N-alkylimidazolium Salts Functionalized with p-Coumaric and Cinnamic Acid: A Study of Their Antimicrobial and Antibiofilm Effects
Abstract
1. Introduction
2. Results and Discussion
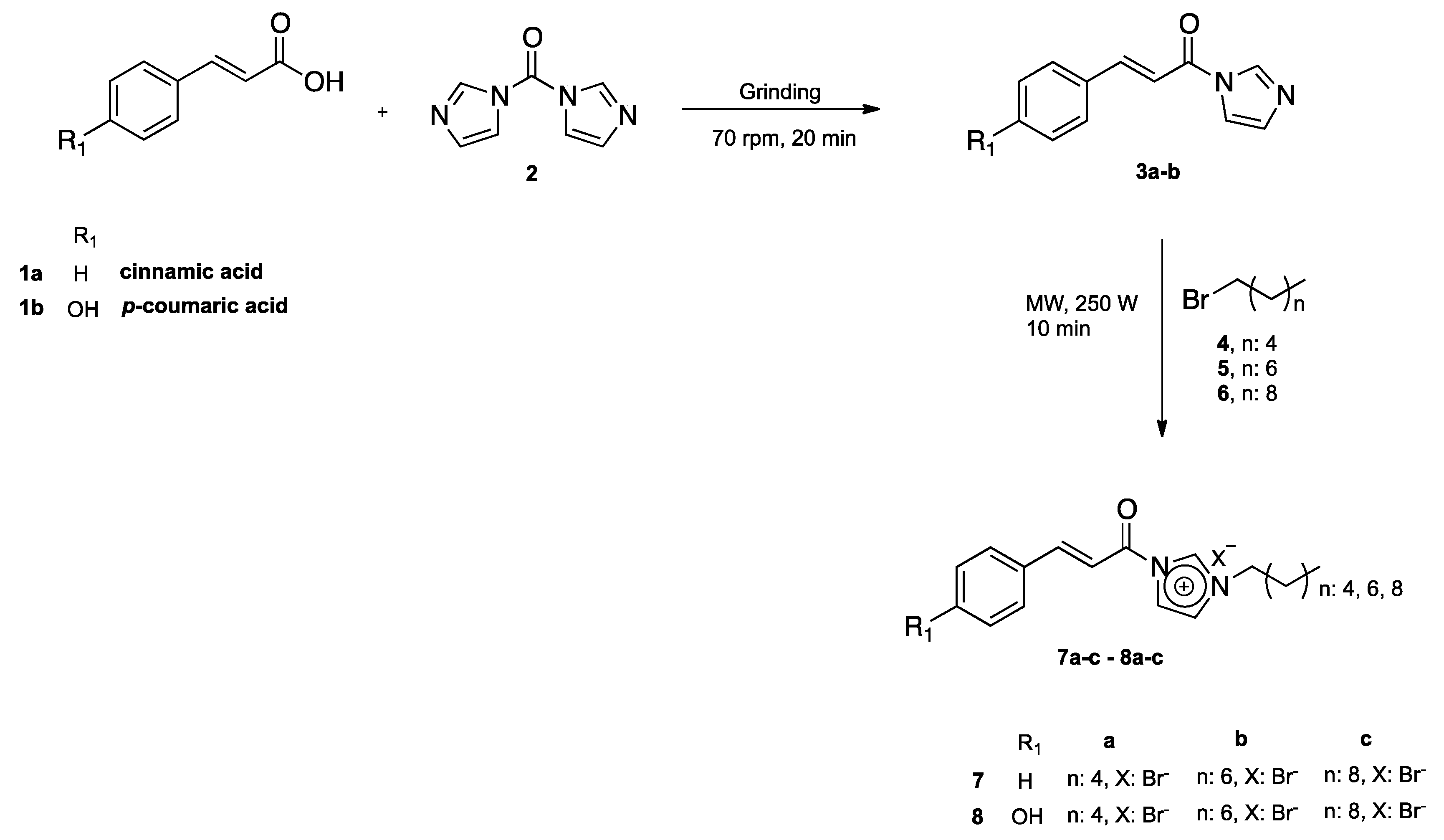
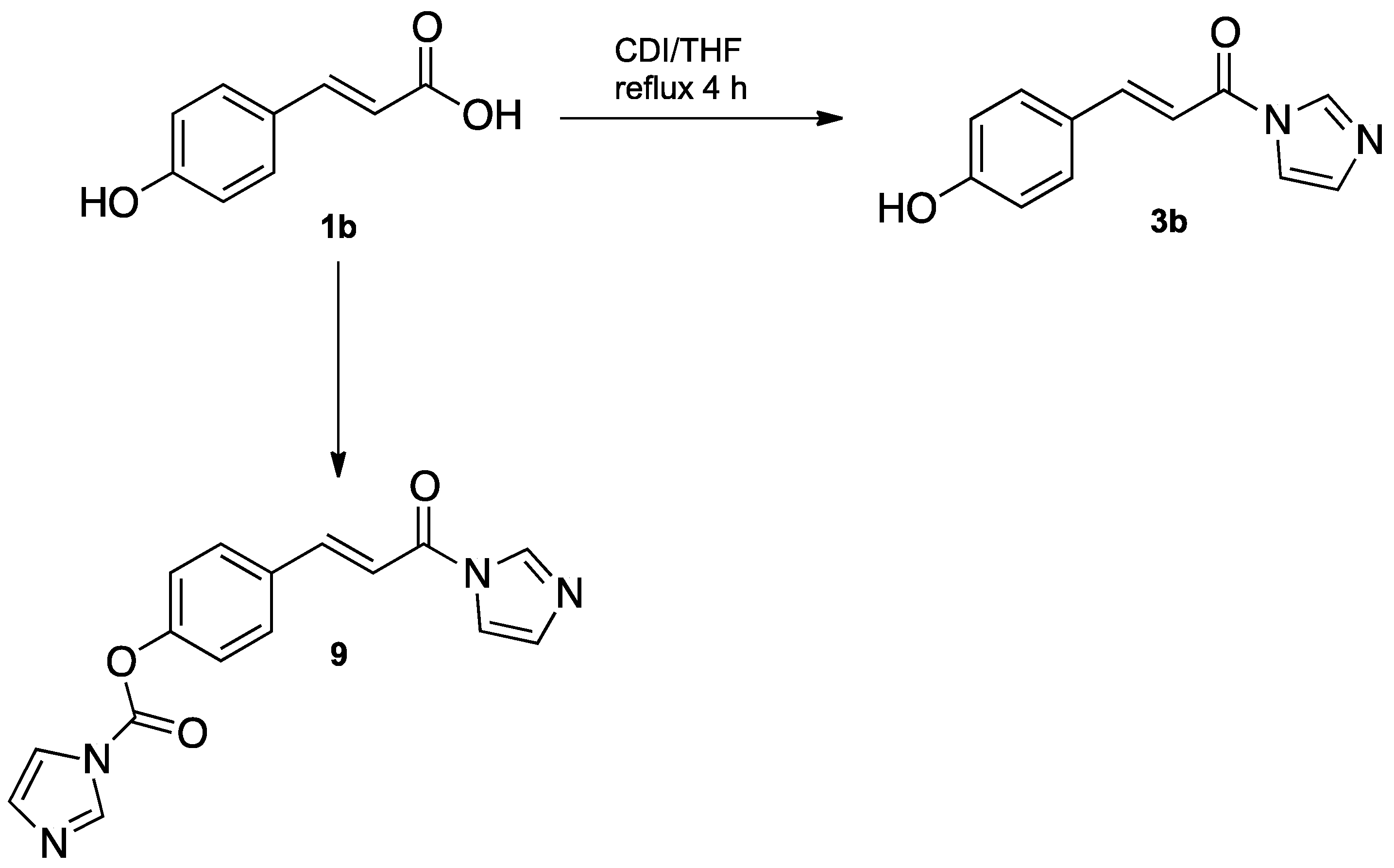
2.1. Chemistry
2.2. Determination of the Minimum Inhibitory Concentration (MIC) by Microdilution in a 96-Well Plate
2.3. Determination of Antibiofilm Activity in 96-well Plates
2.4. Molecular Dynamic (MD) Simulations Analysis
2.5. In Silico ADME Properties of N-alkylimidazolium Salts 7a–c and 8a–c
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure for N-cinnamoylimidazole Synthesis
3.2.2. General Procedure for the Synthesis of Different N-cinnamoylimidazolium Salts
3.3. Bacterial Strains
3.4. Determination of the Minimum Inhibitory Concentration (MIC) by Microdilution in a 96-Well Plate
3.5. Determination of Antibiofilm Activity in 96-Well Plates
3.6. Molecular Dynamic (MD) Simulations Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Messali, M.; Moussa, Z.; Alzahrani, A.Y.; El-Naggar, M.Y.; El Douhaibi, A.S.; Judeh, Z.M.A.; Hammouti, B. Synthesis, characterization and the antimicrobial activity of new eco-friendly ionic liquids. Chemosphere 2013, 91, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Gai, Q.-Y.; Fu, Y.-J.; Zu, Y.-G.; Luo, M.; Zhao, C.-J.; Li, C.-Y. Microwave-assisted ionic liquids treatment followed by hydro-distillation for the efficient isolation of essential oil from Fructus forsythiae seed. Sep. Purif. Technol. 2013, 107, 228–237. [Google Scholar] [CrossRef]
- Romero, A. Líquidos Iónicos a Temperatura Ambiente: Un Nuevo Medio para las Reacciones Químicas. Rev. R. Acad. Cienc. Exact. Fís. Nat. 2008, 102, 79–90. [Google Scholar]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Silva Pereira, C. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.V.; Kumar, V. A profile of the in vitro anti-tumor activity of imidazolium-based ionic liquids. Bioorg. Med. Chem. Lett. 2010, 20, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Blesic, M.; Marques, M.H.; Plechkova, N.V.; Seddon, K.R.; Rebelo, L.P.N.; Lopes, A. Self-aggregation of ionic liquids: Micelle formation in aqueous solution. Green Chem. 2007, 9, 481–490. [Google Scholar] [CrossRef]
- Pernak, J.; Skrzypczak, A.; Lota, G.; Frackowiak, E. Synthesis and Properties of Trigeminal Tricationic Ionic Liquids. Chem. Eur. J. 2007, 13, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, J.; Jungnickel, C.; Łącka, I.; Stolte, S.; Hupka, J. Antimicrobial and surface activity of 1-alkyl-3-methylimidazolium derivatives. Green Chem. 2010, 12, 593–601. [Google Scholar] [CrossRef]
- Vraneš, M.; Tot, A.; Ćosić, J.; Papović, S.; Panić, J.; Gadžurić, S.; Janković, N.; Vrandečić, K. Correlation between lipophilicity of newly synthesized ionic liquids and selected Fusarium genus growth rate. RSC Adv. 2019, 9, 19189–19196. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gilmore, B.F. The antimicrobial potential of ionic liquids: A source of chemical diversity for infection and biofilm control. Int. J. Antimicrob. Agents 2015, 46, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.S. Skin and soft tissue infection: Microbiology and epidemiology. Int. J. Antimicrob. Agents 2009, 34 Suppl 1, S2–S7. [Google Scholar] [CrossRef]
- Barie, P.S.; Wilson, S.E. Impact of Evolving Epidemiology on Treatments for Complicated Skin and Skin Structure Infections: The Surgical Perspective. J. Am. Coll. Surgeons 2015, 220, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Nazar, J. Biofilms bacterianos. J. Nazar. 2007, 67, 161–172. [Google Scholar] [CrossRef]
- Sharma, P. Cinnamic acid derivatives: A new chapter of various pharmacological activities. J. Chem. Pharm. Res. 2011, 3, 403–423. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Ferulic and p-coumaric acids extraction by alkaline hydrolysis of brewer’s spent grain. Ind. Crop. Prod. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Huang, S.-T.; Sun, F.-M.; Chiang, Y.-L.; Chiang, C.-J.; Tsai, C.-M.; Weng, C.-J. Transformation of cinnamic acid from trans- to cis-form raises a notable bactericidal and synergistic activity against multiple-drug resistant Mycobacterium tuberculosis. Eur. J. Pharm. Sci. 2011, 43, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Turner, L.B.; Mueller-Harvey, I.; McAllan, A.B. Light-induced isomerization and dimerization of cinnamic acid derivatives in cell walls. Phytochemistry 1993, 33, 791–796. [Google Scholar] [CrossRef]
- Patel, V.; Ray, D.; Aswal, V.K.; Bahadur, P. Triton X-100 micelles modulated by solubilized cinnamic acid analogues: The pH dependant micellar growth. Colloid Surface A 2014, 450, 106–114. [Google Scholar] [CrossRef]
- Cappelli, A.; Grisci, G.; Paolino, M.; Giuliani, G.; Donati, A.; Mendichi, R.; Artusi, R.; Demiranda, M.; Zanardi, A.; Giorgi, G. Hyaluronan derivatives bearing variable densities of ferulic acid residues. J. Mat. Chem. B 2014, 2, 4489–4499. [Google Scholar] [CrossRef]
- Verma, S.K.; Ghorpade, R.; Pratap, A.; Kaushik, M.P. Solvent free, N,N′-carbonyldiimidazole (CDI) mediated amidation. Tetrahedron Lett. 2012, 53, 2373–2376. [Google Scholar] [CrossRef]
- Patil, P.R.; Ravindranathan Kartha, K.P. Application of Ball Milling Technology to Carbohydrate Reactions: I. Regioselective Primary Hydroxyl Protection of Hexosides and Nucleoside by Planetary Ball Milling. J. Carbohyd. Chem. 2008, 27, 279–293. [Google Scholar] [CrossRef]
- Forero Doria, O.; Castro, R.; Gutierrez, M.; Gonzalez Valenzuela, D.; Santos, L.; Ramirez, D.; Guzman, L. Novel Alkylimidazolium Ionic Liquids as an Antibacterial Alternative to Pathogens of the Skin and Soft Tissue Infections. Molecules 2018, 23, 2354. [Google Scholar] [CrossRef]
- Raya-Cruz, M.; Ferullo, I.; Arrizabalaga-Asenjo, M.; Nadal-Nadal, A.; Díaz-Antolín, M.P.; Garau-Colom, M.; Payeras-Cifre, A. Infecciones de piel y partes blandas en pacientes hospitalizados: Factores epidemiológicos, microbiológicos, clínicos y pronósticos. Enfermedades Infecciosas y Microbiología Clínica 2014, 32, 152–159. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Docherty, K.M.; Kulpa, J.C.F. Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem. 2005, 7, 185–189. [Google Scholar] [CrossRef]
- Wei, Q.-Y.; Jiang, H.; Zhang, J.-X.; Zhang, C.; Guo, P.-F. Antimicrobial Activities of the Cinnamoyl Amide of Amino Acid Derivatives. Asian J. Chem. 2012, 24, 2383–2388. [Google Scholar]
- Santos, A.G.; Ribeiro, B.D.; Alviano, D.S.; Coelho, M.A.Z. Toxicity of ionic liquids toward microorganisms interesting to the food industry. RSC Adv. 2014, 4, 37157–37163. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Perez, L.; Manresa, A.; Comelles, F. Self-assembly and antimicrobial activity of long-chain amide-functionalized ionic liquids in aqueous solution. Colloid. Surface. B 2014, 123, 318–325. [Google Scholar] [CrossRef]
- Venkata, Y.; Reddy, G.K.; Lalithamanasa, P.; Venugopalan, V.P. The ionic liquid 1-alkyl-3-methylimidazolium demonstrates comparable antimicrobial and antibiofilm behavior to a cationic surfactant. Biofouling 2012, 28, 1141–1149. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Rodriguez, J.C.; Álvarez, L.; Artacho, A.; Royo, G.; Mira, A. Effect of antibiotics on biofilm inhibition and induction measured by real-time cell analysis. J. Appl. Microbiol. 2017, 122, 640–650. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| S. aureus | S. epidermidis | A. baumannii | P. aeruginosa | |
|---|---|---|---|---|
| μM | ||||
| 3a | >2000 | >2000 | >2000 | >2000 |
| 7a | 2000 | 2000 | 2000 | >2000 |
| 7b | 250 | 125 | 250 | >2000 |
| 7c | 62.5 | 31.25 | 250 | 2000 |
| 3b | >2000 | >2000 | >2000 | >2000 |
| 8a | 2000 | 1000 | 2000 | >2000 |
| 8b | 62.5 | 31.25 | 1000 | 2000 |
| 8c | 31.25 | <15.625 | 500 | 1000 |
| S. aureus | S. epidermidis | A. baumannii | ||||
|---|---|---|---|---|---|---|
| MIC × 2 | MIC × 1/2 | MIC × 2 | MIC × 1/2 | MIC × 2 | MIC × 1/2 | |
| 7b | 52 ± 4% ** | 20 ± 6% | 28 ± 5% | 3 ± 2% *** | 34 ± 4% * | 1 ± 0.8% * |
| 8b | 34 ± 3% | 33 ± 7% | 35 ± 6% | 33 ± 6% | 20 ± 5% | 20 ± 4% |
| Comp. | MW a | MlogP b | nHBA c | nHBD d | nRB e | TPSA (Å2) f |
|---|---|---|---|---|---|---|
| 3a | 198.22 | 1.70 | 2 | 0 | 3 | 34.89 |
| 7a | 363.29 | 3.57 | 1 | 0 | 8 | 25.88 |
| 7b | 391.35 | 4.02 | 1 | 0 | 10 | 25.88 |
| 7c | 419.40 | 4.45 | 1 | 0 | 12 | 25.88 |
| 3b | 214.22 | 1.12 | 3 | 1 | 3 | 55.12 |
| 8a | 379.29 | 2.99 | 2 | 1 | 8 | 46.11 |
| 8b | 407.34 | 3.44 | 2 | 1 | 10 | 46.11 |
| 8c | 435.40 | 3.87 | 2 | 1 | 12 | 46.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forero-Doria, O.; Araya-Maturana, R.; Barrientos-Retamal, A.; Morales-Quintana, L.; Guzmán, L. N-alkylimidazolium Salts Functionalized with p-Coumaric and Cinnamic Acid: A Study of Their Antimicrobial and Antibiofilm Effects. Molecules 2019, 24, 3484. https://doi.org/10.3390/molecules24193484
Forero-Doria O, Araya-Maturana R, Barrientos-Retamal A, Morales-Quintana L, Guzmán L. N-alkylimidazolium Salts Functionalized with p-Coumaric and Cinnamic Acid: A Study of Their Antimicrobial and Antibiofilm Effects. Molecules. 2019; 24(19):3484. https://doi.org/10.3390/molecules24193484
Chicago/Turabian StyleForero-Doria, Oscar, Ramiro Araya-Maturana, Anggela Barrientos-Retamal, Luis Morales-Quintana, and Luis Guzmán. 2019. "N-alkylimidazolium Salts Functionalized with p-Coumaric and Cinnamic Acid: A Study of Their Antimicrobial and Antibiofilm Effects" Molecules 24, no. 19: 3484. https://doi.org/10.3390/molecules24193484
APA StyleForero-Doria, O., Araya-Maturana, R., Barrientos-Retamal, A., Morales-Quintana, L., & Guzmán, L. (2019). N-alkylimidazolium Salts Functionalized with p-Coumaric and Cinnamic Acid: A Study of Their Antimicrobial and Antibiofilm Effects. Molecules, 24(19), 3484. https://doi.org/10.3390/molecules24193484







