The Influence of Zeolite (Sokyrnytsya Deposit) on the Physical and Chemical Resistance of a Magnesium Potassium Phosphate Compound for the Immobilization of High-Level Waste
Abstract
1. Introduction
2. Results and Discussion
2.1. Mineral and Chemical Composition of Zeolite
2.2. Mechanical and Thermal Stability of the Magnesium Potassium Phosphate Compound
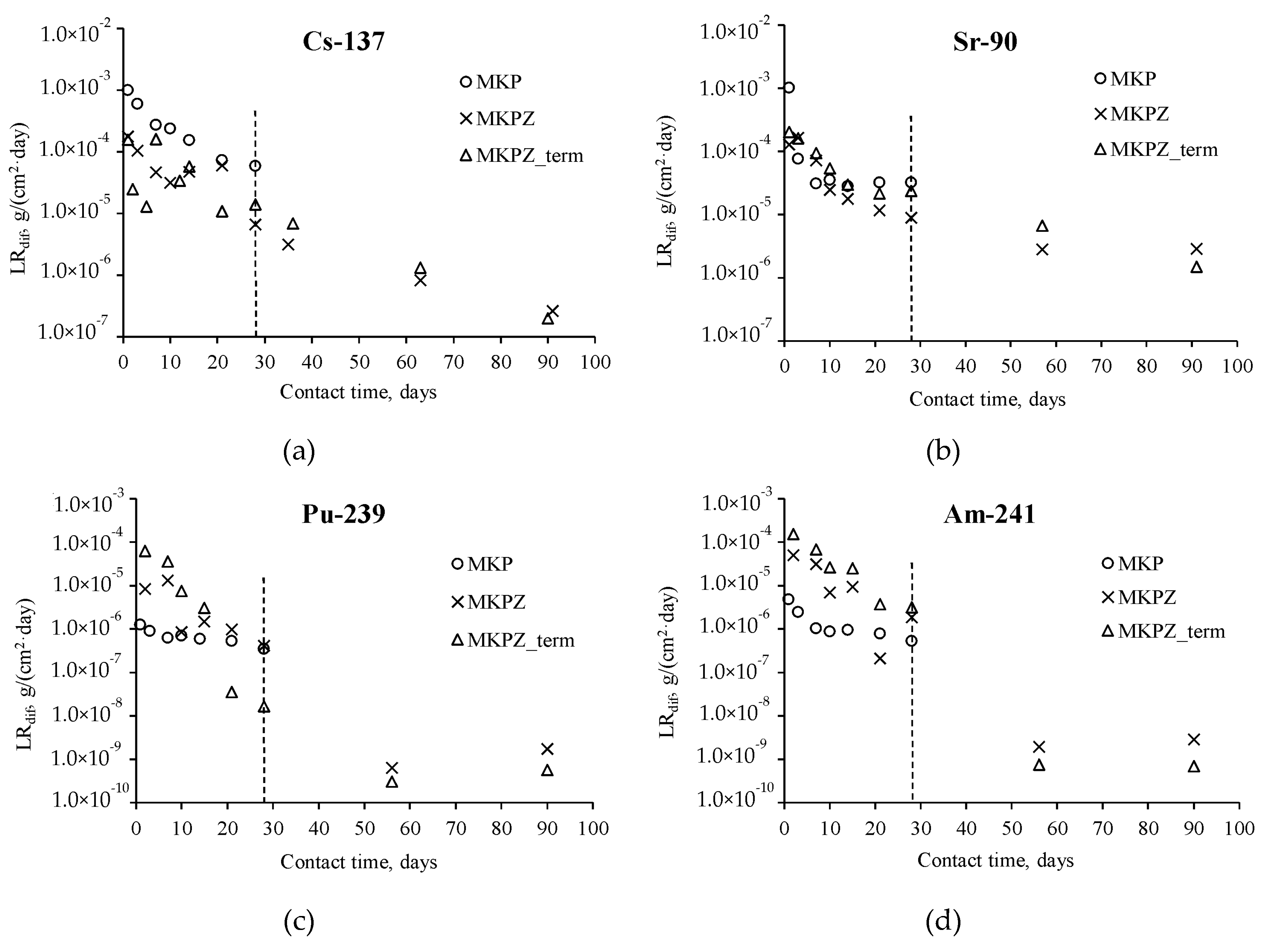
2.3. Hydrolytic Stability of the Magnesium Potassium Phosphate Compound
3. Materials and Methods
3.1. Chemicals and Procedures
3.2. Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ojovan, M.I.; Lee, W.E.; Kalmykov, S.N. An Introduction to Nuclear Waste Immobilisation, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–512. ISBN 978-0-08-102702-8. [Google Scholar] [CrossRef]
- Stefanovsky, S.V.; Yudintsev, S.V.; Vinokurov, S.E.; Myasoedov, B.F. Chemical-technological and mineralogical-geochemical aspects of the radioactive waste management. Geochem. Int. 2016, 54, 1136–1156. [Google Scholar] [CrossRef]
- Graeser, S.; Postl, W.; Bojar, H.-P.; Berlepsch, P.; Armbruster, T.; Raber, T.; Ettinger, K.; Walter, F. Struvite-(K), KMgPO4·6H2O, the potassium equivalent of struvite—A new mineral. Eur. J. Miner. 2008, 20, 629–633. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics: Twenty-First Century Materials with Diverse Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–422. ISBN 978-0-08-100380-0. [Google Scholar]
- Singh, D.; Mandalika, V.R.; Parulekar, S.J.; Wagh, A.S. Magnesium potassium phosphate ceramic for 99Tc immobilization. J. Nucl. Mater. 2006, 348, 272–282. [Google Scholar] [CrossRef]
- Wagh, A.S.; Strain, R.; Jeong, S.Y.; Reed, D.; Kraus, T.; Singh, D. Stabilization of Rocky Flats Pu-contaminated ash within chemically bonded phosphate ceramics. J. Nucl. Mater. 1999, 265, 295–307. [Google Scholar] [CrossRef]
- Zhenyu, L.; Hongtao, W.; Yang, H.; Tao, Y.; Zhongyuan, L.; Shuzhen, L.; Haibin, Z. Rapid solidification of Highly Loaded High-Level Liquid Wastes with magnesium phosphate cement. Ceram. Int. 2019, 45, 5050–5057. [Google Scholar] [CrossRef]
- Shkuropatenko, V.A. High level wastes immobilization in ceramic and hydrated phosphate matrix. East Eur. J. Phys. 2016, 3, 49–60. [Google Scholar]
- Vinokurov, S.E.; Kulyako, Y.M.; Slyunchev, O.M.; Rovny, S.I.; Myasoedov, B.F. Low-temperature immobilization of actinides and other components of high-level waste in magnesium potassium phosphate matrices. J. Nucl. Mater. 2009, 385, 189–192. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulyako, Y.M.; Slyunchev, O.M.; Rovnyi, S.I.; Wagh, A.S.; Maloney, M.D.; Myasoedov, B.F. Magnesium potassium phosphate matrices for immobilization of high-level liquid wastes. Radiochemistry 2009, 51, 65–72. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Magnesium Potassium Phosphate Compound for Immobilization of Radioactive Waste Containing Actinide and Rare Earth Elements. Materials 2018, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Solidification of high level waste using magnesium potassium phosphate compound. Nucl. Eng. Technol. 2019, 51, 755–760. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Hydrolytic and thermal stability of magnesium potassium phosphate compound for immobilization of high level waste. J. Radioanal. Nucl. Chem. 2018, 318, 2401–2405. [Google Scholar] [CrossRef]
- Federal Norms and Rules in the Field of Atomic Energy Use “Collection, Processing, Storage and Conditioning of Liquid Radioactive Waste. Safety Requirements” (NP-019-15). Rostekhnadzor, Russia, 2015; 1–22.
- Sayenko, S.Y.; Shkuropatenko, V.A.; Dikiy, N.P.; Tarasov, R.V.; Ulybkina, K.A.; Surkov, O.Y.; Litvinenko, L.M. Clinoptilolite with cesium immobilization to potassium magnesium phosphate matrix. East Eur. J. Phys. 2017, 4, 37–43. [Google Scholar] [CrossRef][Green Version]
- Kuenzel, C.; Cisneros, J.F.; Neville, T.P.; Vandeperre, L.J.; Simons, S.J.R.; Bensted, J.; Cheeseman, C.R. Encapsulation of Cs/Sr contaminated clinoptilolite in geopolymers produced from metakaolin. J. Nucl. Mater. 2015, 466, 94–99. [Google Scholar] [CrossRef]
- Munthali, M.W.; Johan, E.; Aono, H.; Matsue, N. Cs+ and Sr2+ adsorption selectivity of zeolites in relation to radioactive decontamination. J. Asian Ceram. Soc. 2015, 3, 245–250. [Google Scholar] [CrossRef]
- Borai, E.H.; Harjula, R.; Malinen, L.; Paajanen, A. Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J. Hazard. Mater. 2009, 172, 416–422. [Google Scholar] [CrossRef]
- Plecas, I.; Dimovic, S.; Smiciklas, I. Influence of bentonite and zeolite in cementation of dry radioactive evaporator concentrates. Appl. Clay Sci. 2009, 43, 9–12. [Google Scholar] [CrossRef]
- Tyupina, E.A.; Sazonov, A.B.; Sergeecheva, Y.V.; Shestakov, I.A.; Tuchkova, A.I.; Nikitin, A.V. Application of thermally expanded graphite for the cementation of cesium and tritium containing waste oils. Inorg. Mater. Appl. Res. 2016, 7, 196–203. [Google Scholar] [CrossRef]
- Woszuk, A.; Franus, W. A review of the application of zeolite materials in Warm Mix Asphalt technologies. Appl. Sci. 2019, 7, 293. [Google Scholar] [CrossRef]
- Misaelides, P. Clay minerals and zeolites for radioactive waste immobilization and containment: A concise overview. In Modified Clay and Zeolite Nanocomposite Materials; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–274. ISBN 978-0-12-814617-0. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Z.; Wu, D.; Peng, X.; Xu, Y.; Li, N.; Qi, Y.; Li, P. Immobilization of strontium-loaded zeolite A by metakaolin based-geopolymer. Ceram. Int. in press. [CrossRef]
- Suna, Q.; Li, J.; Wanga, J. Solidification of borate radioactive resins using sulfoaluminate cement blending with zeolite. Nucl. Eng. Des. 2011, 241, 5308–5315. [Google Scholar] [CrossRef]
- Juoi, J.M.; Ojovan, M.I.; Lee, W.E. Microstructure and leaching durability of glass composite wasteforms for spent clinoptilolite immobilization. J. Nucl. Mater. 2008, 372, 358–366. [Google Scholar] [CrossRef]
- Kimura, R.; Inagaki, Y.; Idemitsu, K.; Arima, T. Vitrification processes of simulated cesium sorbing zeolite waste. Prog. Nucl. Energy 2018, 108, 497–502. [Google Scholar] [CrossRef]
- Woszuk, A.; Franus, W. Properties of the Warm Mix Asphalt involving clinoptilolite and Na-P1 zeolite additives. Constr. Build. Mater. 2016, 114, 556–563. [Google Scholar] [CrossRef]
- Markin, T.; Sobol, K.; Franus, M.; Franus, W. Mechanical and durability properties of concretes incorporating natural zeolite. Arch. Civ. Mech. Eng. 2016, 16, 554–562. [Google Scholar] [CrossRef]
- Mola-Abasi, H.; Shooshpasha, I. Influence of zeolite and cement additions on mechanical behavior of sandy soil. J. Rock Mech. Geotech. Eng. 2016, 8, 746–752. [Google Scholar] [CrossRef]
- GOST R 52126-2003. Radioactive Waste. Long Time Leach Testing of Solidified Radioactive Waste Forms; Gosstandart: Moscow, Russia, 2003; pp. 1–8. [Google Scholar]
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Danilov, S.S.; Gromyak, I.N.; Myasoedov, B.F. Investigation of the leaching behavior of components of the magnesium potassium phosphate matrix after high salt radioactive waste immobilization. J. Radioanal. Nucl. Chem. 2018, 315, 481–486. [Google Scholar] [CrossRef]
- Post, J.E.; Bish, D.L. Rietveld refinement of crystal structures using powder X-ray diffraction data. In Modern Powder Diffraction; Bish, D.L., Post, J.E., Eds.; De Gruyter: Berlin, Germany, 1989; pp. 277–308. ISBN 978-1-5015-0901-8. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- GOST 310.4-81. Cements. Methods of Bending and Compression Strength Determination; INTERSTATE STANDARD, Gosstroy 151: Moscow, Russia, 1981; pp. 1–11. [Google Scholar]
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Myasoedov, B.F. Magnesium Potassium Phosphate Compound for Radioactive Waste Immobilization: Phase Composition, Structure, and Physicochemical and Hydrolytic Durability. Radiochemistry 2018, 60, 70–78. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
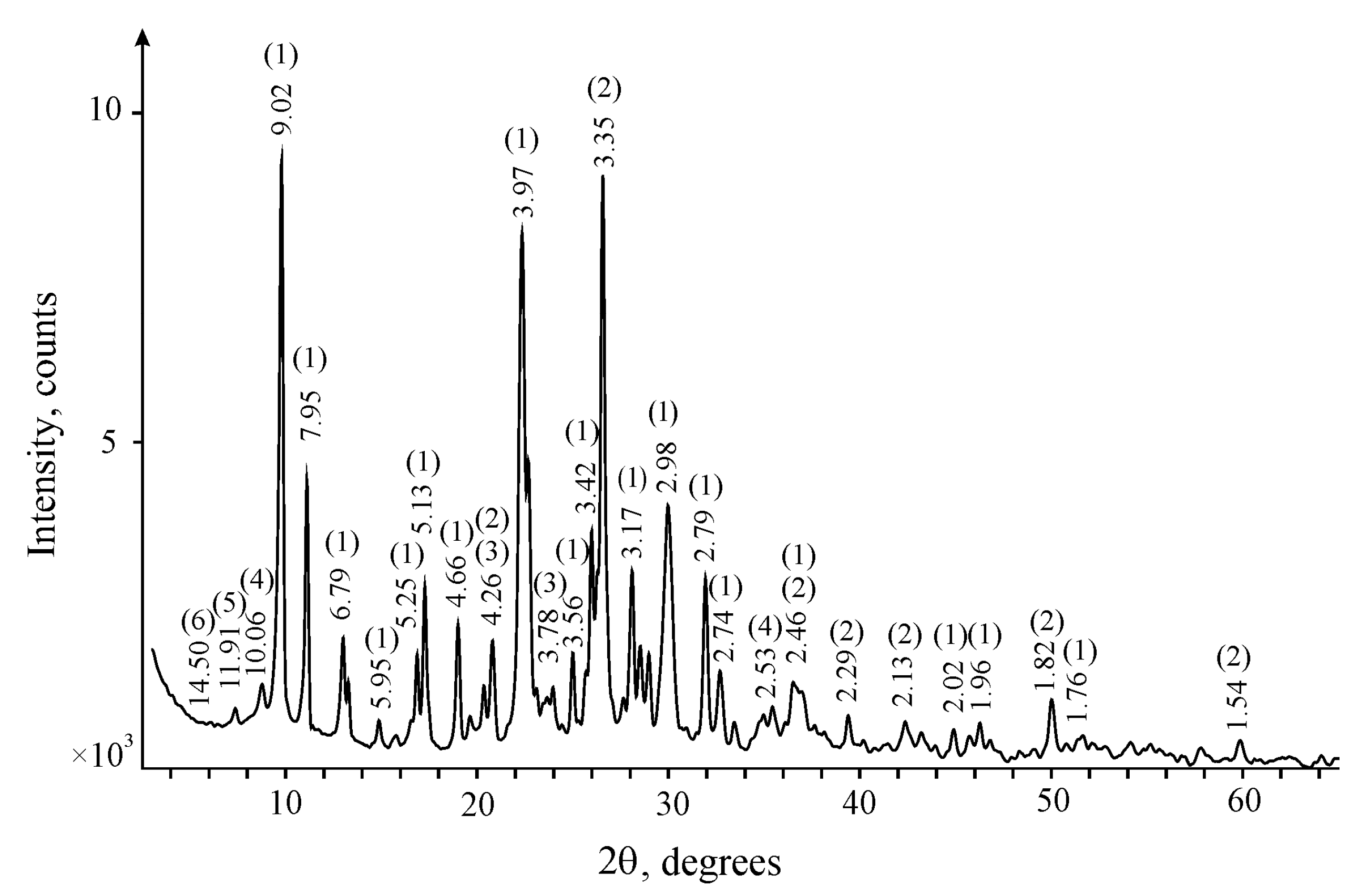
| Compound Formula | Concentration (%) | Compound Formula | Concentration (%) |
|---|---|---|---|
| SiO2 | 78.165 | SrO | 0.053 |
| Al2O3 | 11.530 | MnO | 0.050 |
| Na2O | 3.012 | P2O5 | 0.039 |
| K2O | 2.232 | Rb2O | 0.022 |
| MgO | 2.054 | BaO | 0.015 |
| CaO | 1.622 | ZrO2 | 0.009 |
| Fe2O3 | 0.984 | Y2O3 | 0.001 |
| TiO2 | 0.212 | Total | 100.000 |
| Zeolite Content (wt %) | Compressive Strength (MPa) |
|---|---|
| 0 | 12.0 ± 3.0 |
| 4.2 | 23.8 ± 1.7 |
| 16.7 | 26.6 ± 4.0 |
| 23.0 | 22.3 ± 2.0 |
| 28.6 | 25.6 ± 3.4 |
| Radionuclide | Specific Activity (Bq/g) |
|---|---|
| 137Cs | 1.7 × 104 |
| 90Sr | 1.5 × 104 |
| 239Pu | 6.8 × 104 |
| 241Am | 9.3 × 103 |
| Radionuclide | Compound | LRint (g/(cm2∙day)) | E (%) |
|---|---|---|---|
| 137Cs | MKPZ | 1.5 × 10−5 | 0.22 |
| MKPZ_term | 1.2 × 10−5 | 0.22 | |
| 90Sr | MKPZ | 1.3 × 10−5 | 0.19 |
| MKPZ_term | 1.9 × 10−5 | 0.34 | |
| 239Pu | MKPZ | 1.1 × 10−6 | 0.02 |
| MKPZ_term | 3.8 × 10−6 | 0.07 | |
| 241Am | MKPZ | 3.8 × 10−6 | 0.05 |
| MKPZ_term | 1.0 × 10−5 | 0.18 |
| Specific Activity of Actinides (Bq/L) | Metal Content (g/L) |
|---|---|
| 239Pu: 2.8 × 108 241Am: 3.8 × 107 137Cs: 7.1 × 107 90Sr: 5.8 × 107 | Na – 83.9; Sr – 3.0; Zr – 5.6; Mo – 0.8; Pd – 4.1; Cs – 7.4; Ba – 1.2; Nd – 28.2; Fe – 0.8; Cr – 2.3; Ni – 0.4; U – 2.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, S.A.; Vinokurov, S.E. The Influence of Zeolite (Sokyrnytsya Deposit) on the Physical and Chemical Resistance of a Magnesium Potassium Phosphate Compound for the Immobilization of High-Level Waste. Molecules 2019, 24, 3421. https://doi.org/10.3390/molecules24193421
Kulikova SA, Vinokurov SE. The Influence of Zeolite (Sokyrnytsya Deposit) on the Physical and Chemical Resistance of a Magnesium Potassium Phosphate Compound for the Immobilization of High-Level Waste. Molecules. 2019; 24(19):3421. https://doi.org/10.3390/molecules24193421
Chicago/Turabian StyleKulikova, Svetlana A., and Sergey E. Vinokurov. 2019. "The Influence of Zeolite (Sokyrnytsya Deposit) on the Physical and Chemical Resistance of a Magnesium Potassium Phosphate Compound for the Immobilization of High-Level Waste" Molecules 24, no. 19: 3421. https://doi.org/10.3390/molecules24193421
APA StyleKulikova, S. A., & Vinokurov, S. E. (2019). The Influence of Zeolite (Sokyrnytsya Deposit) on the Physical and Chemical Resistance of a Magnesium Potassium Phosphate Compound for the Immobilization of High-Level Waste. Molecules, 24(19), 3421. https://doi.org/10.3390/molecules24193421






