Inhibitory Effects of Novel 7-Substituted 6-iodo-3-O-Flavonol Glycosides against Cholinesterases and β-secretase Activities, and Evaluation for Potential Antioxidant Properties
Abstract
:1. Introduction
2. Results and Discussion
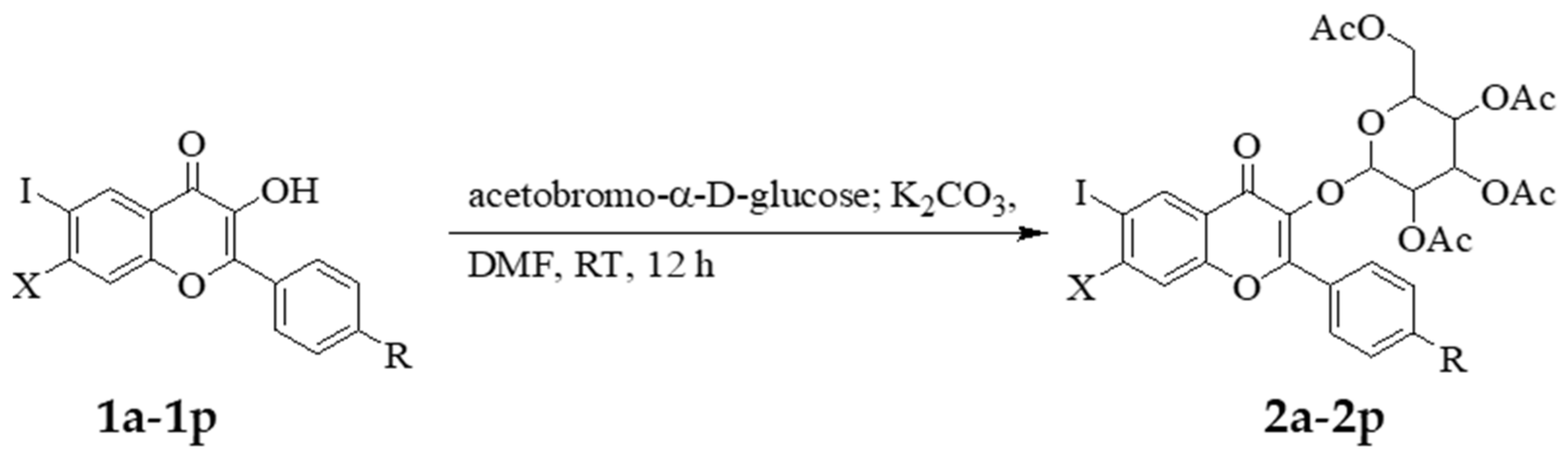
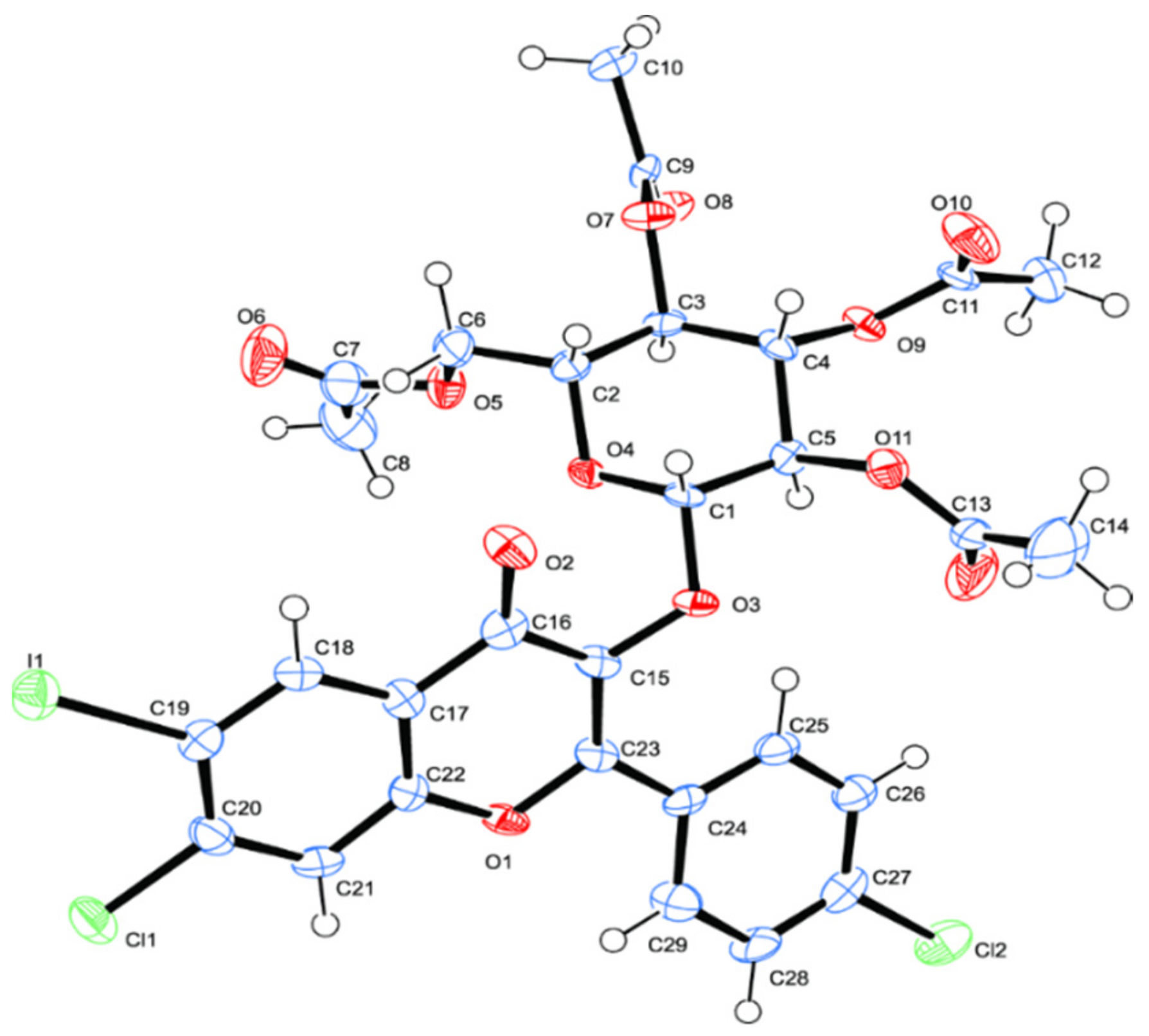
2.1. Chemistry
2.2. Inhibitory Activity of the 3-O-flavonol Glycosides Against AChE, BChE, and β-Secretase
2.3. Kinetic Studies Against Cholinesterases and β-Secretase
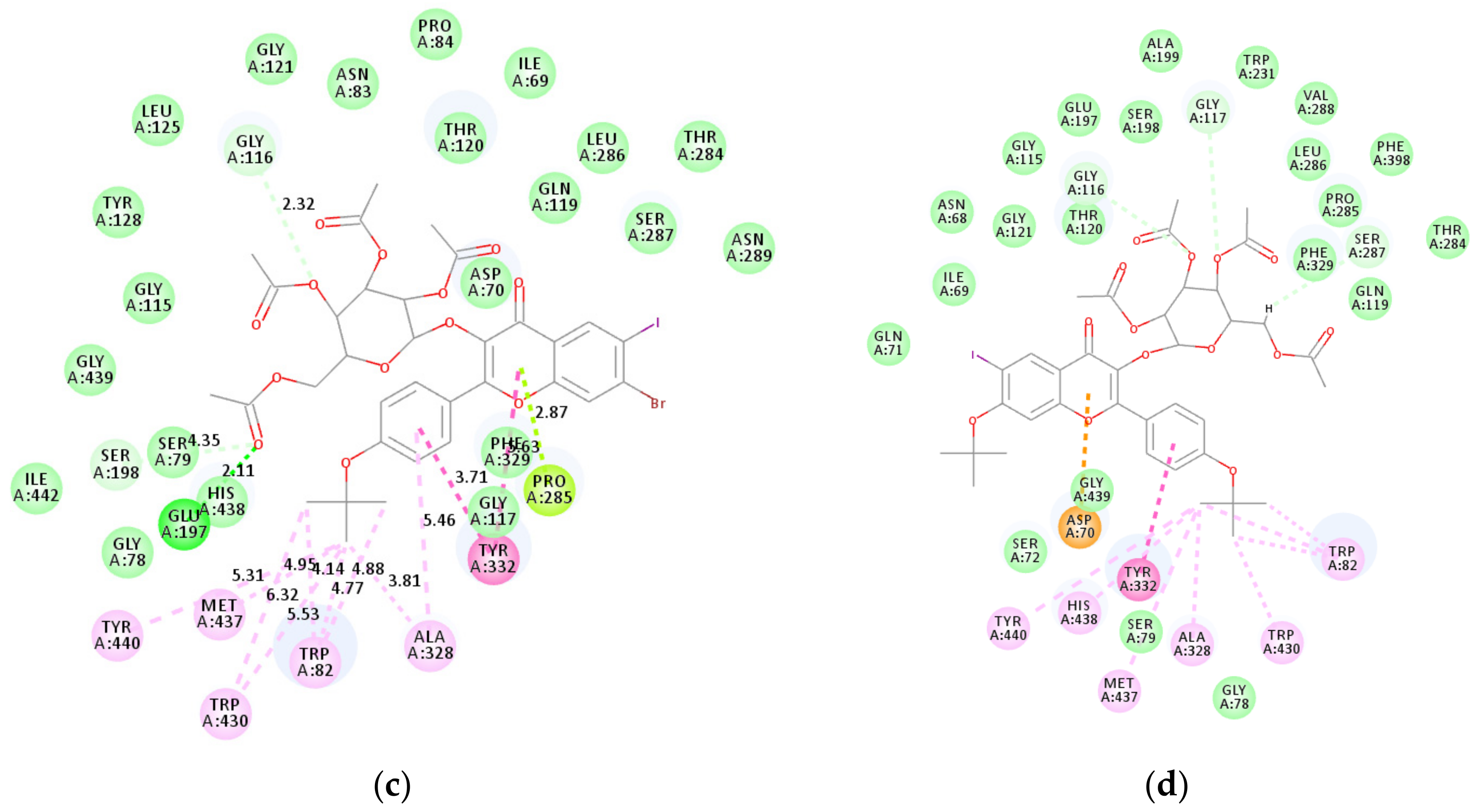
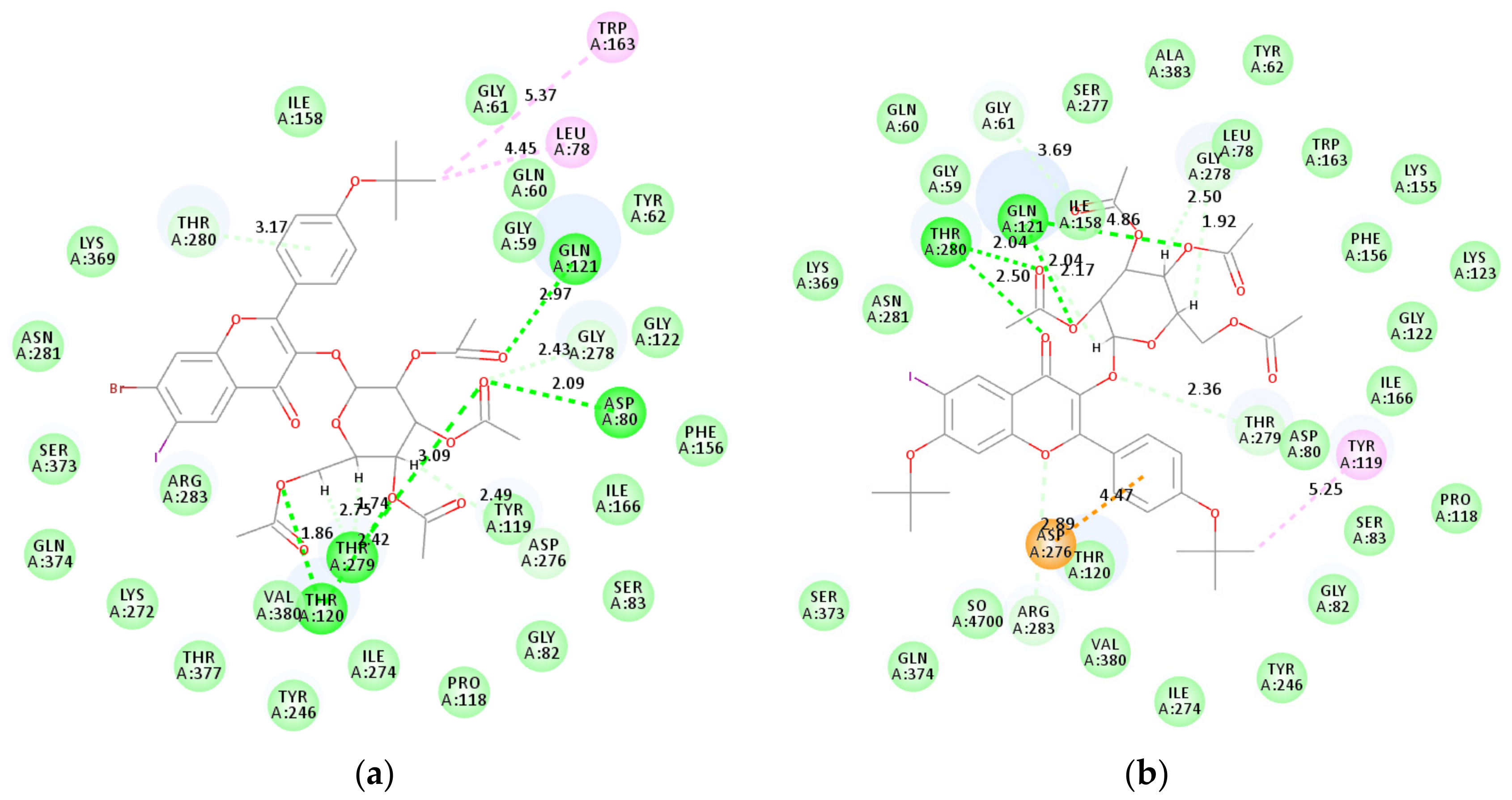
2.4. Molecular Docking Studies into AChE, BChE and β-Secretase Active Sites
2.4.1. Molecular Docking Studies of Compounds 2k, 2l, 2n, and 2p into AChE Active Sites
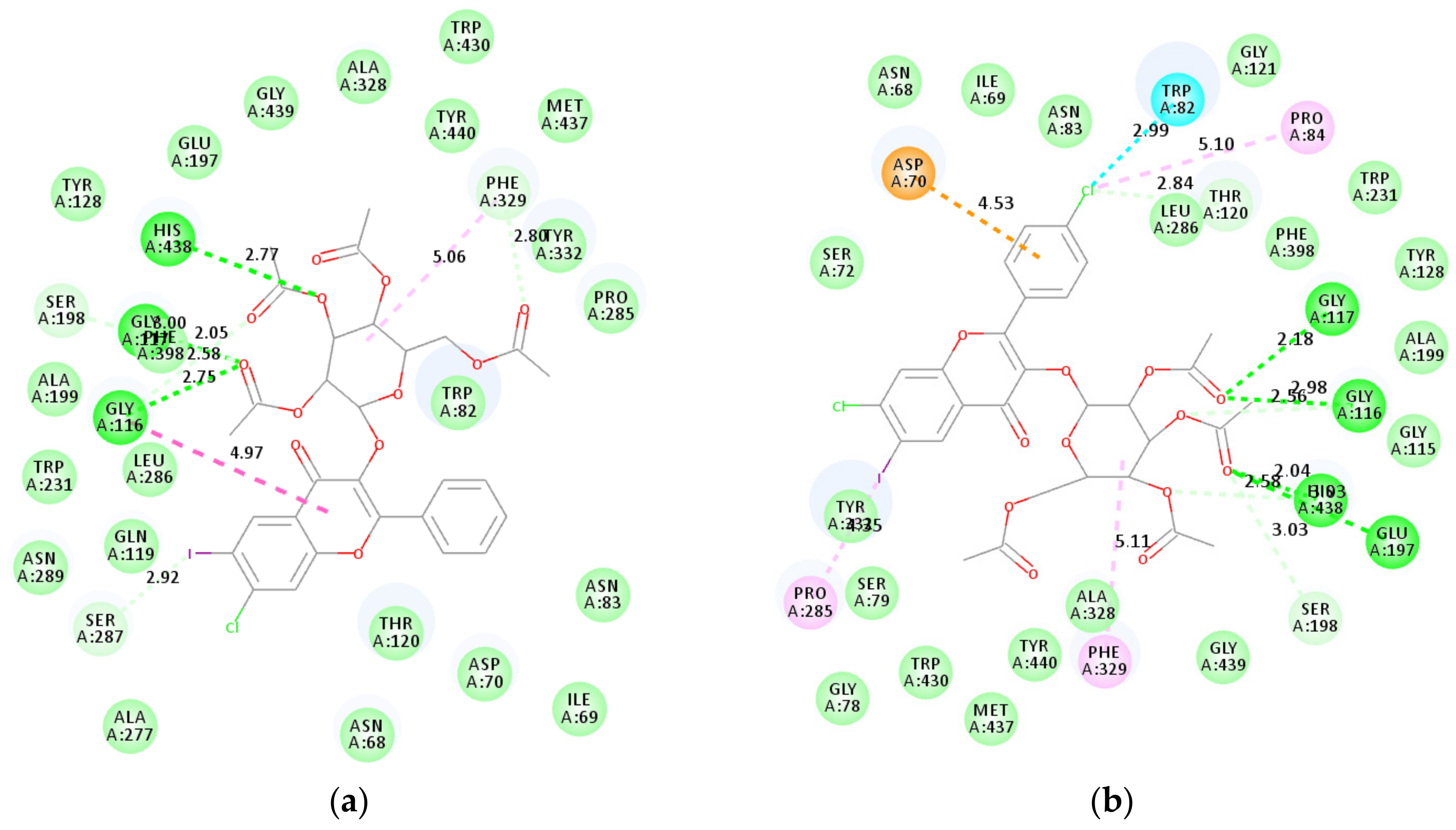
2.4.2. Molecular Docking Studies of 2e, 2g, 2l, and 2p into BChE Active Sites
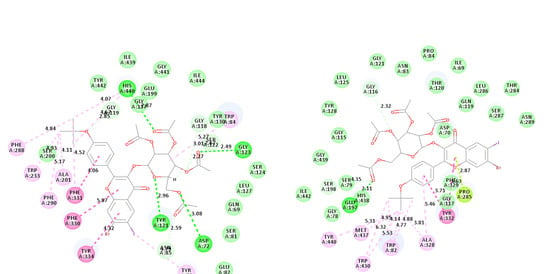
2.4.3. Molecular Docking Studies of Compounds 2l and 2p into β-Secretase Active Sites
2.5. Antioxidant Activity of Compounds 2g, 2k, 2l, 2n, and 2p
3. Materials and Methods
3.1. General
3.2. Typical Procedure for the Synthesis of the 7-substituted-6-iodo-2-phenyl-chromen-4-one-3-O-2,3,4,6-O-tetraacetyl-β-d-glucopyranoside Derivatives (2a–2p)
3.3. In Vitro Cholinesterase (AChE and BChE) Inhibition Assays
3.4. In Vitro β-Secretase Inhibitory Assays
3.5. Kinetic Studies of 2l and 2p against ChEs and β-Secretase
3.6. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity Assay
3.7. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hui-Ming, G.; Jau-Shyong, H. Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar]
- Ortiz, G.G.; Moisés, F.P.P.; Mireles-Ramírez, M.; Flores-Alvarado, L.J.; González-Usigli, H.; Sánchez-González, V.J.; Rivero-Moragrega, P. Oxidative stress: Love and hate history in central nervous system. Adv. Protein Chem. Struct. Biol. 2017, 108, 1–31. [Google Scholar]
- Marco-Contelles, J.; Unzeta, M.; Bolea, I.; Esteban, G.; Ramsay, R.R.; Romero, A.; Martinez-Murillo, R.; Carreiras, M.C.; Ismaili, L. Ass234, as a new multi-target directed propargylamine for Alzheimer’s disease therapy. Front. Neurosci. 2016, 10, 294. [Google Scholar] [CrossRef]
- Gocer, H.; Topal, F.; Topal, M.; Küçük, M.; Teke, D.; Gulcin, I.; Alwasel, S.H.; Supuran, C.T. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J. Enzym. Inhib. Med. Chem. 2016, 31, 441–447. [Google Scholar] [CrossRef]
- Verdile, G.; Fuller, S.J.; Martins, R.N. The role of type 2 diabetes in neurodegeneration. Neurobiol. Dis. 2015, 84, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.J.; Lee, J.E.; Wilson, J.G.; Everhart, A.L.; Brown, C.M.; Hoofnagle, A.N.; Jansen, M.; Vitek, M.P.; Gunn, M.D.; Colton, C.A. Arginine deprivation and immune suppression in a mouse model of Alzheimer’s disease. J. Neurosci. 2015, 35, 5969–5982. [Google Scholar] [CrossRef] [PubMed]
- Christen, Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000, 71, 621S–629S. [Google Scholar] [CrossRef]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [PubMed]
- Bajda, M.; Guzior, N.; Ignasik, M.; Malawska, B. Multi-target-directed ligands in Alzheimer’s disease treatment. Curr. Med. Chem. 2011, 18, 4949–4975. [Google Scholar] [CrossRef]
- Chen, X.; Decker, M. Multi-target compounds acting in the central nervous system designed from natural products. Curr. Med. Chem. 2013, 20, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeuticutility for Alzheimer disease. Free Rad. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cellbased and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta 2008, 1780, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Luisa Helena, C.; Leila, Z.; Elga Heloisa, A.; Maria Santos Reis Bonorino, F.; Poliane, F.; Rosangela Guollo, D.; Moacir Geraldo, P.; Fatima Regina Mena Barreto, S. Flavonoids: Prospective Drug Candidates. Mini-Rev. Med. Chem. 2008, 8, 1429–1440. [Google Scholar]
- Hayes, M.R.; Pietruszka, J. Synthesis of glycosides by glycosynthases. Molecules 2017, 22, 1434. [Google Scholar] [CrossRef]
- Guzzi, C.; Colombo, L.; De Luigie, A.; Salmona, M.; Nicotra, F.; Airoldi, C. Flavonoids and their glycosides as anti-amyloidogenic compounds: Ab1–42 interaction studies to gain new insights into their potential for Alzheimer’s disease prevention and therapy. Chem. Asian. J. 2017, 12, 67–75. [Google Scholar] [CrossRef]
- Rahman, A.; Ali, M.T.; Shawan, M.M.A.K.; Sarwar, M.G.; Khan, M.A.K.; Halim, M.A. Halogen-directed drug design for Alzheimer’s disease: A combined density functional and molecular docking study. SpringerPlus 2016, 5, 1346–1359. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, T.; Wang, Y.; Yang, H.; Yan, X.; Luo, X.; Jiang, H.; Zhu, W. Halogen bonding– A novel interaction for rational drug design? J. Med. Chem. 2009, 52, 2854–2862. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Xu, Z.; Li, H.; Liu, H.; Zhu, W. Halogen bonding for rational drug design and new drug discovery. Expert Opin. Drug Discov. 2012, 7, 375–383. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem. 2012, 56, 1363–1388. [Google Scholar] [CrossRef]
- Mughal, E.U.; Javid, A.; Sadiq, A.; Murtaza, S.; Zafar, M.N.; Khan, B.A.; Sumra, S.H.; Tahir, M.N.; Kanwal; Khan, K.M. Synthesis, structure-activity relationship and molecular docking studies of 3-O-flavonol glycosides as cholinesterase inhibitors. Bioorg. Med. Chem. 2018, 26, 3696–3706. [Google Scholar]
- Mphahlele, M.J.; Agbo, E.N.; Gildenhuys, S. Synthesis and evaluation of the 4-substituted 2-hydroxy-5-iodochalcones and their 7-substituted 6-iodoflavonol derivatives for inhibitory effect on cholinesterases and β-secretase. Int. J. Mol. Sci. 2018, 19, 4112. [Google Scholar] [CrossRef] [PubMed]
- CCDC 1915892 Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 18 September 2019).
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.G. Advances in the treatment of Alzheimer’s disease: Benefits of dual cholinesterase inhibition. Eur. Neurol. 2002, 47, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ayllon, M.S.; Small, D.H.; Avila, J.; Saez-Valero, J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: Cross-talk with P.-tau and β-amyloid. Front. Mol. Neurosci. 2011, 22, 1–9. [Google Scholar]
- Guillozet, A.L.; Smiley, J.F.; Mash, D.C.; Mesulam, M.-M.C. Butyrycholinesterase in the life cycle of amyloid plaques. Ann. Neurol. 1997, 42, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Park, J.-H.; Lee, J.; Jeong, W.-S.; Ho, C.-T.; Jun, M. The identification of biochanin A as a potent and selective β-site app-cleaving enzyme 1 (Bace1) inhibitor. Nutrients 2016, 8, 637. [Google Scholar] [CrossRef]
- Kang, J.E.; Cho, J.K.; Curtis-Long, M.J.; Ryu, H.W.; Kim, J.H.; Kim, H.J.; Yuk, H.K.; Kim, D.W.; Park, K.H. Inhibitory evaluation of sulfonamide chalcones on β-secretase and acylcholinesterase. Molecules 2013, 18, 140–153. [Google Scholar] [CrossRef]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective effect of quercetin in primary neurons against α,β(1–42): Relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.M.; Li, X.M.; Li, F.; Wu, J.-J.; Kong, L.-Y.; Wang, X.-B. Synthesis and evaluation of multi-target-directed ligands for the treatment of Alzheimer’s disease based on the fusion of donepezil and melatonin. Bioorg. Med. Chem. 2016, 24, 4324–4338. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.A.; Sousa, E. Bace-1 and γ-secretase as therapeutic targets for Alzheimer’s disease. Pharmaceuticals 2019, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Butini, S.; Brogi, S.; Novellino, E.; Campiani, G.; Ghosh, A.K.; Brindisi, M.; Gemma, S. The structural evolution of β-secretase inhibitors: A focus on the development of small-molecule inhibitors. Curr. Top. Med. Chem. 2013, 13, 1787–1807. [Google Scholar]
- Yadav, P.; Parshad, B.; Manchanda, P.; Sharma, S. Chromones and their derivatives as radical scavengers: A remedy for cell impairment. Curr. Top. Med. Chem. 2004, 14, 2552–2575. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Lee, C.Y. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef]
- Arduini, F.; Errico, I.; Amine, A.; Micheli, L.; Palleschi, G.; Moscone, D. Enzymatic spectrophotometric method for aflatoxin B detection based on acetylcholinesterase inhibition. Anal. Chem. 2007, 79, 3409–3415. [Google Scholar] [CrossRef] [PubMed]
- Akıncıoğlu, K.; Akıncıoğlu, H.; Gülcin, í.; Durdagi, S.; Supuran, C.T.; Suleyman, G. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: Novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg. Med. Chem. 2015, 23, 3592–3602. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, H.; Qian, H. Antioxidant and free radical scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006, 41, 1296–1302. [Google Scholar] [CrossRef]
- Köse, L.P.; ˙Gülcin¸, I.; Gören, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC–MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crops Prod. 2015, 74, 712–721. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Güçlcin, I.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzyme Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar]
Sample Availability: Samples of the compounds 2a–2p are available from the authors. |


| R | Designation of X for 2a–2p | |||
|---|---|---|---|---|
| H | F (2a) | Cl (2e) | Br (2i) | OCH3 (2m) |
| F | F (2b) | Cl (2f) | Br (2j) | OCH3 (2n) |
| Cl | F (2c) | Cl (2g) | Br (2k) | OCH3 (2o) |
| OCH3 | F (2d) | Cl (2h) | Br (2l) | OCH3 (2p) |
| Compound | IC50 (µM) | SI | ||
|---|---|---|---|---|
| AChE | BChE | BChE/AChE | AChE/BChE | |
| 2a | 18.72 ± 0.03 | 26.17 ± 0.01 | 1.40 | 0.71 |
| 2b | 28.2 ± 0.02 | 21.9 ± 0.04 | 0.78 | 1.3 |
| 2c | 24.74 ± 0.03 | 30.84 ± 0.04 | 1.25 | 0.80 |
| 2d | 60.40 ± 0.05 | 19.25 ± 0.11 | 0.32 | 3.13 |
| 2e | 34.44 ± 0.03 | 9.03 ± 0.12 | 0.26 | 3.85 |
| 2f | 70.66 ± 0.01 | 31.09 ± 0.04 | 0.44 | 2.27 |
| 2g | 31.44 ± 0.13 | 7.15 ± 0.01 | 0.23 | 4.35 |
| 2h | 25.92 ± 0.02 | 10.10 ± 0.06 | 0.39 | 2.56 |
| 2i | 70.56 ± 0.03 | 9.79 ± 0.04 | 0.14 | 7.14 |
| 2j | 31.20 ± 0.05 | 40.93 ± 0.04 | 1.31 | 0.76 |
| 2k | 5.18 ± 0.01 | 29.95 ± 0.02 | 5.78 | 0.17 |
| 2l | 5.05 ± 0.02 | 7.24 ± 0.03 | 1.43 | 0.70 |
| 2m | 56.21 ± 0.01 | 29.80 ± 0.06 | 0.53 | 1.89 |
| 2n | 6.23 ± 0.05 | 34.21 ± 0.02 | 5.49 | 0.18 |
| 2o | 28.12 ± 0.06 | 36.10 ± 0.02 | 1.28 | 0.78 |
| 2p | 9.19 ± 0.03 | 5.21 ± 0.03 | 0.57 | 1.75 |
| Donepezil | 4.82 ± 0.01 | 6.01 ± 0.02 | 1.25 | 0.80 |
| Compounds | IC50 (µM) |
|---|---|
| 2l | 18.3 ± 0.01 |
| 2p | 25.2 ± 0.15 |
| Quercetin | 13.7 ± 0.02 |
| Compounds | IC50 (µM) |
|---|---|
| 2g | 13.1 ± 0.01 |
| 2k | 11.4 ± 0.02 |
| 2l | 21.6 ± 0.03 |
| 2n | 14.4 ± 0.02 |
| 2p | 17.9 ± 0.04 |
| Ascorbic acid Quercetin | 5.5 ± 0.02 3.1 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbo, E.N.; Gildenhuys, S.; Mphahlele, M.J. Inhibitory Effects of Novel 7-Substituted 6-iodo-3-O-Flavonol Glycosides against Cholinesterases and β-secretase Activities, and Evaluation for Potential Antioxidant Properties. Molecules 2019, 24, 3500. https://doi.org/10.3390/molecules24193500
Agbo EN, Gildenhuys S, Mphahlele MJ. Inhibitory Effects of Novel 7-Substituted 6-iodo-3-O-Flavonol Glycosides against Cholinesterases and β-secretase Activities, and Evaluation for Potential Antioxidant Properties. Molecules. 2019; 24(19):3500. https://doi.org/10.3390/molecules24193500
Chicago/Turabian StyleAgbo, Emmanuel N., Samantha Gildenhuys, and Malose J. Mphahlele. 2019. "Inhibitory Effects of Novel 7-Substituted 6-iodo-3-O-Flavonol Glycosides against Cholinesterases and β-secretase Activities, and Evaluation for Potential Antioxidant Properties" Molecules 24, no. 19: 3500. https://doi.org/10.3390/molecules24193500
APA StyleAgbo, E. N., Gildenhuys, S., & Mphahlele, M. J. (2019). Inhibitory Effects of Novel 7-Substituted 6-iodo-3-O-Flavonol Glycosides against Cholinesterases and β-secretase Activities, and Evaluation for Potential Antioxidant Properties. Molecules, 24(19), 3500. https://doi.org/10.3390/molecules24193500