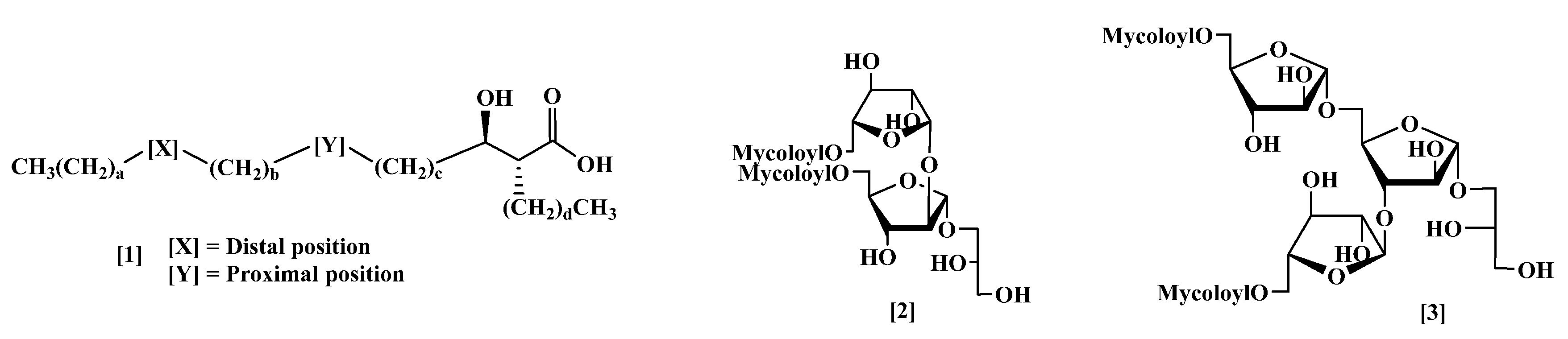
3. Methods
3.1. General
The chemicals used were obtained from commercial suppliers (Sigma-Aldrich, Lee on Solent, UK, and Alfa Aesar, Heysham, UK) or prepared from them by the methods described. Ether and tetrahydrofuran were dried over sodium wire and benzophenone under nitrogen, while dichloromethane was dried over calcium hydride. The petroleum spirit (petrol) used had boiling point of 40–60 °C. All reagents and solvents used were of reagent grade unless otherwise stated. Silica gel (Merck 7736) used for column and thin-layer chromatography was obtained from Sigma; separated components were detected variously using UV light, I2 and phosphomolybdic acid solution in IMS followed by charring. Anhydrous MgSO4 was used to dry organic solutions. Infrared (IR) spectra were carried out on a Perkin–Elmer 1600 F.T.I.R. spectrometer using liquid films or a KBr disc (solid). NMR spectra were carried out on Bruker Avance 400 or 500 spectrometers. [α]D values were recorded in CHCl3 on a POLAAR 2001 optical activity polarimeter. Matrix-assisted laser desorption/ionization (MALDI) mass spectra were provided by the Engineering and Physical Science Research Council Mass Spectrometry Service in Swansea University.
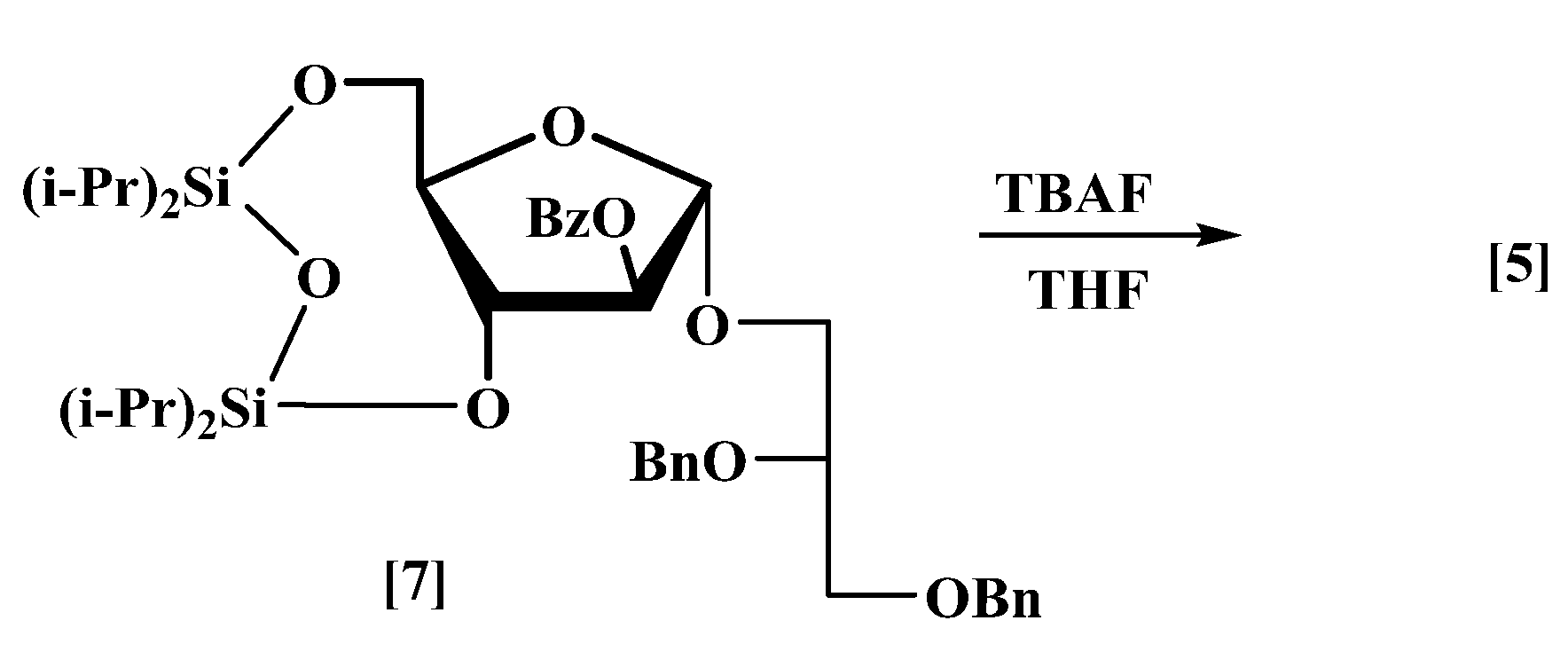
3.2. 2′,3′-Di-O-benzyl-l-glycerol-(1′→1)-2-O-benzoyl-α-d-arabinofuranoside (5)
Tetrabutylammonium fluoride (17.8 mL, 0.0616 mol, 1.0 M in THF) was added dropwise with stirring to 2′,3′-di-
O-benzyl-
l-glycerol-(1′→1)-2-
O-benzoyl-3,5-
O-(tetraisopropylsiloxane-1,3-diyl)-α-
d-arabinofuranoside (
7) [
20] (6.7 g, 0.0089 mol) in anhydrous THF (50 mL) at 0 °C under nitrogen. The mixture was allowed to reach room temperature (rt) and was stirred for 4 h, and it was then diluted with EtOAc (100 mL) and water (50 mL). The aqueous layer was re-extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with sat. aq. NH
4Cl (50 mL), brine (50 mL), dried and concentrated. Column chromatography eluting with petrol/EtOAc (1:1) gave compound (
5) as a colourless thick oil (4.3 g, 95%) [MALDI: Found (M + Na)
+: 531.2; C
29H
32NaO
8 requires: 531.2],
+53 (
c 4.0, CHCl
3), which showed δ
H (400 MHz, CDCl
3): 7.98–7.93 (2H, m), 7.53 (1H, t,
J 7.4 Hz), 7.39 (2H, t,
J 7.7 Hz), 7.33–7.17 (10H, m), 5.20 (1H, br. s), 5.04 (1H, br. s), 4.65 (1H, d,
J 12.0 Hz), 4.61 (1H, d,
J 12.0 Hz), 4.51 (1H, d,
J 12.2 Hz), 4.48 (1H, d,
J 12.2 Hz), 4.10 (2H, br. s), 3.85 (1H, dd,
J 5.6, 10.3 Hz), 3.80 (1H, m), 3.76 (1H, br. p,
J 5.2 Hz), 3.72–3.66 (1H, m), 3.63 (1H, dd,
J 4.3, 10.3 Hz), 3.60–3.52 (2H, including br. d,
J 4.8 Hz at 3.58), 2.59–2.31 (2H, incl. 2 × OH groups); δ
C (101 MHz, CDCl
3): 166.6, 138.3, 138.0, 133.6, 129.8, 129.0, 128.5, 128.4, 128.3, 127.8, 127.6, 105.3, 85.9, 84.2, 76.6, 76.4, 73.4, 72.2, 69.7, 67.1, 61.9; ν
max: 3405, 3065, 3031, 2945, 2868,1715, 1465, 1105, 884, 712 cm
−1.
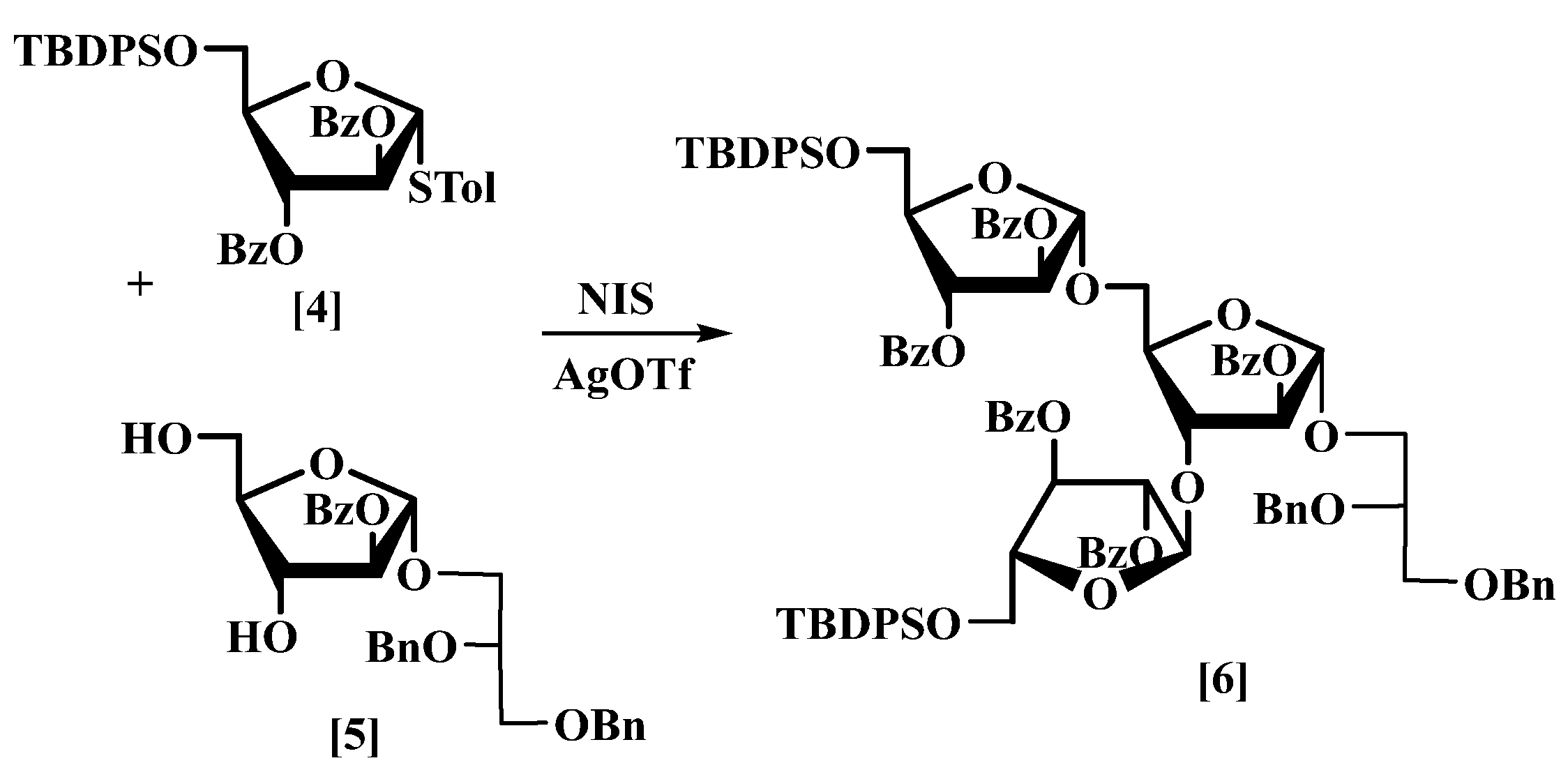
3.3. 2′,3′-Di-O-benzyl-l-glycerol-(1′→1)-2,3-di-O-benzoyl-5-O-tert-butyldiphenylsilyl-α-d-arabinofuranosyl-(1→3)-[2,3-di-O-benzoyl-5-O-tert-butyldiphenylsilyl-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranoside (6)
Molecular sieves 4 Å (1.5 g) was added with stirring to furanoside (
4) [
18] (16.7 g, 28.9 mmol) and furanoside (
5) (4.2 g, 8.2 mmol) in dry CH
2Cl
2 (50 mL) at rt under nitrogen. The mixture was stirred for 30 min., then cooled to −36 °C and
N-iodosucinimide (9.1 g, 0.037 mol) was added followed by silver triflate (2.1 g, 8.2 mmol). The mixture was stirred until it turned dark brown, quenched by the addition of triethylamine (2 mL) until it turned yellow, then diluted with CH
2Cl
2 (100 mL) and filtered through celite. The filtrate was evaporated under reduced pressure; column chromatography eluting with hexane/EtOAc (5:2) gave compound (
6) as a colourless thick oil (13.0 g, 91%) [MALDI: Found (M + NH
4)
+: 1682.6679; C
99H
104NO
20Si
2 requires: 1682.6685] (
Figure S3),
−1.4 (
c 2.8, CHCl
3); δ
H (400 MHz, CDCl
3): 8.01–7.92 (10H, m), 7.70–7.67 (4H, m), 7.65–7.61 (4H, m), 7.58–7.53 (2H, m), 7.50–7.44 (3H, m), 7.41–7.21 (32H, m), 5.66–5.62 (2H, incl. br. d,
J 4.8 Hz at 5.63), 5.61 (1H, br.s), 5.55 (1H, br.d,
J 1.3 Hz), 5.51 (1H, br.d,
J 0.9 Hz), 5.43 (1H, br.d,
J 0.9 Hz), 5.31 (1H, br.s), 5.22 (1H, br.s), 4.68 (2H, br.s), 4.53 (1H, d,
J 12.0 Hz), 4.49 (1H, d,
J 12.0 Hz), 4.47 (1H, br.s), 4.40 (1H, dd,
J 5.2, 9.7 Hz), 4.38–4.32 (2H, incl. br. dd,
J 5.8, 10.5 Hz at 4.36), 4.04 (1H, dd,
J 4.9, 11.4 Hz), 3.95–3.89 (5H, m), 3.85 (1H, dd,
J 2.2, 11.6 Hz), 3.81 (1H, dd,
J 5.1, 10.1 Hz), 3.68 (1H, dd,
J 4.7, 10.4 Hz), 3.65–3.59 (2H, incl. br. t,
J 4.7 Hz at 3.63), 1.00 (9H, s), 0.96 (9H, s); δ
C (101 MHz, CDCl
3): 165.5, 165.4, 165.2, 165.1, 138.6, 138.3, 135.7, 135.6, 135.5, 133.3, 133.2, 133.15, 133.1, 133.0, 130.0, 129.9, 129.8, 129.75, 129.7, 129.6, 129.35, 129.3, 129.25, 129.2, 128.4, 128.35, 128.3, 128.25, 128.2, 127.8, 127.7, 127.6, 127.55, 127.5, 127.4, 126.3, 106.1, 105.2, 83.7, 83.4, 82.3, 82.2, 82.1, 81.8, 80.5, 77.2, 76.6, 73.3, 72.2, 70.1, 67.2, 66.1, 63.3, 26.7, 26.6, 19.3, 19.2; ν
max: 3069, 3010, 2932, 2857, 1723, 1602, 1452, 1072, 706 cm
−1.
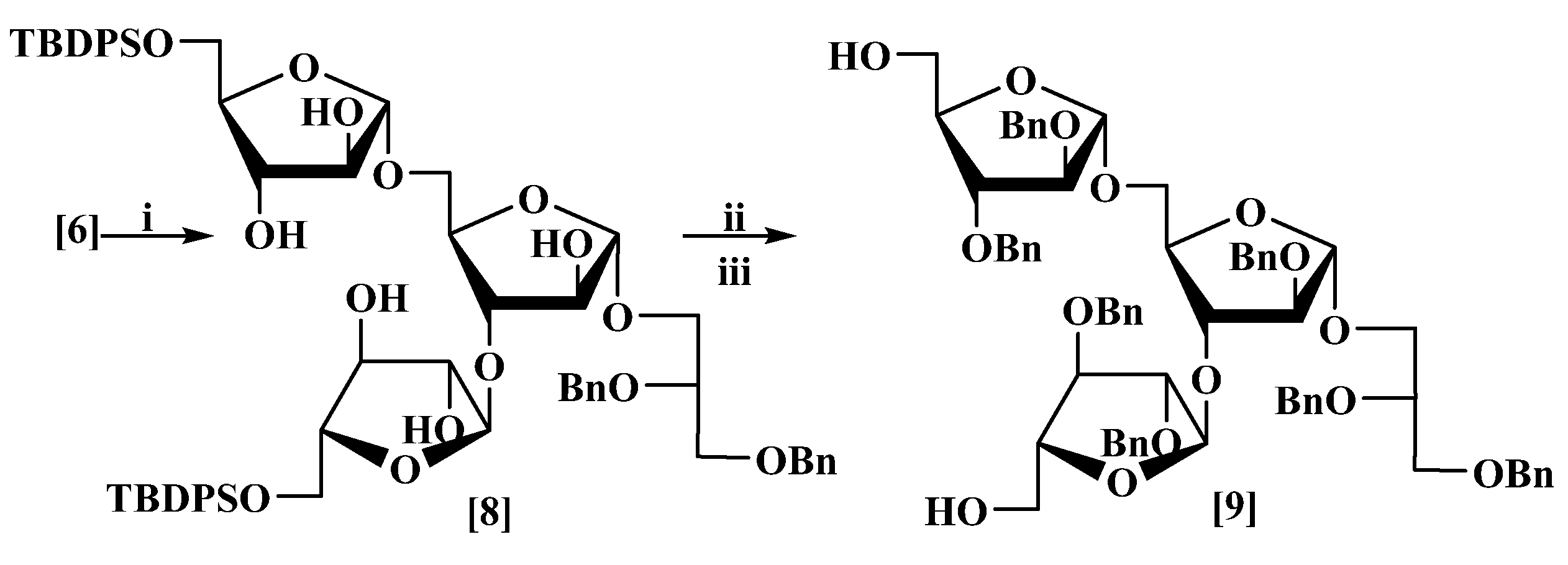
3.4. 2′,3′-Di-O-benzyl-l-glycerol-(1′→1)-2,3-di-O-benzyl-α-d-arabinofuranosyl-(1→3) [2,3-di-O-benzyl-α-d-arabinofuranosyl-(1→5)]-2-O-benzyl-α-d-arabinofuranoside (9)
(a) Sodium methoxide (25 mL, 1M, in methanol) was added to a stirred solution of furanoside (6) (9.1 g, 5.4 mmol) in dry CH2Cl2:MeOH (1:1, 50 mL) at rt until a pH of 11 was obtained. The mixture was stirred for 90 min, then neutralized by the addition of acetic acid. The solvent was evaporated under reduced pressure to give an oil; column chromatography eluting with chloroform/methanol (1:1) gave 2′,3′-di-O-benzyl-L-glycerol-(1′→1)-5-O-tert-butyldiphenyl-silyl-α-d-arabino-furanosyl-(1→3)-[5-O-tert-butyldiphenylsilyl-α-d-arabinofuranosyl-(1→5)]-α-d-arabino-furanoside (8) as a colourless thick oil (5.1 g, 83%) [MALDI: Found (M + Na)+: 1167.4913; C64H80NaO15Si2 requires: 1167.4928], +35 (c 6.7, CHCl3) which showed δH (400 MHz, CDCl3): 7.69–7.62 (7H, m), 7.51–7.18 (23H, m), 5.18 (1H, br.s), 5.12 (1H, br.s), 4.93 (1H, br. s), 4.67 (1H, d, J 12.1 Hz), 4.63 (1H, d, J 12.1 Hz), 4.55 (1H, d, J 12.1 Hz), 4.51 (1H, d, J 12.1 Hz), 4.18 (1H, br. d, J 3.4 Hz), 4.16 (1H, br. dd, J 3.7, 6.1 Hz), 4.12 (1H, br. d, J 3.8 Hz), 4.07 (2H, br. s), 4.03–3.98 (2H, br. m), 3.98–3.93 (2H, incl. br. d, J 2.0 Hz at 3.96), 3.80 (1H, dd, J 3.3, 8.8 Hz), 3.78–3.68 (4H, m), 3.67–3.64 (1H, m), 3.63–3.59 (2H, incl. br. dd, J 3.0, 8.1 Hz at 3.60), 3.57 (1H, dd, J 5.2, 9.9 Hz), 3.02–2.55 (7H, br. m), 1.05 (9H, s), 1.03 (9H, s); δC (101 MHz, CDCl3): 138.4, 138.2, 135.6, 135.5, 131.9, 131.8, 131.7, 131.6, 130.2, 130.1, 130.0, 128.4, 128.35, 128.3, 128.0, 127.9, 127.8, 127.7, 127.6, 127.55, 127.5, 108.6, 108.4, 108.3, 87.8, 87.4, 83.7, 82.3, 79.5, 78.8, 78.5, 77.8, 77.7, 76.7, 76.6, 73.3, 71.9, 69.7, 67.0, 66.0, 64.0, 63.8, 26.7, 26.6, 19.0, 18.9; νmax: 3418, 3071, 2933, 2858, 1454, 1053, 822 cm−1.
(b) A solution of the above α-D-rabinofuranoside (8) (5.0 g, 4.0 mmol) in dry DMF was added dropwise to a stirred suspension of NaH (1.0 g, 43 mmol) (60% w/w, dispersion in mineral oil) at rt under nitrogen. The mixture was stirred for 10 min then benzyl bromide (5.2 g, 3.6 mL, 30 mmol) in dry DMF (2 mL) was added, stirred at rt for 6 h, then quenched by the slow addition of methanol (2 mL), and water (10 mL). The aqueous layer was re-extracted with EtOAc (2 × 50 mL). The combined organic layers were washed with water (25 mL) and brine (25 mL), dried and evaporated under reduced pressure. Column chromatography eluting with petrol/EtOAc (5:1) gave 2′,3′-di-O-benzyl-l-glycerol-(1′→1)-2,3-di-O-benzyl-5-O-tert-butyldiphenylsilyl-α-d-arabinofurano-syl-(1→3)-[2,3-di-O-benzyl-5-O-tert-butyldiphenylsilyl-α-d-arabinofuranosyl-(1→5)]-2-O-benzyl-α-d-arabinofuranoside as a colourless thick oil (4.5 g, 65%) [MALDI: Found (M+Na)+: 1617.7; C99H110NaO15Si2 requires: 1617.7], +43 (c 1.9, CHCl3) which showed δH (400 MHz, CDCl3): 7.71–7.57 (8H, m), 7.48–7.13 (47H, m), 5.18 (2H, br. d, J 2.2 Hz), 5.07 (1H, br. s), 4.70 (1H, d, J 12.0 Hz), 4.66 (1H, d, J 12.0 Hz), 4.58–4.50 (7H, m), 4.47 (2H, d, J 11.9 Hz), 4.46 (1H, d, J 11.9 Hz), 4.39 (1H, d, J 11.9 Hz), 4.38 (1H, d, J 11.9 Hz), 4.30 (1H, br.dd, J 2.8, 6.8 Hz), 4.18 (2H, br. m), 4.13–4.07 (4H, m), 4.06–3.99 (2H, incl. br. dd, J 4.2, 10.6 Hz at 4.03), 3.95 (1H, dd, J 4.7, 11.9 Hz), 3.87 (1H, dd, J 5.0, 10.5 Hz), 3.84–3.80 (2H, incl. br. dd, J 4.0, 11.0 Hz at 3.82), 3.79–3.74 (4H, m), 3.68–3.58 (3H, m), 1.03 (18H, s); δC (101 MHz, CDCl3): 138.6, 138.3, 138.2, 138.0, 137.9, 137.6, 137.5, 135.75, 135.67, 135.65, 135.6, 133.55, 133.5, 133.4, 133.3, 129.6, 129.55, 129.5, 128.4, 128.35, 128.3, 128.25, 128.2, 127.9, 127.85, 127.8, 127.75, 127.7, 127.65, 127.6, 127.55, 127.5, 127.45, 127.4, 106.6, 106.4, 105.4, 88.6, 88.5, 88.0, 83.0, 82.8, 82.3, 81.8, 81.3, 80.3, 73.4, 72.2, 72.0, 71.9, 71.8, 71.7, 71.6, 70.4, 67.0, 66.0, 63.4, 63.2, 26.8, 26.7, 19.3, 19.2; νmax: 3067, 3031, 2929, 2857, 1495,1455, 1111, 698 cm−1.
(c) TBAF (14.3 mL, 0.0493 mol, in 1.0 M THF) was added dropwise to a stirred solution of the above furanoside (3.8 g, 2.3 mmol) in anhydrous THF (25 mL) at 0 °C under nitrogen. The mixture was allowed to reach rt and stirred for 8 h then diluted with EtOAc (100 mL) washed with sat. aq. NH4Cl (50 mL) and brine (50 mL). The organic layer was dried and concentrated; column chromatography eluting with hexane/EtOAc (1:1) gave the title compound (9) as a colourless thick oil (2.3 g, 87%) [MALDI–Found (M+Na)+: 1141.5, C67H74NaO15 requires: 1141.5], +53 (c 4.7, CHCl3) which showed δH (400 MHz, CDCl3): 7.34–7.16 (35H, m), 5.10 (1H, br. s), 5.07 (1H, br. s), 5.04 (1H, br. d, J 0.7 Hz), 4.64 (2H, br. s), 4.55–4.39 (11H, m), 4.31 (1H, d, J 11.7 Hz), 4.27 (1H, dd, J 3.8, 7.4 Hz), 4.23–4.17 (1H, m), 4.07 (1H, br. d, J 2.2 Hz), 4.06 (1H, br. dd, J 4.0, 7.6 Hz), 4.02 (1H, br. dd, J 2.2, 5.9 Hz), 3.98 (1H, br. dd, J 1.3, 3.8 Hz), 3.96 (1H, br. dd, J 1.2, 3.6 Hz), 3.88 (1H, br. dd, J 4.1, 12.3 Hz), 3.85 (1H, br. dd, J 3.2, 6.5 Hz), 3.82 (1H, d, J 5.2 Hz), 3.78 (1H, dd, J 3.7, 7.4 Hz), 3.76–3.72 (2H, m), 3.71–3.64 (2H, incl. br. dd, J 12.3, 2.4 Hz at 3.68), 3.61 (1H, dd, J 4.8, 7.3 Hz), 3.59–3.55 (3H, incl. br. d, J 5.1 Hz at 3.58), 3.53 (1H, dd, J 5.9, 12.3 Hz), 1.5 (2H, br s); δC (101 MHz, CDCl3): 138.5, 138.2, 137.7, 137.6, 137.5, 137.4, 137.2, 128.5, 128.45, 128.4, 128.35, 128.3, 128.0, 127.95, 127.9, 127.85, 127.8, 127.75, 127.7, 127.6, 127.5, 106.1, 106.0, 105.9, 88.7, 88.3, 87.4, 83.0, 82.9, 82.4, 81.9, 80.8, 79.8, 73.4, 72.3, 72.25, 72.2, 72.0, 71.9, 71.8, 70.2, 67.2, 64.8, 62.7; νmax: 3459, 3064, 3030, 2921, 2860, 1605, 1496, 1115, 820 cm−1.
3.5. 2′,3′-Di-O-benzyl-l-glycerol-(1′→1)-2,3-di-O-benzyl-5-O-methanesulfonyl-α-d-arabinofuranosyl-(1→3)-[2,3-di-O-benzyl-5-O-methanesulfonyl-α-d-arabinofuranosyl-(1→5)]-2-O-benzyl-α-d-arabinofuranoside (10)
Methanesulfonyl chloride (0.57 g, 0.40 mL, 0.0050 mol) and DMAP (0.05g, 0.43 mmol) were added to a stirred solution of furanoside (9) (0.56 g, 0.50 mmol) in dry pyridine (5 mL) under nitrogen at rt. After 16 h it was quenched by the addition of water (1 mL); the organic layer was diluted with CH2Cl2 (10 mL) then washed with 1M aq. HCl (2 × 10 mL), sat. aq. NaHCO3 (2 × 10 mL), dried and evaporated to give a thick oil; column chromatography eluting with petrol/EtOAc (3:1) gave the title compound (10) as a colourless thick oil (0.55 g, 87%) [MALDI: Found (M+Na)+: 1297.4; C69H78NaO19S2 requires: 1297.4], +59 (c 0.60, CHCl3), which showed δH (400 MHz, CDCl3): 7.40–7.15 (35H, m), 5.16 (1H, br. s), 5.13 (1H, br. s), 5.07 (1H, br. s), 4.68 (2H, br. s), 4.57–4.43 (11H, m), 4.40 (1H, d, J 11.3 Hz), 4.38–4.34 (1H, m), 4.33 (1H, br.dd, J 3.9, 7.3 Hz), 4.30–4.26 (2H, incl. br. dd, J 3.4, 7.3 Hz at 4.29), 4.24 (1H, d, J 5.4 Hz), 4.22–4.12 (2H, m), 4.11–4.07 (2H, m), 4.02–3.97 (2H, m), 3.91–3.84 (4H, m), 3.80 (1H, br. p, J 4.8 Hz), 3.73 (1H, br. dd, J 1.5, 11.5 Hz), 3.67–3.59 (3H, incl. br. dd, J 4.8, 8.7 Hz at 3.64), 2.94 (3H, s), 2.89 (3H, s); δC (101 MHz, CDCl3): 138.5, 138.2, 137.4, 137.3, 137.2, 137.0, 128.6, 128.55, 128.5, 128.45, 128.4, 128.3, 128.1, 128.05, 128.0, 127.95, 127.9, 127.7, 127.65, 127.6, 127.55, 106.5, 106.1, 105.8, 88.3, 87.7, 87.5, 82.9, 82.8, 80.3, 80.2, 79.3, 79.2, 77.2, 73.4, 72.35, 72.3, 72.2, 72.1, 72.0, 71.9, 70.2, 68.8, 68.5, 67.2, 65.2, 37.6, 37.5; νmax: 3088, 3065, 3031, 2933, 2871, 1586, 1454, 1177, 745 cm−1.
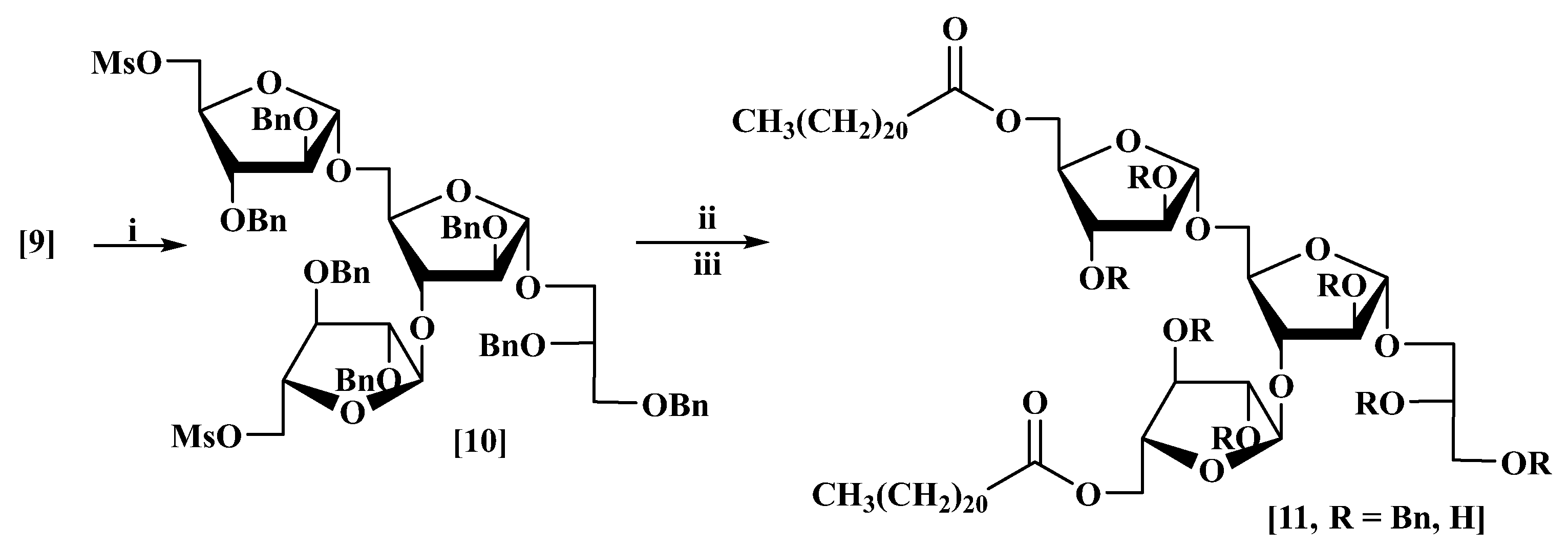
3.6. l-Glycerol-(1′→1)-5-O-behenate-α-d-arabinofuranosyl-(1→3)-5-O-behenate-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranoside (11, R = H)
(a) CsHCO3 (0.076 g, 0.39 mmol) was added to a stirred solution of dimesylate (10) (0.05 g, 0.03 mmol) and behenic acid (0.03 g, 0.09 mmol) in dry DMF:THF (1:5, 1 mL) at rt under nitrogen. The mixture was stirred at 70 °C for 3 days, then diluted with EtOAc (25 mL) and water (5 mL). The aqueous layer was re-extracted with EtOAc (2 × 10 mL). The combined organic layers were washed with water (5 mL) and brine (5 mL), dried and evaporated under reduced pressure to give a thick oil; column chromatography eluting with petrol/EtOAc (3:1) gave 2′,3′-di-O-benzyl-l-glycerol-(1′→1)-2,3-di-O-benzyl-5-O-behenate-α-d-arabinofuranosyl-(1→3)-[2,3-di-O-benzyl-5-O-behenate-α-d-arabinofuranosyl-(1→5)]-2-O-benzyl-α-d-arabinofuranoside (11, R = Bn) as a colourless thick oil (55 mg, 80%) [MALDI: Found (M + Na)+: 1786.1; C111H158NaO17 requires: 1786.1], +36 (c 1.0, CHCl3) which showed δH (400 MHz, CDCl3): 7.31–7.11 (35H, m), 5.09 (1H, br. s), 5.06 (1H, br. s), 4.97 (1H, br. s), 4.59 (2H, br. s), 4.51–4.35 (10H, m), 4.33 (1H, d, J 11.8 Hz), 4.26 (1H, d, J 11.8 Hz), 4.20 (1H, br. dd, J 3.3, 7.3 Hz), 4.12 (6H, br. m), 4.04 (1H, br. dd, J 2.8, 6.9 Hz), 4.00 (1H, br. d, J 2.5 Hz), 3.95–3.89 (2H, m), 3.84 (1H, dd, J 4.3, 11.8 Hz), 3.81–3.76 (2H, incl. br. dd, J 4.1, 8.5 Hz at 3.78), 3.75 (1H, br. d, J 3.4 Hz), 3.74–3.68 (1H, p, J 5.0 Hz), 3.66 (1H, br. dd, J 2.1, 11.7 Hz), 3.58–3.49 (3H, incl. br. q, J 4.6 Hz at 3.53), 2.17 (4H, t, J 7.6 Hz), 1.56–1.00 (76H, m), 0.81 (6H, t, J 6.8 Hz); δC (101 MHz, CDCl3): 173.6, 173.5, 138.6, 138.3, 137.7, 137.6, 137.5, 137.4, 137.3, 128.5, 128.4, 128.35, 128.3, 128.0, 127.95, 127.9, 127.85, 127.8, 127.75, 127.7, 127.65, 127.6, 127.5, 106.5, 106.2, 105.5, 88.3, 88.2, 88.1, 83.4, 83.3, 80.8, 80.3, 79.2, 78.9, 76.9, 73.4, 72.3, 72.2, 72.1, 72.0, 71.9, 71.7, 70.3, 67.1, 65.6, 63.3, 63.2, 34.1, 34.0, 31.9, 29.7, 29.6, 29.5, 29.4, 29.35, 29.3, 29.2, 24.8, 22.7, 14.1; νmax: 3064, 3031, 2923, 2853, 1740, 1718, 1455, 1066, 698 cm−1.
(b) A general procedure for debenzylation was used throughout: Palladium hydroxide on activated charcoal (Pd(OH)2-C/20% (1.1 fold by weight) was added to a stirred solution of the benzyl protected compound (0.010 mmol) in CH2Cl2:MeOH:THF (1:1:1.5, 2 mL) at rt under hydrogen. The mixture was stirred for 36 h then filtered through celite and the solvent was evaporated under reduced pressure to give a residue; column chromatography eluting with chloroform/methanol (10:1) afforded the desired compound. In this way, compound (11, R = H) was obtained as a colourless thick oil (29 mg, 82%) [MALDI: Found (M + Na)+: 1155.8; C62H1116NaO17 requires: 1155.8], −21 (c 1.1, CHCl3), which showed δH (400 MHz, CDCl3+few drops CD3OD): 5.01 (1H, br. s), 4.97 (1H, br. s), 4.90 (1H, br. s), 4.28–4.20 (2H, incl. br. dd J 3.2, 11.7 Hz at 4.24), 4.19–4.13 (3H, incl. br. dd J 5.0, 11.8 Hz at 4.17), 4.12 (1H, br. d, J 4.3 Hz), 4.11–4.08 (1H, m), 4.04 (1H, br. q, J 5.5 Hz), 4.01–3.95 (3H, br. m), 3.94 (1H, dd, J 3.6, 11.5 Hz), 3.83–3.76 (3H, m), 3.73 (1H, br. dd, J 4.8, 10.1 Hz), 3.67–3.62 (1H, m), 3.62–3.57 (2H, incl. br. d, J 3.1 Hz at 3.60), 3.56–3.51 (1H, m), 2.30 (4H, t, J 7.6 Hz), 1.64–1.02 (83H, m), 0.83 (6 H, t, J 6.5 Hz); δC (101 MHz, CDCl3): 174.05, 174.0, 108.0, 107.7, 83.3, 83.0, 82.4, 81.8, 81.3, 80.7, 79.0, 77.6, 75.8, 69.9, 69.1, 66.4, 63.9, 63.7, 63.5, 34.0, 33.95, 31.8, 29.6, 29.5, 29.45, 29.4, 29.3, 29.25, 29.2, 29.1, 29.0, 24.8, 24.7, 22.6, 13.9.; νmax: 3374, 2920, 2852, 1730, 1723, 1180, 757 cm−1.
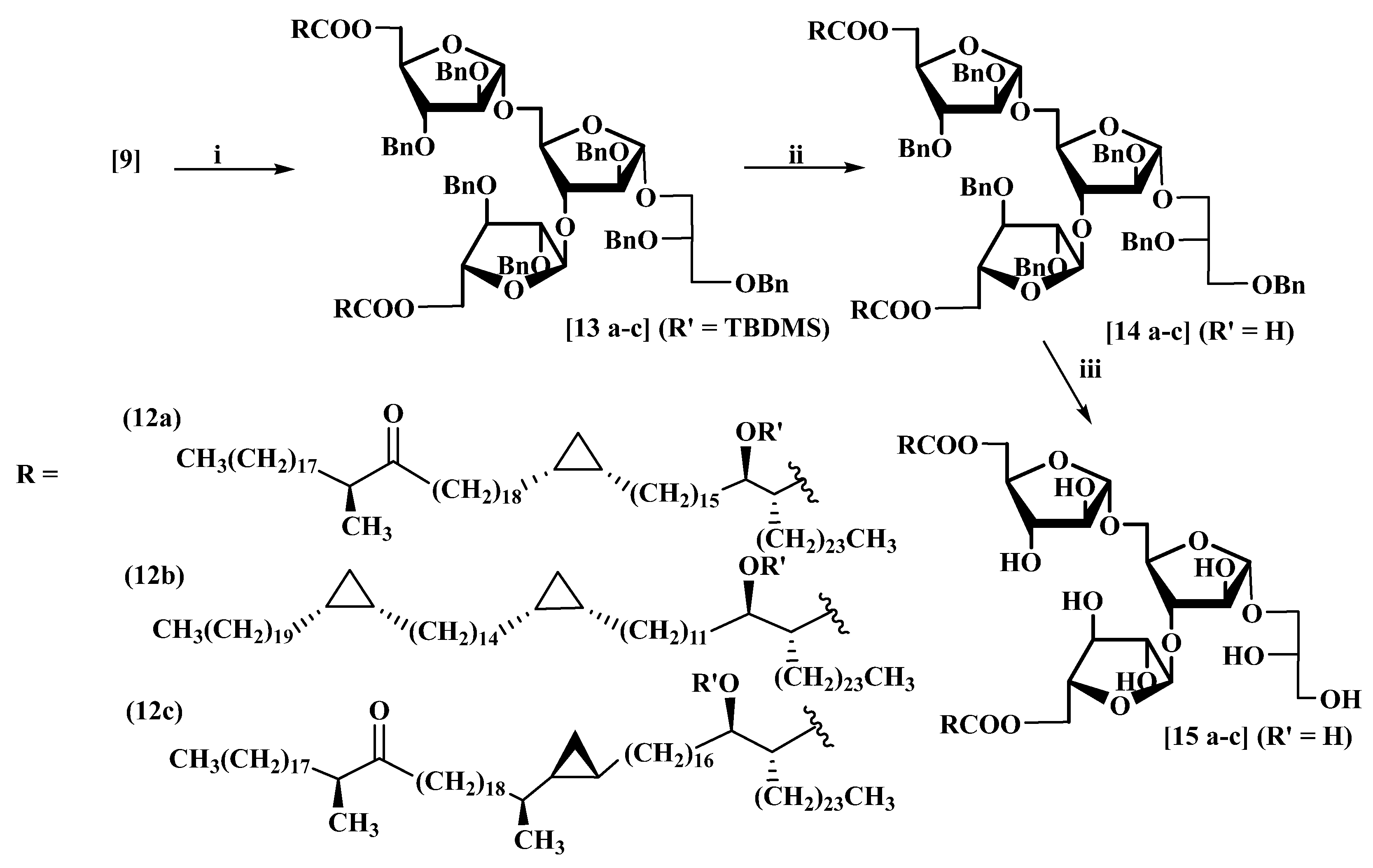
3.7. 12l-Glycerol-(1′→1)-5-O-(2R)-2-(1-hydroxy-16-((1S,2R)-2-((S)-20-methyl-19-oxooctatria-contyl)cyclopropyl)hexadecyl)hexacosanoate-α-d-arabinofuranosyl-(1→3)-[5-O-((2R)-2-(1-hydroxy-16-((1S,2R)-2-((S)-20-methyl-19-oxooctatriacontyl)cyclopropyl)hexadecyl)hexacosan-oate-α-d-arabinofuranosyl-(1→5)]-α-d-arabinofuranoside (15a)
(a) 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) (60 mg, 0.31 mmol), in dry CH
2Cl
2 (1 mL) was added to a stirred solution of α-D-arabinofuranoside (
9) (35 mg, 0.031 mmol), molecular sieves 4 Å (50 mg), DMAP (38 mg, 0.31) (2
R)-2-(1-((
tert-butyldimethyl-silyl)oxy)-16-((1
S,2
R)-2-((
S)-20-methyl-19-oxooctatriacontyl)cyclopropyl)hexadecyl)hexacosanoic acid (
12a) (85 mg, 0.062 mmol) [
30] in dry CH
2Cl
2 (1 mL) at rt under nitrogen. After 4 days, the precipitate was filtered off and washed with CH
2Cl
2 (10 mL), and the solvent was evaporated; column chromatography eluting with hexane/EtOAc (10:1) gave compound (
13a) as a colourless thick oil (60 mg, 51%) [MALDI: Found (M + Na)
+: 3808.2; C
247H
426NaO
21Si
2 requires: 3808.2],
+18 (
c 5.0, CHCl
3), which showed δ
H (400 MHz, CDCl
3): 7.69–6.95 (35H, m), 5.16 (1H, br. s), 5.13 (1H, br. s), 5.04 (1H, br. s), 4.68 (1H, d,
J 12.1 Hz), 4.65 (1H, d,
J 12.1 Hz), 4.58–4.45 (9H, m), 4.45 (1H, d,
J 11.9 Hz), 4.38 (1H, d,
J 11.9 Hz), 4.33 (1H, d,
J 11.8 Hz), 4.29–4.22 (5H, incl. br. dd
J 4.0, 9.1 Hz at 4.25), 4.21–4.16 (2H, m), 4.13 (1H, br. dd,
J 5.7, 8.2 Hz), 4.06 (1H, br. d,
J 2.7 Hz), 4.01–3.96 (2H, m), 3.94–3.87 (5H, m), 3.85 (1H, br. dd,
J 4.8, 10.7 Hz), 3.78 (1H, br. p,
J 5.1 Hz), 3.76–3.71 (1H, m), 3.65–3.56 (3H, incl. br. dd,
J 4.8, 8.2 Hz at 3.60), 2.53 (4H, incl. sextet,
J 5.4 Hz), 2.42 (4H, t,
J 7.6 Hz), 1.68–1.11 (288H, m), 1.06 (6H, d,
J 6.9 Hz), 0.89 (12H, t,
J 6.8 Hz), 0.85 (9H, s), 0.84 (9H, s), 0.71–0.61 (4H, m), 0.57 (2H, dt,
J 4.0, 8.5 Hz), 0.03 (6H, s), 0.01 (6H, s), −0.32 (2H, br.q,
J 5.1 Hz); δ
C (101 MHz, CDCl
3): 215.2, 174.3, 174.2, 138.5, 138.2, 137.8, 137.7, 137.6, 137.4, 137.3, 128.5, 128.45, 128.4, 128.35, 128.3, 128.2, 127.9, 127.85, 127.8, 127.75, 127.7, 127.65, 127.6, 127.55, 127.5, 106.6, 106.3, 105.2, 88.3, 88.2, 87.8, 83.7, 83.6, 81.2, 80.2, 79.4, 78.9, 77.2, 73.4, 73.1, 72.3, 72.2, 72.0, 71.9, 71.6, 70.3, 67.0, 65.9, 63.0, 62.7, 51.6, 46.3, 41.1, 33.6, 33.5, 33.0, 31.9, 30.3, 30.2, 29.9, 29.8, 29.75, 29.7, 29.65, 29.6, 29.55, 29.5, 29.45, 29.4, 29.3, 28.7, 27.9, 27.8, 27.3, 27.2, 27.1, 25.8, 24.0, 23.7, 22.7, 22.6, 18.0, 16.4, 15.8, 14.1, 10.9, −4.4, −4.5, −4.7, −4.8; ν
max: 3063,3031, 2920, 2851, 1741, 1714, 1467, 1106, 836, 699 cm
−1.
(b) The protected furanoside (13a) (53 mg, 0.014 mmol) was dissolved in dry THF (10 mL) in a dry polyethylene vial equipped with an acid-proof rubber septum, followed by the addition of pyridine (0.1 mL) at rt under nitrogen. The mixture was cooled to 0 °C, and then HF–pyridine complex (70% w, 1.5 mL) was added dropwise. The mixture was stirred at 43 °C for 24 h, then poured slowly into sat. aq. NaHCO3 and stirred until no more CO2 was liberated. The aqueous layer was re-extracted with chloroform (3 × 10 mL). The combined organic layers were dried and evaporated. Column chromatography eluting with hexane/EtOAc (10:1) afforded compound (14a) as a colourless thick oil (38 mg, 76%) [MALDI: Found (M+Na)+: 3579.9; C235H398NaO21 requires: 3579.9], +19 (c 1.2, CHCl3); δH (400 MHz, CDCl3): 7.36–7.15 (35H, m), 5.16 (1H, br. s), 5.13 (1H, br. s), 5.05 (1H, br. s), 4.67 (2H, br. s), 4.55 (1H, d, J 11.8 Hz), 4.53–4.47 (7H, m), 4.45 (3H, d, J 11.8 Hz), 4.40 (1H, d, J 11.8 Hz), 4.36–4.22 (7H, m), 4.17 (1H, br. dd, J 3.7, 6.9 Hz), 4.11 (1H, br. dd, J 3.3, 6.3 Hz), 4.07 (1H, br. d, J 2.1 Hz), 4.00–3.96 (2H, m), 3.94–3.88 (2H, m), 3.88–3.82 (2H, incl. br. dd J 4.5, 9.8Hz at 3.86), 3.81–3.75 (2H, m), 3.73 (1H, br. d, J 11.7 Hz), 3.67–3.51 (5H, incl. br. d J 5.3 Hz at 3.61), 2.51 (2H, sextet, J 6.7 Hz), 2.42 (6H, incl. t J 7.2 Hz), 2.05–1.10 (288H, m), 1.06 (6H, d, J 6.9 Hz), 0.89 (12H, t, J 6.7 Hz), 0.69–0.61 (4H, m), 0.57 (2H, dt, J 4.1, 8.5 Hz), -0.33 (2H, br. q, J 5.0 Hz); δC (101 MHz, CDCl3): 215.2, 175.1, 175.0, 138.6, 138.2, 137.6, 137.5, 137.45, 137.4, 137.2, 128.5, 128.4, 128.35, 128.3, 128.2, 128.1, 128.0, 127.95, 127.9, 127.85, 127.8, 127.75, 127.7, 127.6, 127.5, 106.3, 106.2, 105.5, 88.2, 88.0, 87.9, 83.6, 80.7, 80.3, 79.3, 79.2, 77.2, 73.4, 72.4, 72.2, 72.1, 72.0, 71.7, 70.2, 68.0, 67.1, 65.4, 63.0, 62.9, 51.9, 51.7, 46.3, 41.1, 35.3, 35.2, 33.0, 31.9, 30.3, 30.2, 29.8, 29.7, 29.65, 29.6, 29.55, 29.5, 29.45, 29.4, 29.3, 29.2, 28.7, 27.5, 27.4, 27.3, 25.7, 25.6, 23.7, 22.7, 16.4, 15.8, 14.1, 10.9; νmax: 3511, 3063,3030, 2918, 2851, 1737, 1714, 1467, 1105, 754, 698 cm−1.
(c) Using the generalised procedure for debenzylation, compound (15a) was obtained as a colourless thick oil (25 mg, 86%) [MALDI: Found (M + Na)+: 2949.7; C186H356NaO21 requires: 2949.7], +19 (c 2.2, CHCl3); δH (400 MHz, CDCl3 + few drops CD3OD): 5.00 (1H, br. s), 4.96 (1H, br. s), 4.90 (1H, br. s), 4.41 (1H, dd, J 4.2, 11.6 Hz), 4.36 (1H, dd, J 5.1, 11.9 Hz), 4.26 (1H, dd, J 11.9, 5.4 Hz), 4.21 (1H, d, J 4.0 Hz), 4.17 (1H, dd, J 3.8, 10.3 Hz), 4.12 (1H, br. d, J 4.8 Hz), 4.08 (1H, br. q, J 6.6 Hz), 4.04 (1H, d, J 5.1 Hz), 4.01–3.96 (3H, m), 3.92 (1H, dd, J 3.8, 8.0 Hz), 3.90–3.86 (2H, m), 3.77 (1H, dd, J 2.7, 8.4 Hz), 3.72 (1H, d, J 5.1 Hz), 3.62 (5H, br. m), 3.54 (1H, dd, J 2.6, 10.5 Hz), 2.52–2.44 (2H, m), 2.38 (6H, incl. t, J 7.5 Hz), 1.65–1.05 (297 H, m), 1.01 (6H, d, J 6.9 Hz), 0.84 (12H, t, J 6.8 Hz), 0.66–0.57 (4H, m), 0.52 (2H, dt, J 4.2, 8.2 Hz), -0.37 (2H, br. q, J 4.7 Hz); δC (101 MHz, CDCl3): 216.2, 175.05, 174.9, 109.2, 108.7, 81.95, 81.2, 79.4, 78.0, 77.6, 77.2, 76.5, 72.5, 71.9, 65.0, 63.5, 63.4, 61.5, 52.6, 35.0, 32.8, 31.8, 30.1, 29.6, 29.55, 29.5, 29.4, 29.3, 29.25, 29.2, 28.9, 28.6, 27.3, 27.2, 26.1, 25.3, 23.5, 22.6, 16.1, 15.7, 14.0, 10.8.; νmax: 3511, 3063,3030, 2918, 2851, 1737, 1714, 1467, 1105, 754, 698 cm−1.
3.8. l-Glycerol-(1′→1)-5-O-(2R)-2-((1R)-1-hydroxy-12-((2R)-2-(14-((2R)-2-eicosylcyclo-propyl)tetradecyl)cyclopropyl)dodecyl)hexacosanoate-α-d-arabinofuranosyl-(1→3)-[5-O-(2R)-2-((1R)-1-hydroxy-12-((2R)-2-(14-((2R)-2-icosylcyclopropyl)tetradecyl)cyclopropyl)-dodecyl)hexacosanoate-α-d-arabinofuranosyl-(1→5)]-α-d-arabinofuranoside (15b)
(a) A solution of EDCI (48 mg, 0.25 mmol) in dry CH
2Cl
2 (1 mL) was added with stirring to furanoside (
9) (28 mg, 0.025 mmol), molecular sieves 4 Å (50 mg), DMAP (30 mg, 0.24 mmol) and (2
R)-2-((1
R)-1-((
tert-butyldimethylsilyl)oxy)-12-((2
R)-2-(14-((2
R)-2-eicosylcyclopropyl)tetradecyl)cyclopropyl)-dodecyl)hexacosanoic acid (
12b) (62 mg, 0.049 mmol) [
31] in dry CH
2Cl
2 (1 mL) at rt under nitrogen. After 4 days, the precipitate was filtered and washed with CH
2Cl
2 (10 mL), the solvent was evaporated and the residue was purified by column chromatography eluting with hexane/EtOAc (10:1) to afford compound (
13b) as a colourless thick oil (75 mg, 84%) [MALDI: Found (M+Na)
+: 3607.9; C
235H
402NaO
19Si
2 requires: 3607.9],
+20 (
c 0.90, CHCl
3), which showed δ
H (400 MHz, CDCl
3): 7.94–6.85 (35H, m), 5.16 (1H, br. s), 5.13 (1H, br. s), 5.04 (1H, br. s), 4.68 (1H, d,
J 12.1 Hz), 4.65 (1H, d,
J 12.1 Hz), 4.57–4.45 (9H, m), 4.45 (1H, d,
J 11.8 Hz), 4.38 (1H, d,
J 11.8 Hz), 4.32 (1H, d,
J 11.8 Hz), 4.28–4.20 (5H, m), 4.18 (1H, br. dd,
J 3.6, 7.4 Hz), 4.15–4.09 (1H, m), 4.06 (1H, br. d,
J 2.9 Hz), 4.01–3.96 (2H, m), 3.90 (3H, br. m), 3.87–3.81 (2H, incl. br. dd,
J 4.8, 10.7 Hz at 3.85), 3.78 (1H, br. p,
J 5.1 Hz), 3.76–3.71 (1H, m), 3.64–3.49 (3H, incl. br. dd,
J 4.8, 8.3 Hz at 3.60), 2.60–2.47 (2H, m), 1.67–1.06 (270H, m), 0.89 (12H, t,
J 6.8 Hz), 0.85 (9H, s), 0.84 (9H, s), 0.74–0.62 (8H, m), 0.57 (4H, td,
J 4.1 Hz), 0.02 (6H, s), 0.00 (6H, s), −0.33 (4H, br. q,
J 4.9 Hz); δ
C (101 MHz, CDCl
3): 174.3, 174.2, 138.5, 138.3, 137.8, 137.7, 137.6, 137.4, 137.3, 128.5, 128.45, 128.4, 128.35, 128.3, 127.9, 127.85, 127.8, 127.75, 127.7, 127.65, 127.6, 127.55, 127.5, 106.6, 106.3, 105.2, 88.3, 88.2, 87.8, 83.7, 83.6, 81.2, 80.2, 79.4, 78.9, 77.2, 73.4, 73.1, 72.3, 72.2, 71.9, 71.6, 70.3, 67.0, 65.9, 63.0, 62.7, 51.6, 33.6, 33.5, 31.9, 30.3, 30.2, 29.9, 29.8, 29.7, 29.6, 29.5, 29.4, 28.7, 27.9, 27.8, 27.2, 27.1, 25.9, 25.8, 24.0, 22.7, 18.0, 15.8, 14.1, 11.0, 10.9, −4.4, −4.5, −4.7, −4.8; ν
max: 3063, 2925, 2854, 1737, 1456, 1101, 770 cm
−1.
(b) The protected glycolipid α-d-arabinofuranoside (13b) (70 mg, 0.019 mmol) was dissolved in dry THF (10 mL) in a dry polyethylene vial equipped with an acid-proof rubber septum, followed by the addition of pyridine (0.1 mL) at rt under nitrogen. The mixture was cooled to 0 °C, and then HF–pyridine complex (70% w, 1.5 mL) was added dropwise. The mixture was stirred at 43 °C for 24 h, then poured slowly into sat. aq. NaHCO3 and stirred until no more CO2 was liberated. The aqueous layer was re-extracted with chloroform (3 × 10 mL). The combined organic layers were dried and evaporated; column chromatography eluting with hexane/EtOAc (10:1) afforded compound (14b) as a colourless thick oil (33 mg, 51%) [MALDI: Found (M+Na)+: 3379.8; C223H374NaO19 requires: 3379.8], +22 (c 0.90, CHCl3), which showed δH (400 MHz, CDCl3): 7.37–7.17 (35H, m), 5.16 (1H, br. s), 5.13 (1H, br. s), 5.05 (1H, br. s), 4.67 (2H, br. s), 4.55 (1H, d, J 11.9 Hz), 4.53–4.47 (8H, m), 4.46 (2H, d, J 11.8 Hz), 4.41 (1H, d, J 11.9 Hz), 4.35–4.23 (6H, m), 4.17 (1H, br. dd, J 3.7, 6.9 Hz), 4.15–4.08 (3H, incl. br. t J 7.0 Hz at 4.12), 4.07 (1H, br. d, J 2.1 Hz), 4.00–3.95 (2H, m), 3.94–3.87 (2H, m), 3.95–3.87 (2H, incl. br. dd, J 4.6, 10.3 Hz at 3.86), 3.79 (1H, p, J 4.8 Hz), 3.75–3.69 (1H, m), 3.67–3.55 (3H, incl. br. d, J 5.3 Hz at 3.61), 2.44–2.37 (2H, m), 1.67–1.03 (270H, m), 0.89 (12H, t, J 7.1 Hz), 0.69–0.61 (8H, m), 0.57 (4H, dt, J 4.2, 8.5 Hz), −0.32 (4H, br. q, J 4.9 Hz); δC (101 MHz, CDCl3): 175.1, 175.0, 138.5, 138.2, 137.6, 137.5, 137.4, 137.4, 137.2, 128.5, 128.4, 128.35, 128.3, 128.0, 127.95, 127.9, 127.85, 127.8, 127.75, 127.7, 127.6, 127.5, 106.3, 106.2, 105.5, 88.2, 88.0, 87.9, 83.6, 80.7, 80.3, 79.3, 79.2, 77.2, 73.4, 72.4, 72.2, 72.0, 71.9, 71.7, 70.3, 67.1, 65.4, 63.1, 63.0, 62.0, 51.9, 51.8, 35.3, 35.2, 34.2, 31.9, 30.3, 30.2, 29.8, 29.75, 29.7, 29.65, 29.6, 29.5, 29.4, 28.7, 27.5, 27.4, 25.8, 25.7, 24.9, 22.7, 22.6, 20.4, 15.8, 14.1, 10.9; νmax: 3511, 3062, 2921, 2854, 1733,1725, 1456, 1115, 754 cm−1.
(c) Using the generalised debenzylation procedure, compound (15b) was obtained as a colourless thick oil (22 mg, 88%) [MALDI: Found (M+Na)+: 2749.5; C174H332NaO19 requires: 2749.5], +18 (c 2.2, CHCl3), which showed δH (400 MHz, CDCl3+few drops CD3OD): 5.00 (1H, br. s), 4.96 (1H, br. s), 4.90 (1H, br. s), 4.41 (1H, dd, J 4.6, 11.7 Hz), 4.37 (1H, br. dd, J 5.1, 11.9 Hz), 4.26 (1H, dd, J 5.0, 11.7 Hz), 4.20 (1H, dd, J 4.7, 11.8 Hz), 4.16 (1H, br. q, J 4.8 Hz), 4.12 (1H, br. d, J 6.2 Hz), 4.10–4.06 (2H, incl. br. t, J 7.4 Hz at 4.08), 4.05 (1H, br. d, J 5.6 Hz), 4.02–3.96 (3H, incl. br. d, J 8.5 Hz at 3.99), 3.95–3.86 (3H, m), 3.77 (1H, dd, J 4.2, 7.4 Hz), 3.72 (1H, d, J 5.4 Hz), 3.67–3.57 (4H, incl. br. d, J 10.5 Hz at 3.63), 3.54 (1H, dd, J 2.6, 9.7 Hz), 2.42–2.35 (2H, m), 1.64–1.04 (277H, m), 0.84 (12H, t, J 6.7 Hz), 0.64–0.56 (8H, m), 0.52 (4H, dt, J 4.1, 8.4 Hz), −0.37 (4H, br. q, J 4.9 Hz); δC (101 MHz, CDCl3+ few drops CD3OD): 175.0, 174.9, 108.7, 108.0, 81.7, 81.3, 79.7, 79.4, 77.9, 77.5, 77.2, 76.3, 72.4, 71.9, 69.7, 69.15, 68.9, 67.6, 65.1, 63.4, 62.2, 61.4, 57.8, 52.6, 34.9, 31.8, 30.1, 29.55, 29.5, 29.45, 29.4, 29.3, 29.2, 29.15, 29.1, 28.6, 27.3, 25.3, 22.5, 20.5, 15.6, 13.9, 10.7.; νmax: 3399, 2920, 2851, 1734, 1467, 1043 cm−1.
3.9. l-Glycerol-(1′→1)-5-O-(2R)-2-((1R)-1-hydroxy-17-((1S,2R)-2-((22S)-22-methyl-21-oxotetracontan-2-yl)cyclopropyl)heptadecyl)hexacosanoate-α-d-arabinofuranosyl-(1→3)-[-5-O-(2R)-2-((1R)-1-hydroxy-17-((1S,2R)-2-((22S)-22-methyl-21-oxotetracontan-2-yl)cyclo-propyl)heptadecyl)hexacosanoate-α-d-arabinofuranosyl-(1→5)]-α-d-arabinofuranoside (15c)
(a) A solution of EDCI (35 mg, 0.18 mmol) in dry CH
2Cl
2 (1 mL) was added to a stirred solution of α-D-arabinofuranoside (
9) (22 mg, 0.019 mmol), molecular sieves 4 Å (25 mg), DMAP (23 mg, 0.18 mmol) and (2
R)-2-((1
R)-1-((
tert-butyldimethylsilyl)oxy)-17-((1
S,2
R)-2-((22
S)-22-methyl-21-oxotetra-contan-2yl)cyclopropyl)-heptadecyl)hexacosanoic acid (
12c) (50 mg, 0.035 mmol) [
32] in dry CH
2Cl
2 (1 mL) at rt under nitrogen. The mixture was stirred for 4 days, then the precipitate was filtered off and washed with CH
2Cl
2 (10 mL), the solvent was evaporated and the residue was purified by column chromatography eluting with hexane/EtOAc (10:1) to afford the title compound (
13c) as a colourless thick oil (35 mg, 46%) [MALDI: Found (M + Na)
+: 3892.2; C
253H
438NaO
21Si
2 requires: 3892.2],
+14 (
c 3.0, CHCl
3), which showed δ
H (400 MHz, CDCl
3): 7.54–7.00 (35H, m), 5.16 (1H, br. s), 5.13 (1H, br. s), 5.04 (1H, br. s), 4.68 (1H, d,
J 12.1 Hz), 4.65 (1H, d,
J 12.1 Hz), 4.58–4.47 (9H, m), 4.46 (1H, d,
J 11.9 Hz), 4.39 (1H, d,
J 11.8 Hz), 4.33 (1H, d,
J 11.9 Hz), 4.30–4.22 (5H, incl. br. dd,
J 3.9, 10.4 Hz at 4.26), 4.22–4.16 (2H, m), 4.13 (1H, br. dd,
J 5.6, 8.4 Hz), 4.06 (1H, br. d,
J 2.9 Hz), 4.01–3.97 (2H, m), 3.95–3.87 (5H, m), 3.85 (1H, br. dd,
J 3.8, 9.4 Hz), 3.78 (1H, br. p,
J 4.9 Hz), 3.76–3.72 (1H, m), 3.65–3.55 (3H, incl. br. dd
J 4.8, 8.3 Hz at 3.60), 2.53 (4H, incl. sextet,
J 5.6 Hz), 2.42 (4H, t,
J 7.5 Hz), 1.62–1.14 (292 H, m), 1.06 (6H, d,
J 6.9 Hz), 0.89 (12H, t,
J 6.8 Hz), 0.88 (6H, d,
J 6.8 Hz), 0.85 (9H, s), 0.84 (9H, s), 0.75–0.62 (2H, m), 0.51–0.40 (2H, m), 0.24–0.08 (6H, m), 0.03 (6H, s), 0.01 (6H, s); δ
C (101 MHz, CDCl
3): 215.1, 174.2, 174.1, 138.6, 138.3, 137.8, 137.7, 137.6, 137.55, 137.5, 128.45, 128.4, 128.35, 128.3, 128.25, 128.2, 127.9, 127.85, 127.8, 127.75, 127.7, 127.65, 127.6, 127.55, 127.5, 106.6, 106.3, 105.2, 88.3, 88.2, 87.8, 83.8, 83.7, 81.2, 80.2, 79.5, 78.9, 77.2, 73.4, 73.2, 72.3, 72.2, 72.0, 71.9, 71.6, 70.3, 67.0, 65.9, 63.0, 62.7, 51.6, 46.3, 41.1, 38.1, 37.4, 34.5, 33.7, 33.6, 33.0, 31.9, 30.1, 29.9, 29.8, 29.75, 29.7, 29.65, 29.6, 29.55, 29.5, 29.45, 29.4, 29.35, 29.3, 27.8, 27.4, 27.3, 27.2, 27.1, 26.1, 25.9, 25.8, 24.1, 23.7, 22.7, 22.6, 19.7, 18.6, 18.0, 16.4, 14.1, 10.5, −4.4, −4.5, −4.7, −4.8; ν
max: 3063,3032, 2919, 2851, 1739, 1714, 1467, 1105, 836, 698 cm
−1.
(b) The protected glycolipid furanoside (13c) (31 mg, 0.0080 mmol) was dissolved in dry THF (10 mL) in a dry polyethylene vial equipped with an acid-proof rubber septum, followed by the addition of pyridine (0.1 mL) at rt under nitrogen. The mixture was cooled to 0 °C, and then HF–pyridine complex as (70% w, 1.5 mL) was added dropwise. The mixture was stirred at 43 °C for 24 h, then poured slowly into sat. aq. NaHCO3 and stirred until no more CO2 was liberated. The aqueous layer was re-extracted with chloroform (3 × 10 mL). The combined organic layers were dried and evaporated; column chromatography eluting with hexane/EtOAc (10:1) afforded compound (14c) as a colourless thick oil (19 mg, 66%) [MALDI: Found (M + Na)+: 3664.1; C241H410NaO21 requires: 3664.1], +25 (c 1.1, CHCl3), which showed δH (400 MHz, CDCl3): 7.39–7.18 (35H, m), 5.16 (1H, br. s), 5.13 (1H, br. s), 5.04 (1H, br. s), 4.67 (2H, br. s), 4.55 (1H, d, J 11.9 Hz), 4.53–4.47 (8H, m), 4.45 (2H, d, J 11.8 Hz), 4.40 (1H, d, J 11.8 Hz), 4.29 (6H, br. m), 4.17 (1H, br. dd, J 3.8, 6.9 Hz), 4.11 (1H, br. dd, J 3.4, 6.2 Hz), 4.07 (1H, br. d, J 2.5 Hz), 3.99–3.95 (2H, m), 3.94–3.87 (2H, m), 3.87–3.82 (2H, incl. br. dd, J 4.6, 10.3 Hz at 3.85), 3.79 (1H, br. p, J 4.9 Hz), 3.72 (1H, br.dd, J 1.7, 11.6 Hz), 3.64–3.57 (5H, m), 2.51 (2H, sextet, J 6.8 Hz), 2.42 (6H, incl. t, J 7.5 Hz), 1.87–1.08 (300H, m), 1.05 (6H, d, J 6.9 Hz), 0.89 (12H, t, J 7.3 Hz), 0.72–0.62 (2H, m), 0.50–0.38 (2H, m), 0.25–0.05 (6H, m); δC (101 MHz, CDCl3): 215.2, 175.1, 175.0, 138.6, 138.3, 137.7, 137.5, 137.45, 137.4, 137.2, 128.5, 128.4, 128.35, 128.3, 128.2, 128.0, 127.95, 127.9, 127.85, 127.8, 127.75, 127.7, 127.6, 106.3, 106.2, 105.4, 88.2, 88.0, 87.9, 83.6, 80.7, 80.3, 79.3, 79.2, 77.2, 73.4, 72.4, 72.2, 72.1, 72.0, 71.9, 71.7, 70.3, 67.1, 65.4, 63.0, 62.9, 51.9, 51.7, 46.3, 41.1, 38.1, 37.4, 35.3, 35.2, 34.5, 33.0, 31.9, 30.1, 29.75, 29.7, 29.65, 29.6, 29.55, 29.5, 29.45, 29.4, 29.3, 27.5, 27.4, 27.35, 27.3, 26.1, 25.7, 23.7, 22.7, 19.7, 18.6, 16.4, 14.1, 10.5; νmax: 3457, 3063,3031, 2919, 2852, 1737, 1715, 1464, 1104,734, 698 cm−1.
(c) Using the generalised debenzylation procedure, compound (15c) was obtained as a colourless oil (9.0 mg, 82%) [MALDI: Found (M+Na)+: 3033.8; C192H368NaO21 requires: 3033.8], +26 (c 0.90, CHCl3); δH (400 MHz, CDCl3+ few drops CD3OD): 5.01 (1H, br. s), 4.96 (1H, br. s), 4.91 (1H, br. s), 4.42 (1H, dd, J 4.9, 12.1 Hz), 4.37 (1H, dd, J 4.6, 11.8 Hz), 4.27 (1H, dd, J 4.7, 11.1Hz), 4.22 (1H, br. d, J 4.7 Hz), 4.18 (1H, dd, J 4.2, 9.0 Hz), 4.13 (1H, br. q, J 5.4 Hz), 4.09 (1H, t, J 7.0 Hz), 4.06 (1H, d, J 5.1 Hz), 4.02–3.97 (3H, br. m), 3.93 (1H, br. dd, J 3.3, 7.3 Hz), 3.89 (2H, br. m), 3.80–3.72 (2H, m), 3.67–3.58 (5H, m), 3.55 (1H, dd, J 2.4, 9.6 Hz), 2.53–2.44 (2H, sextet, J 6.6 Hz), 2.39 (6H, incl. t, J 7.4 Hz), 1.57–1.06 (210 H, m), 1.01 (6H, d, J 6.9 Hz), 0.85 (12H, t, J 7.5 Hz), 0.67–0.57 (2H, m), 0.47–0.34 (2H, m), 0.21–0.02 (6H, m); δC (101 MHz, CDCl3): 215.8, 175.0, 174.75, 109.3, 108.7, 87.1, 82.95, 82.9, 80.9, 79.4, 78.8, 78.2, 77.9, 77.2, 72.6, 71.7, 68.8, 65.0, 64.0, 63.3, 61.8, 52.5, 46.3, 41.1, 38.1, 37.4, 35.1, 34.4, 33.0, 32.9, 31.9, 30.0, 29.7, 29.6, 29.55, 29.5, 29.45, 29.4, 29.35, 29.3, 27.4, 27.35, 27.3, 27.25, 27.2, 26.1, 25.45, 25.4, 23.6, 23.55, 22.6, 19.6, 18.6, 16.3, 16.2, 14.1, 10.4.; νmax: 3434, 2919, 2852, 1736, 1717, 1467, 1104,735, 699 cm−1.