Migration Studies of Two Common Components of UV-curing Inks into Food Simulants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mathematical Modelling
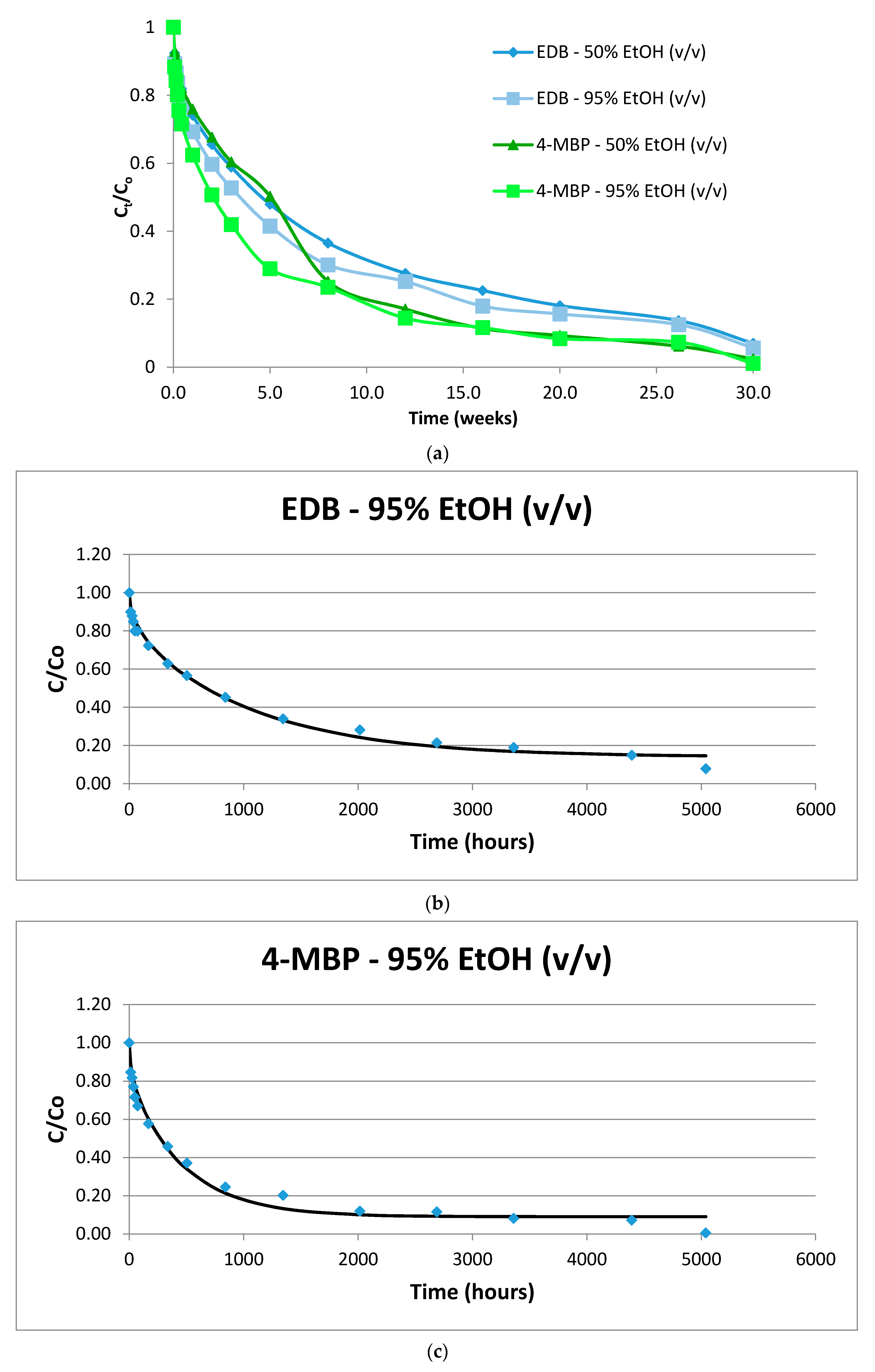
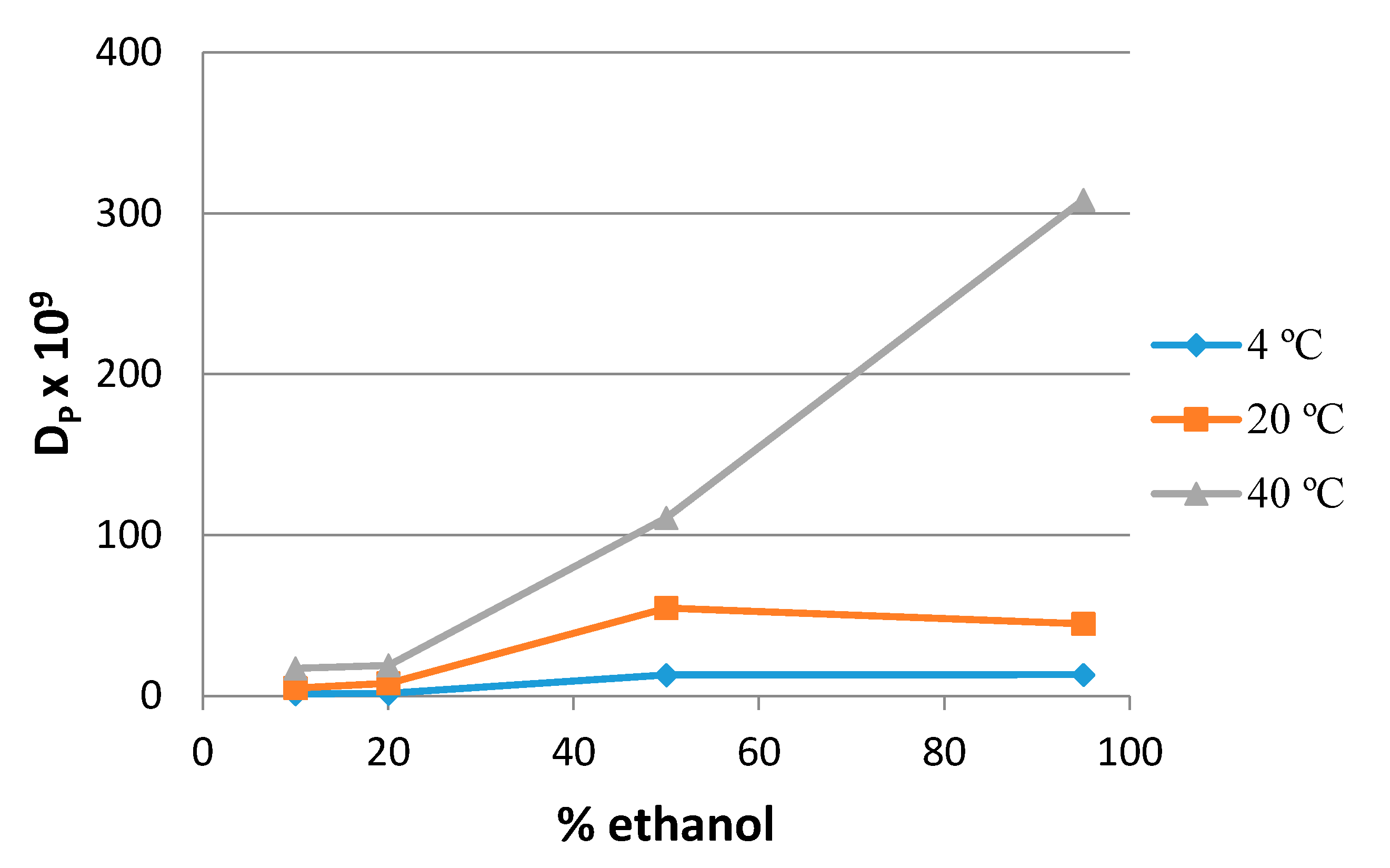
2.2. 4-MBP
2.3. Ethyl-4-(dimethylamino) Benzoate (EDB)
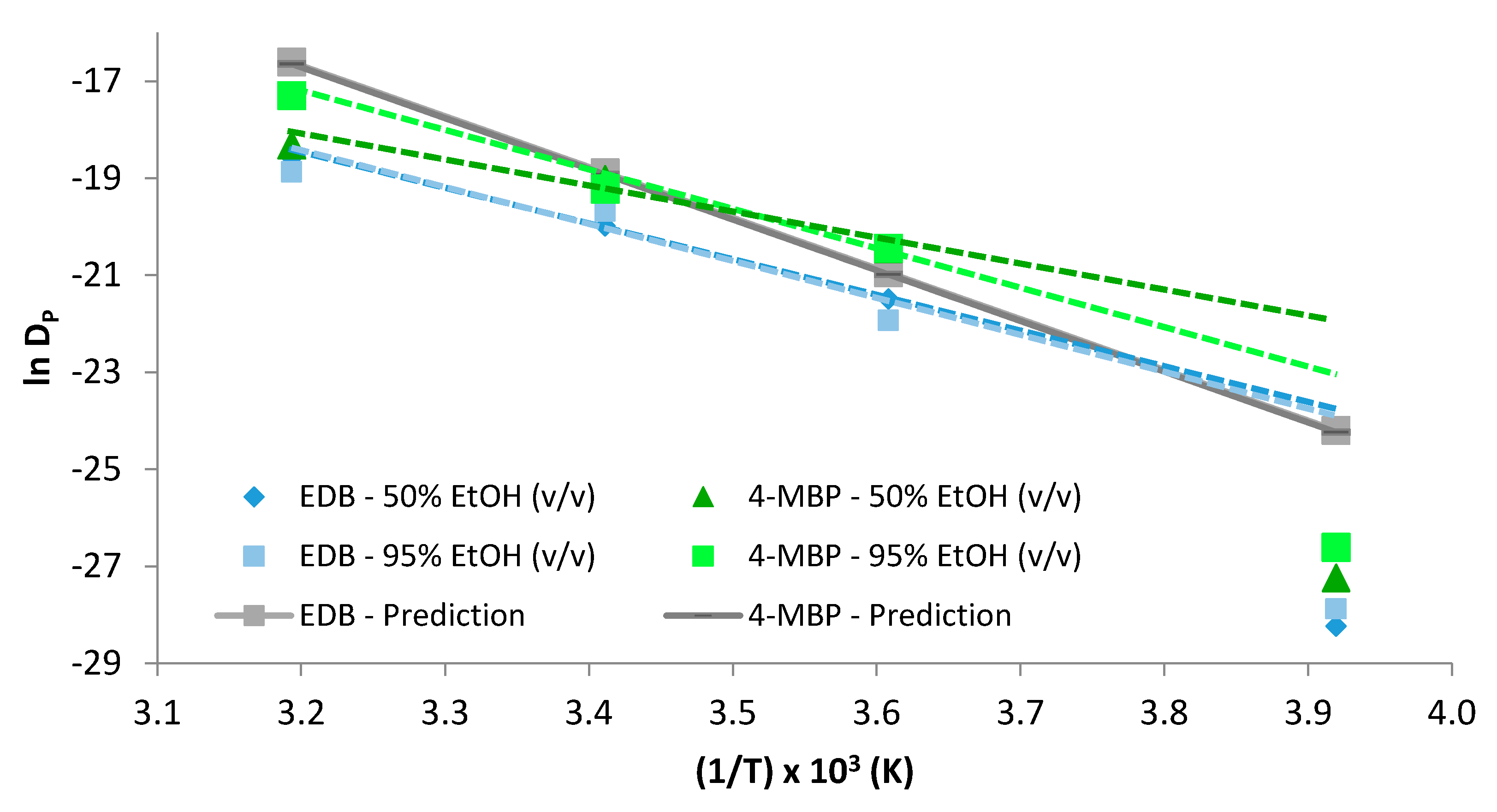
2.4. Diffusion Coefficient Linearity
2.5. Worst Case Prediction
3. Materials and Methods
3.1. Reagents and Standards
3.2. Migration Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castle, L.; Bradley, E.L. Residues of food contact materials. In Handbook of Dairy Foods Analysis, 1st ed.; Nollet, L.N.L., Toldrá, F., Eds.; Taylor & Francis: Boca Ratón, FL, USA, 2010; pp. 755–775. [Google Scholar]
- Simoneau, C. Food contact materials. In Comprehensive Analytical Chemistry. Food Contaminants and Residue Analysis, 1st ed.; Picó, Y., Ed.; Elsevier: Oxford, UK, 2008; Volume 51, pp. 733–774. [Google Scholar]
- Lau, O.W.; Wong, S.K. Contamination in food from packaging material. J. Chromatogr. A 2000, 882, 255–270. [Google Scholar] [CrossRef]
- Brandsch, J.; Mercea, P.; Rüter, M.; Tosa, V.; Piringer, O. Migration modelling as a tool for quality assurance of food packaging. Food. Addit. Contam. 2002, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Helmroth, I.E.; Rijk, R.; Dekker, M.; Jongen, W. Predictive modelling of migration from packaging materials into food products for regulatory purposes. Trends Food Sci. Technol. 2002, 13, 102–109. [Google Scholar] [CrossRef]
- Helmroth, I.E.; Dekker, M.; Hankemeier, T. Influence of solvent absorption on the migration of Irganox 1076 from LDPE. Food Addit. Contam. 2002, 19, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.H.; Trier, X.T.; Fabech, B. Mathematical modelling of migration: A suitable tool for the enforcement authorities? Food Addit. Contam. 2005, 22, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Sanches Silva, A.T.; Sendón García, R.; Cooper, I.; Franz, R.; Paseiro, P. Compilation of analytical methods and guidelines for the determination of selected model migrants from plastic packaging. Trends Food. Sci. Technol. 2006, 17, 535–546. [Google Scholar] [CrossRef]
- Sanches Silva, A.T.; Cruz, J.M.; Sendón García, R.; Franz, R.; Paseiro, P. Kinetic migration studies from packaging films into meat products. Meat Sci. 2007, 77, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Sanches Silva, A.T.; Cruz Freire, J.M.; Sendón, R.; Franz, R.; Paseiro Losada, P. Migration and diffusion of diphenylbutadiene from packages into foods. J. Agric. Food Chem. 2009, 57, 10225–10230. [Google Scholar] [CrossRef] [PubMed]
- Sanches Silva, A.T.; Cruz Freire, J.M.; Paseiro Losada, P. Study of the diffusion coefficients of diphenylbutadiene and triclosan into and within meat. Eur. Food Res. Technol. 2010, 230, 957–964. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89.
- Commission Regulation (EU) No 1935/2004 of 27 October 2004 on materials and articles intended to come into contact with food. Off. J. Eur. Union 2004, L338/4.
- Bustos, J.; Martin, P.; Lago, M.A.; Sendón, R.; Rodríguez Bernaldo de Quirós, A.; Puga, M.A.; Basadre Pampín, M.I.; Sánchez, J.J. GC/MS screening of ink compounds (photoinitiators) in food packaging. In Proceedings of the 5th International Symposium on Food Packaging: Scientific Developments Supporting Safety and Innovation, Berlin, Germany, 14–16 November 2012. [Google Scholar]
- RASFF Portal. Available online: https://webgate.ec.europa.eu/rasff-window/portal/ (accessed on 20 February 2015).
- Lago, M.A.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Bustos, J.; Nieto, M.T.; Paseiro, P. Photoinitiators: A food safety review. Food Addit. Contam. A 2015, 32, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bernaldo de Quirós, A.; Paseiro-Cerrato, R.; Pastorelli, S.; Koivikko, R.; Simoneau, C.; Paseiro-Losada, P. Migration of photoinitiators by gas phase into dry foods. J. Agric. Food Chem. 2009, 57, 10211–10215. [Google Scholar] [CrossRef] [PubMed]
- Van Den Houwe, K.; Van Heyst, A.; Evrard, C.; Van Loco, J.; Bolle, F.; Lynen, F.; Van Hoeck, E. Migration of 17 photoinitiators from printing inks and cardboard into packaged food—Results of a Belgian market survey. Packag. Technol. Sci. 2016, 29, 121–131. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, C.; Zhang, Y.; Chen, Y. Simultaneous determination of 10 photoinitiators in milk by solid-phase microextraction coupled with gas chromatography/mass spectrometry. J. Food Sci. 2016, 81, T1336–T1341. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon: Oxford, UK, 1975; pp. 44–68. [Google Scholar]
- Piringer, O.G. Evaluation of plastics for food packaging. Food Addit. Contam. 1994, 11, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Pastorelli, S.; Cruz, J.M.; Simoneau, C.; Castanheira, I.; Paseiro-Losada, P. Development of a method to study the migration of six photoinitiators into powdered milk. J. Agric. Food Chem. 2008, 56, 2722–2726. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, C. Applicability of Generally Recognised Diffusion Models for the Estimation of Specific Migration in Support of EU Directive 2002/72/EC. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/111111111/14935/1/reqno_jrc59476_mathmod_v10_cs_2010_09_24_final.pdf%5B1%5D.pdf (accessed on 6 January 2015).
- Lago Crespo, M.A. Printing Inks for Food Packaging. Study of the Key Parameters in the Migration of Phthoinitiators. Ph.D. Thesis, University of Santiago de Compostela, Santiago de Compostela, Spain, 29 July 2016. [Google Scholar]
- Brandsch, J.; Mercea, P.; Piringer, O. Possibilities and limitations of migration modelling. In Packaging Materials for Food. Plastic Barrier Function, Mass Transport, Quality Assurance and Legislation, 1st ed.; Piringer, O.G., Baner, A.L., Eds.; Wiley-VCH: Weinheim, Germany; New York, NY, USA, 2000; pp. 445–468. [Google Scholar]
- Maia, J.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Cruz, J.M.; Seiler, A.; Franz, R.; Simoneau, C.; Castle, C.; Driffield, M.; Mercea, P.; et al. The Determination of key diffusion and partition parameters and their use in migration modelling of benzophenone from low density polyethylene (LDPE) into different foodstuffs. Food Addit. Contam. A 2016, 33, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Council Directive (EU) No 85/572/ECC of 19 December 1985 laying down the list of simulants to be used for testing migration of constituents of plastic materials and articles intended to come into contact with foodstuffs. Off. J. Eur. Comm. 1985, L372/14.
- Lago, M.A.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Bustos, J.; Santillana, M.I.; Paseiro, P. Simultaneous chromatographic analysis of photoinitiators and amine synergists in food contact materials. Anal. Bioanal. Chem. 2014, 406, 4252–4259. [Google Scholar] [CrossRef] [PubMed]
| Food/Food Simulant | Temperature (°C) | 4-MBP | EDB | ||||
|---|---|---|---|---|---|---|---|
| DP (cm2∙s−1) | KP/F (w/v) | RMSE (%) | DP (cm2∙s−1) | KP/F (w/v) | RMSE (%) | ||
| Water | 4 | 1.0 × 10−10 | 309.0 | 3.4 | 2.6 × 10−10 | 60.3 | 3.9 |
| 20 | 1.5 × 10−10 | 365.5 | 3.0 | 8.9 × 10−10 | 47.1 | 2.2 | |
| 40 | 3.2 × 10−10 | 302.5 | 2.5 | 3.0 × 10−9 | 119.4 | 3.5 | |
| 3% Acetic acid (w/v) | 4 | 1.9 × 10−10 | 279.7 | 3.5 | 4.6 × 10−10 | 22.6 | 2.2 |
| 20 | 2.4 × 10−10 | 407.0 | 3.1 | 2.1 × 10−9 | 22.2 | 4.3 | |
| 40 | 4.1 × 10−10 | 353.3 | 3.9 | 6.8 × 10−9 | 20.0 | 3.4 | |
| 10% Ethanol (v/v) | 4 | 1.3 × 10−10 | 257.7 | 6.9 | 3.2 × 10−10 | 42.7 | 3.7 |
| 20 | 4.9 × 10−10 | 239.8 | 3.8 | 1.3 × 10−9 | 38.5 | 5.1 | |
| 40 | 1.7 × 10−9 | 148.6 | 3.9 | 5.1 × 10−9 | 30.2 | 4.7 | |
| 20% Ethanol (v/v) | 4 | 1.6 × 10−10 | 148.2 | 5.5 | 3.9 × 10−10 | 18.2 | 4.4 |
| 20 | 7.8 × 10−10 | 82.7 | 3.7 | 1.5 × 10−9 | 23.5 | 4.5 | |
| 40 | 1.9 × 10−9 | 78.9 | 6.3 | 1.2 × 10−8 | 13.0 | 2.5 | |
| 50% Ethanol (v/v) | −18 | 1.5 × 10−12 | 13.9 | 4.7 | 5.5 × 10−13 | 25.4 | 2.1 |
| 4 | 1.3 × 10−9 | 3.3 | 4.3 | 4.7 × 10−10 | 5.1 | 5.7 | |
| 20 | 5.5 × 10−9 | 3.5 | 4.8 | 2.1 × 10−9 | 3.9 | 6.0 | |
| 40 | 1.1 × 10−8 | 4.9 | 8.9 | 8.5 × 10−9 | <2.3 * | 5.4 | |
| 95% Ethanol (v/v) | −18 | 2.8 × 10−12 | 20.6 | 3.6 | 7.8 × 10−13 | 37.1 | 3.4 |
| 4 | 1.3 × 10−9 | <1.5 * | 3.6 | 4.0 × 10−10 | 4.9 | 4.1 | |
| 20 | 4.5 × 10−9 | 1.9 | 3.3 | 2.8 × 10−9 | <1.5 * | 4.0 | |
| 40 | 3.1 × 10−8 | 2.6 | 3.8 | 6.4 × 10−9 | <1.4 * | 6.3 | |
| Food/Food Simulant | Migrant | EA (kJ∙mol−1) | D0 (cm2∙s−1) | R2 |
|---|---|---|---|---|
| H2O | 4-MBP | 22.35 | 1.63 × 10−6 | 0.98009 |
| EDB | 48.62 | 3.89 × 10−1 | 0.99877 | |
| 3% Acetic Acid | 4-MBP | 15.75 | 1.67 × 10−7 | 0.95748 |
| EDB | 53.81 | 6.94 | 0.98835 | |
| 10% Ethanol | 4-MBP | 52.11 | 8.76 × 10−1 | 0.99722 |
| EDB | 55.05 | 8.02 | 0.99752 | |
| 20% Ethanol | 4-MBP | 48.70 | 2.84 × 10−1 | 0.96626 |
| EDB | 69.20 | 3.91 × 103 | 0.99036 | |
| 50% Ethanol | 4-MBP | 42.57 | 1.59 × 10−1 | 0.95148 |
| EDB | 58.09 | 4.32 × 101 | 0.99733 | |
| 95% Ethanol | 4-MBP | 63.32 | 1.03 × 103 | 0.99003 |
| EDB | 60.73 | 1.11 × 102 | 0.91564 | |
| Theoretical Prediction (Piringer) | 4-MBP | 86.9 | 1.86 × 107 | - |
| EDB | 1.93 × 107 |
| Structure | CAS nr. | Common Name | Mw | Log Ko/w | Mp (°C) | Bp (°C) | PI Type |
|---|---|---|---|---|---|---|---|
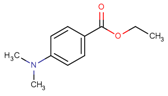 | 10287-53-3 | EDB | 193.24 | 2.51 b | 63.50 a | 296.50 b | Amine synergist |
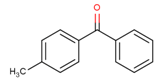 | 134-84-9 | 4-MBP | 196.24 | 3.69 b | 59.50 a | 328.10 b | II |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lago, M.A.; Sendón, R.; Bustos, J.; Nieto, M.T.; Paseiro Losada, P.; Rodríguez-Bernaldo de Quirós, A. Migration Studies of Two Common Components of UV-curing Inks into Food Simulants. Molecules 2019, 24, 3607. https://doi.org/10.3390/molecules24193607
Lago MA, Sendón R, Bustos J, Nieto MT, Paseiro Losada P, Rodríguez-Bernaldo de Quirós A. Migration Studies of Two Common Components of UV-curing Inks into Food Simulants. Molecules. 2019; 24(19):3607. https://doi.org/10.3390/molecules24193607
Chicago/Turabian StyleLago, Miguel A., Raquel Sendón, Juana Bustos, María T. Nieto, Perfecto Paseiro Losada, and Ana Rodríguez-Bernaldo de Quirós. 2019. "Migration Studies of Two Common Components of UV-curing Inks into Food Simulants" Molecules 24, no. 19: 3607. https://doi.org/10.3390/molecules24193607
APA StyleLago, M. A., Sendón, R., Bustos, J., Nieto, M. T., Paseiro Losada, P., & Rodríguez-Bernaldo de Quirós, A. (2019). Migration Studies of Two Common Components of UV-curing Inks into Food Simulants. Molecules, 24(19), 3607. https://doi.org/10.3390/molecules24193607







