. Anti-Platelet Properties of Phenolic Extracts from the Leaves and Twigs of Elaeagnus rhamnoides (L.) A. Nelson. Molecules. 2019; 24(19):3620. https://doi.org/10.3390/molecules24193620
Chicago/Turabian StyleSkalski, Bartosz, Bogdan Kontek, Agata Rolnik, Beata Olas, Anna Stochmal, and Jerzy Żuchowski. 2019. "Anti-Platelet Properties of Phenolic Extracts from the Leaves and Twigs of Elaeagnus rhamnoides (L.) A. Nelson" Molecules 24, no. 19: 3620. https://doi.org/10.3390/molecules24193620
APA StyleSkalski, B., Kontek, B., Rolnik, A., Olas, B., Stochmal, A., & Żuchowski, J. (2019). Anti-Platelet Properties of Phenolic Extracts from the Leaves and Twigs of Elaeagnus rhamnoides (L.) A. Nelson. Molecules, 24(19), 3620. https://doi.org/10.3390/molecules24193620