A New Class of β–Pyrrolidino-1,2,3-Triazole Derivatives as β-Adrenergic Receptor Inhibitors: Synthesis, Pharmacological, and Docking Studies
Abstract
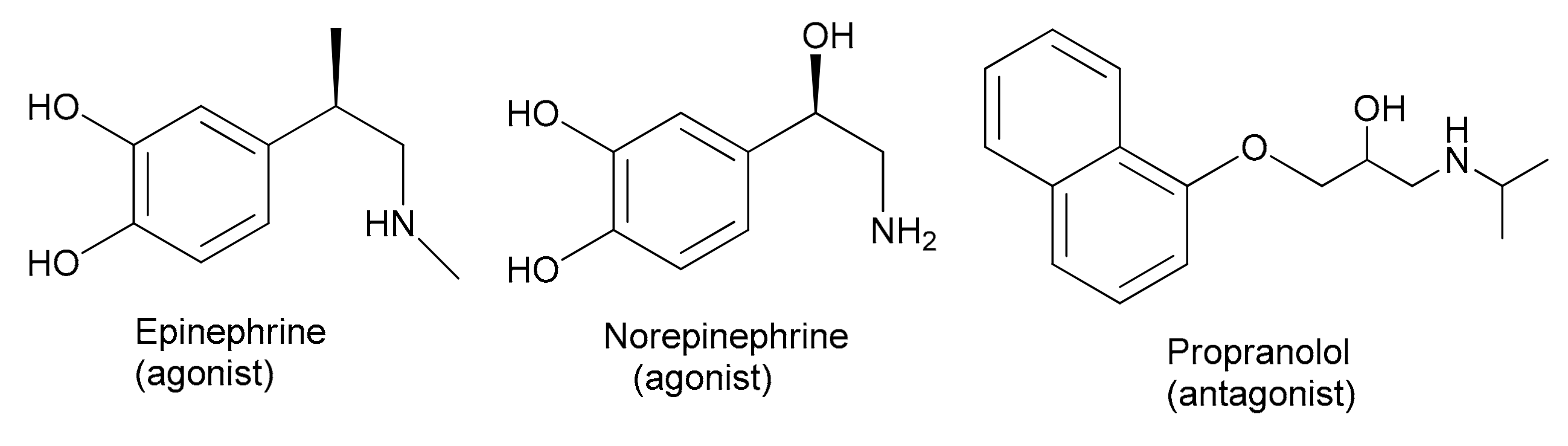
1. Introduction
2. Results and Discussions
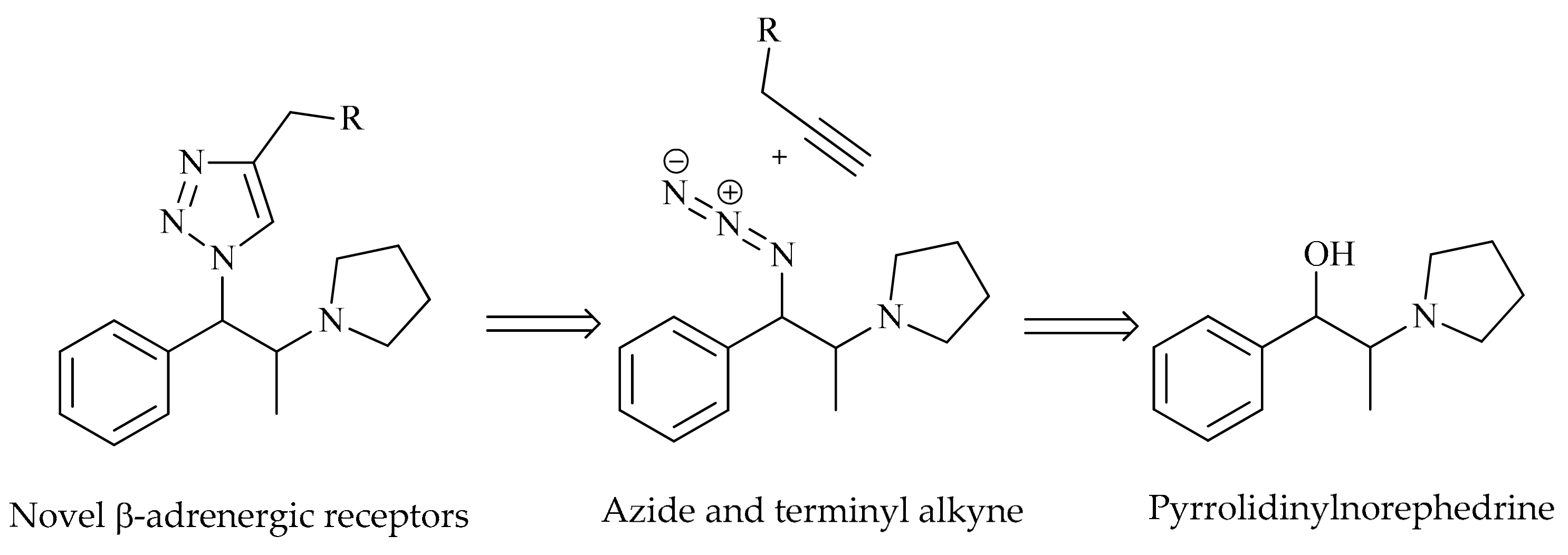
2.1. Chemistry
2.2. Antimicrobial Activity
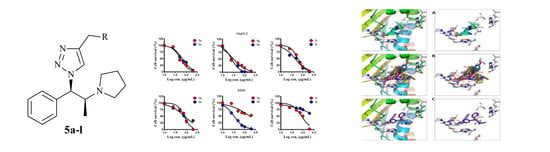
2.3. Anticancer Results
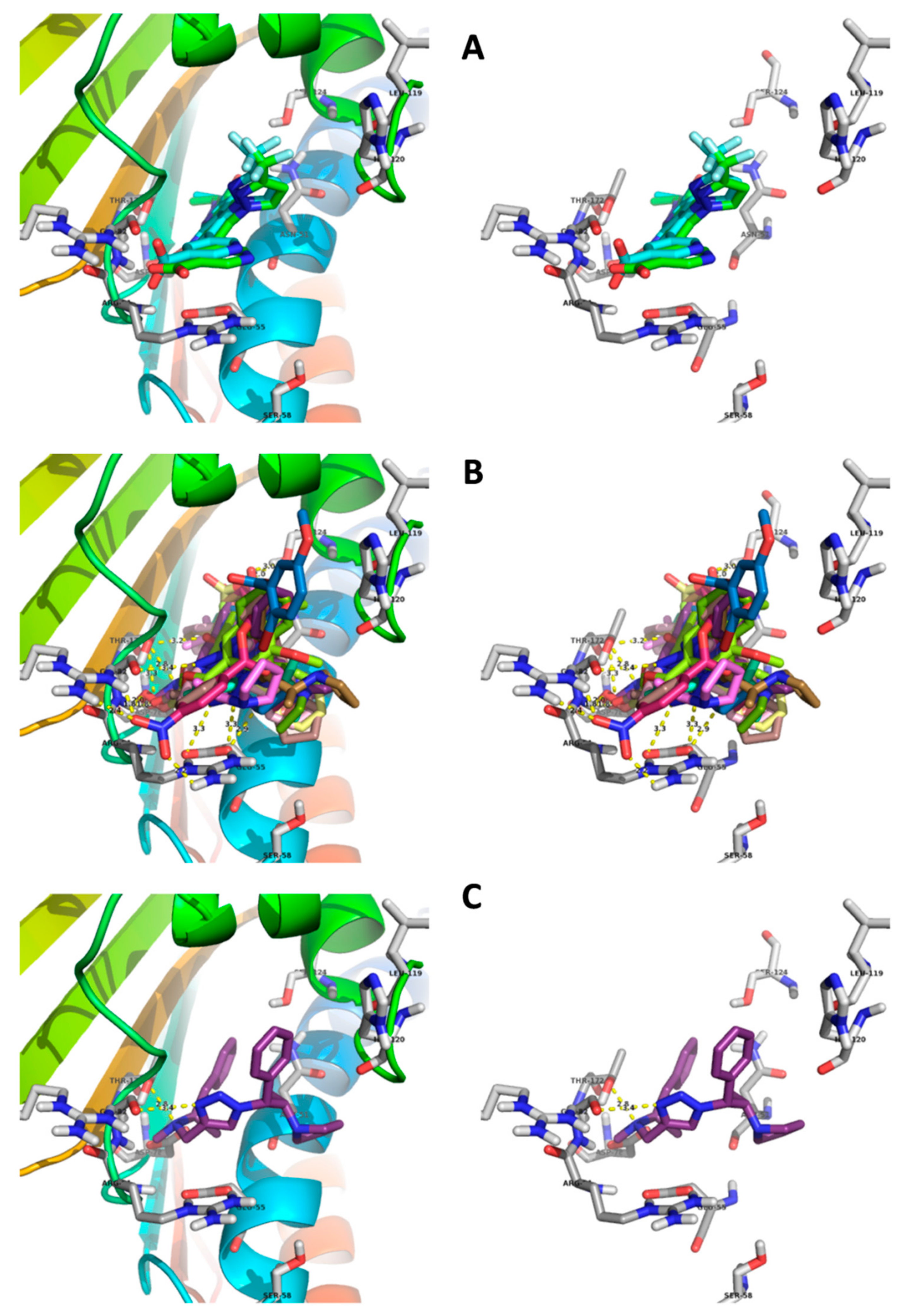
2.4. Molecular Docking Studies
3. Materials and Methods
3.1. Pharmacological Activities
3.2. Minimum Inhibitory Concentration (MIC)
3.3. Cytotoxicity Properties
3.4. General Procedure for the Synthesis of β-pyrrolidino-1,2,3-triazoles 5a–l:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyd, N.M.; Reade, P.C. Mechanisms of carcinogenesis with particular reference to the oral mucosa. J. Oral Pathol. 1988, 17, 193–201. [Google Scholar] [CrossRef]
- Rajeevan, M.S.; Vernon, S.D.; Taysavang, N.; Unger, E.R. Validation of array-based gene expression profiles by real-time (kinetic) RT-PCR. J. Mol. Diagn. 2001, 3, 26–31. [Google Scholar] [CrossRef]
- Meinhardt, G.; Wendtner, C.M.; Hallek, M. Molecular pathogenesis of chronic lymphocytic leukemia: Factors and signaling pathways regulating cell growth and survival. J. Mol. Med. 1999, 77, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.S.; Karpova, Y.; Prokopovich, S.; Smith, A.J.; Essau, B.; Gersappe, A. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J. Biol. Chem. 2007, 282, 14094–14100. [Google Scholar] [CrossRef]
- Entschladen, F.; Lang, K.; Drell, T.L.; Joseph, J.; Zaenker, K.S. Neurotransmitters are regulators for the migration of tumor cells and leukocytes. Cancer Immunol. Immunother. 2002, 51, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Drell, T.L.; Lindecke, A.; Niggemann, B.; Kaltschmidt, C.; Zaenker, K.S. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int. J. Cancer 2004, 112, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.J.; Liu, K.; Liang, D.F. Expression of beta2-adrenergic receptor in oral squamous cell carcinoma. J. Oral Pathol. Med. 2009, 38, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.; Wong, H.P.; Luo, C.K.; Huang, F.Y.; Hui, M.K. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone from cigarette smoke stimulates colon cancer growth via beta-adrenoceptors. Cancer Res. 2005, 65, 5272–5277. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.P.; Yu, L.; Lam, E.K.; Tai, E.K.; Wu, W.K.; Cho, C.H. Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation. Toxicol. Sci. 2007, 97, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Steimer, F.; Carmona, A.T.; Moreno-Vargas, A.J.; Caffa, I.; Cea, M.; Montecucco, F.; Nencioni, A.; Vogel, P.; Robina, I. Synthesis of Pyrrolidine 3,4-Diol Derivatives with Anticancer Activity on Pancreatic Tumor Cells. Heterocycles 2014, 88, 1445–1464. [Google Scholar]
- Jung, S.H.; Choi, K.; Pae, A.N.; Kyun Lee, J.; Choo, H.; Keum, G.; Cho, Y.S.; Min, S.J. Facile diverted synthesis of pyrrolidinyl triazoles using organotrifluoroborate: Discovery of potential mPTP blockers. Org. Biomol. Chem. 2014, 12, 9674–9682. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Bailen, M.; Carmona, A.T.; Nirebi-Clavijo, E.; Robina, I.; Ide, D.; Kato, A.; Moreno-Vargas, A.J. Tuning of β-glucosidase and α-galactosidase inhibition by generation and in situ screening of a library of pyrrolidine-triazole hybrid molecules. Eur. J. Med. Chem. 2017, 138, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lewis, W.G.; Green, L.G.; Grynszpan, F.; Radic, Z.; Carlier, P.R.; Taylor, P.; Finn, M.G.; Sharpless, K.B. Click Chemistry In Situ: Acetylcholinesterase as a Reaction Vessel for the Selective Assembly of a Femtomolar Inhibitor from an Array of Building Blocks. Angew. Chem. Int. Ed. 2002, 41, 1053–1057. [Google Scholar] [CrossRef]
- Easwaramoorthi, K.; Jeya Rajendran, A.; Chennakesava Rao, K.; Arun, Y.; Balachandran, C.; Perumal, P.T.; Emi, N.; Mahalingam, S.M.; Duraipandiyan, V.; Al-Dhabi, N.A. Synthesis of novel 1,4-disubstituted 1,2,3-triazolo-bosentan derivatives—Evaluation of antimicrobial and anticancer activities and molecular docking. RSC Adv. 2015, 5, 105266–105278. [Google Scholar] [CrossRef]
- Sandip, A.G.; Shrikant, P.G.; Swapna, G.M.; Vandana, P.S. Alumina-Supported Copper Iodide: An Efficient and Recyclable Catalyst for Microwave-Assisted Synthesis of 1,4-Disubstituted 1,2,3-Triazoles via Three-Component Reaction in Water. Asian J. Org. Chem. 2015, 4, 943–951. [Google Scholar]
- Roberts, B.A.; Strauss, C.R. Toward Rapid, “Green”, Predictable Microwave-Assisted Synthesis. Acc. Chem. Res. 2005, 38, 653–661. [Google Scholar] [CrossRef]
- Prakasam, T.; Ramana, D.V.; Srinivasan, P.S.; Kumar, B.N.H.; Arabindoo, B. A process for the preparation of N-alkylaminophenyl(pyrrolidinyl)propane hydrochloride derivatives. Indian patent IN2007CH01379; Chennai, India, 2007. [Google Scholar]
- Balachandran, C.; Arun, Y.; Duraipandiyan, V.; Ignacimuthu, S.; Balakrishna, K.; Al-Dhabi, N.A. Antimicrobial and cytotoxicity properties of 2,3-dihydroxy-9,10-anthraquinone isolated from Streptomyces galbus (ERINLG-127). Appl. Biochem.Biotechnol. 2014, 17, 3513–3528. [Google Scholar] [CrossRef]
- Balachandran, C.; Duraipandiyan, V.; Balakrishna, K.; Lakshmi Sundaram, R.; Vijayakumar, A.; Ignacimuthu, S.; Al-Dhabi, N.A. Synthesis and medicinal properties of plant-derived vilangin. Environ. Chem. Lett. 2013, 11, 303–308. [Google Scholar] [CrossRef]
- Balachandran, C.; Chennakesava Rao, K.; Arun, Y.; Emi, N.; Yamamoto, N.; Inaguma, Y.; Okamoto, A.; Easwaramoorthi, K.; Perumal, P.T. Synthetic investigation on chirally pure Mannich derivatives of pseudophenylpropanolamine and their anticancer properties against HepG-2 cells with inhibition of JAK2/STAT3. RSC Adv. 2016, 6, 96946–96962. [Google Scholar] [CrossRef]
- Chennakesava Rao, K.; Arun, Y.; Easwaramoorthi, K.; Balachandran, C.; Prakasam, T.; Yuvaraj, T.E.; Perumal, P.T. Synthesis, antimicrobial and molecular docking studies of enantiomerically pure N-alkylated β-amino alcohols from phenylpropanolamines. Bioorg. Med. Chem. Lett. 2014, 24, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Chennakesava Rao, K.; Easwaramoorthi, K.; Arun, Y.; Balachandran, C.; Muralidhara Rao, K.S.; Govindhan, M.; Emi, N.; Prakasam, T.; Perumal, P.T. Synthesis of BF3 catalyzed Mannich derivatives with excellent ee from phenylpropanolamine, study of their antimicrobial activity and molecular docking. Bioorg. Med. Chem. Lett. 2015, 25, 4232–4238. [Google Scholar] [CrossRef] [PubMed]
- Arun, Y.; Saranraj, K.; Balachandran, C.; Perumal, P.T. Novel spirooxindole-pyrrolidine compounds: Synthesis, anticancer and molecular docking studies. Eur. J. Med. Chem. 2014, 74, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graphics Modell. 1999, 17, 57–61. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bnk. Available online: http://www.rcsb.org/pdb (accessed on 24 September 2019).
Sample Availability: Samples of the compounds are available from the authors. |



 | ||||||
|---|---|---|---|---|---|---|
| Entry | Base (1.2 mol equiv.) | Catalyst (mol%) | Solvent | Temp (°C) | Time (h) | 5a Yield (%) |
| 1 | DIPEA | CuI (10) | n-BuOH | 25–30 | 10 | 75 |
| 2 | DIPEA | CuBr (10) | n-BuOH | 25-30 | 10 | 15 |
| 3 | DIPEA | CuI (5) | n-BuOH | 25–30 | 10 | 46 |
| 4 | DIPEA | CuI (20) | n-BuOH | 25–30 | 10 | 85 |
| 5 | DIPEA | CuI (10) | H2O | 25–30 | 10 | 48 |
| 6 | DIPEA | CuI (10) | n-BuOH/H2O(1:1) | 25–30 | 10 | 80 |
| 7 | DIPEA | CuI (10) | EtOH | 25–30 | 10 | Trace |
| 8 | DIPEA | CuI (10) | EtOH | 45–50 | 20 | 15 |
| 9 | DIPEA | CuI (10) | EtOH/H2O(1:1) | 45–50 | 20 | 27 |
| 10 | DIPEA | CuI (10) | MeOH/THF/water(1:1:1) | 25–30 | 10 | 82 |
| 11 | DIPEA | 10% CuI/Al2O3 (5) | MeOH/THF/water(1:1:1) | 25–30 | 10 | 95 |
| 12 | DIPEA | 10% CuI/Al2O3 (10) | MeOH/THF/water(1:1:1) | 25–30 | 10 | 92 |
| 13 | DIPEA | 10% CuI/Al2O3 (5) | EtOH/THF/water(1:1:1) | 25 | 10 | 89 |
| 14 | TEA | 10% CuI/Al2O3 (10) | n-BuOH/water(1:1:1) | 25 | 10 | 65 |
| 15 | DIPEA | CuI (10) | THF | 25–30 | 20 | 60 |
| 16 | TEA | CuI (10) | n-BuOH | 25–30 | 10 | Trace |
| 17 | TEA | CuI (10) | THF | 25–30 | 10 | Trace |
| 18 | KOH | CuI (20) | n-BuOH | 25–30 | 10 | Trace |
| 19 | KOH | CuI (20) | n-BuOH | 50–55 | 20 | Trace |
| Entry | Terminal Alkyne | β-pyrrolidino-1,2,3-triazole | Yield% | Entry | Terminal Alkyne | β-pyrrolidino-1,2,3-triazole | Yield % | |
|---|---|---|---|---|---|---|---|---|
| 1 | 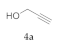 4a | 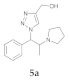 5a | 95 | 7 | 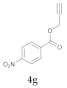 4g | 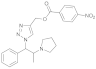 5g | 80 | |
| 2 | 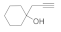 4b | 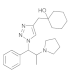 5b | 84 | 8 | 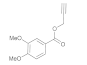 4h | 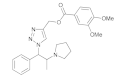 5h | 86 | |
| 3 |  4c |  5c | 88 | 9 | 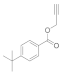 4i |  5i | 90 | |
| 4 |  4d |  5d | 96 | 10 | 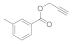 4j | 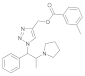 5j | 84 | |
| 5 | 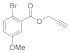 4e | 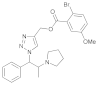 5e | 92 | 11 | 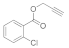 4k |  5k | 79 | |
| 6 | 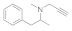 4f |  5f | 82 | 12 | 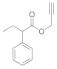 4l | 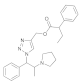 5l | 70 | |
| Organism | 5a | 5b | 5c | 5d | 5e | 5f | 5g | 5h | 5i | 5j | 5k | 5l | C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | |||||||||||||
| Enterobacter aerogenes | 14 | 12 | 14 | 10 | 10 | - | 22 | 17 | 14 | - | 22 | - | 22 |
| Staphylococcus aureus | 12 | 10 | 16 | 13 | 12 | - | 16 | 15 | 16 | 15 | 18 | - | 14 |
| Staphylococcus epidermidis | 14 | 10 | 15 | 13 | 10 | 17 | 28 | 16 | 11 | 14 | - | - | 26 |
| Staphylococcus aureus-MRSA | 13 | 12 | 18 | 16 | 10 | 15 | 24 | 20 | 14 | 14 | 17 | - | 30 |
| Salmonella paratyphi-B | 14 | 11 | 14 | 14 | 10 | 14 | 20 | 19 | 12 | 18 | 18 | - | 18 |
| Salmonella typhimurium | 14 | 11 | 12 | 12 | 12 | - | 22 | 22 | - | 20 | 15 | - | 24 |
| Proteus vulgaris | 17 | 12 | 10 | - | 13 | - | 26 | 23 | - | 20 | 16 | - | 30 |
| Micrococcus luteus | 28 | 13 | 16 | 19 | 15 | 13 | 17 | 14 | 16 | 16 | - | - | 26 |
| Klebsiella pneumoniae | 20 | 10 | 12 | - | 10 | 18 | 24 | 20 | 12 | 22 | 14 | - | 20 |
| Shigella flexneri | 22 | - | 13 | 13 | 15 | 19 | 25 | 18 | 14 | 22 | 17 | - | 30 |
| Fungi | C | ||||||||||||
| Candida albicans | 14 | - | - | 13 | 15 | - | 13 | - | 12 | 13 | - | - | 28 |
| Malassezia pachydermatis | 12 | - | - | 10 | 14 | 12 | - | - | 10 | 11 | - | 12 | 26 |
| Organism | 5a | 5c | 5d | 5f | 5g | 5h | 5i | 5j | 5k | C |
|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | ||||||||||
| Enterobacter aerogenes | 250 | 250 | 500 | - | 62.5 | 125 | 250 | - | 62.5 | 25 |
| Staphylococcus aureus | 500 | 125 | 250 | - | 125 | 250 | 125 | 250 | 125 | 6.25 |
| Staphylococcus epidermidis | 250 | 250 | 250 | 125 | 31.25 | 125 | 500 | 250 | - | 25 |
| Staphylococcus aureus-MRSA | 250 | 125 | 125 | 250 | 31.25 | 62.5 | 250 | 250 | 125 | 6.25 |
| Salmonella paratyphi-B | 250 | 250 | 250 | 250 | 62.5 | 62.5 | 500 | 125 | 125 | 30 |
| Salmonella typhimurium | 250 | 500 | 500 | - | 62.5 | 62.5 | - | 62.5 | 250 | 6.25 |
| Proteus vulgaris | 125 | 500 | - | - | 31.25 | 62.5 | - | 62.5 | 125 | 6.25 |
| Micrococcus luteus | 31.25 | 125 | 62.5 | 250 | 125 | 250 | 125 | 125 | - | 6.25 |
| Klebsiella pneumoniae | 62.5 | 500 | - | 125 | 31.25 | 62.5 | 500 | 62.5 | 250 | 25 |
| Shigella flexneri | 62.5 | 250 | 250 | 62.5 | 31.25 | 125 | 250 | 62.5 | 125 | 6.25 |
| Compound | A549 | HepG-2 | IMR90 |
|---|---|---|---|
| 5a | 190 ± 1.35 | 275 ± 1.52 | >250 |
| 5e | 130 ± 0.65 | 164 ± 2.01 | >250 |
| 5g | 72 ± 3.21 | >300 | >250 |
| 5h | 58 ± 2.31 | 73 ± 3.88 | >250 |
| 5i | 134 ± 1.05 | 197 ± 2.34 | >250 |
| 5j | 111 ± 1.82 | >300 | >250 |
| Cisplatin (µM) | 16.4 ± 3.19 | 22.1 ± 3.08 | NT |
| Compound | Binding Energy (kcal/mol) a | |
|---|---|---|
| DNA Topoisomerase IV (4EMV) | Anaplastic Lymphoma Kinase (2XP2) | |
| 5a | −7.31 | −6.55 |
| 5b | −8.64 | NC |
| 5c | −8.76 | NC |
| 5d | −8.84 | NC |
| 5e | −7.63 | −7.79 |
| 5f | −8.93 | NC |
| 5g | −8.06 | −8.12 |
| 5h | −7.24 | −7.10 |
| 5i | −8.98 | −8.28 |
| 5j | −7.63 | −8.72 |
| 5k | −8.75 | NC |
| 5l | −7.71 | NC |
| CL | −9.80 | −8.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Easwaramoorthi, K.; A. Rajendran, J.; Chennakesava Rao, K.; Balachandran, C.; Arun, Y.; Mahalingam, S.M.; Arumugam, N.; I. Almansour, A.; Suresh Kumar, R.; Al-thamili, D.M.; et al. A New Class of β–Pyrrolidino-1,2,3-Triazole Derivatives as β-Adrenergic Receptor Inhibitors: Synthesis, Pharmacological, and Docking Studies. Molecules 2019, 24, 3501. https://doi.org/10.3390/molecules24193501
Easwaramoorthi K, A. Rajendran J, Chennakesava Rao K, Balachandran C, Arun Y, Mahalingam SM, Arumugam N, I. Almansour A, Suresh Kumar R, Al-thamili DM, et al. A New Class of β–Pyrrolidino-1,2,3-Triazole Derivatives as β-Adrenergic Receptor Inhibitors: Synthesis, Pharmacological, and Docking Studies. Molecules. 2019; 24(19):3501. https://doi.org/10.3390/molecules24193501
Chicago/Turabian StyleEaswaramoorthi, Kaliyappan, Jeya A. Rajendran, Kella Chennakesava Rao, Chandrasekar Balachandran, Yuvaraj Arun, Sakkarapalayam M. Mahalingam, Natarajan Arumugam, Abdulrahman I. Almansour, Raju Suresh Kumar, Dhaifallah M. Al-thamili, and et al. 2019. "A New Class of β–Pyrrolidino-1,2,3-Triazole Derivatives as β-Adrenergic Receptor Inhibitors: Synthesis, Pharmacological, and Docking Studies" Molecules 24, no. 19: 3501. https://doi.org/10.3390/molecules24193501
APA StyleEaswaramoorthi, K., A. Rajendran, J., Chennakesava Rao, K., Balachandran, C., Arun, Y., Mahalingam, S. M., Arumugam, N., I. Almansour, A., Suresh Kumar, R., Al-thamili, D. M., & Aoki, S. (2019). A New Class of β–Pyrrolidino-1,2,3-Triazole Derivatives as β-Adrenergic Receptor Inhibitors: Synthesis, Pharmacological, and Docking Studies. Molecules, 24(19), 3501. https://doi.org/10.3390/molecules24193501