Effects of Pretreatment with Ionic Liquids on Cellulose Hydrolysis under Hydrothermal Conditions
Abstract
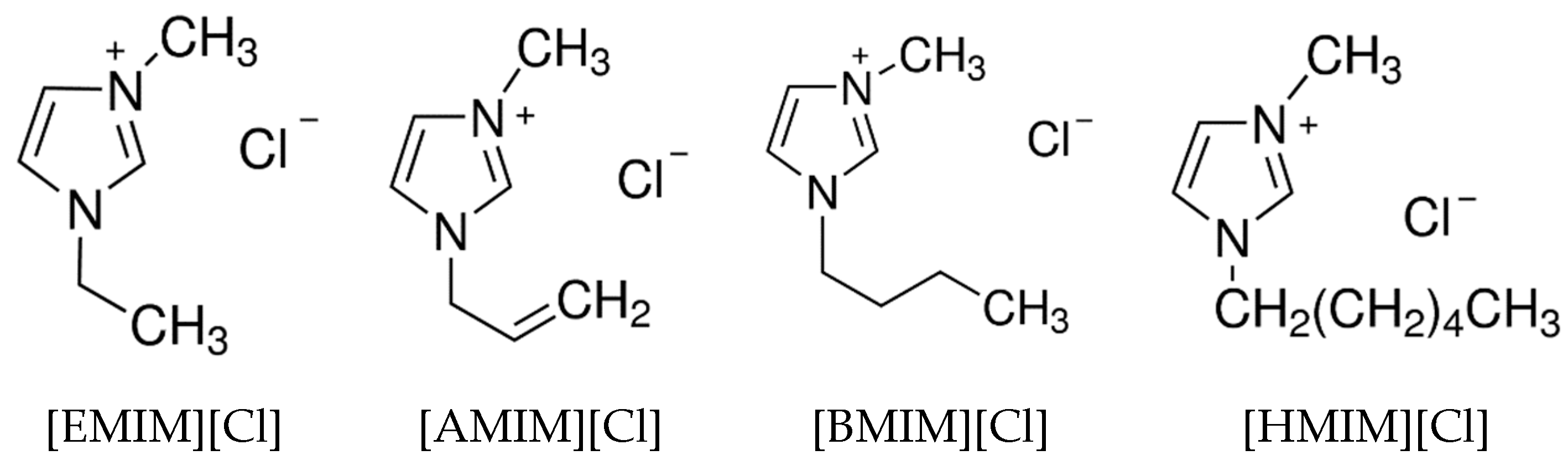
1. Introduction
2. Results and Discussion
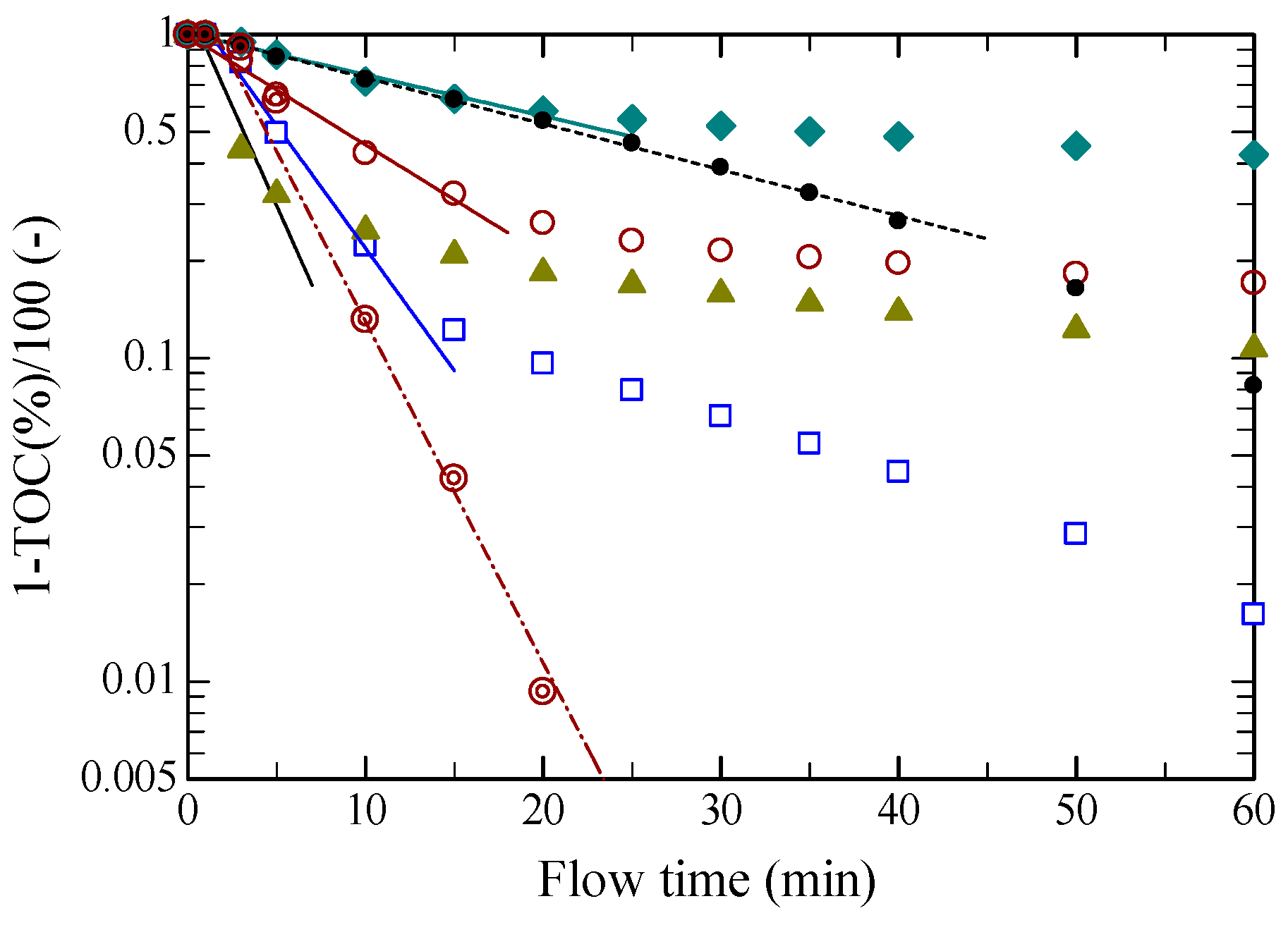
2.1. Dissolution Rate in Hydrothermal Hydrolysis
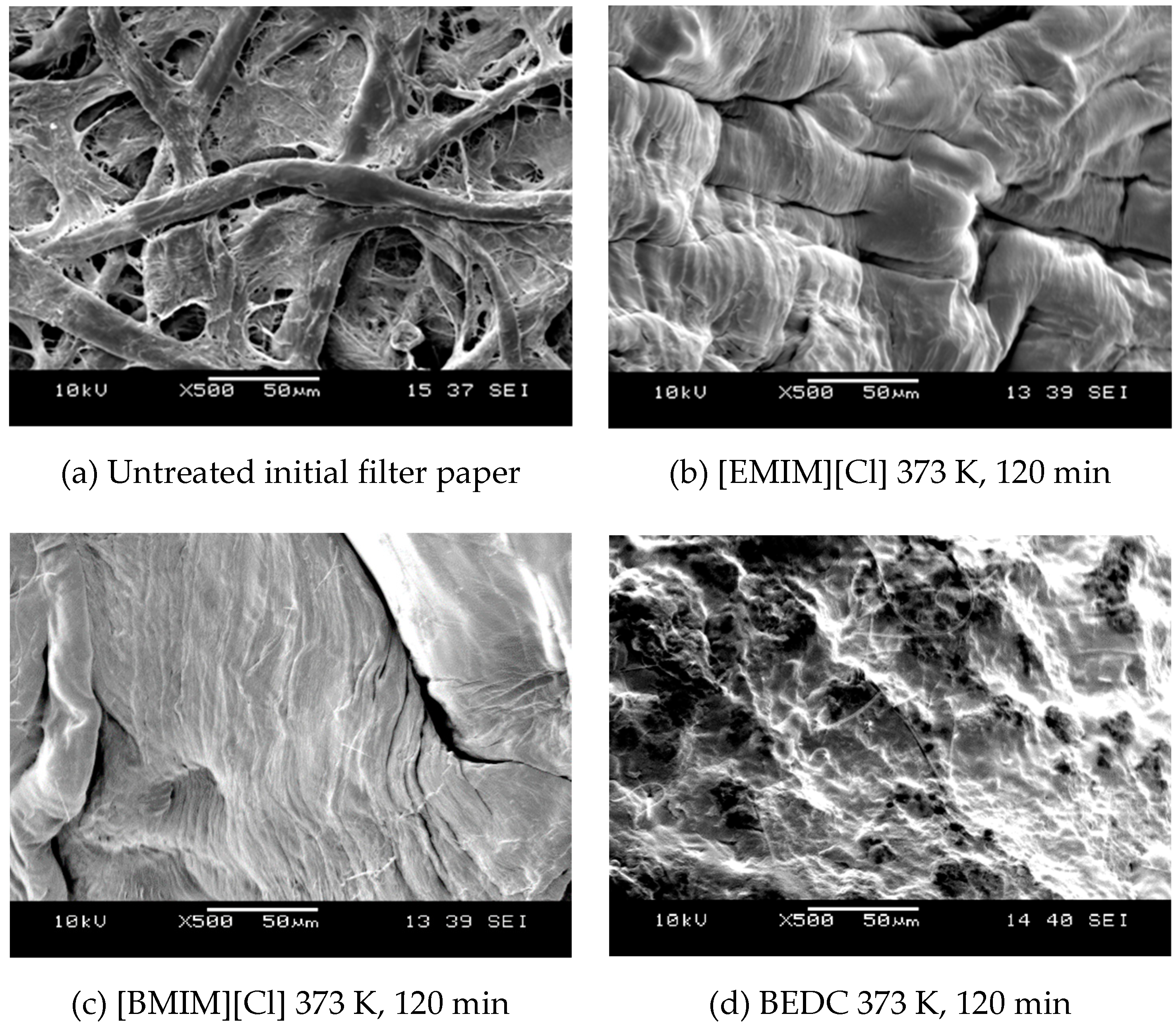
2.2. Scanning Electron Microscope
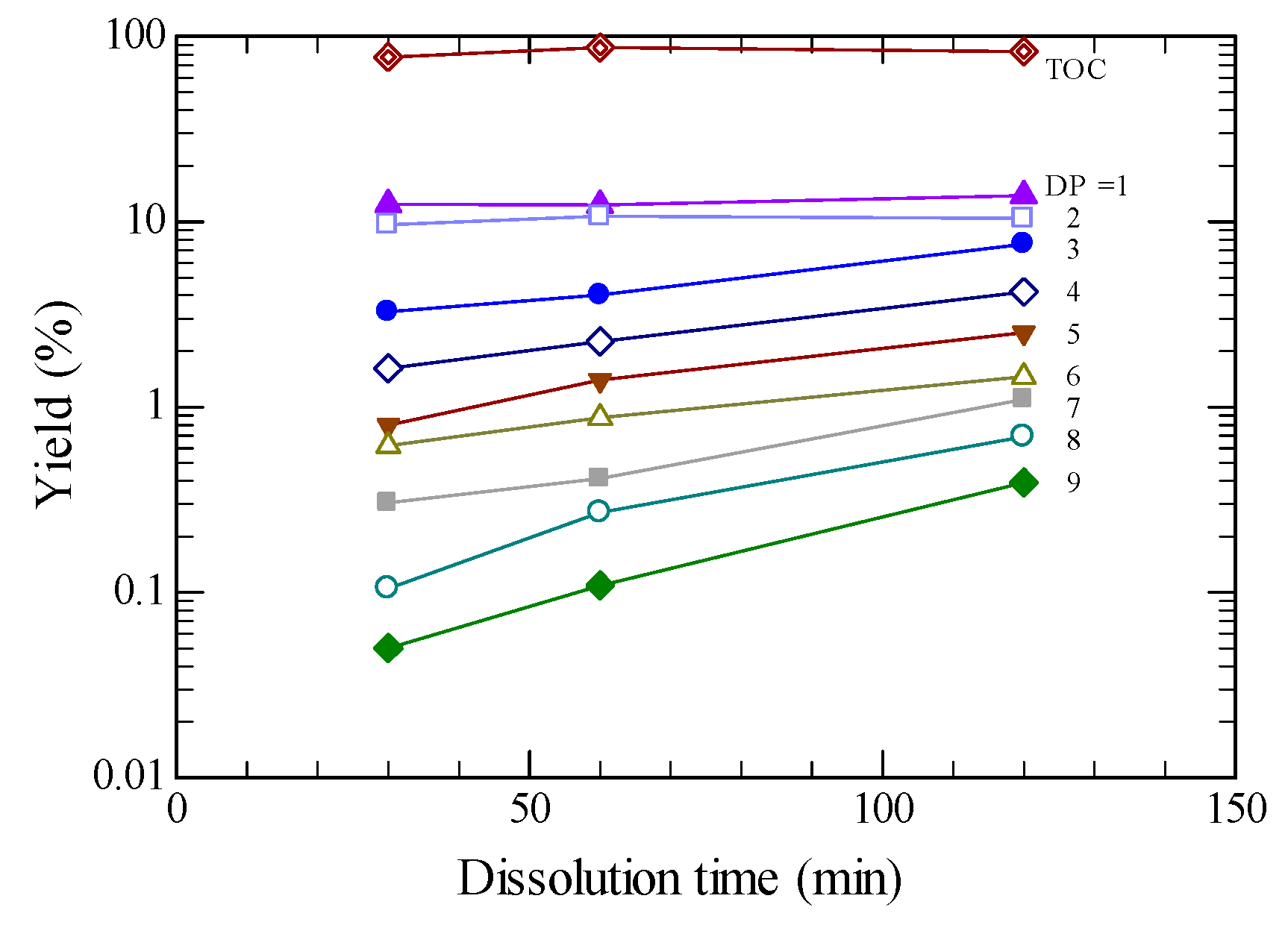
2.3. Product Yields
2.4. Effect of Pretreatment Time for [BMIM][Cl]
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Procedures
3.2.1. Pretreatment with Ionic Liquids and BEDC
3.2.2. Hydrolysis in a Semi-Batch Reactor
3.2.3. Analyses
Degree of crystallinity
Definition of yield
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Satari, B.; Karimi, K.; Kumar, R. Cellulose solvent-based pretreatment for enhance second-generation biofuel production: A review. Sustain. Energy Fuels 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Bobleter, O. Hydrothermal degradation of polymers derived from plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Olsson, L.; Jørgensen, H.; Krog, K.B.R.; Roca, C. Bioethanol production from lignocellulosic material. In Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; Dumiotriu, S., Ed.; Marcel Dekker: New York, NY, USA, 2005; pp. 957–993. [Google Scholar]
- Wyman, C.E.; Decker, S.R.; Himmel, M.E.; Brady, J.W.; Skopec, C.E.; Viikari, L. Hydrolysis of cellulose and hemicellulose. In Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; Dumiotriu, S., Ed.; Marcel Dekker: New York, NY, USA, 2005; pp. 995–1033. [Google Scholar]
- Mousdale, D.M. Biofuels: Biotechnology, Chemistry, and Sustainable Development; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9781420051247. [Google Scholar]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustan. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Pena, C.A.; Soto, A.; King, A.W.; Rodriguez, H. Improved reactivity of cellulose via its crystallinity reduction by Nondissolving pretreatment with an ionic liquid. Sustain. Chem. Eng. 2019, 7, 9164–9171. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B.; Levec, J. Kinetic model of homogeneous lignocellulosic biomass solvolysis in glycerol and imidazolium-based ionic liquids with subsequent heterogeneous hydrodeoxygenation over NiMo/Al2O3 catalyst. Catal. Today 2015, 256, 302–314. [Google Scholar] [CrossRef]
- Asaoka, Y.; Funazukuri, T. Hydrothermal saccharification of cotton cellulose in dilute aqueous formic acid solution. Res. Chem. Intermed. 2011, 37, 233–242. [Google Scholar] [CrossRef]
- Hirajima, K.; Taguchi, M.; Funazukuri, T. Semibatch hydrothermal hydrolysis of cellulose in a filter paper by dilute organic acids. Ind. Eng. Chem. Res. 2015, 54, 6052–6059. [Google Scholar] [CrossRef]
- Funazukuri, T.; Asaoka, Y.; Hirajima, K.; Taguchi, M. Correlation of the product yield with total organic carbon dioxide yield in the hydrothermal conversion of pure celluloses in the absence of additives. Ind. Eng. Chem. Res. 2016, 55, 9372–9377. [Google Scholar] [CrossRef]
- Nagamori, M.; Funazukuri, T. Glucose production by hydrolysis of starch under hydrothermal conditions. J. Chem. Technol. Biotechnol. 2004, 79, 229–233. [Google Scholar] [CrossRef]
- Miyazawa, T.; Ohtsu, S.; Nakagawa, Y.; Funazukuri, T. Solvothermal treatment of starch for the production of glucose and maltooligosaccharides. J. Mater. Sci. 2006, 41, 1489–1494. [Google Scholar] [CrossRef]
- Miyazawa, T.; Ohtsu, S.; Funazukuri, T. Hydrothermal degradation of polysaccharides in a semi-batch reactor: Product distribution as a function of severity parameter. J. Mater. Sci. 2008, 43, 2447–2451. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Schwikal, K.; Barthel, S. Ionic liquids as reaction medium in cellulose functionalization. Macromol. Biosci. 2005, 5, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose dissolution with polar ionic liquids under mild conditions: Required factors for anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Li, C.; Wang, Q.; Zhao, Z.K. Acid in ionic liquid: An efficient system for hydrolysis of lignocelluloses. Green Chem. 2008, 10, 177–182. [Google Scholar] [CrossRef]
- Rinaldi, R.; Palkovits, R.; Schuth, F. Depolymerization of cellulose using solid catalysts in ionic liquids. Angew. Chem. Int. Ed. 2008, 47, 8047–8050. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lian, Y.; Yan, L.; Qi, X.; Smith, R.L. Cellulose-derived superparamagnetic carbonaceous solid acid catalyst for cellulose hydrolysis in an ionic liquid or aqueous reaction system. Green Chem. 2013, 15, 2167–2174. [Google Scholar] [CrossRef]
- Qi, X.; Lian, Y.; Yan, L.; Smith, R.L. One-step preparation of carbonaceous solid acid catalysts by hydrothermal carbonization of glucose for cellulose hydrolysis. Catal. Commun. 2014, 57, 50–54. [Google Scholar] [CrossRef]
- Dadi, A.P.; Varanasi, S.S.; Sasidhar, S.C.A. Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol. Bioeng. 2006, 95, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, I.; Xie, H.; King, A.; Granstrom, M.; Heikkinen, S.; Argyropoulos, D.S. Dissolution of wood in ionic liquids. J. Agric. Food Chem. 2007, 55, 9142–9148. [Google Scholar] [CrossRef]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2009, 102, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jones, C.L.; Baker, G.A.; Xia, S.; Olubajo, O.; Person, V.N. Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J. Biotechnol. 2009, 139, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.V.; Auer, M.; Vogel, K.P.; Simmons, B.A.; Singh, S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef] [PubMed]
- Samayam, I.P.; Schall, C.A. Saccharification of ionic liquid pretreated biomass with commercial enzyme mixtures. Bioresour. Technol. 2010, 101, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-A.; Kim, K.R.; Han, S.J.; Cho, H.Y.; Kim, J.W.; Park, S.M.; Park, J.C.; Sim, S.J. Pretreatment of rice straw with ammonia and ionic liquid for lignocellulose conversion to fermentable sugars. Bioresour. Technol. 2010, 101, 7432–7438. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Mladenov, R.; Wulfhorst, H.; Jäger, G.; Spiess, A.C. Point by point analysis: How ionic liquid affects the enzymatic hydrolysis of native and modified cellulose. Green Chem. 2010, 12, 1959–1966. [Google Scholar] [CrossRef]
- Bose, S.; Armstrong, D.W.; Petrich, J.W. Enzyme-catalyzed hydrolysis of cellulose in ionic liquids: A green approach toward the production of biofuels. J. Phys. Chem. B 2010, 114, 8221–8227. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Holmes, B.; Park, J.I.; Chen, Z.; Dibble, D.C.; Hadi, M. Ionic liquid tolerant hyperthermophilic cellulases for biomass pretreatment and hydrolysis. Green Chem. 2010, 12, 338–345. [Google Scholar] [CrossRef]
- JIS P8215, Measurement of Degree of Polymerization of Cellulose at the Infinite Dilution—Method of with Copper Bis (Diethylene Diamine) Solution, Japan Industrial Standard. Available online: https://kikakurui.com/p/P8215-1998-02.html (accessed on 30 May 2019).
- Muranaka, Y.; Suzuki, T.; Sawanishi, H.; Hasegawa, I.; Mae, K. Effective production of levulinic acid from biomass through pretreatment using phosphoric acid, hydrochloric acid, or ionic liquid. Ind. Eng. Chem. Res. 2014, 53, 11611–11621. [Google Scholar] [CrossRef]
- Kimura, H.; Nakahara, M.; Matubayasi, N. In situ kinetic study on hydrothermal transformation of D-glucose into 5-hydroxymethylfurfural through D-fructose with 13C NMR. J. Phys. Chem. A 2011, 115, 14012–14021. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating he degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 27, 786–794. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
 : [EMIM][Cl],
: [EMIM][Cl],  : [AMIM][Cl],
: [AMIM][Cl],  : [BMIM][Cl],
: [BMIM][Cl],  : [HMIM][Cl],
: [HMIM][Cl],  : BEDC,
: BEDC,  : untreated.
: untreated.
 : [EMIM][Cl],
: [EMIM][Cl],  : [AMIM][Cl],
: [AMIM][Cl],  : [BMIM][Cl],
: [BMIM][Cl],  : [HMIM][Cl],
: [HMIM][Cl],  : BEDC,
: BEDC,  : untreated.
: untreated.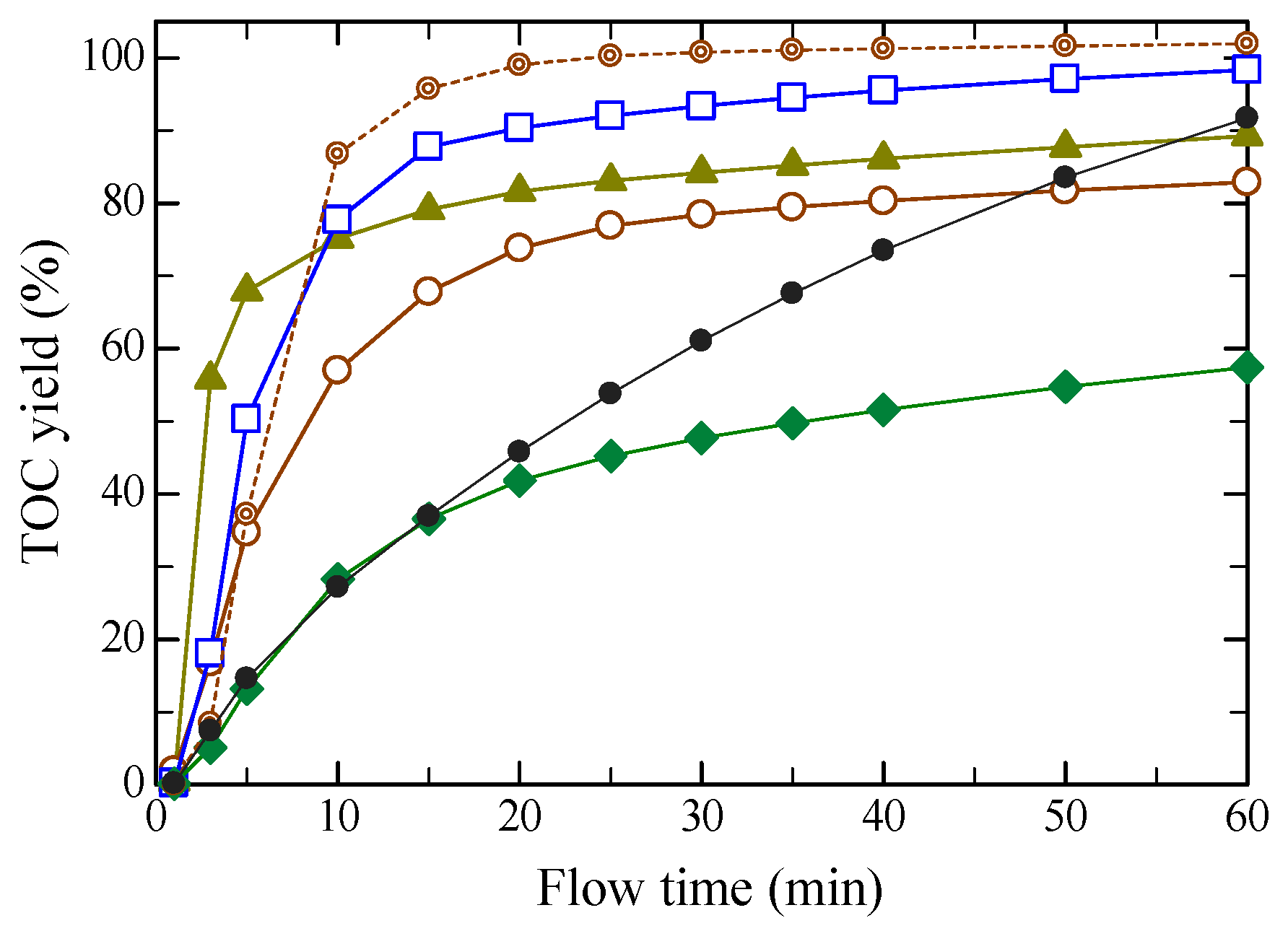
 : [EMIM][Cl],
: [EMIM][Cl], 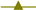 : [AMIM][Cl],
: [AMIM][Cl], 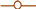 : [BMIM][Cl],
: [BMIM][Cl],  : [HMIM][Cl],
: [HMIM][Cl], 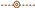 : BEDC,
: BEDC,  : untreated.
: untreated.
 : [EMIM][Cl],
: [EMIM][Cl],  : [AMIM][Cl],
: [AMIM][Cl],  : [BMIM][Cl],
: [BMIM][Cl],  : [HMIM][Cl],
: [HMIM][Cl],  : BEDC,
: BEDC,  : untreated.
: untreated.


| Pre-Treatment | ||||||
| IL or BEDC | [EMIM][Cl] | [AMIM][Cl] | [BMIM][Cl] | [HMIM][Cl] | none | BEDC a |
| temperature (K) | 373 | 373 | 373 | 373 | - | 298 |
| time (min) | 120 | 120 | 120 | 120 | - | 60 |
| C.I. XCI | 45 | 44 | 47 | 48 | 87 | - |
| Hydrolysis | ||||||
| temperature (K) | 533 | 533 | 533 | 533 | 533 | 533 |
| operation time (min) | 60 | 60 | 60 | 60 | 60 | 60 |
| TOC (%) | 98.4 | 89.3 | 82.9 | 57.4 | 91.77 | 102.0 |
| glucose | 14.4 | 28.6 | 13.9 | 9.33 | 14.11 | 26.91 |
| oligomer DP = 2 to 9 | 27.3 | 19.8 | 28.3 | 21.28 | 27.99 | 21.84 |
| sugar b | 41.7 | 48.5 | 42.2 | 30.61 | 42.10 | 48.75 |
| levoglucosan | 1.19 | 3.16 | 1.34 | 0.93 | 2.01 | 24.0 |
| 5-HMF | 3.67 | 4.88 | 2.67 | 1.58 | 5.35 | 5.85 |
| fructose | 2.50 | 1.89 | 2.05 | 1.36 | 3.00 | 3.47 |
| DP = 2 | 8.84 | 6.49 | 10.40 | 7.89 | 16.56 | 10.76 |
| 3 | 6.30 | 5.97 | 7.59 | 4.72 | 4.63 | 5.89 |
| 4 | 4.19 | 3.28 | 4.19 | 3.26 | 3.03 | 2.54 |
| 5 | 3.06 | 1.67 | 2.52 | 2.21 | 1.52 | 1.22 |
| 6 | 1.98 | 1.02 | 1.46 | 1.28 | 1.01 | 0.73 |
| 7 | 1.52 | 0.71 | 1.10 | 0.98 | 0.55 | 0.41 |
| 8 | 0.85 | 0.33 | 0.69 | 0.59 | 0.41 | 0.18 |
| 9 | 0.58 | 0.37 | 0.39 | 0.35 | 0.29 | 0.10 |
| Not identified product c | 49.3 | 30.9 | 34.7 | 23.0 | 39.3 | 1.6 |
| Compound | Purity (%) |
|---|---|
| 1-Ethyl-3-methylimidazolium chloride ([EMIM][Cl]) | 98 |
| 1-Allyl-3-methylimidazolium chloride ([AMIM][Cl]) | 97.0 |
| 1-Butyl-3-methylimidazolium chloride ([BMIM][Cl]) | 98.0 |
| 1-Hexyl-3-methylimidazolium chloride ([HMIM][Cl]) | 97.0 |
| d-(+)Glucose | 99.5 |
| d-(−)-Fructose | 99.0 |
| d-(+)-Cellobiose | 98 |
| d-(+)-Cellotriose | 98 |
| 5-Hydroxylmethyl-2-furaldehyde | 98 |
| 1,6-Anhydro-β-d-glucose | 99 |
| Bis(ethylenediamine)copper(II) solution (BEDC) | 95 |
| Cellulose filter paper | ashless, α-cellulose |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funazukuri, T.; Ozawa, S. Effects of Pretreatment with Ionic Liquids on Cellulose Hydrolysis under Hydrothermal Conditions. Molecules 2019, 24, 3572. https://doi.org/10.3390/molecules24193572
Funazukuri T, Ozawa S. Effects of Pretreatment with Ionic Liquids on Cellulose Hydrolysis under Hydrothermal Conditions. Molecules. 2019; 24(19):3572. https://doi.org/10.3390/molecules24193572
Chicago/Turabian StyleFunazukuri, Toshitaka, and Shingo Ozawa. 2019. "Effects of Pretreatment with Ionic Liquids on Cellulose Hydrolysis under Hydrothermal Conditions" Molecules 24, no. 19: 3572. https://doi.org/10.3390/molecules24193572
APA StyleFunazukuri, T., & Ozawa, S. (2019). Effects of Pretreatment with Ionic Liquids on Cellulose Hydrolysis under Hydrothermal Conditions. Molecules, 24(19), 3572. https://doi.org/10.3390/molecules24193572






