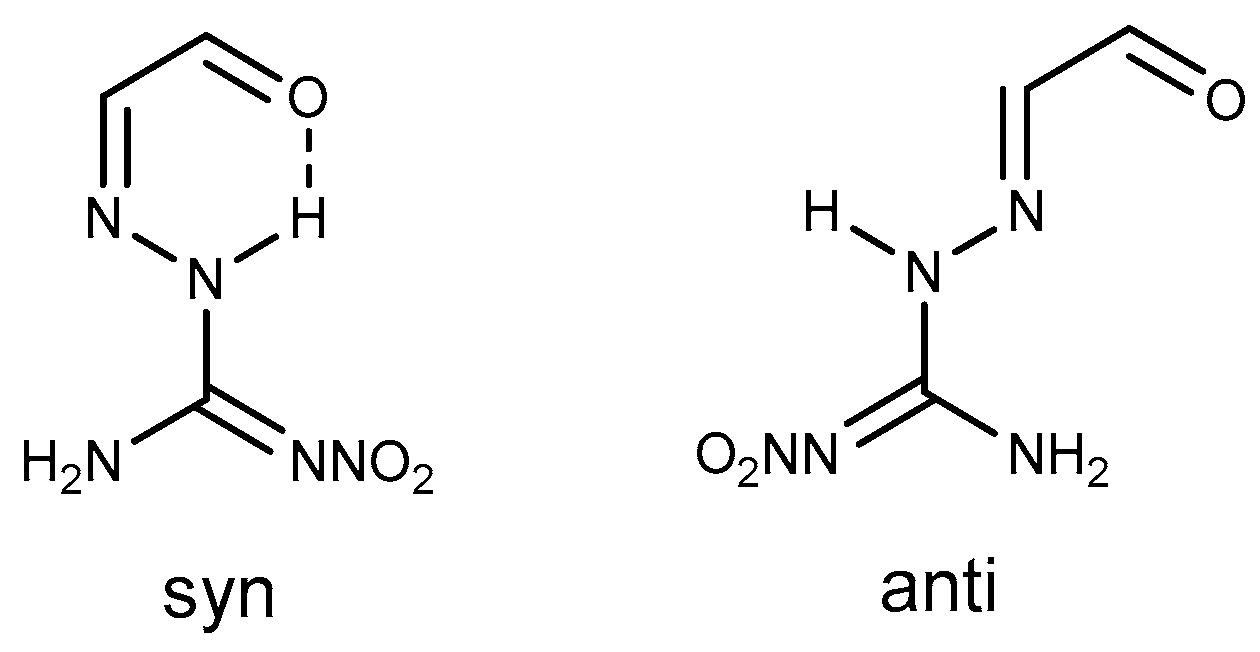
A Review on the Reactivity of 1-Amino-2-Nitroguanidine (ANQ)
Abstract
:1. Introduction
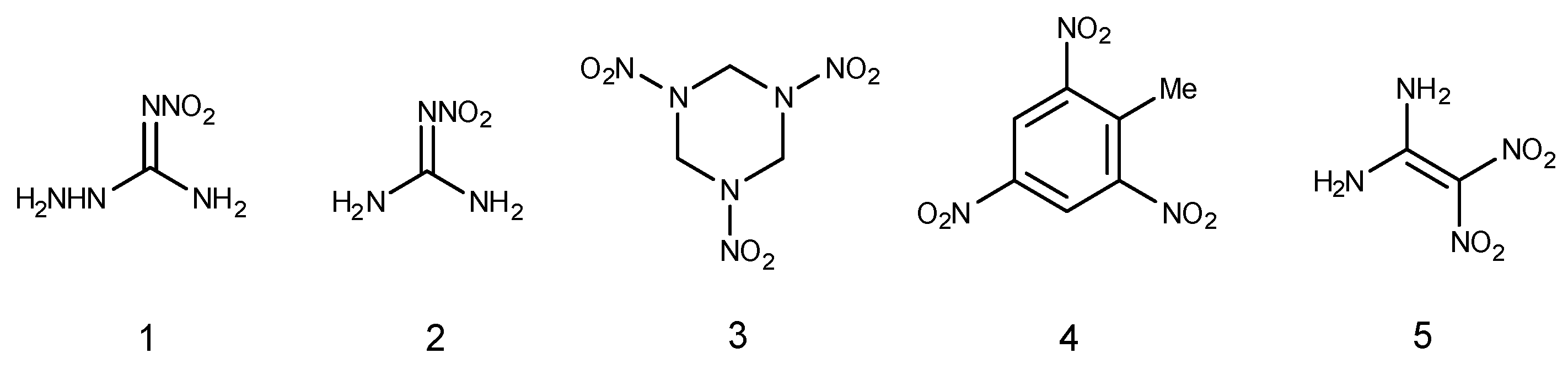
2. Reactivity of ANQ
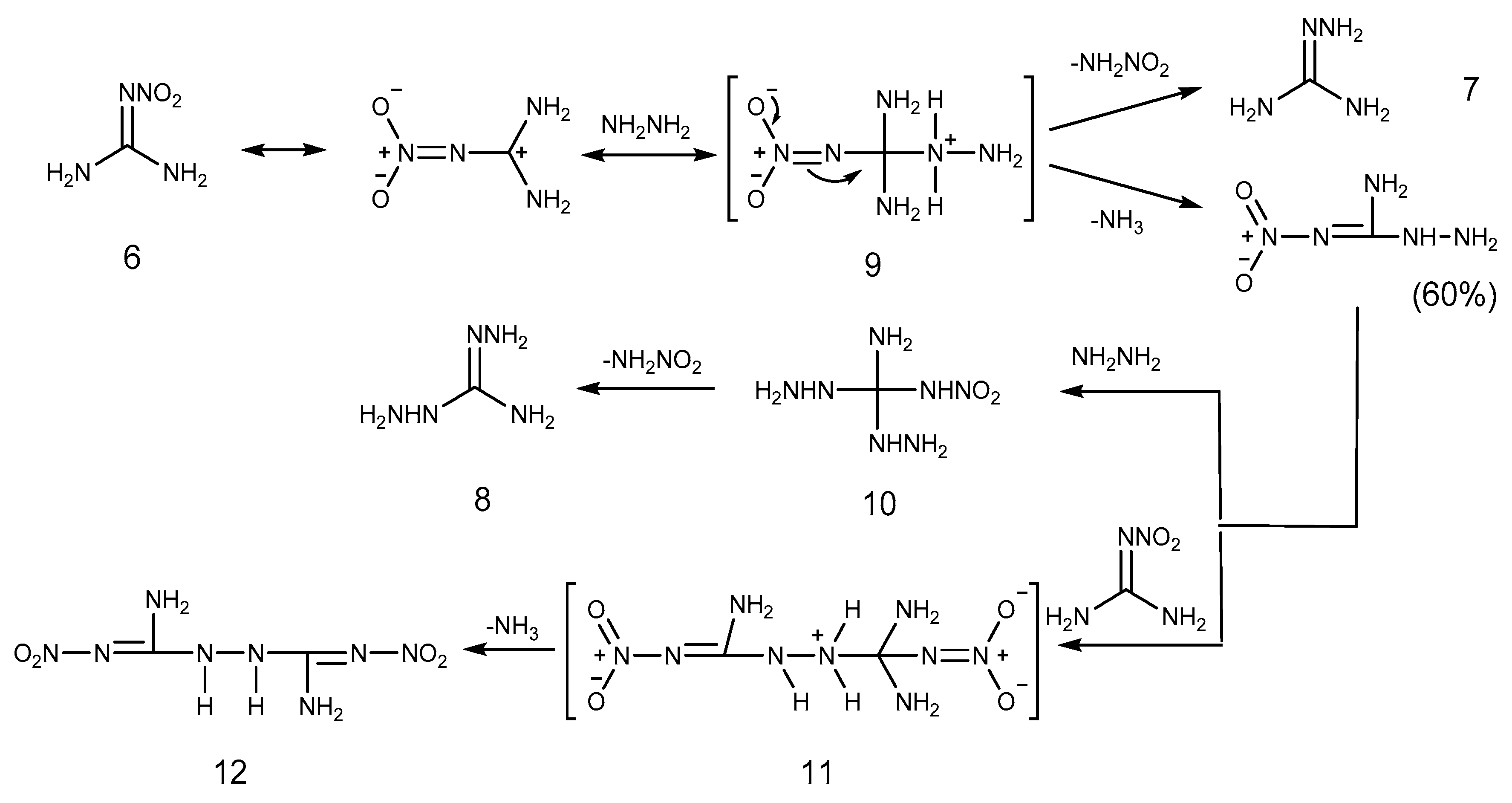
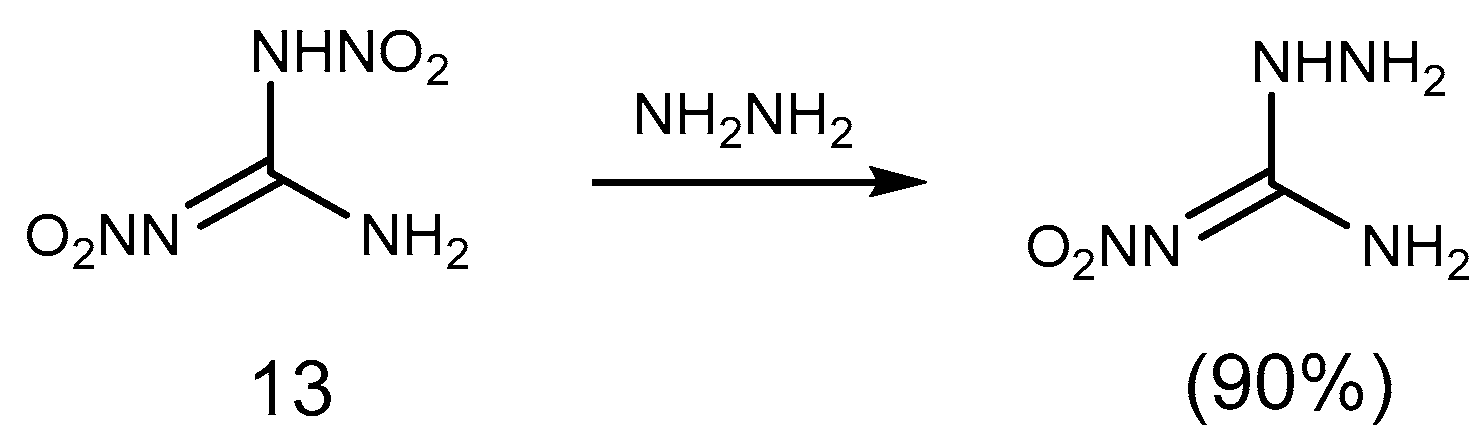
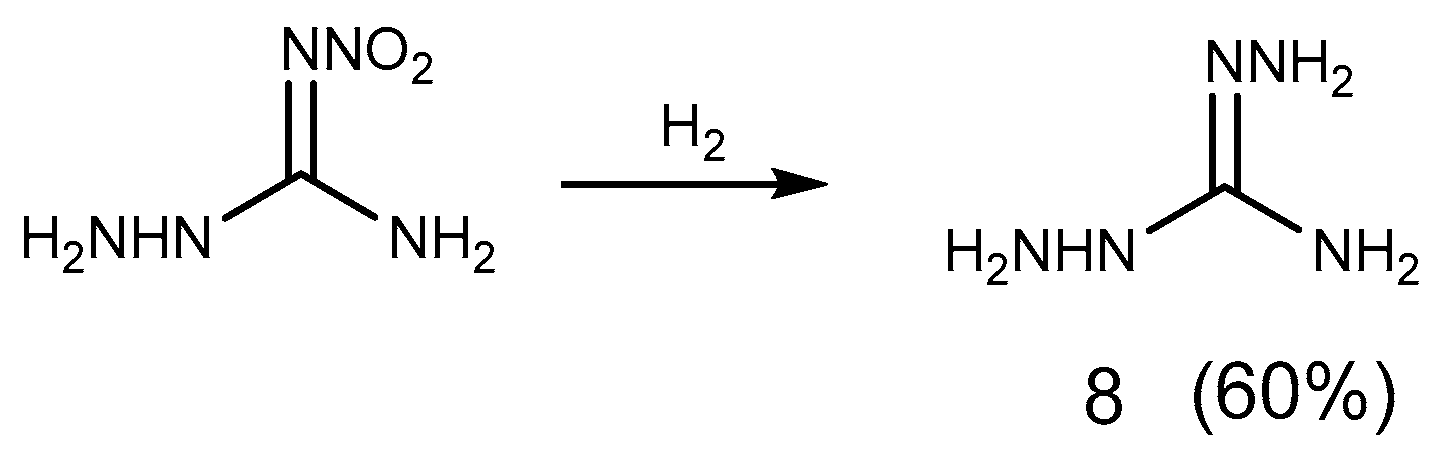
2.1. Reduction Reaction
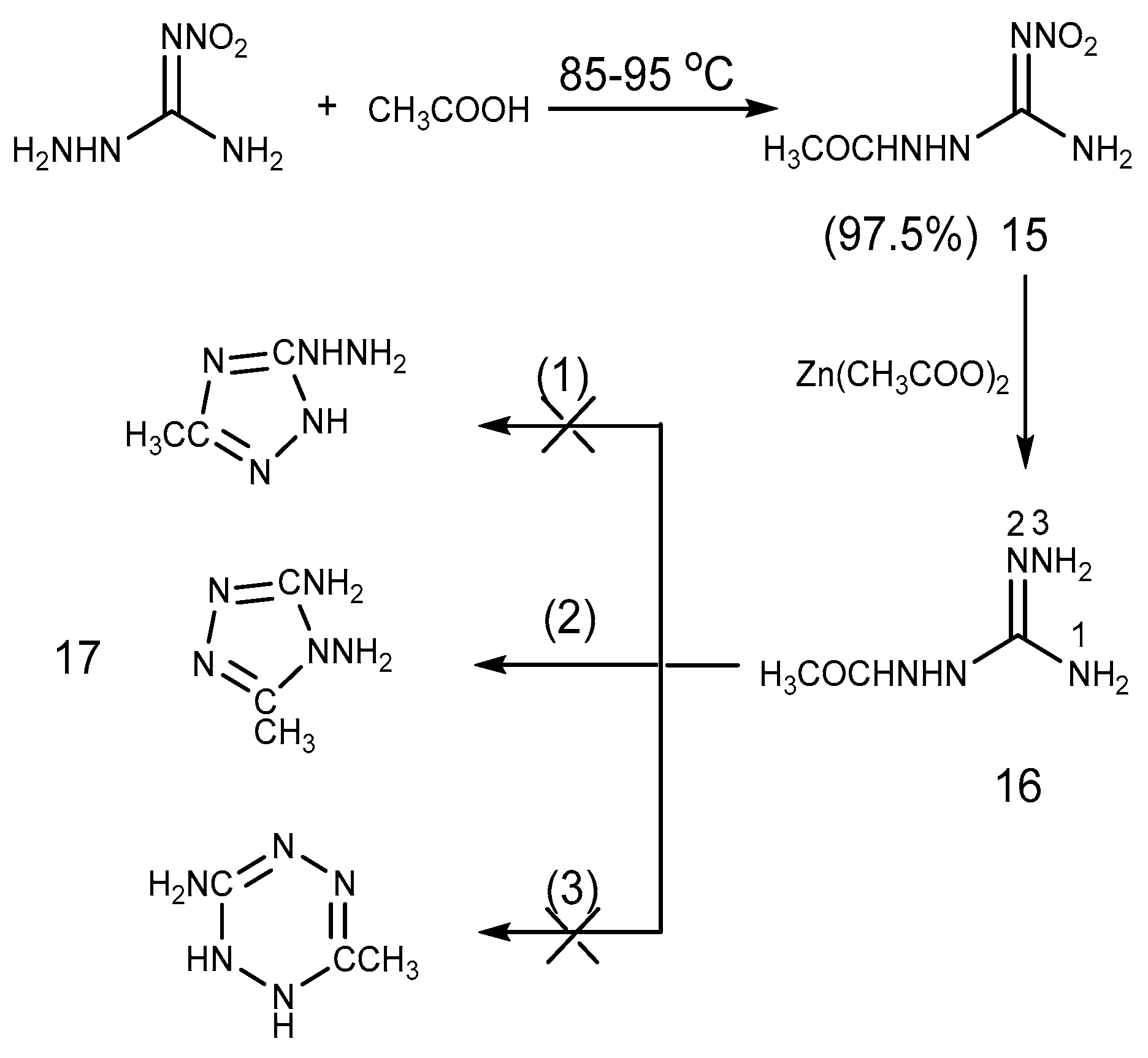
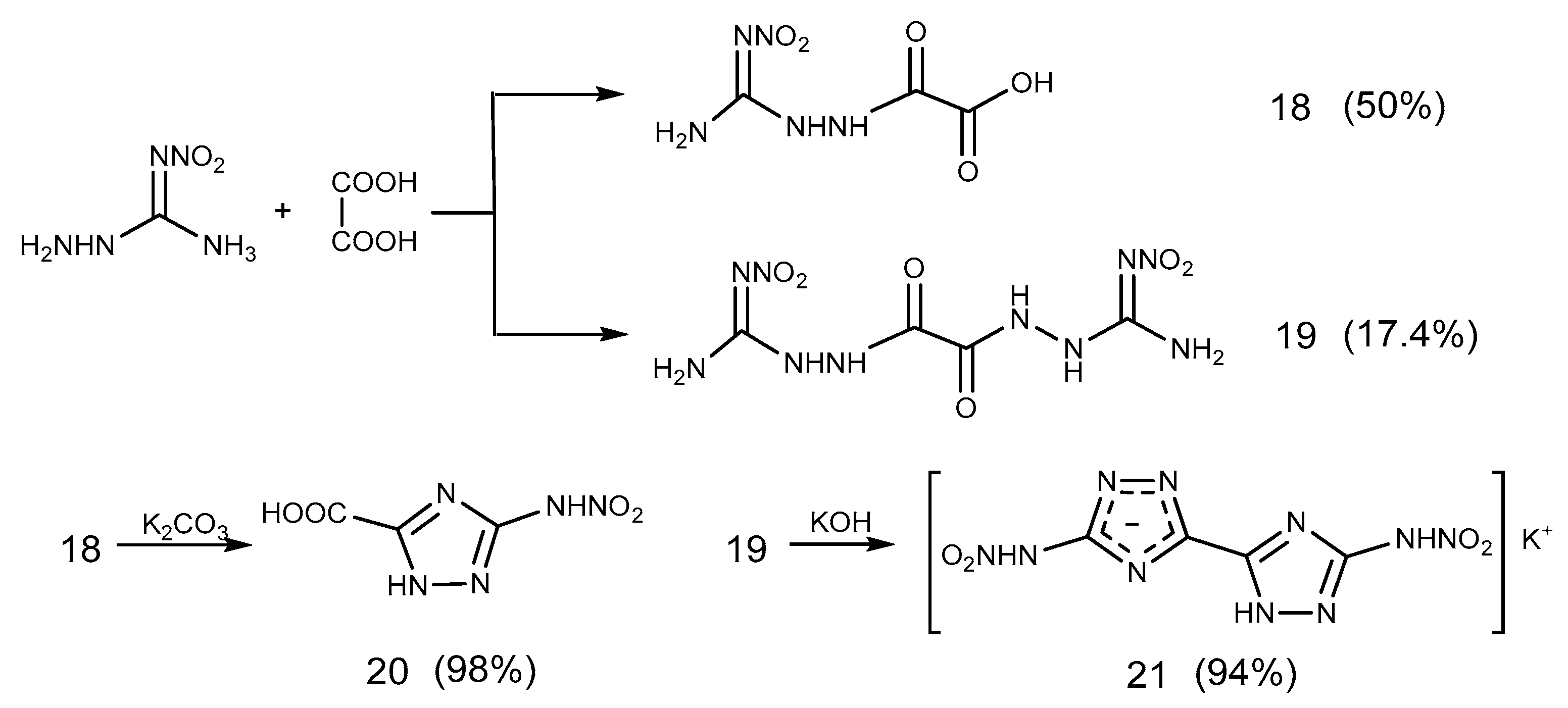
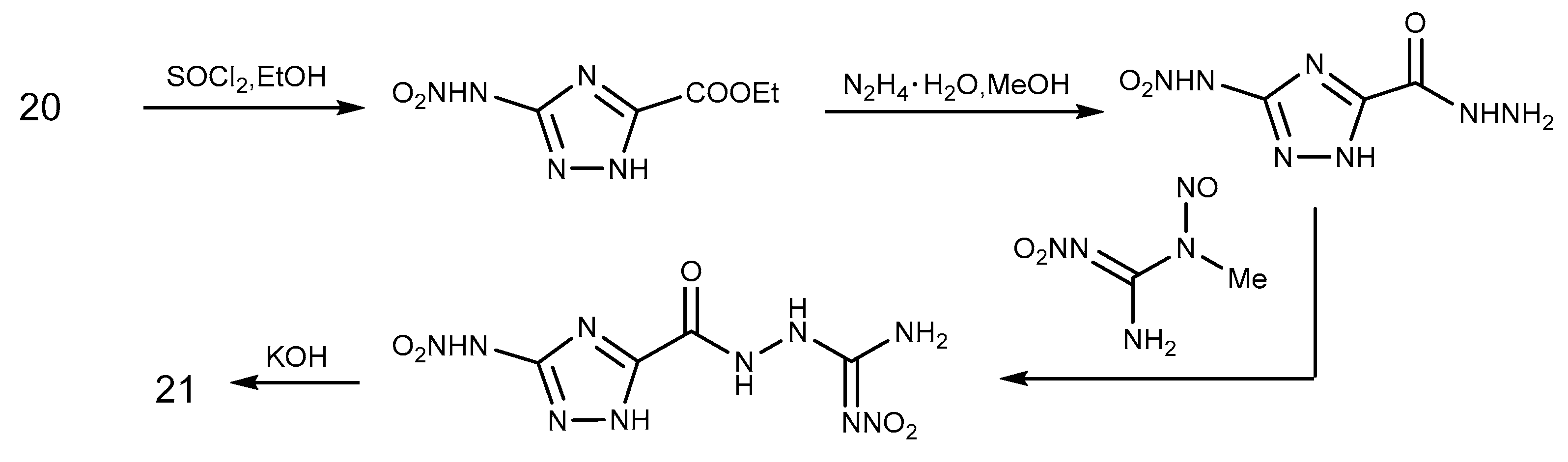
2.2. Acylation Reaction
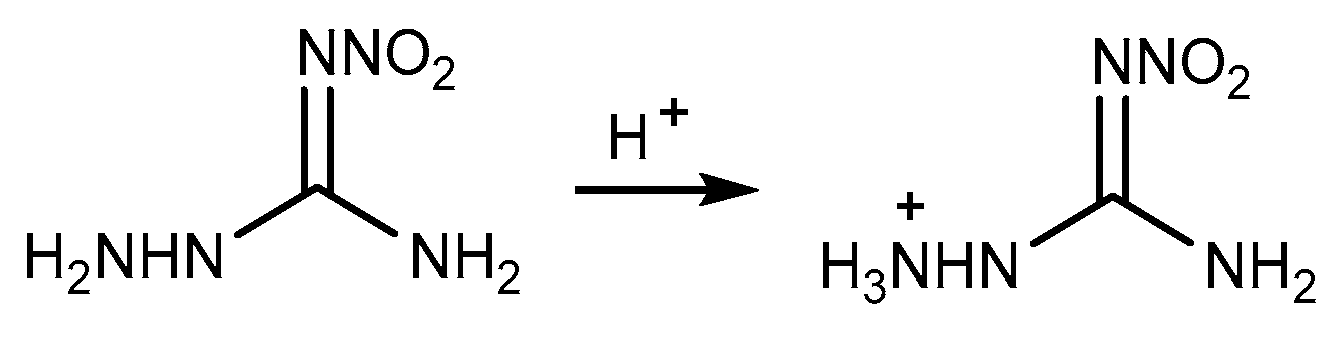
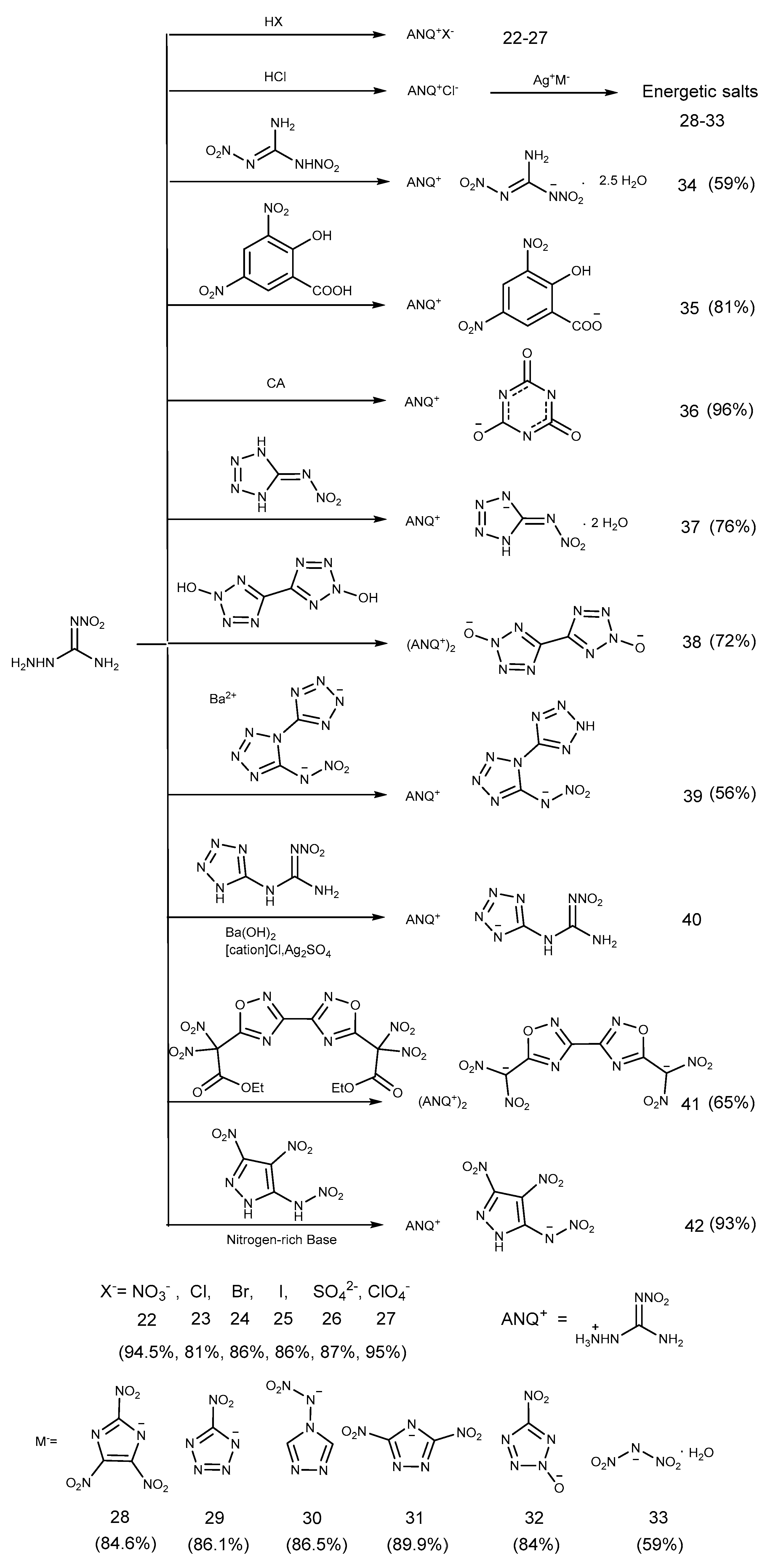
2.3. Salification Reaction
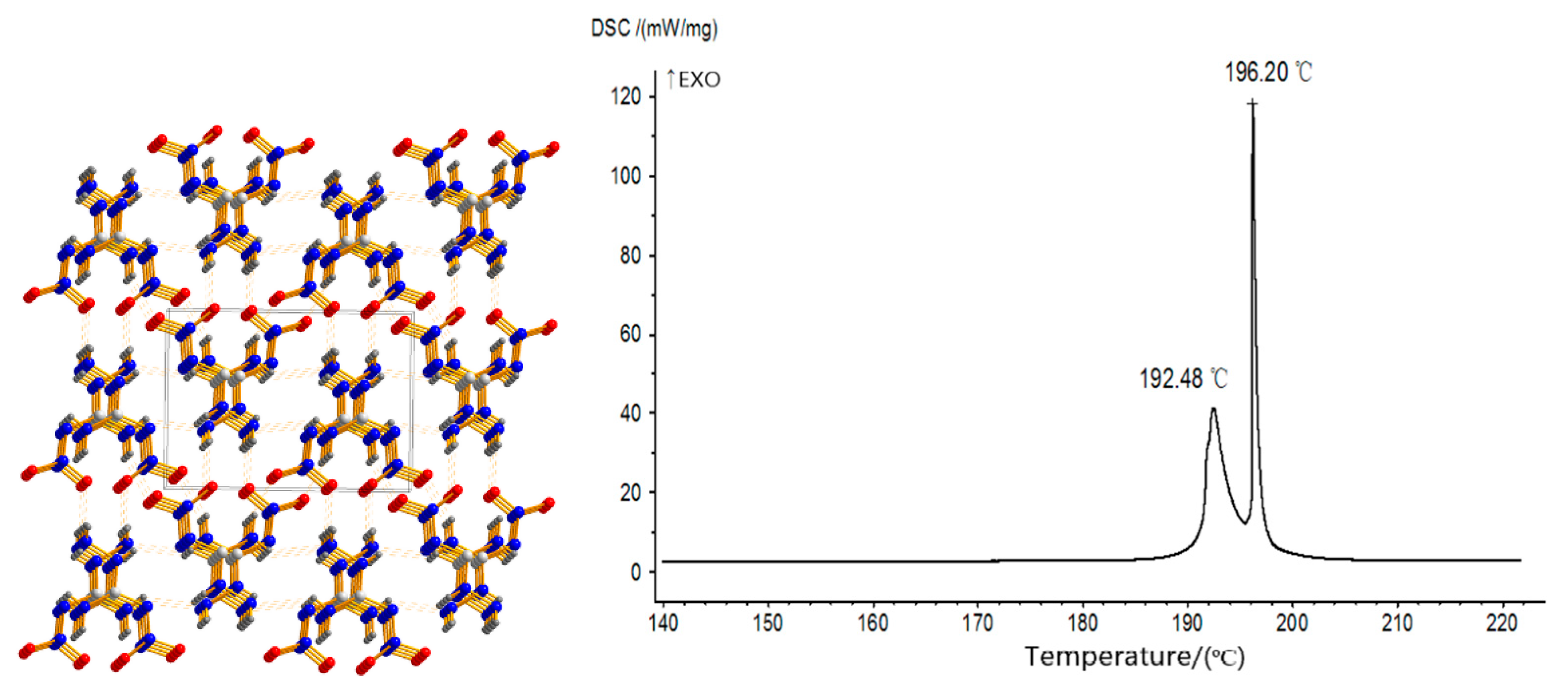
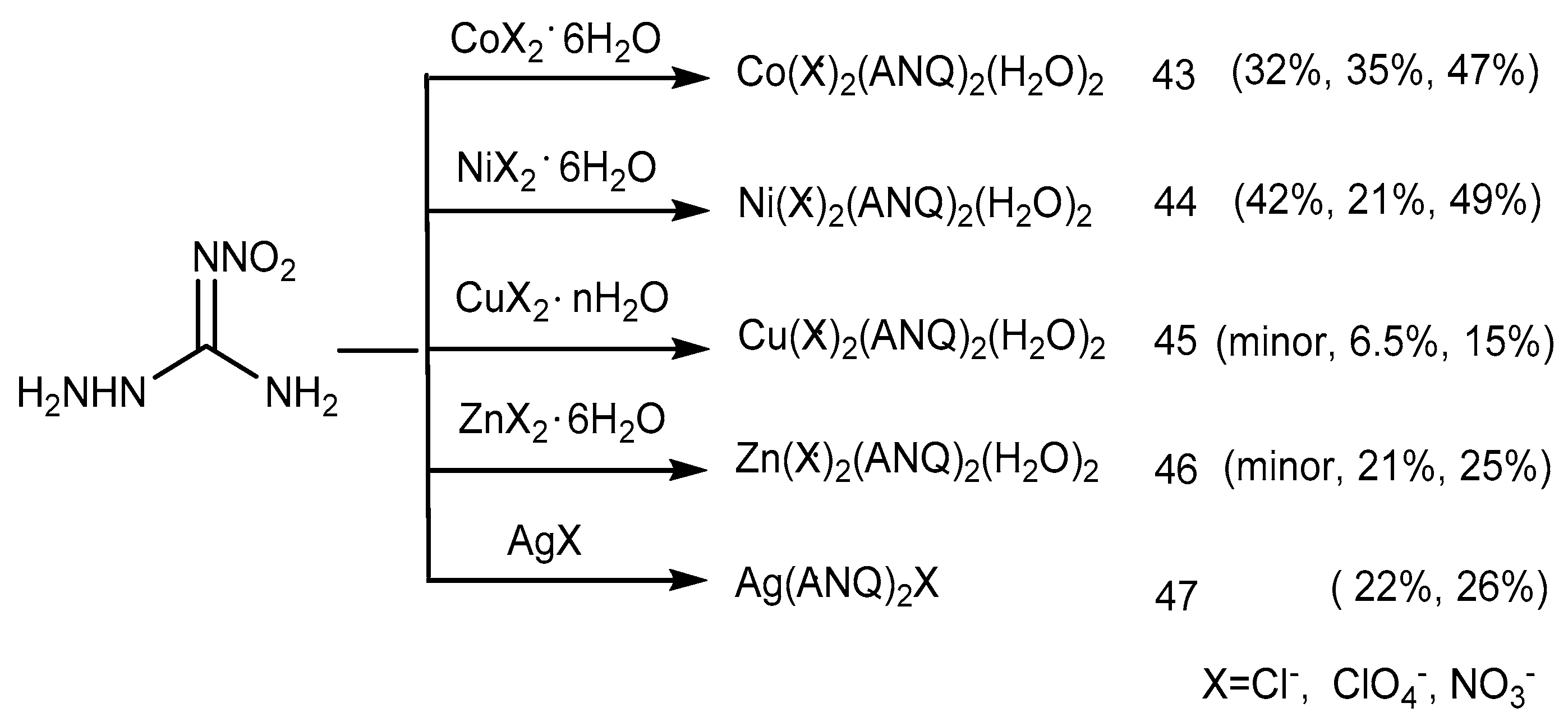
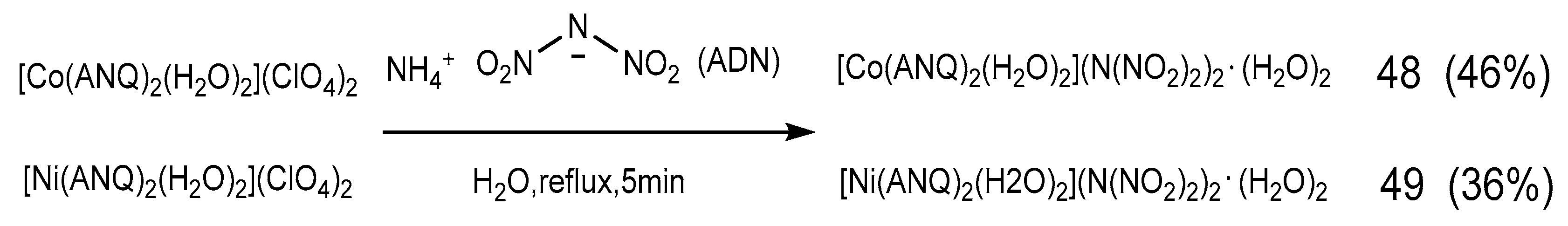
2.4. Coordination Reaction
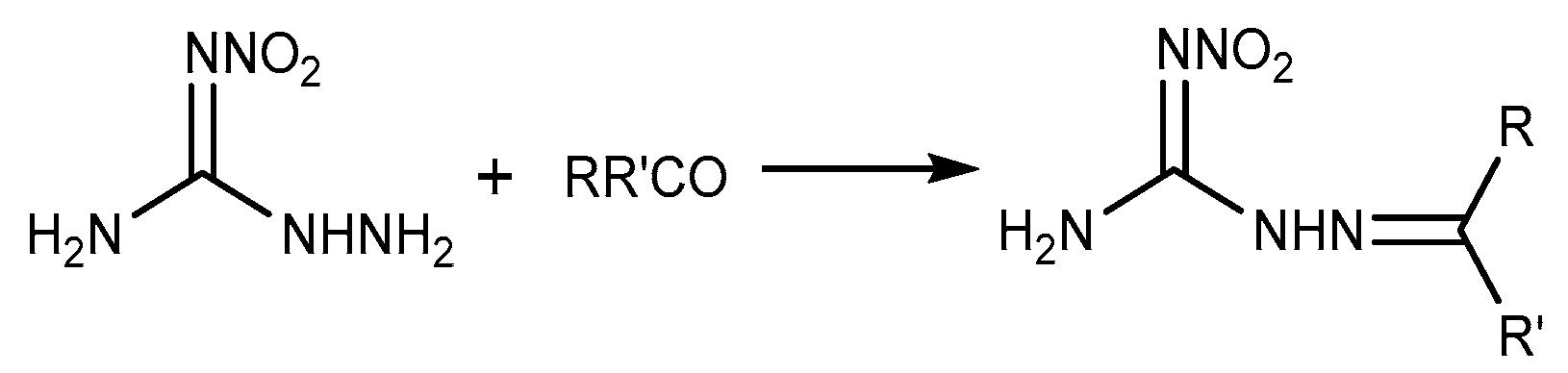
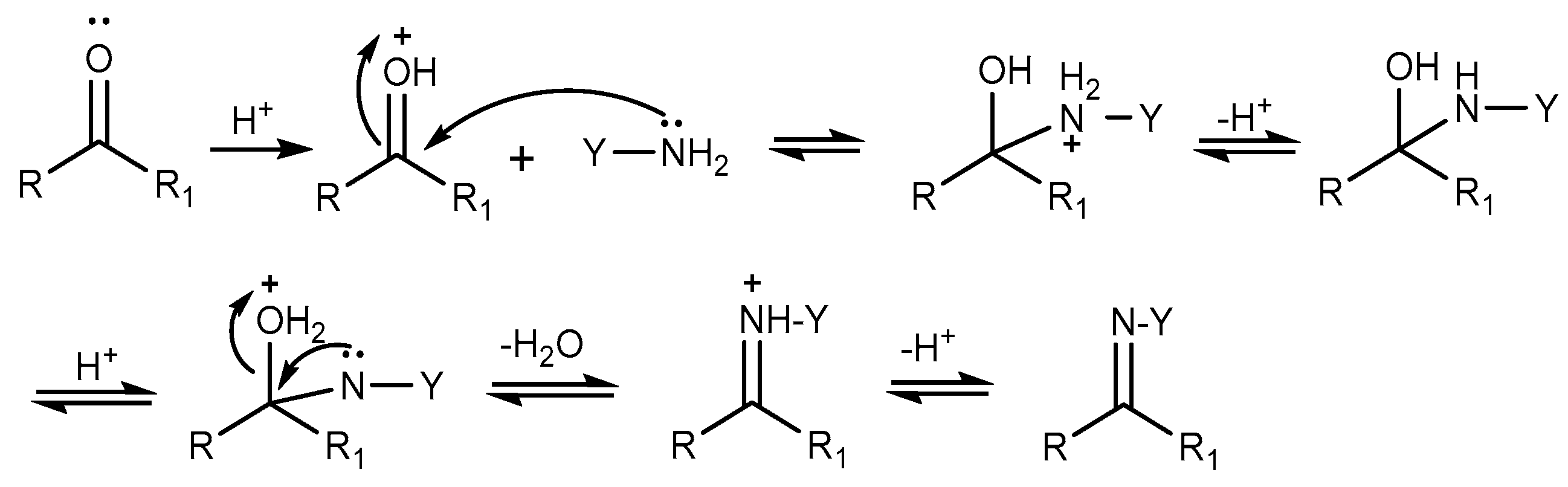
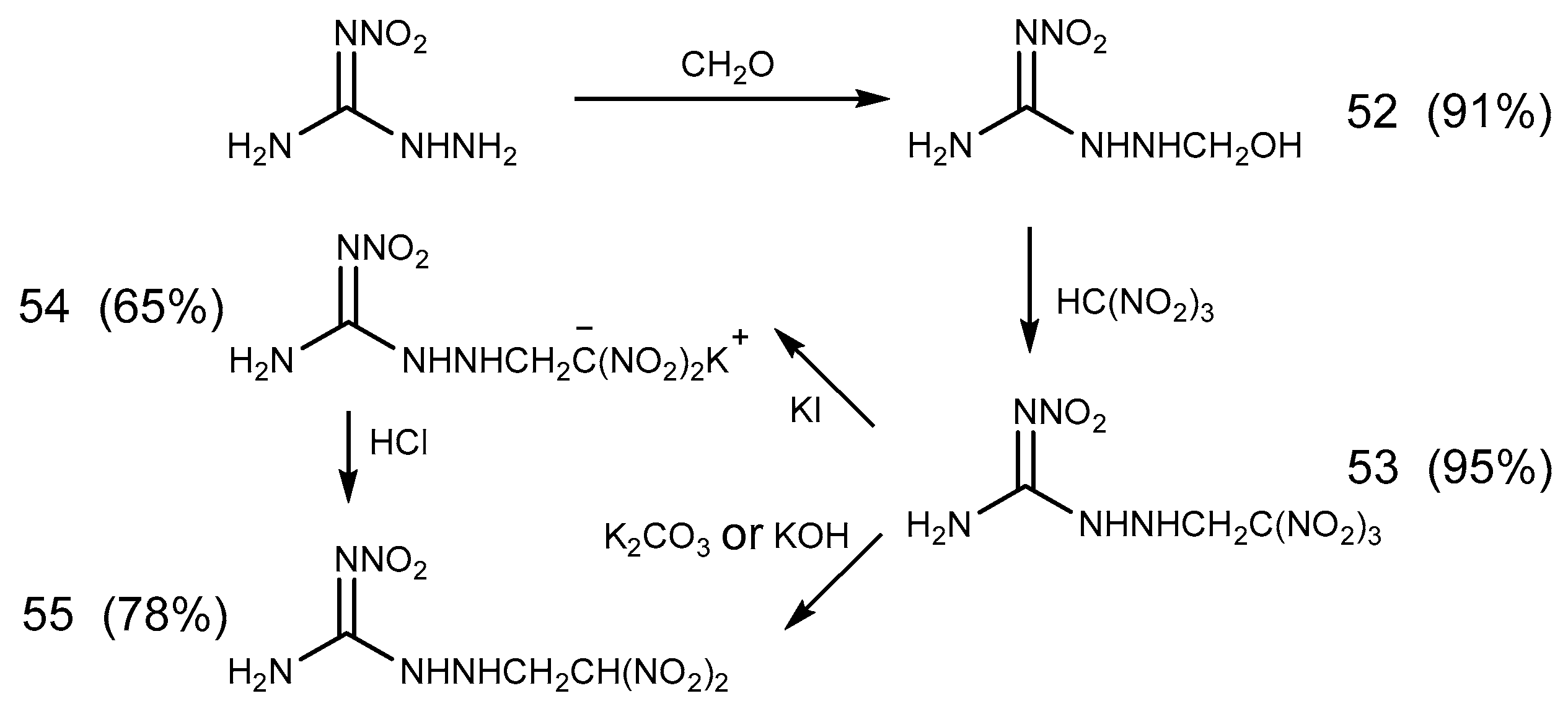
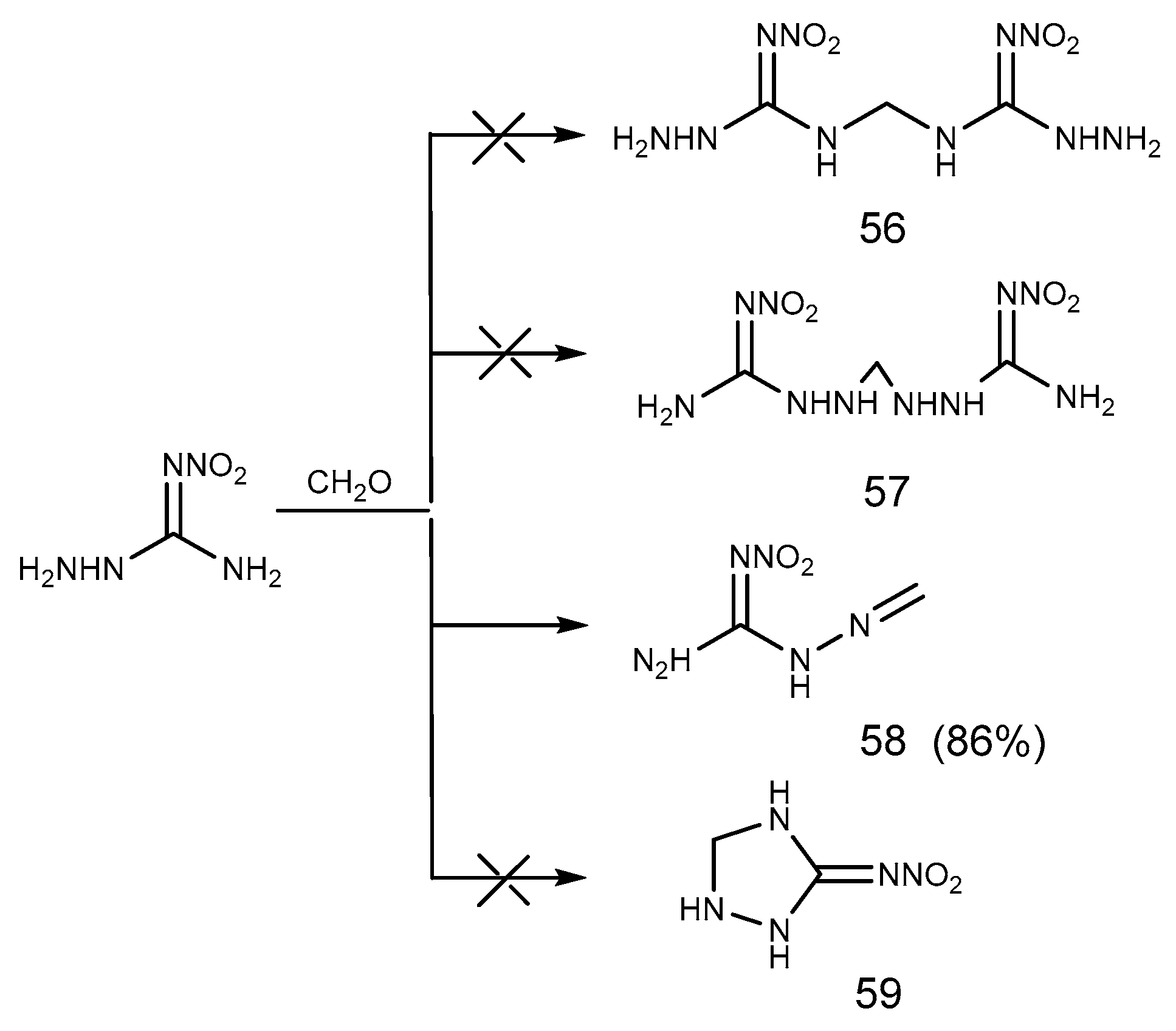
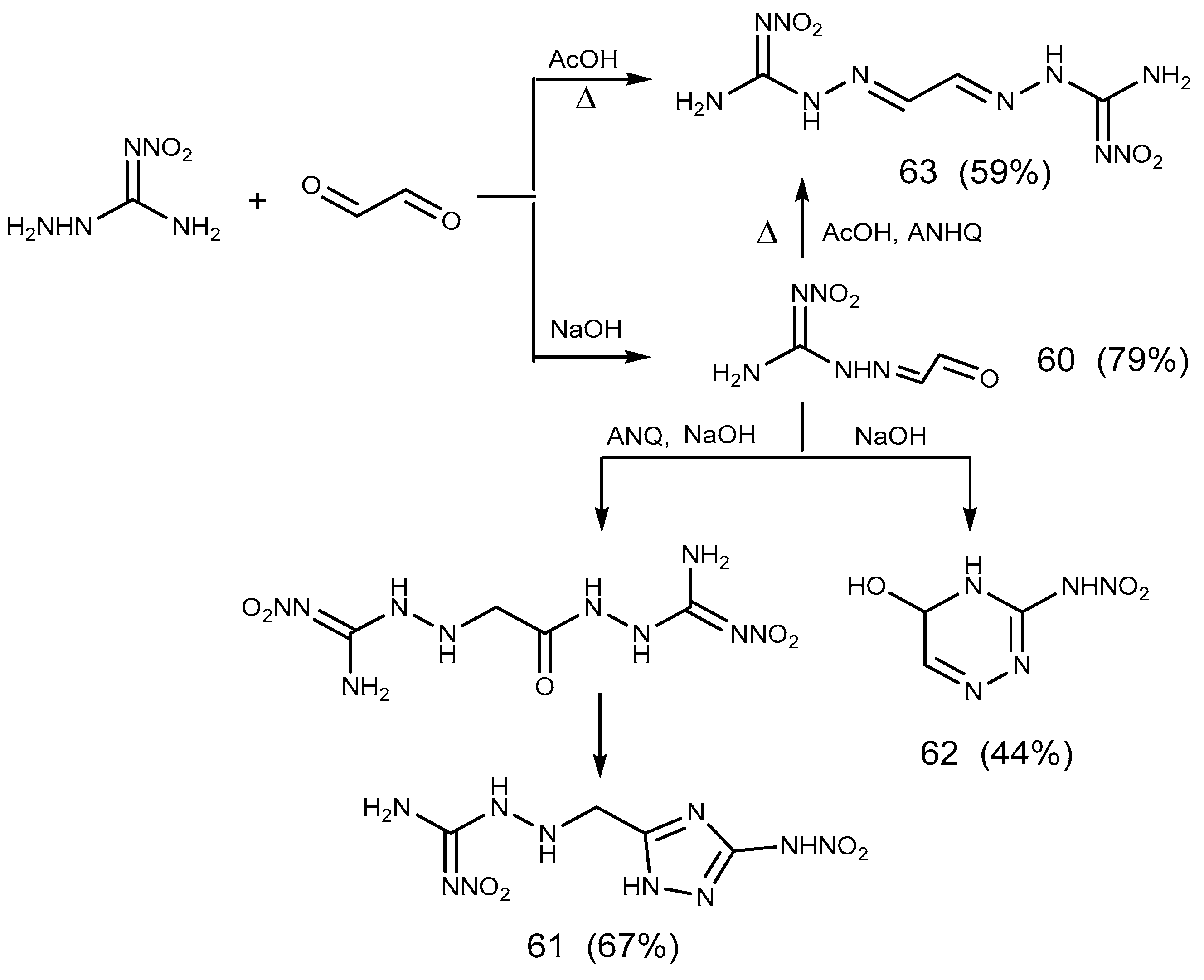
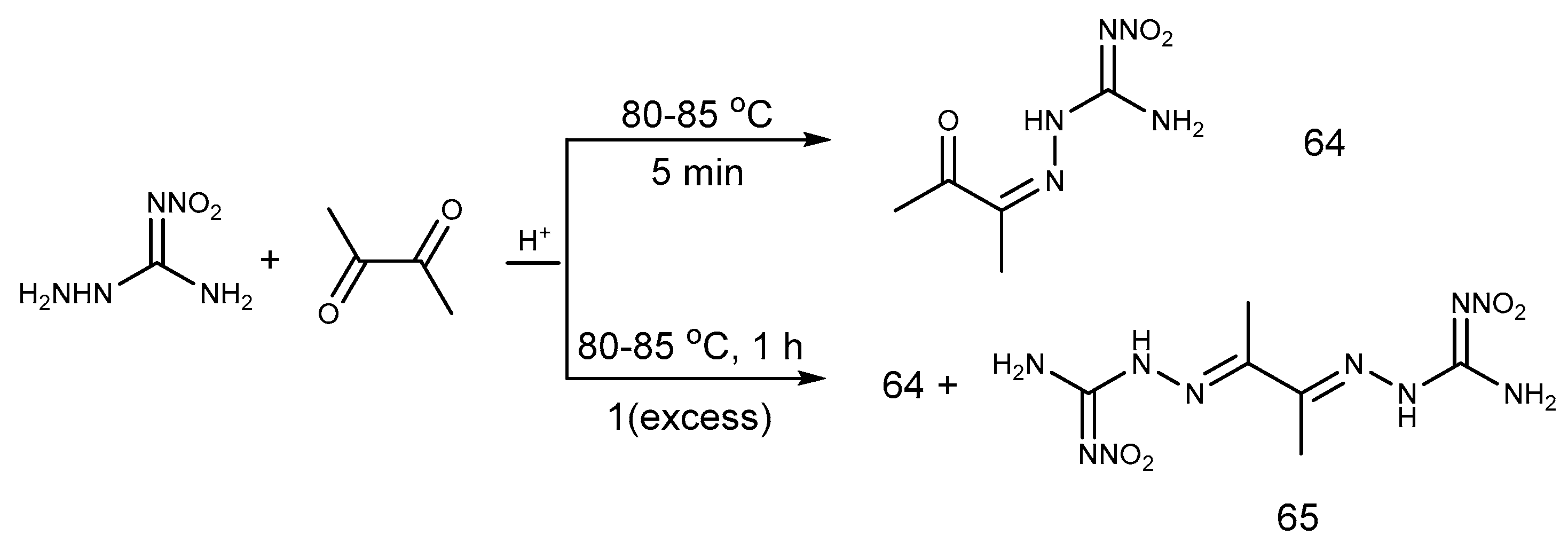
2.5. Aldimine Condensation Reaction
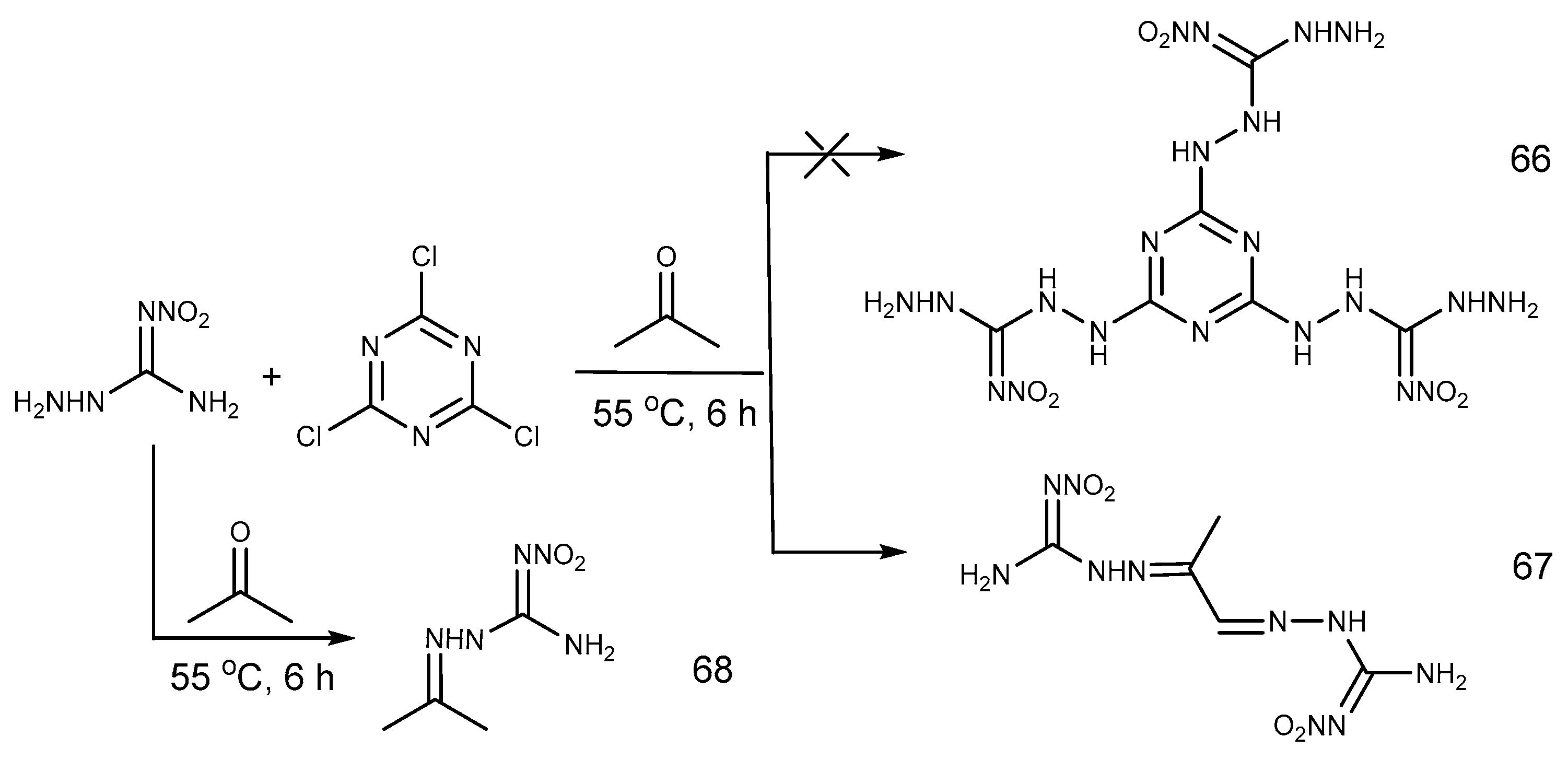
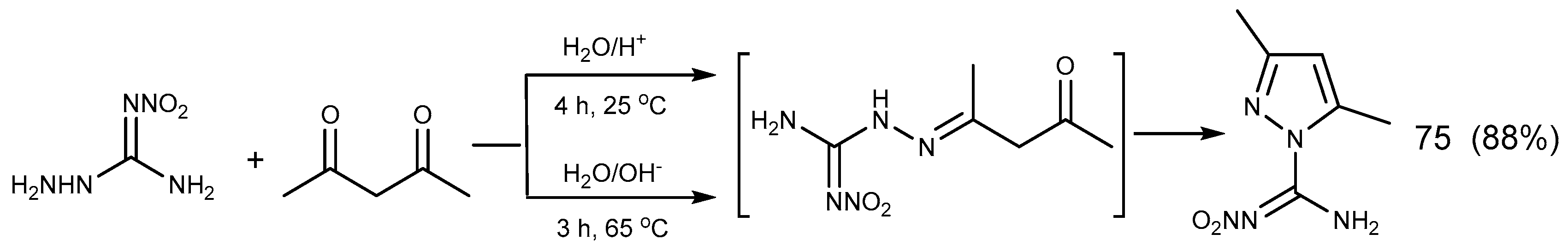
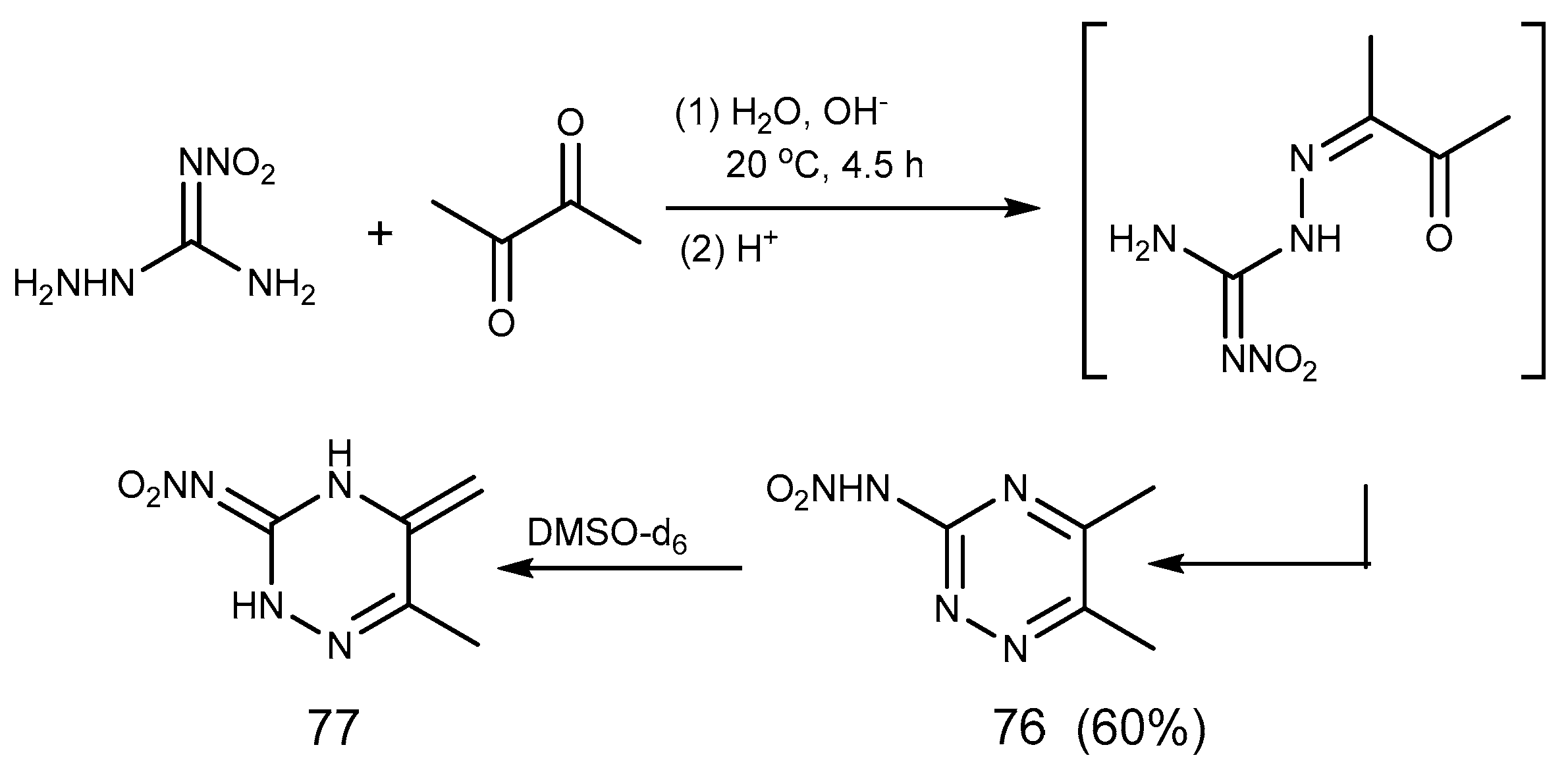
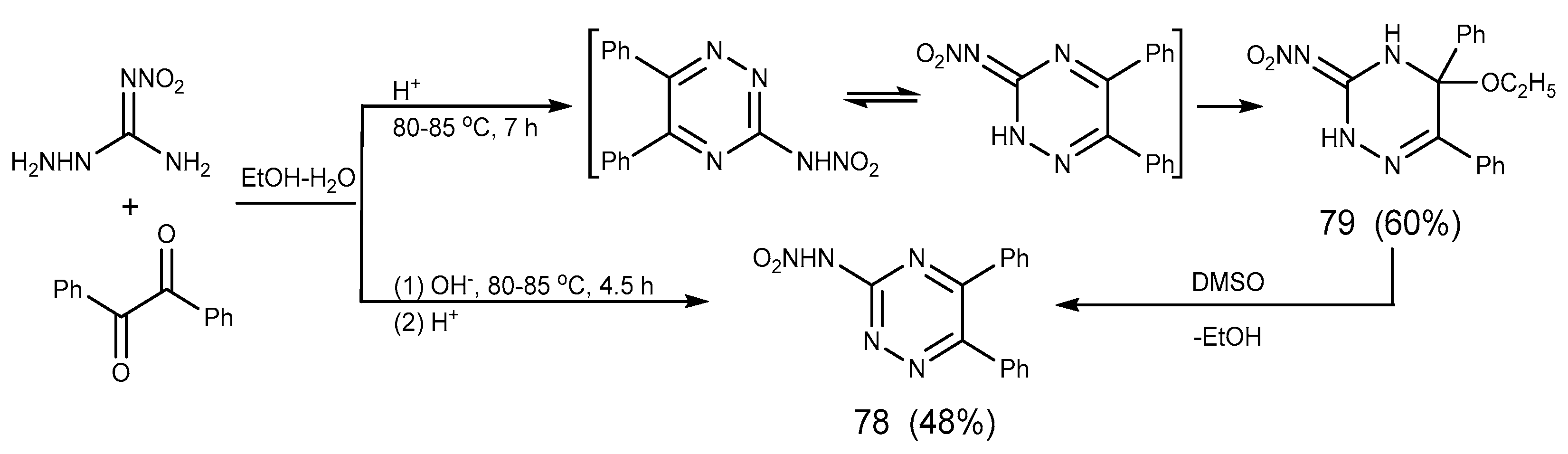
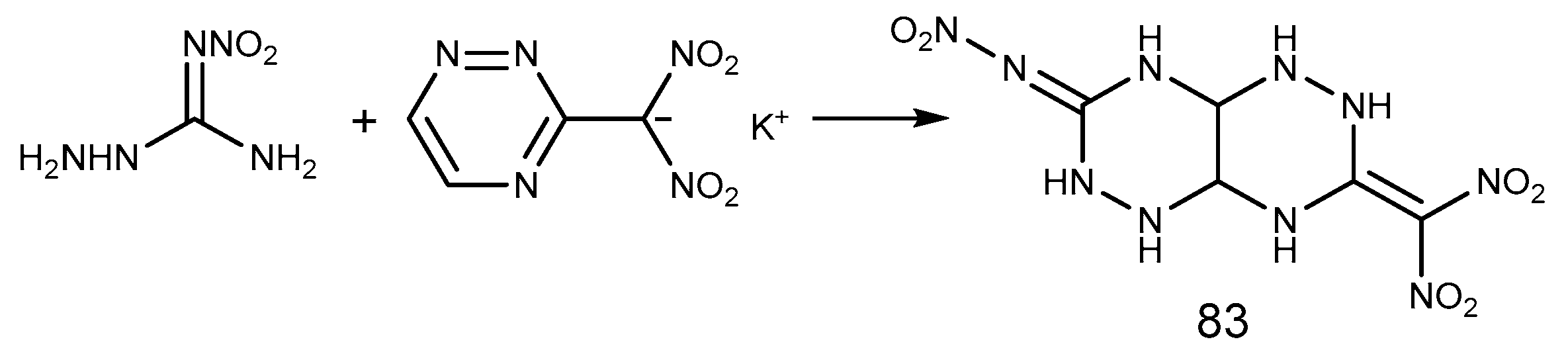
2.6. Cyclization Reaction
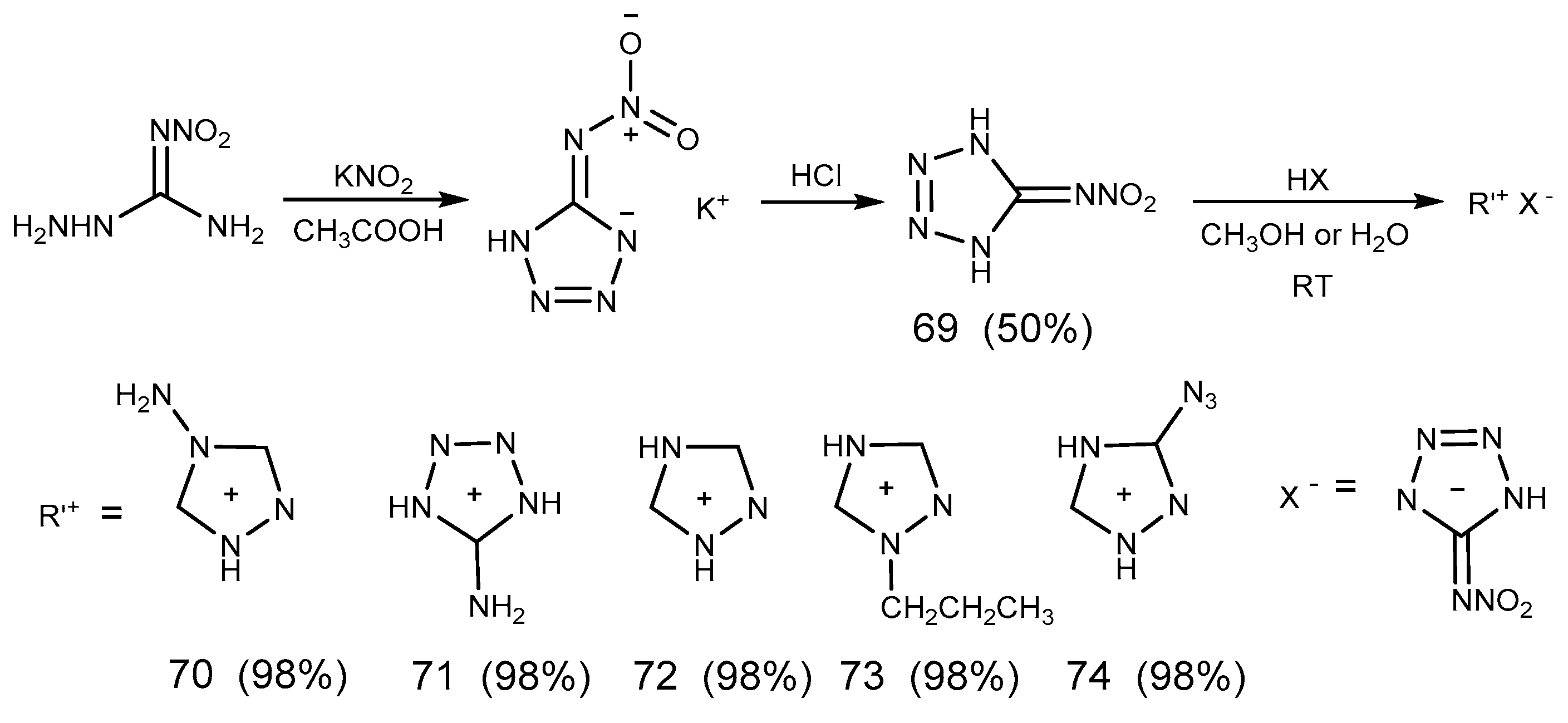
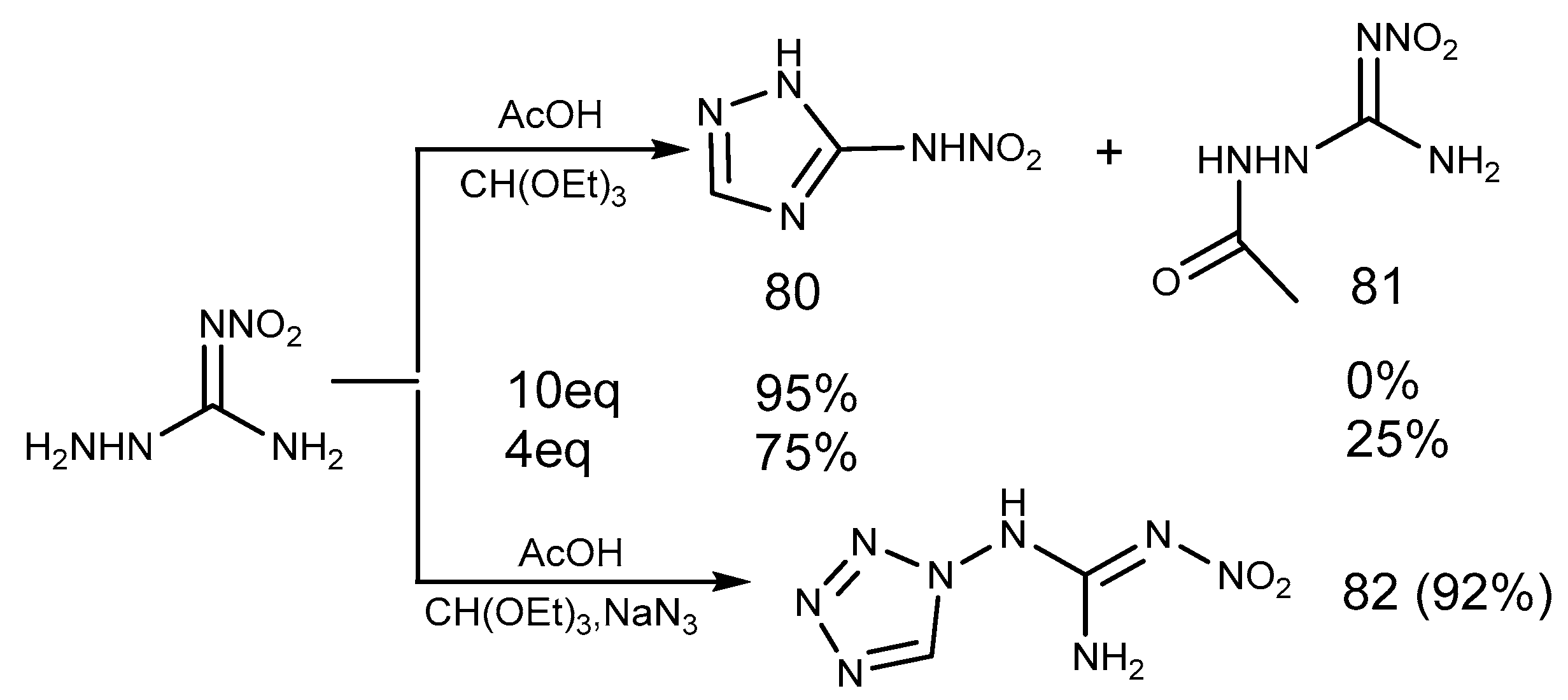
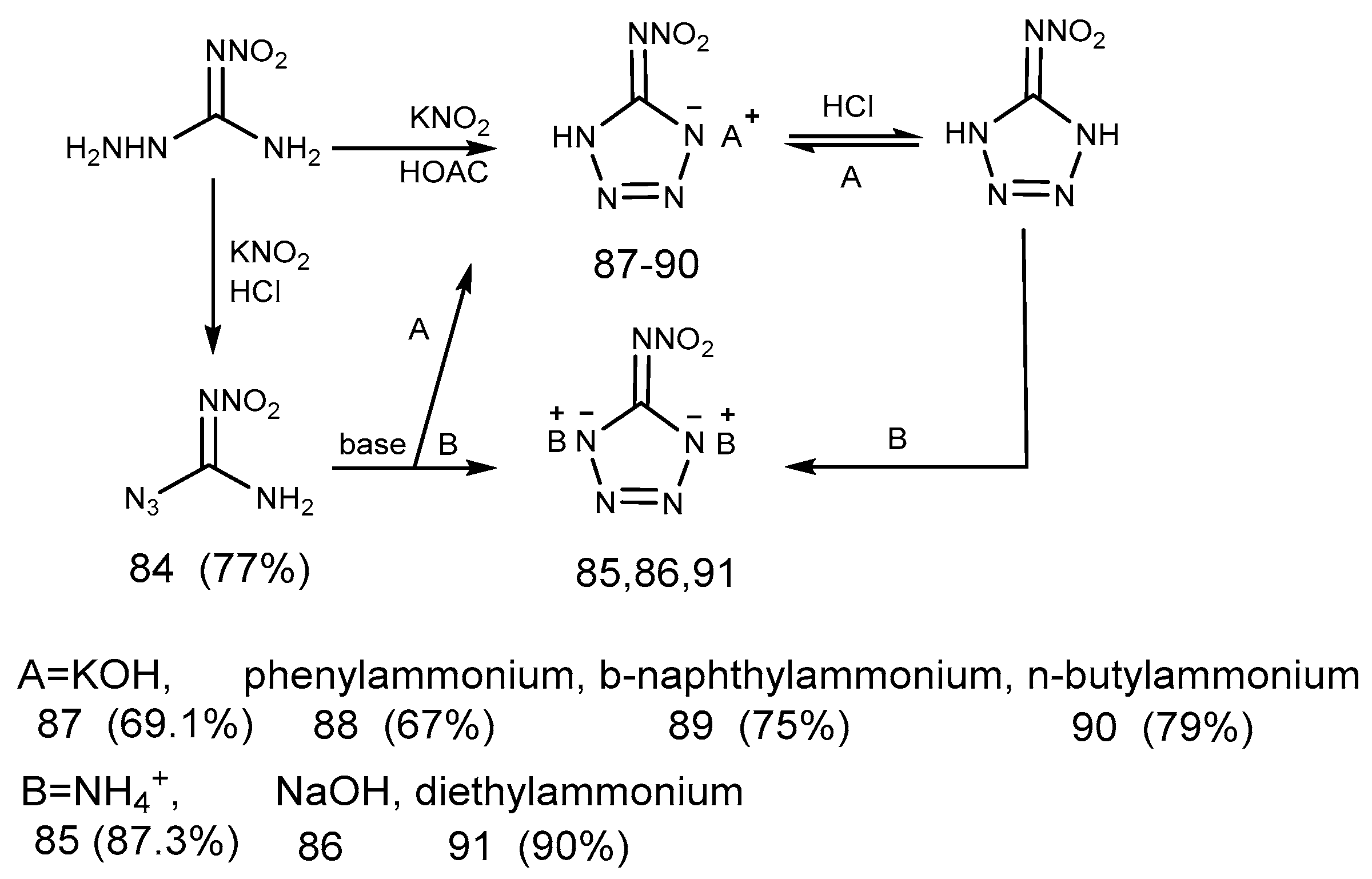
2.7. Azide Reaction
2.8. Detonation Properties
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Phillips, R.; Williams, J.F. Nitro-Aminoguanidine. J. Am. Chem. Soc. 1928, 50, 2465–2470. [Google Scholar] [CrossRef]
- Yaginuma, S.; Asahi, A.; Takada, M.; Hayashi, M.; Fukukawa, K. Synthesis or Isolation of Estatin A and Estatin B and Their Use as Enzyme Inhibitors. US Patent 4732910, 22 March 1988. [Google Scholar]
- Qin, Z.H.; Ma, Y.Q.; Su, W.C.; Zhao, B.B.; Fang, J.S. 2,5-Disubstituted-3-Nitroimino-1,2,4-Triazolines as Insecticide and Their Preparation. WO Patent 2013003977, 10 January 2013. [Google Scholar]
- Qin, Z.H.; Ma, Y.Q.; Su, W.C.; Wang, L.; Zhang, Z.; Zhao, B.B.; Fang, J.S. Preparation of Condensed 1-Amino-2-Nitroguanidine Compounds as Botanical Insecticides. US Patent 20110306639, 15 December 2011. [Google Scholar]
- Liberatore, A.M.; Bigg, D.; Pons, D.; Prevost, G. New 2-(Phenoxymethyl)-4-(Phenoxyphenyl)-1H-Imidazole Derivatives, Their Preparation, and Their Use as Inhibitors of Tubulin Polymerization for Treating Neoplasm. US Patent 8188133, 29 May 2012. [Google Scholar]
- Westphal, G.; Scheybal, A.; Lipke, B.; Weber, F.G. Preparation of Several 3-Hetaryl-2- Quinoxalinones. Pharmazie 1977, 32, 563–565. [Google Scholar]
- Efimova, T.P.; Ozerova, O.Y.; Belik, I.V.; Novikova, T.A.; Berestovitskaya, V.M. β-Nitro- and β-Bromo-β-nitrostyrenes in the Reactions with Aminonitroguanidine. Russ. J. Gen. Chem. 2012, 82, 1409–1415. [Google Scholar] [CrossRef]
- Bierowska-Charytonowicz, D.; Konieczny, M. Search for New Aminoguanidine Derivatives with Immunosuppressive and Cytostatic Properties. I. Reactions of Amino-, Nitroamino- and Diaminoguanidine with Acetylpyruvic Acid Ethyl Ester. Arch. Immunol. Ther. Exp. 1976, 24, 871–881. [Google Scholar]
- Efimova, T.P.; Ozerova, O.Y.; Novikova, T.A.; Baichurin, R.I.; Berestovitskaya, V.M. Reactions of 1-Amino-2-nitroguanidine with 2-Aryl(hetaryl)-1-nitro-1-ethoxycarbonyl (benzoyl)ethenes. Russ. J. Gen. Chem. 2014, 84, 1496–1499. [Google Scholar] [CrossRef]
- Sun, C.W.; Wang, H.F.; Zhu, J.; Yang, D.R.; Jin, J.; Xing, J.H. Synthesis, Insecticidal Activities, and Molecular Docking Studies of 1,5-Disubstituted-1,3,5-hexahydrotriazine-2-(N-nitro)imines. J. Heterocycl. Chem. 2011, 48, 829–835. [Google Scholar] [CrossRef]
- Su, W.C.; Zhou, Y.H.; Ma, Y.Q.; Wang, L.; Zhang, Z.; Rui, C.H.; Duan, H.X.; Qin, Z.H. N′-Nitro-2-hydrocarbylidenehydrazinecarboximidamides: Design, Synthesis, Crystal Structure, Insecticidal Activity, and Structure-Activity Relationships. J. Agric. Food Chem. 2012, 60, 5028–5034. [Google Scholar] [CrossRef] [PubMed]
- Berestovitskaya, V.M.; Ozerova, O.Y.; Efimova, T.P.; Gurzhiy, V.V.; Novikova, T.A. A New Synthesis of a Nitroimino-Containing 1,2,4-Triazin-5-one from 3-Bromo-3-nitropropenoates. Mendeleev Commun. 2016, 26, 323–325. [Google Scholar] [CrossRef]
- Serebryannikova, A.V.; Lapshina, L.V.; Efremova, I.E.; Gurzhiy, V.V.; Ryabinin, A.E. 2-Benzylidene-3-methyl-4-nitro-3-thiolene-1,1-dioxide and its Analogs in Aza-Michael Reaction. Russ. J. Gen. Chem. 2018, 88, 1612–1617. [Google Scholar] [CrossRef]
- Sorba, G.; Fruttero, R.; Calvino, R.; Gasco, A.; Orsetti, M. Potential Histamine H2-Receptor Antagonists: Ranitidine Analogues Containing “Semicarbazono Equivalent” Groups. Arch. Pharm. 1984, 317, 496–501. [Google Scholar] [CrossRef]
- Vaillancourt, V.A.; Larsen, S.D.; Tanis, S.P.; Burr, J.E.; Connell, M.A.; Cudahy, M.M.; Evans, B.R.; Fisher, P.V.; May, P.D.; Meglasson, M.D.; et al. Synthesis and Biological Activity of Aminoguanidine and Diaminoguanidine Analogues of the Antidiabetic/Antiobesity Agent 3-Guanidinopropionic Acid. J. Med. Chem. 2001, 44, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Berestovitskaya, V.M.; Ozerova, O.Y.; Efimova, T.P.; Novikova, T.A. Reaction of 2,3-Dibromo-3-nitroacrylates with N-amino-N”-nitroguanidine. Russ. J. Org. Chem. 2015, 51, 1797–1798. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Stierstorfer, J. Current Advances in RDX Replacements. II. In Proceedings of the 27th Army Science Conference, Orlando, FL, USA, 29 November–2 December 2010. [Google Scholar]
- Fischer, N.; Klapötke, T.M.; Stierstorfer, J. 1-Amino-3-nitroguanidine (ANQ) in High- Performance Ionic Energetic Materials. Z. Naturforsch. B A J. Chem. Sci. 2012, 67, 573–588. [Google Scholar] [CrossRef]
- Li, Y.F. Synthesis, Characterization and Thermal Behavior of ANQ Derivatives (in Chinese). Master’s Dissertation, Northwest University, Xi’an, China, 2017. [Google Scholar]
- Li, Y.F.; Wang, M.J.; Xu, K.Z.; Song, J.R.; Zhao, F.Q. Thermal Behaviors of 1-Amino- 2-nitroguanidine. Chin. J. Energ. Mater. 2016, 24, 848–852. [Google Scholar]
- Licht, H.H.; Ritter, H.; Wanders, B. Novel explosives: Nitrotriazoles. Syntheses and Explosive Properties. In Proceedings of the 25th International Annual Conference of ICT, Weil am Rhein, Germany, 28 June–1 July 1994. [Google Scholar]
- Feng, Z.C.; Guan, X.G.; Xu, K.Z.; Zhai, L.J.; Zhao, F.Q. Three New Energetic Compounds Based on 1-Amino-2-nitroguanidine (ANQ): Synthesis, Crystal Structure and Properties. J. Mol. Struct. 2018, 1166, 369–376. [Google Scholar] [CrossRef]
- Koch, E.C. Insensitive High Explosives: III. Nitroguanidine-Synthesis-Structure- Spectroscopy-Sensitiveness. Propellants Explos. Pyrotech. 2019, 44, 267–292. [Google Scholar] [CrossRef]
- Wharton, R.K.; Chapman, D. The Relationship between BAM Friction and Rotary Friction Sensitiveness Data for High Explosives. Propellants Explos. Pyrotech. 1997, 22, 71–73. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Penger, A.; Pflüger, C.; Stierstorfer, J.; Sućeska, M. Advanced Open-Chain Nitramines as Energetic Materials: Heterocyclic-Substituted 1,3-Dichloro-2-nitrazapropane. Eur. J. Inorg. Chem. 2013, 2013, 4667–4678. [Google Scholar] [CrossRef]
- Tang, Y.X.; Gao, H.X.; Parrish, D.A.; Shreeve, J.M. 1,2,4-Triazole Links and N-Azo Bridges Yield Energetic Compounds. Chem. Eur. J. 2015, 21, 11401–11407. [Google Scholar] [CrossRef]
- Szala, M.; Sabatini, J.J. 2,4,6-Trinitrotoluene-A Useful Starting Compound in the Synthesis of Modern Energetic Compounds. Z. Anorg. Allg. Chem. 2018, 644, 262–269. [Google Scholar] [CrossRef]
- Xiang, D.L.; Rong, J.L.; He, X. Detonation Performance of Four Groups of Aluminized Explosives. Cent. Eur. J. Energ. Mater. 2016, 13, 903–915. [Google Scholar] [CrossRef]
- Storm, C.B.; Stine, J.R.; Kramer, J.F. Sensitivity Relationships in Energetic Materilas. In Chemistry and Physics of Energetic Materials; Bulusu, S.N., Ed.; NATO ASI Series; Kluwer Acad. Publishers: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Latypov, N.V.; Bergman, J. Synthesis and Reactions of 1,1-Diamino-2,2-dinitroethylene. Tetrahedron 1998, 54, 11525–11536. [Google Scholar] [CrossRef]
- Viswanath, D.S.; Ghosh, T.K.; Boddu, V.M. Emerging Energetic Materials: Synthesis, Physicochemical, and Detonation Properties. In FOX-7 (1,1-Diamino-2,2-Dinitroethylene); Springer Nature: New York, NY, USA, 2018. [Google Scholar]
- Bemm, U.; Ostmark, H. 1,1-Diamino-2,2-dinitroethylene: A Novel Energetic Material with Infinite Layers in Two Dimensions. Acta Cryst. 1998, C54, 1997–1999. [Google Scholar] [CrossRef]
- Talawar, M.B.; Sivabalan, R.; Anniyappan, M.; Gore, G.M.; Asthana, S.N.; Gandhe, B.R. Emerging Trends in Advanced High Energy Materials. Combust. Explos. Shock Waves 2007, 43, 62–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Xu, K.Z.; Song, J.R.; Zhao, F.Q. Review on the Reactivity of 1,1- Diamino-2,2-dinitroethylene (FOX-7). Propellants Explos. Pyrotech. 2016, 41, 35–52. [Google Scholar] [CrossRef]
- Castillo-Meléndez, J.A.; Golding, B.T. Optimisation of the Synthesis of Guanidines from Amines via Nitroguanidines Using 3,5-Dimethyl-N-nitro-1H-pyrazole-1-carboxamidine. Synthesis 2004, 10, 1655–1663. [Google Scholar] [CrossRef]
- Mckay, A.F. Nitroguanidines. Chem. Rev. 1952, 51, 301–346. [Google Scholar] [CrossRef]
- Metelkina, E.L. 2-Nitroguanidine Derivatives: VI. Synthesis and Chemical Properties of Hydrazo- and Azobis(nitroformamidine). Russ. J. Org. Chem. 2004, 40, 928–935. [Google Scholar] [CrossRef]
- Astrat’yev, A.A.; Dashko, D.V.; Kuznetsov, L.L. Synthesis and Some Properties of 1,2- Dinitroguanidine. Russ. J. Org. Chem. 2003, 39, 501–512. [Google Scholar] [CrossRef]
- Astakhov, A.M.; Vasil’ev, A.D.; Gelemurzina, I.V.; Sokolenko, V.A.; Kruglyakova, L.A.; Stepanov, R.S. N-Nitroimines: I. Synthesis, Structure, and Properties of 3,5-Diamino-1- nitroamidino-1,2,4-triazole. Russ. J. Org. Chem. 2003, 39, 120–124. [Google Scholar] [CrossRef]
- Jia, S.Y.; Bi, F.Q.; Wang, B.Z.; Li, Z.X.; Huo, H. Method for Synthesizing 1-Amino-3-nitroguanidine (in Chinese). CN Patent 105503661, 20 April 2016. [Google Scholar]
- Kumler, W.D.; Sah, P.P.T. The Structure of Nitroguanidine and Nitroaminoguanidine. J. Org. Chem. 1953, 18, 669–675. [Google Scholar] [CrossRef]
- Hall, L.M.; Devries, J.E.; Gantz, E.S.C. Basic Equilibrium Constants of Nitroguanidine and Nitroaminoguanidine. J. Am. Chem. Soc. 1955, 77, 6507–6508. [Google Scholar] [CrossRef]
- Lieber, E.; Sherman, E.; Patinkin, S.H. Ultraviolet Absorption Spectra of 5-Nitroaminotetrazole and its Salts. J. Am. Chem. Soc. 1951, 73, 2329–2331. [Google Scholar] [CrossRef]
- Henry, R.A. Some Derivatives of Nitroguanidine. J. Am. Chem. Soc. 1950, 72, 5343–5345. [Google Scholar] [CrossRef]
- Lieber, E.; Schiff, S.; Henry, R.A.; Finnegan, W.G. Acetylation and Ring Closure in Reduction of Nitro- and Nitroaminoguanidine. J. Org. Chem. 1953, 18, 218–228. [Google Scholar] [CrossRef]
- Metelkina, E.L.; Novikova, T.A.; Berdonosova, S.N.; Berdonosov, D.Y. 2-Nitroguanidine Derivatives: IX. Reaction of 1-Amino-2-nitroguanidine with Oxalic Acid as a Method of Synthesis of 3(5)-Nitroamino-1,2,4-triazole-5(3)-carboxylic Acid and 5,5′-Bi(3-nitroamino-1, 2,4-triazole) Salts. Russ. J. Org. Chem. 2005, 41, 440–443. [Google Scholar] [CrossRef]
- Jin, X.H.; Hu, B.C.; Liu, Z.L.; Lv, C.X. Structure and Properties of 1-Amino-2-Nitroguanidinium nitrate. RSC Adv. 2014, 4, 23898–23903. [Google Scholar] [CrossRef]
- Fischer, N.; Klapötke, T.M.; Lux, K.; Martin, F.A.; Stierstorfer, J. Inorganic Amino-Nitro- Guanidinium Derivatives. Crystals 2012, 2, 675–689. [Google Scholar] [CrossRef]
- Jin, X.H.; Hu, B.C.; Liu, Z.L. Synthesis and Properties of Two Energetic Salts Based on 1-Amino-2-nitroguanidine. J. Braz. Chem. Soc. 2015, 26, 124–130. [Google Scholar] [CrossRef]
- Jin, X.H.; Hu, B.C.; Liu, Z.L.; Lv, C.X. Synthesis and Properties of 1-Amino-2- nitroguanidine 4-Nitramino-1,2,4-triazole Salt (in Chinese). Chin. J. Energ. Mater. 2015, 23, 213–217. [Google Scholar]
- Jin, X.H.; Hu, B.C. Synthesis and Properties of 1-Amino-2-nitroguanidine 3,5-Dinitro-1,2,4- triazole Salt (in Chinese). J. QiLu. Uni. Technol. 2016, 30, 20–25. [Google Scholar]
- Gao, S.J. Spheroidization of Nitroguanidine and Synthesis of its Derivatives (in Chinese). Master’s Dissertation, Nanjing University of Science and Technology, Nanjing, China, 2015. [Google Scholar]
- Jin, X.H. Design, Synthesis and Properties of Nitroguanidine-derived Energetic Compounds (in Chinese). Doctoral Dissertation, Nanjing University of Science and Technology, Nanjing, China, 2015. [Google Scholar]
- Liu, Q.Q.; Jin, B.; Peng, R.F.; Guo, Z.C.; Zhao, J.; Zhang, Q.C.; Shang, Y. Synthesis, Characterization and Properties of Nitrogen-rich Compounds based on Cyanuric Acid: A Promising Design in the Development of New Energetic Materials. J. Mater. Chem. A 2016, 4, 4971–4981. [Google Scholar] [CrossRef]
- Fischer, N.; Gao, L.; Klapötke, T.M.; Stierstorfer, J. Energetic Salts of 5,5′-Bis(tetrazole-2- oxide) in a Comparison to 5,5′-Bis(tetrazole-1-oxide) Derivatives. Polyhedron 2013, 51, 201–210. [Google Scholar] [CrossRef]
- Wang, B.S.; Qi, X.J.; Zhang, W.Q.; Wang, K.C.; Li, W.; Zhang, Q.H. Synthesis of 1-(2H- Tetrazol-5-yl)-5-Nitraminotetrazole and its Derivatives from 5-Aminotetrazole and Cyanogen Azide: A Promising Strategy Towards Development of C-N Linked Bistetrazolate Energetic Materials. J. Mater. Chem. A 2017, 5, 20867–20873. [Google Scholar] [CrossRef]
- Wang, R.H.; Guo, Y.; Zeng, Z.; Shreeve, J.M. Nitrogen-rich Nitroguanidyl-Functionalized Tetrazolate Energetic Salts. Chem. Commun. 2009, 19, 2697–2699. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Mayr, N.; Stierstorfer, J.; Weyrauther, M. Maximum Compaction of Ionic Organic Explosives: Bis(hydroxylammonium) 5,5′-Dinitromethyl-3,3′-bis(1,2,4-oxadiazolate) and its Derivatives. Chem. Eur. J. 2014, 20, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Parrish, D.A.; Shreeve, J.M. Energetic Multifunctionalized Nitraminopyrazoles and Their Ionic Derivatives: Ternary Hydrogen-Bond Induced High Energy Density Materials. J. Am. Chem. Soc. 2015, 137, 4778–4786. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Joas, M.; Klapootke, T.M.; Stierstorfer, J. Transition Metal Complexes of 3-Amino-1-nitroguanidine as Laser Ignitible Primary Explosives: Structures and Properties. Inorg. Chem. 2013, 52, 13791–13802. [Google Scholar] [CrossRef] [PubMed]
- Gerasimchuk, A.I.; Mazurenko, Y.A. Metallochelates of Cobalt(II), Nickel(II), and Copper(II) with Salicylidene- and Dimethylaminobenzylidenenitroaminoguanidine. Ukr. Khim. Zh. (Russian Edition) 2004, 70, 21–25. [Google Scholar]
- Xing, Q.Y. Nucleophilic Addition and Conjugate Addition of Aldehydes and Ketones (in Chinese). In Basic Organic Chemistry; Higher Education Press: Beijing, China, 2007; p. 107. [Google Scholar]
- Wu, B.; Yang, H.W.; Tang, Y.X.; Wang, Z.X.; Lu, C.X.; Cheng, G.B. New Energetic Derivatives of 1-Amino-3-Nitroguanidine. J. Energ. Mater. 2015, 33, 180–190. [Google Scholar] [CrossRef]
- Metelkina, E.L.; Novikova, T.A.; Telenyuk, S.E. 2-Nitroguanidine Derivatives. Part 3. Study of Reaction Between 1-Amino-2-nitroguanidine and Formaldehyde. Russ. J. Org. Chem. 1999, 35, 1587–1589. [Google Scholar] [CrossRef]
- Metelkina, E.L.; Novikova, T.A. 2-Nitroguanidine Derivatives. Synthesis and Structure of 1-(2,2,2-Trinitroethylamino)- and 1-(2,2-Dinitroethylamino)-2-nitroguanidines. Russ. J. Org. Chem. 2002, 38, 1378–1379. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Liu, X.B.; Huang, M. Research Progress of Energetic Nitroguanidine Derivatives. J. Energ. Mater. 2013, 5, 668–674. [Google Scholar]
- Glover, D.J.; Kamlet, M.J. Reaction of Trinitromethyl Compounds with Potassium Iodide. J. Org. Chem. 1961, 26, 4734–4735. [Google Scholar] [CrossRef]
- Yang, H.; Feng, Z.C.; Guan, X.G.; Xu, K.Z.; Song, J.R.; Zhao, F.Q. Crystal Structure and Thermal Behavior of Methyleneaminonitroguanidine (MANG) (in Chinese). Chin. J. Energ. Mater. 2019, 27, 125–130. [Google Scholar]
- Ozerova, O.Y.; Efimova, T.P.; Novikova, T.A.; Berestovitskaya, V.M. Reactions of 1-Amino-2-nitroguanidine with Glyoxal. Russ. J. Gen. Chem. 2015, 85, 2583–2591. [Google Scholar] [CrossRef]
- Metelkina, E.L. 2-Nitroguanidine Derivatives: XI. Reactions of N’-Nitrohydrazine carboximidamide and 2-Methylidene-N′-nitrohydrazinecarboximidamide with Glyoxal. Russ. J. Org. Chem. 2008, 44, 495–498. [Google Scholar] [CrossRef]
- Ozerova, O.Y.; Efimova, T.P.; Novikova, T.A. Synthesis of New Nitroamino-Containing 1,2,4-Triazines by Reaction of 1-Amino-2-nitroguanidine with α-Diketones. Russ. J. Org. Chem. 2018, 88, 1381–1384. [Google Scholar] [CrossRef]
- Scott, F.L.; Kennedy, M.T.; Reilly, J. Studies in the Pyrazole Series. II. The 1-Nitroguanyl type. J. Am. Chem. Soc. 1953, 75, 1294–1297. [Google Scholar] [CrossRef]
- Zhang, Q.H.; He, C.L.; Yin, P.; Shreeve, J.M. Insensitive Nitrogen-Rich Materials Incorporating the Nitroguanidyl Functionality. Chem. Asian J. 2014, 9, 212–217. [Google Scholar] [CrossRef]
- Lieber, E.; Sherman, E.; Henry, R.A.; Cohen, J. The Reaction of Nitrous Acid with Nitroaminoguanidine. J. Am. Chem. Soc. 1951, 73, 2327–2329. [Google Scholar] [CrossRef]
- O’Connor, T.E.; Fleming, G.; Reilly, J. Diazotization of Nitroaminoguanidine. J. Soc. Chem. Ind. 1949, 68, 309–310. [Google Scholar] [CrossRef]
- Xue, H.; Gao, H.X.; Twamley, B.; Shreeve, J.M. Energetic Salts of 3-Nitro-1,2,4- triazole-5-one, 5-Nitroaminotetrazole, and Other Nitro-Substituted Azoles. Chem. Mater. 2007, 19, 1731–1739. [Google Scholar] [CrossRef]
- Astakhov, A.M.; Vasiliev, A.D.; Molokeev, M.S.; Sirotinin, A.M.; Kruglyakova, L.A.; Stepanov, R.S. Crystal and Molecular Structure of Nitramino Derivatives of Tetrazole and 1,2,4- Triazole. II. 5-Nitraminotetrazole Diammonium Salt. J. Struct. Chem. 2004, 45, 175–180. [Google Scholar] [CrossRef]
- Vasiliev, A.D.; Astakhov, A.M.; Nefedov, A.A.; Stepano, R.S. Crystal and Molecular Structure of Monoammonium Salt of 5-nitroaminotetrazole. J. Struct. Chem. 2003, 44, 322–325. [Google Scholar] [CrossRef]
- Astakhov, A.M.; Vasiliev, A.D.; Molokeev, M.S.; Sirotinin, A.M.; Stepanov, R.S. Crystal and Molecular Structure of Nitraminotetrazoles and Nitramino-1,2,4-triazoles. V. 5-Nitraminotetrazole methylammonium salt. J. Struct. Chem. 2005, 46, 517–522. [Google Scholar] [CrossRef]
- Vasiliev, A.D.; Astakhov, A.M.; Molokeev, M.S.; Kruglyakova, L.A.; Sirotinin, A.M.; Stepanov, R.S. Crystal and Molecular Structure of the Nitramino Derivatives of Tetrazole and 1,2,4-Triazole. III. 5-Nitraminotetrazole Lithium Salt Monohydrate. J. Struct. Chem. 2004, 45, 360–364. [Google Scholar] [CrossRef]
- Astakhov, A.M.; Vasiliev, A.D.; Molokeev, M.S.; Kruglyakova, L.A.; Sirotinin, A.M.; Stepanov, R.S. Crystal and Molecular Structure of Nitraminotetrazole and Nitramino-1,2,4- triazole. IV. 5-Nitraminotetrazole Sodium Salt Sesquihydrate. J. Struct. Chem. 2004, 45, 537–540. [Google Scholar] [CrossRef]
- Tappan, B.C.; Incarvito, C.D.; Rheingold, A.L.; Brill, T.B. Thermal Decomposition of Energetic Materials 79 Thermal, Vibrational, and X-ray Structural Characterization of Metal Salts of Mono- and Di-anionic 5-Nitraminotetrazole. Thermochim. Acta 2002, 384, 113–120. [Google Scholar] [CrossRef]
- Palopoli, S.F.; Geib, S.J.; Rheingold, A.L.; Brill, T.B. Synthesis and Modes of Coordination of Energetic Nitramine Ligands in Copper (II), Nickel (II), and Palladium (II) Complexes. Inorg. Chem. 1988, 27, 2963–2971. [Google Scholar] [CrossRef]
- Semenov, S.N.; Rogachev, A.Y.; Eliseeva, S.V.; Belousov, Y.A.; Drozdov, A.A.; Troyanov, S.I. 5-Nitroaminotetrazole as a Building Block for Extended Network Structures: Syntheses and Crystal Structures of a Number of Heavy Metal Derivatives. Polyhedron 2007, 26, 4899–4907. [Google Scholar] [CrossRef]
- Ozerova, O.Y.; Efimova, T.P.; Novikova, T.A.; Gurzhii, V.V.; Berestovitskaya, V.M. Structure and Synthesis of 3,5-Dimethyl-N-nitro-1H-pyrazole-1-carboxamidine. Russ. J. Gen. Chem. 2015, 85, 1623–1628. [Google Scholar] [CrossRef]
- Metelkina, E.L.; Novikova, T.A. Substituted 2-Nitroguanidines in Reactions with Hydrazine Hydrate. Zh. Org. Khim. 1993, 12, 2386–2389. [Google Scholar]
- Aboudi, J.; Bayat, Y.; Abedi, Y.; Nabati, M.; Mahkam, M. 3-Nitro, 1-Amino Guanidine and 5-Hydrazino-1HTetrazole Derivatives as New Energetic Materials. Iran. J. Chem. Chem. Eng. 2015, 34, 1–16. [Google Scholar]
- Gao, H.X.; Shreeve, J.M. The Many Faces of FOX-7: A Precursor to High-Performance Energetic Materials. Angew. Chem. Int. Ed. 2015, 54, 6335–6338. [Google Scholar] [CrossRef] [PubMed]
- Impact: Insensitive >40 J, less sensitive >35 J, sensitive >4 J, very sensitive <3 J. Friction: Insensitive >360 N, less sensitive =360 N, sensitive >80 N, very sensitive >10 N, extremely sensitive <10 N. According to the UN Recommendations on the Transport. of Dangerous Goods.
Sample Availability: Samples of the compounds are not available from the authors. |
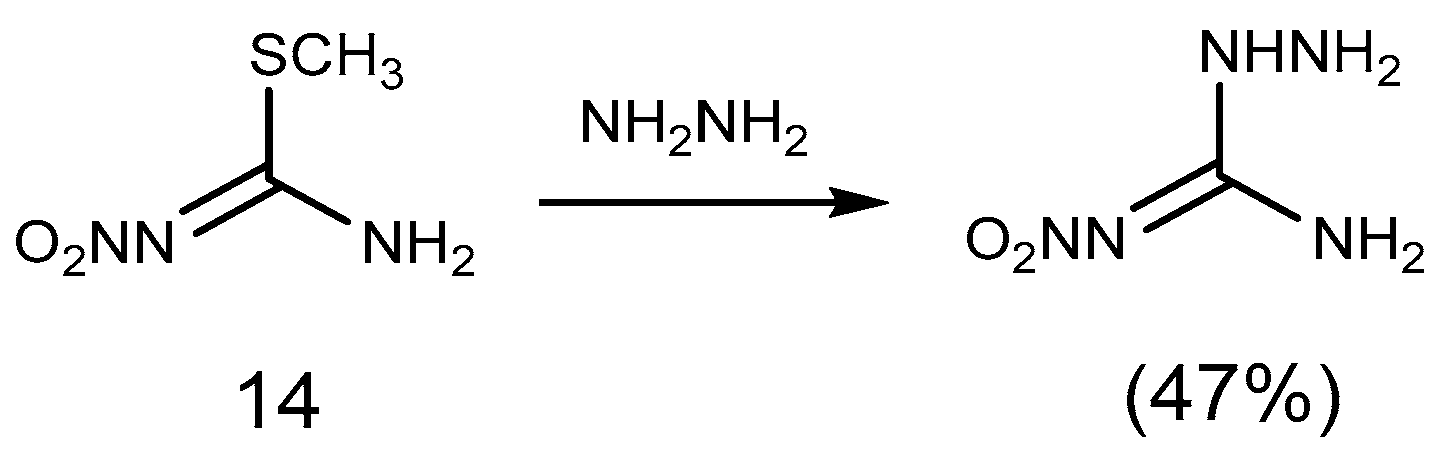

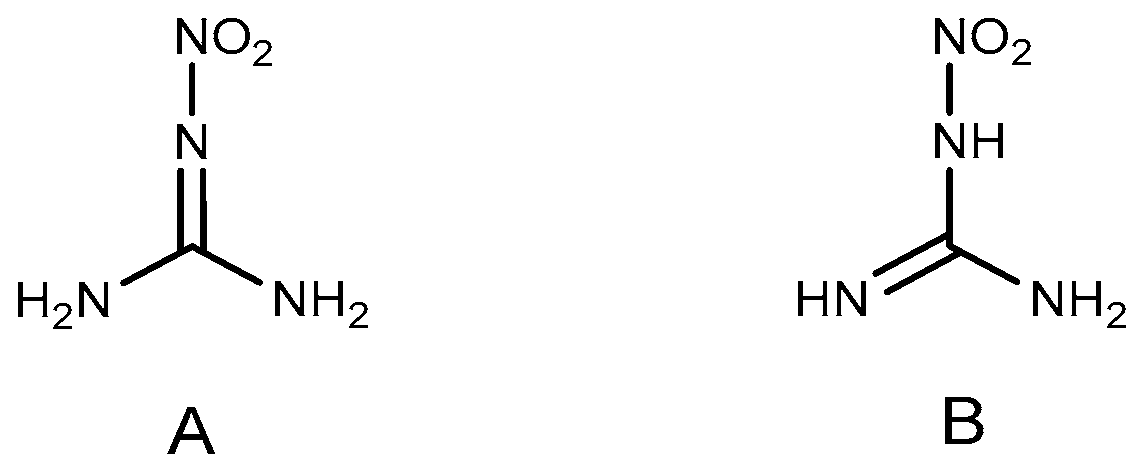
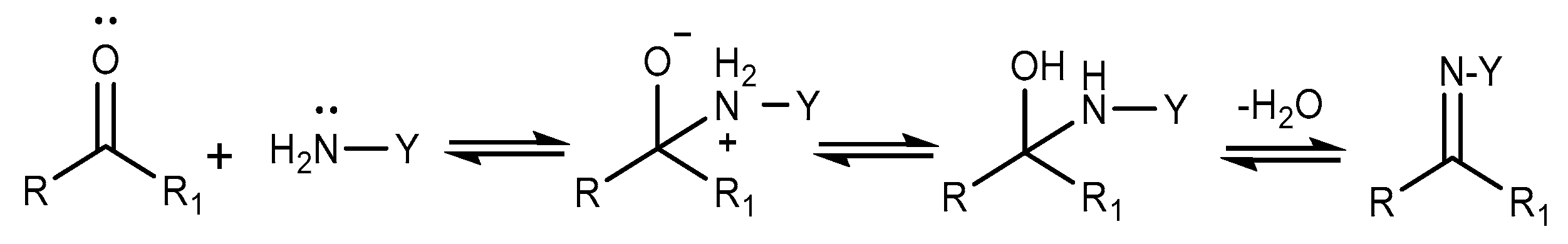

| ANQ | NQ | RDX | TNT | FOX-7 | |
|---|---|---|---|---|---|
| Ma (g mol−1) | 119.08 | 104.07 | 222.12 | 227.13 | 148.08 |
| ρb (g cm−3) | 1.767 | 1.759 | 1.80 | 1.663 | 1.885 |
| ∆fH c (kJ mol−1) | 161.7 | −86 | 70 | −59.4 | −32.0 |
| Tdecd (°C) | 184 | 254 | 205 | 295 | 237.2 |
| Pe (GPa) | 30.7 | 29.0 | 34.9 | 19.1 | 28.4 |
| Vdetf (m s−1) | 8250 | 8344 | 8795 | 6928 | 8325 |
| IS g (J) | 20 | >50 | 7.5 | 39.4 n | 24.7 |
| FS h (N) | 144 | >355 | 120 | >353 o | >350 |
| ESD i (J) | 0.15 | - | 0.2 | 0.25 | 0.45 |
| N j (%) | 58.8 | 53.83 | 37.84 | 18.5 | 43.24 |
| OB k (%) | −33.59 | −30.75 | −21.6 | −74.0 | −21.61 |
| Reference | [18] l,[21,22] | [23] m | [24],[25] l,[26] | [26,27,28,29] | [30,31,32,33,34] |
| IS a (J) | FS b (N) | ESD c (J) | Tdecd (°C) | ∆fH e (kJ mol−1) | Mf (g mol−1) | N g (%) | ρh (g cm−3) | Tdeti (K) | Pj (GPa) | Vdetk (m s−1) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 22 | 10 l | 120 | 0.50 | 130 | 563.4 | 182.10 | 46.2 | 1.905 | 4196 | 42.7 | 9551 | [18] l |
| 23 | 25 | 288 | 0.15 | 80 | - | 173.56 | 39.82 | 1.731 | - | - | - | [48] l |
| 24 | 25 | 288 | 0.20 | 80 | - | 218.03 | 31.76 | 2.024 | - | - | - | [48] l |
| 25 | 25 | 288 | 0.20 | 86 | - | 265.02 | 26.46 | 2.339 | - | - | - | [48] l |
| 26 | 6 | 120 | 0.30 | 144 | - | 336.29 | 41.35 | 1.968 | - | - | - | [48] l |
| 27 | 1 | 20 | 0.15 | 130 | 599.4 | 219.54 | 31.90 | 1.980 | - | - | - | [18] l |
| 28 | - | - | - | 143.0 | 264.7 | - | 43.58 | 1.70 | - | 30.2 | 8398 | [49] |
| 29 | - | - | - | 121.6 | 487.2 | - | 59.82 | 1.64 | - | 29.1 | 8334 | [49] |
| 30 | - | - | - | 175.5 | 551.3 | - | 56.49 | 1.59 | - | 26.6 | 8050 | [50] |
| 31 | - | - | - | 217.1 | 353.7 | - | 50.44 | - | - | 29.8 | 8420 | [51] |
| 32 | - | - | - | - | 467.0 | 590.42 | - | - | - | 31.8 | 8642 | [52] |
| 33 | 10 | 40 | 0.10 | 108 | 511.4 | 244.1 | 45.9 | 1.850 | 3949 | 37.7 | 9175 | [18] l |
| 34 | 12 | 228 | 0.20 | 118 | 40.9 | 313.09 | 44.7 | 1.705 | 3612 | 31.1 | 8656 | [18] l |
| 35 | >40 | - | - | - | - | 347.2 | 32.09 | 1.63 | - | 23.4 | 7500 | [22] m |
| 36 | >60 | - | - | 202.1 | 337.5 | 248.06 | 42.41 | 1.75 | - | 30.7 | 8392 | [54] n |
| 37 | 5 | 96 | 0.40 | 136 | 544.3 | 285.2 | 54.4 | 1.729 | 3607 | 31.7 | 8753 | [18] l |
| 38 | 10 | 48 | 1.5 | 163 | 1043.8 | 408.26 | 61.76 | 1.832 | 4129 | 38.2 | 9350 | [55] l |
| 39 | 7 | 54 | - | 109 | 986.6 | - | 66.2 | 1.79 | - | 36.1 | 9432 | [56] o |
| 40 | - | - | - | 171 | 557.1 | - | - | 1.72 | - | 33.7 | 9171 | [57] |
| 41 | 7 | 120 | 0.07 | 149 | 593.1 | 584.29 | 43.15 | 1.85 | 4343 | 36.6 | 8872 | [58] l |
| 42 | 8 | 80 | - | 130 | 450.0 | - | - | 1.88 | - | 39.5 | 9237 | [59] o |
| 50 | 19 | 128 | 0.268 | 218 | 421.6 | - | 42.8 | 1.65 | - | 24.8 | 7670 | [63] o,p |
| 51 | 24 | 168 | 0.305 | 204 | 306.2 | - | 32.8 | 1.79 | - | 28.1 | 7965 | [63] o,p |
| 58 | >7.9 | - | - | 170.9 | - | - | - | 1.63 | - | 20.9 | 7100 | [68] q |
| 67 | >21.56 | - | - | - | 676.95 | - | - | 1.690 | - | 28.43 | 8390 | |
| 68 | 19.6 | - | - | - | −470.9 | - | - | 1.555 | - | 19.06 | 6860 | |
| 69 | - | - | - | - | 252.3 | - | 64.12 | 2.06 | - | 48.5 | 10,358 | [76] |
| 70 | - | - | - | 184 | 440.6 | - | 65.4 | 1.72 | - | 27.0 | 8506 | [76] |
| 71 | - | - | - | 165 | 487.5 | - | 71.6 | 1.63 | - | 25.5 | 8276 | [76] |
| 72 | - | - | - | 177 | 328.0 | - | 63.3 | 1.74 | - | 26.0 | 8334 | [76] |
| 74 | - | - | - | 135 | 694.2 | - | 70.0 | 1.68 | - | 25.9 | 8230 | [76] |
| 83 | 8 | 360 | - | 123.5 | 304.2 | - | - | 1.73 | - | 29.4 | 8492 | [88] o |
| ANQ | 20 | 144 | 0.15 | 184 | 161.7 | 119.08 | 58.8 | 1.767 | - | 30.7 | 8250 | [18] l, [21,22] |
| RDX | 7.5 | 120 | 0.2 | 205 | 70 | 222.12 | 37.84 | 1.80 | - | 34.9 | 8795 | [24], [25] l, [26] |
| TNT | 39.4 r | >353 o | 0.25 | 295 | −59.4 | 227.13 | 18.5 | 1.663 | - | 19.1 | 6928 | [26,27,28,29] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Cai, M.; Zhao, F.; Xu, K. A Review on the Reactivity of 1-Amino-2-Nitroguanidine (ANQ). Molecules 2019, 24, 3616. https://doi.org/10.3390/molecules24193616
Wang J, Cai M, Zhao F, Xu K. A Review on the Reactivity of 1-Amino-2-Nitroguanidine (ANQ). Molecules. 2019; 24(19):3616. https://doi.org/10.3390/molecules24193616
Chicago/Turabian StyleWang, Jinghua, Meng Cai, Fengqi Zhao, and Kangzhen Xu. 2019. "A Review on the Reactivity of 1-Amino-2-Nitroguanidine (ANQ)" Molecules 24, no. 19: 3616. https://doi.org/10.3390/molecules24193616
APA StyleWang, J., Cai, M., Zhao, F., & Xu, K. (2019). A Review on the Reactivity of 1-Amino-2-Nitroguanidine (ANQ). Molecules, 24(19), 3616. https://doi.org/10.3390/molecules24193616






