The Relationship between the Structure and Properties of Amino Acid Ionic Liquids
Abstract
1. Introduction
2. Results and Discussion
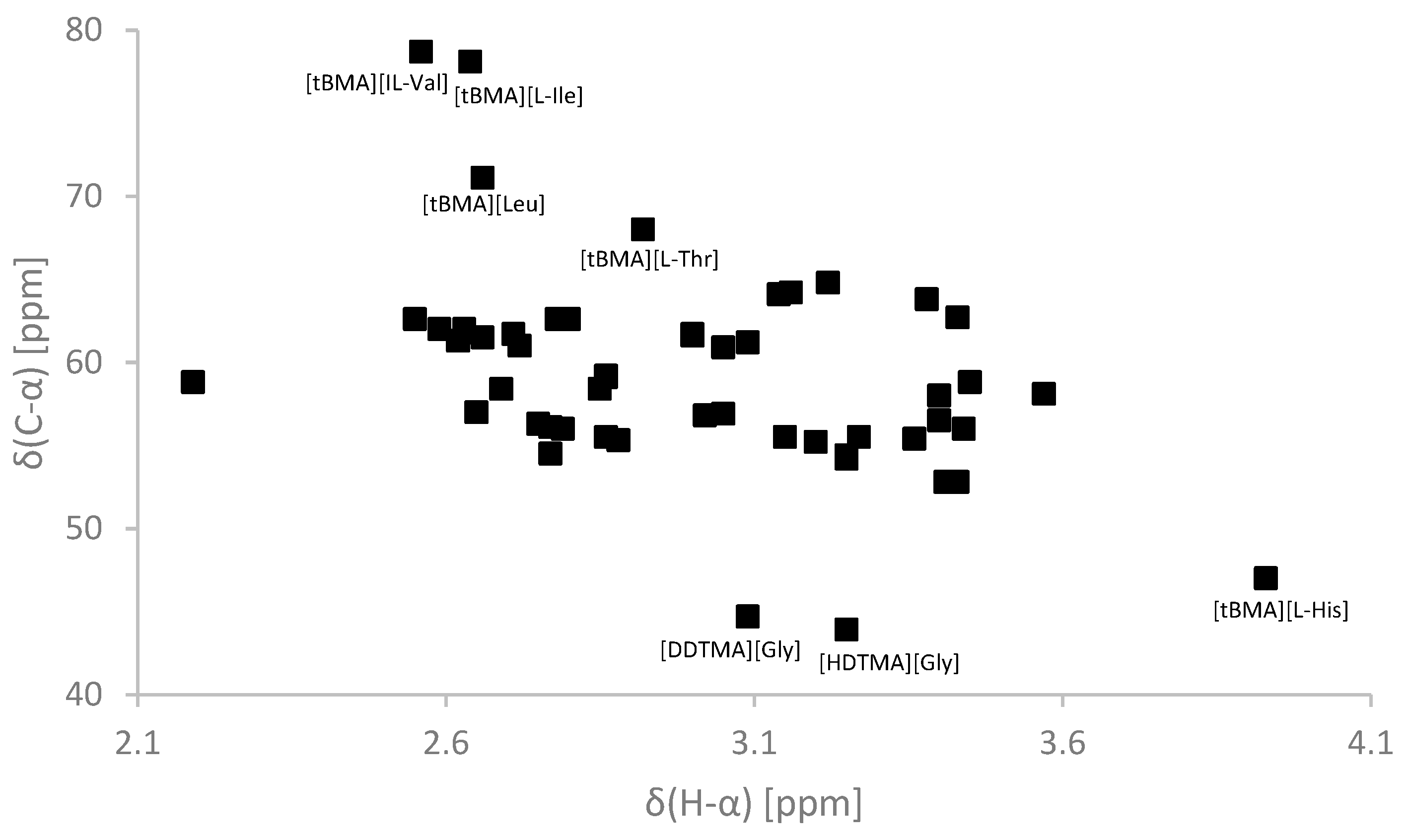
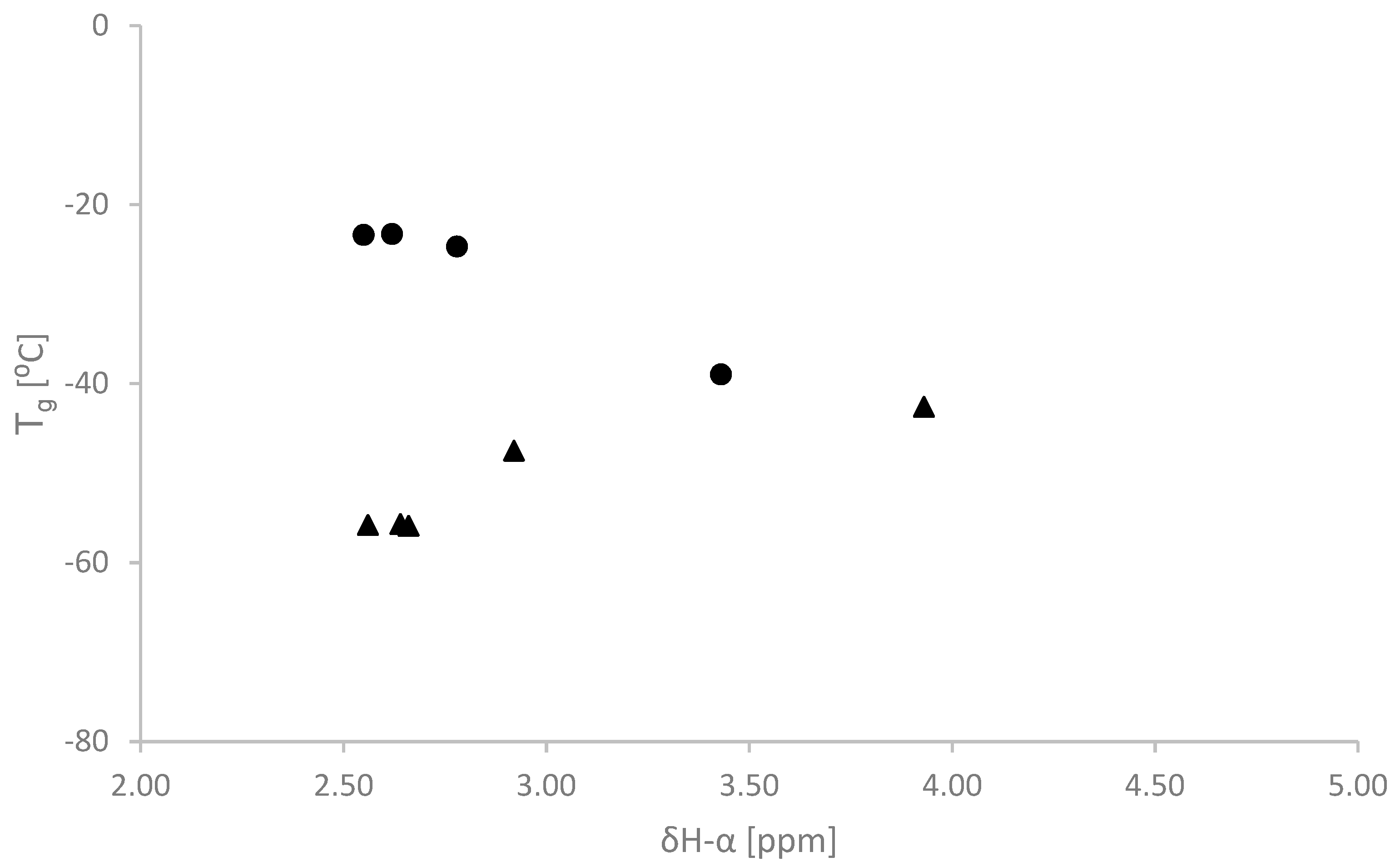
2.1. Spectroscopic Properties
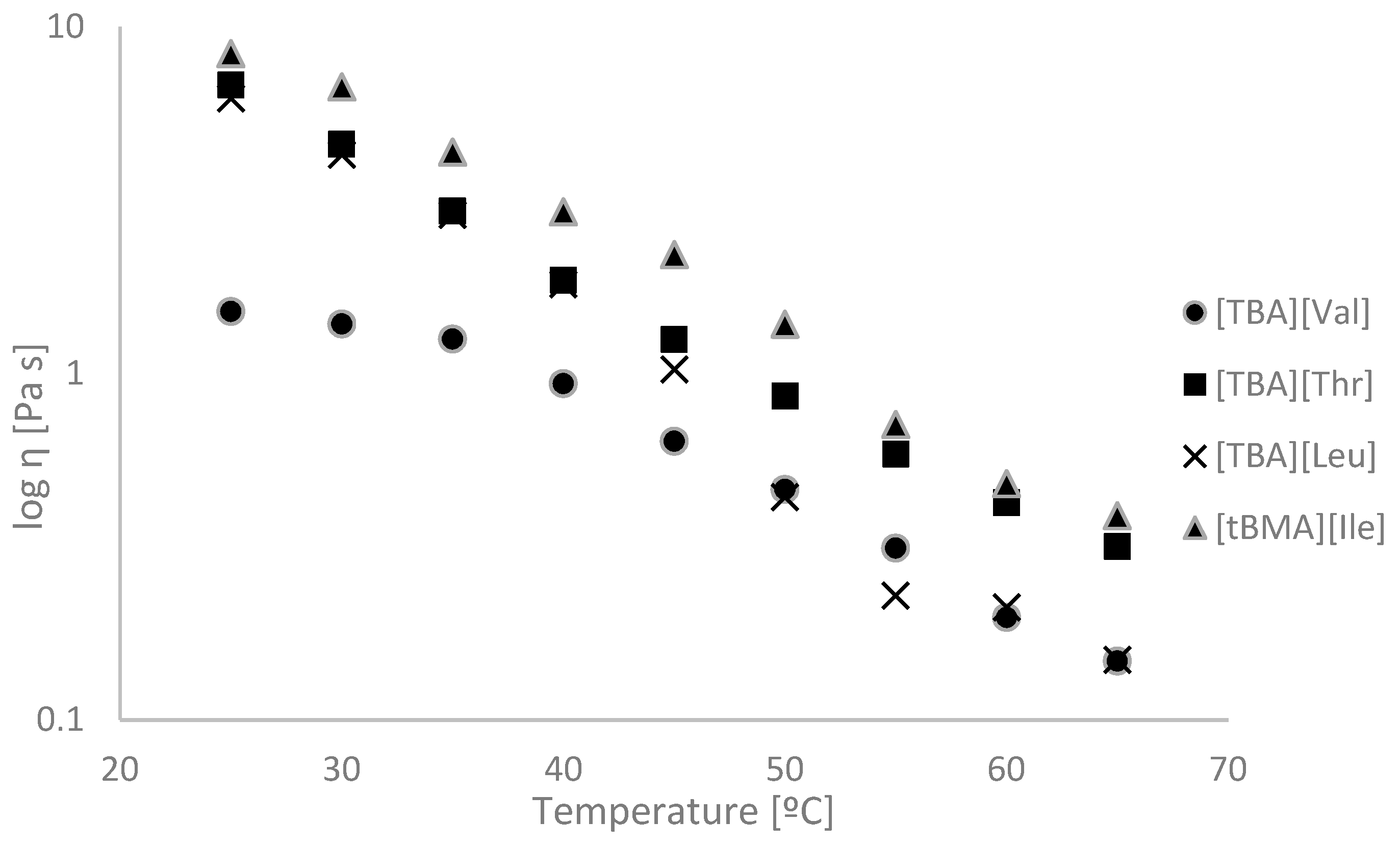
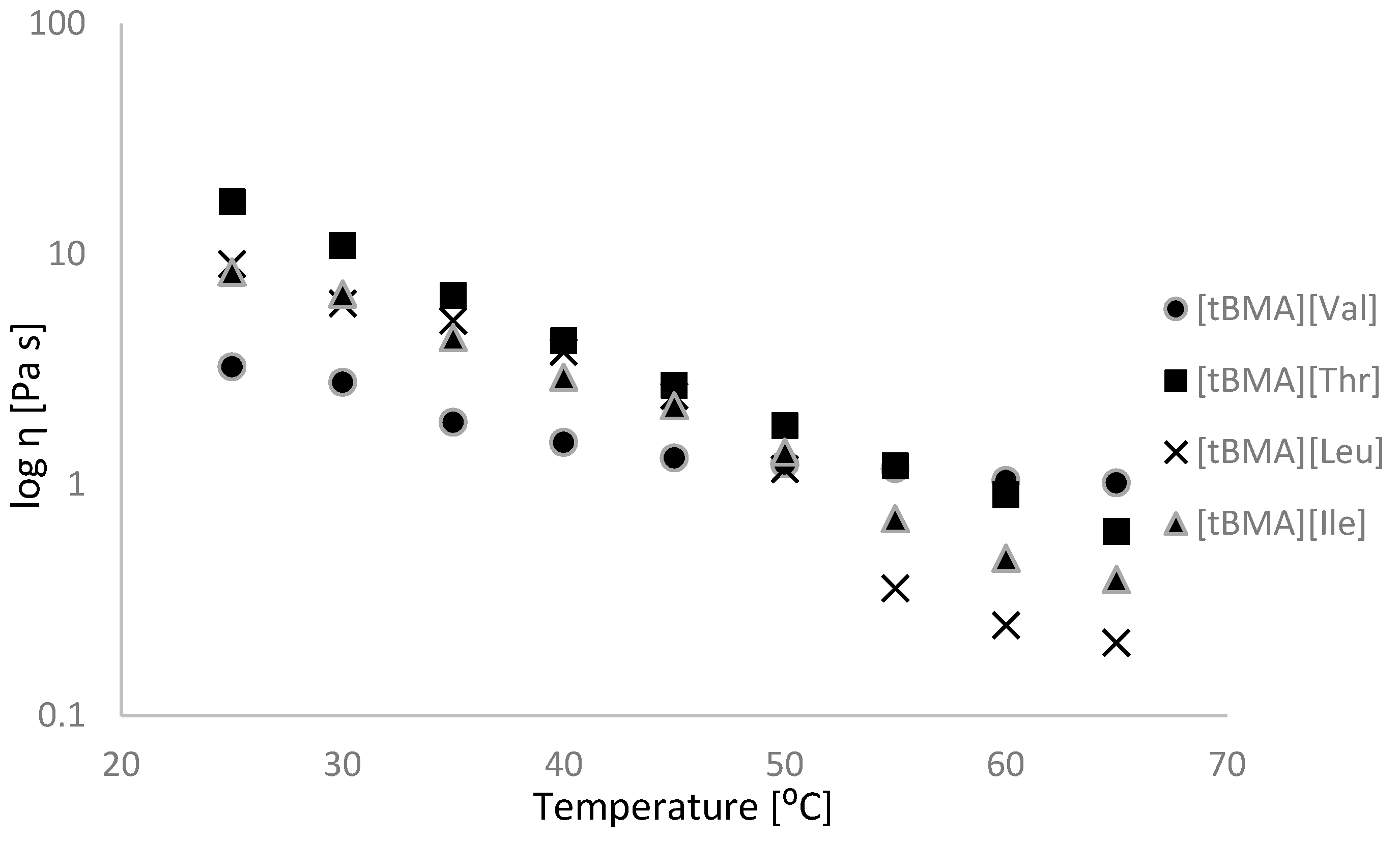
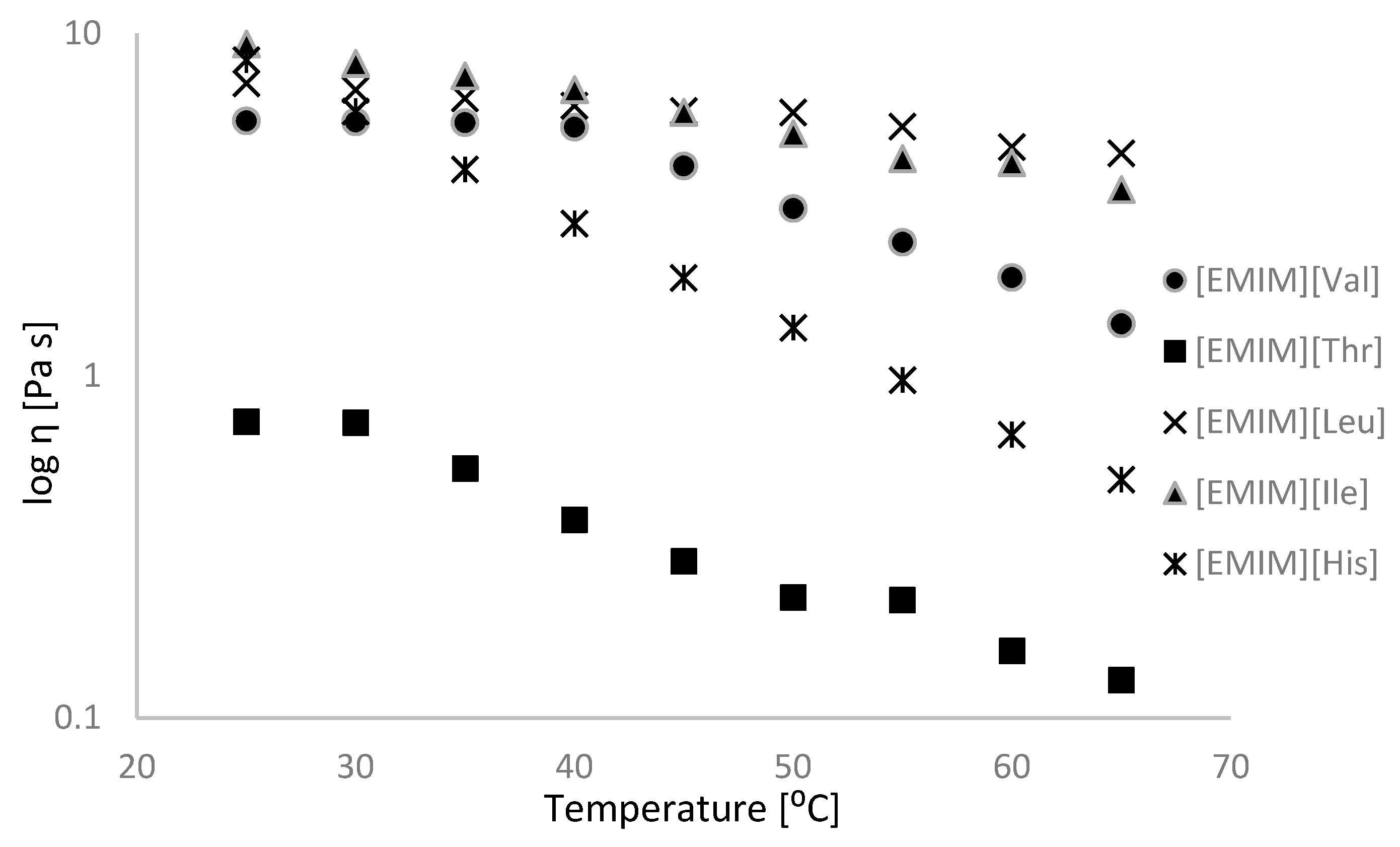
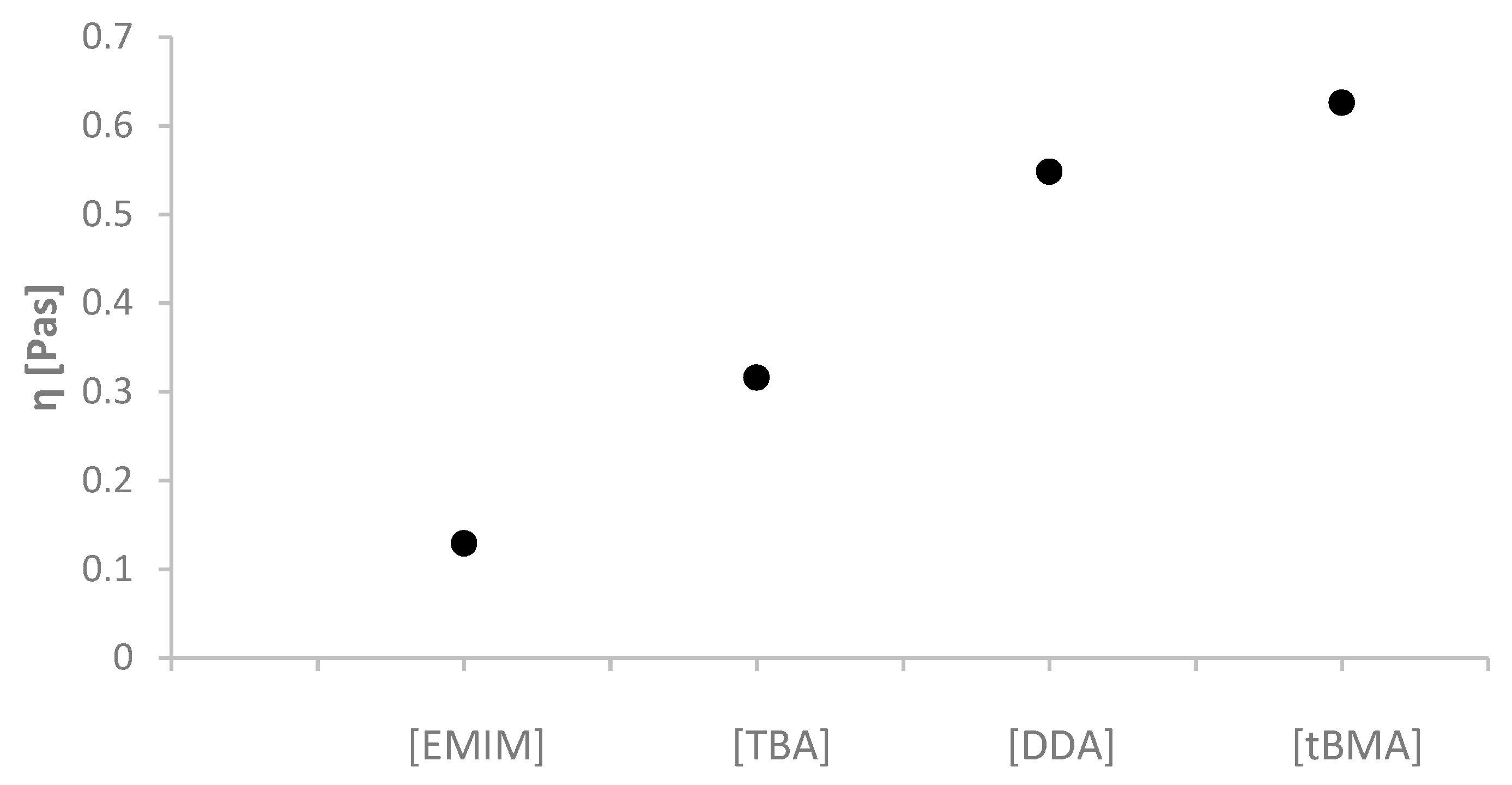
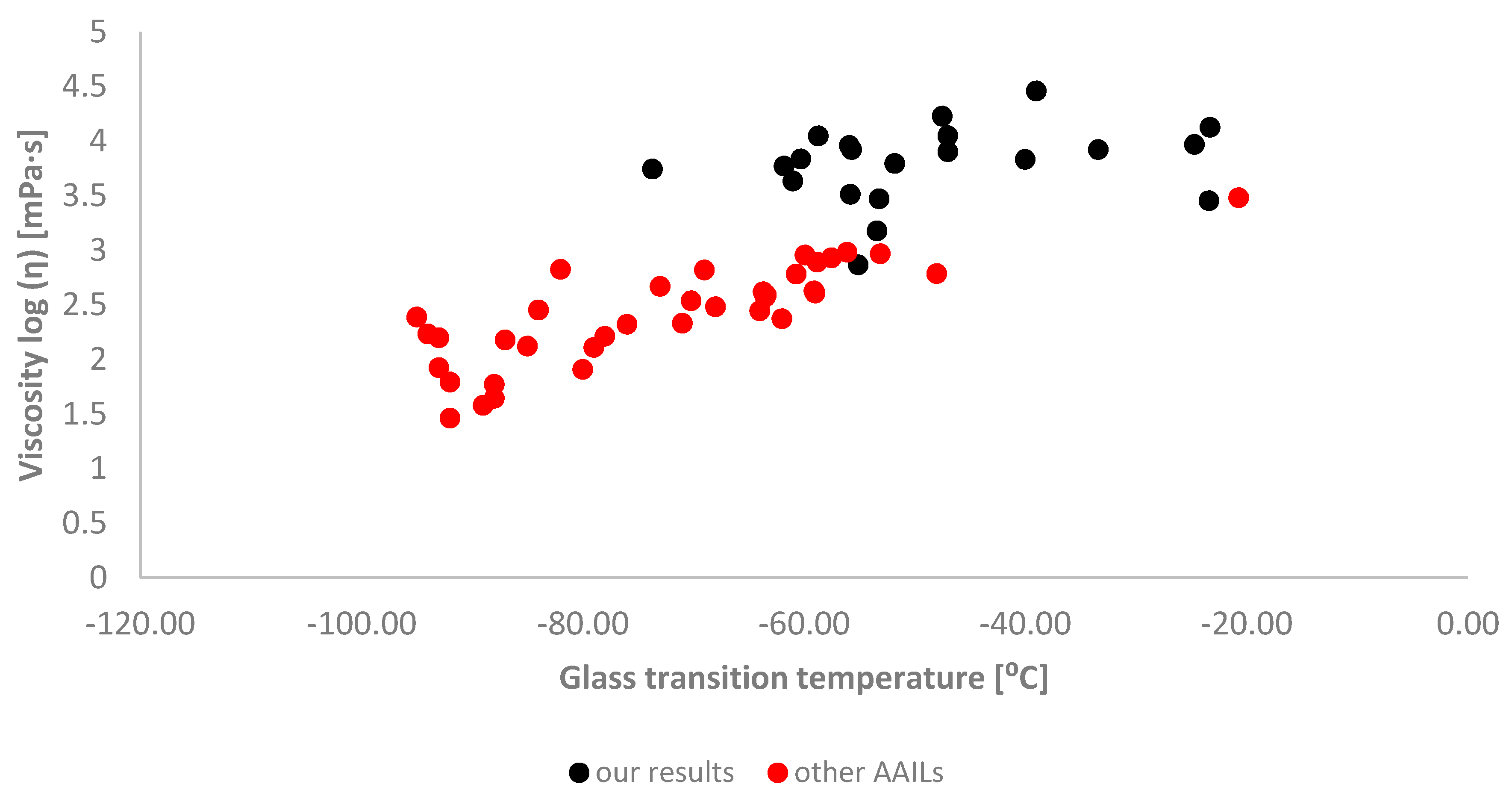
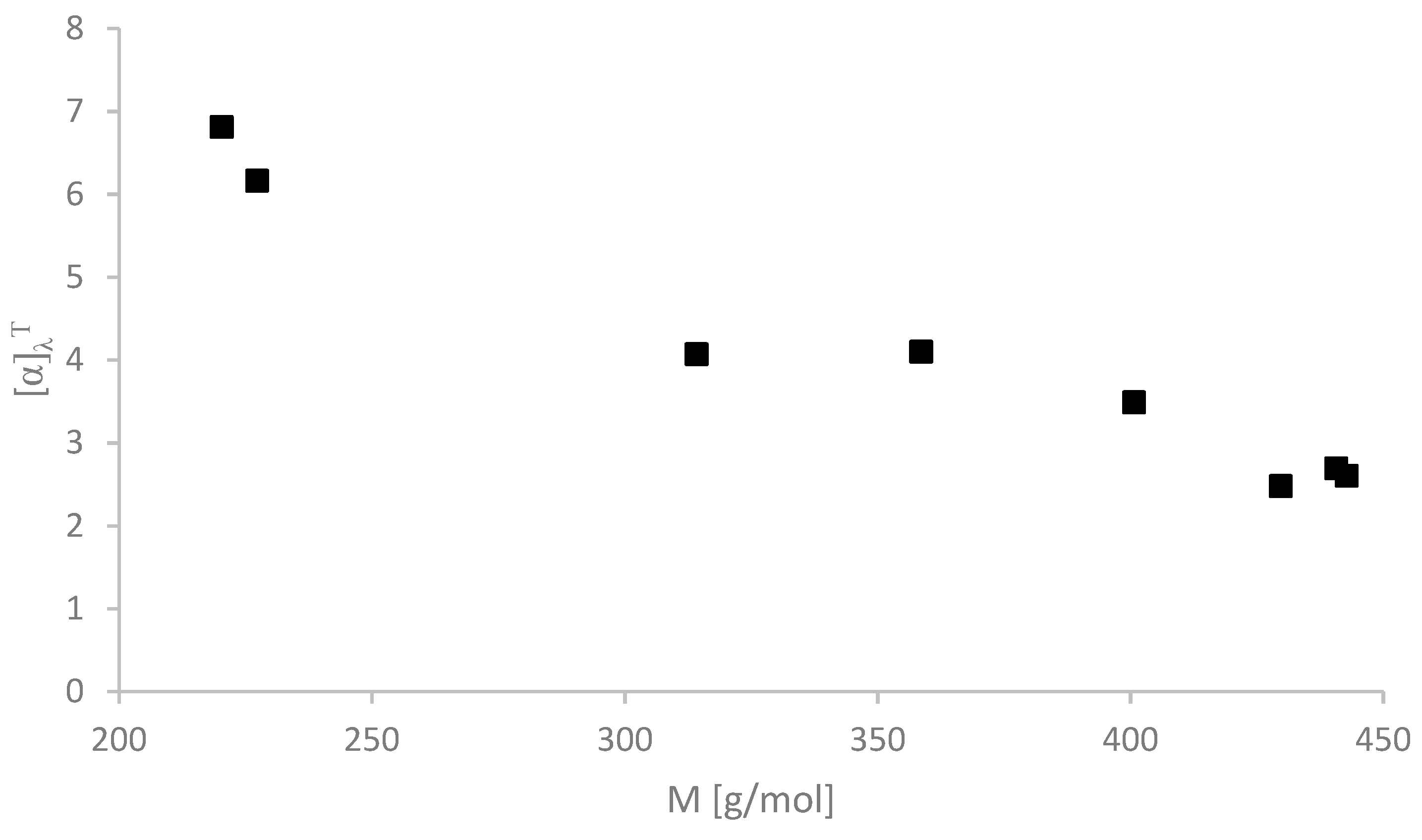
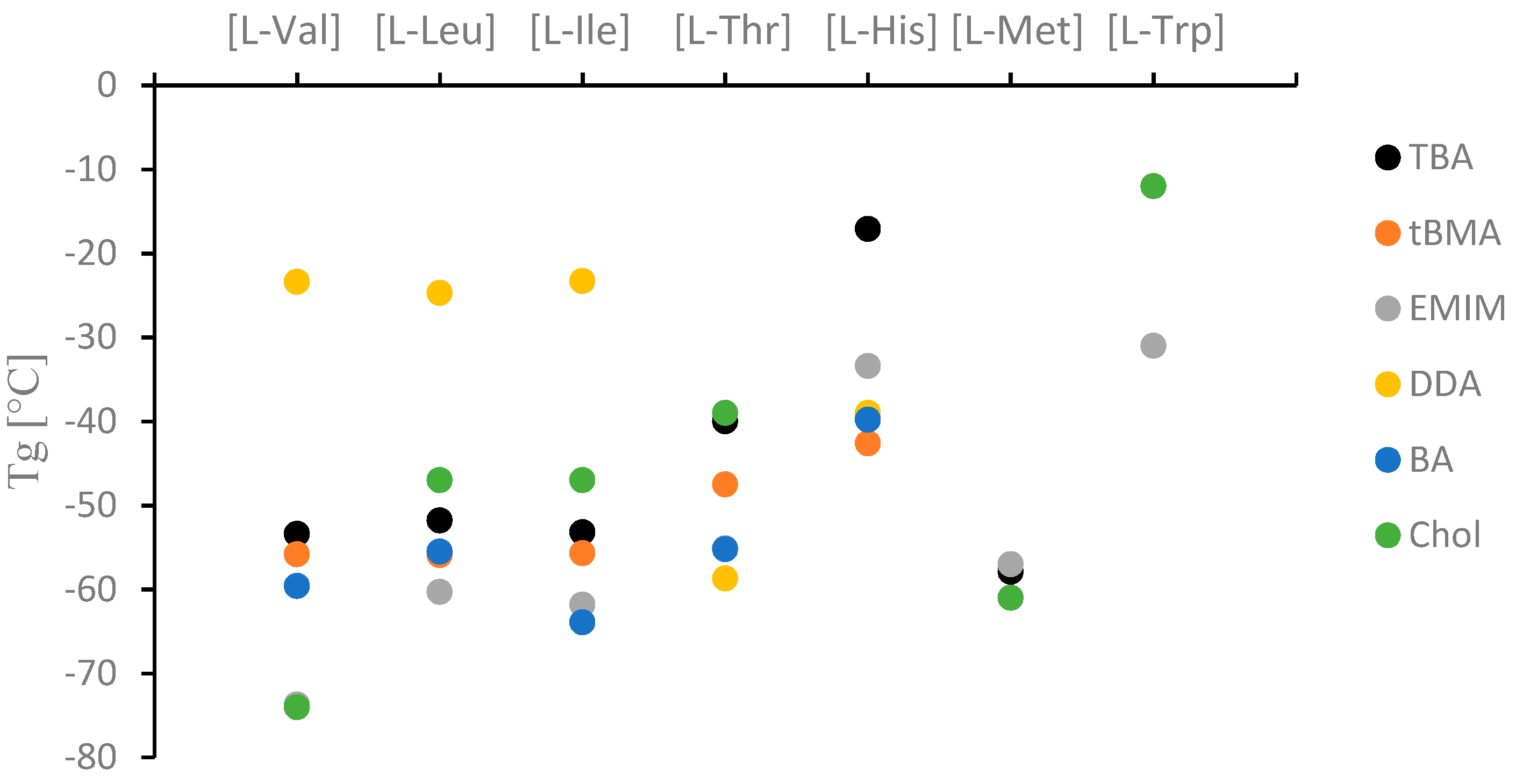
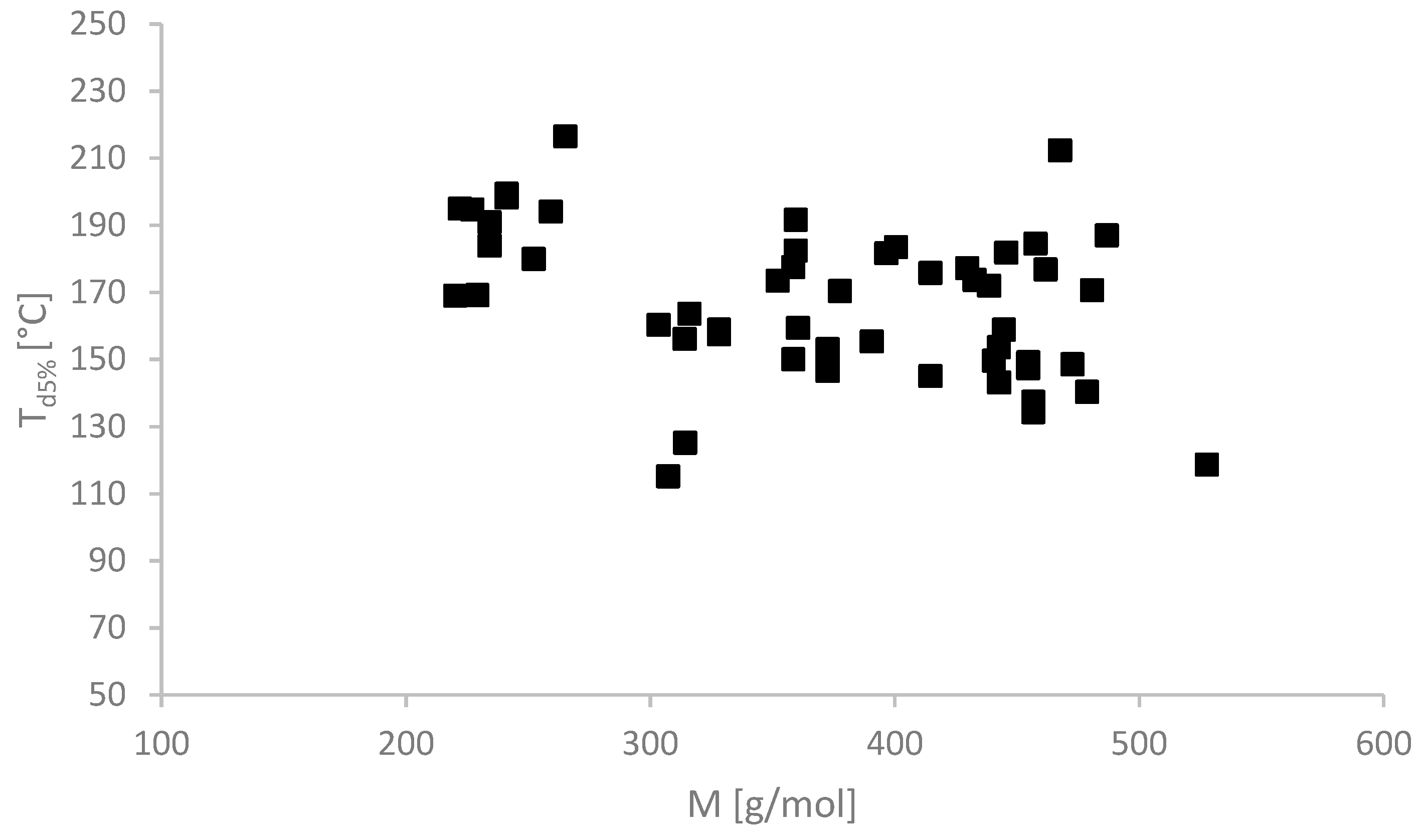
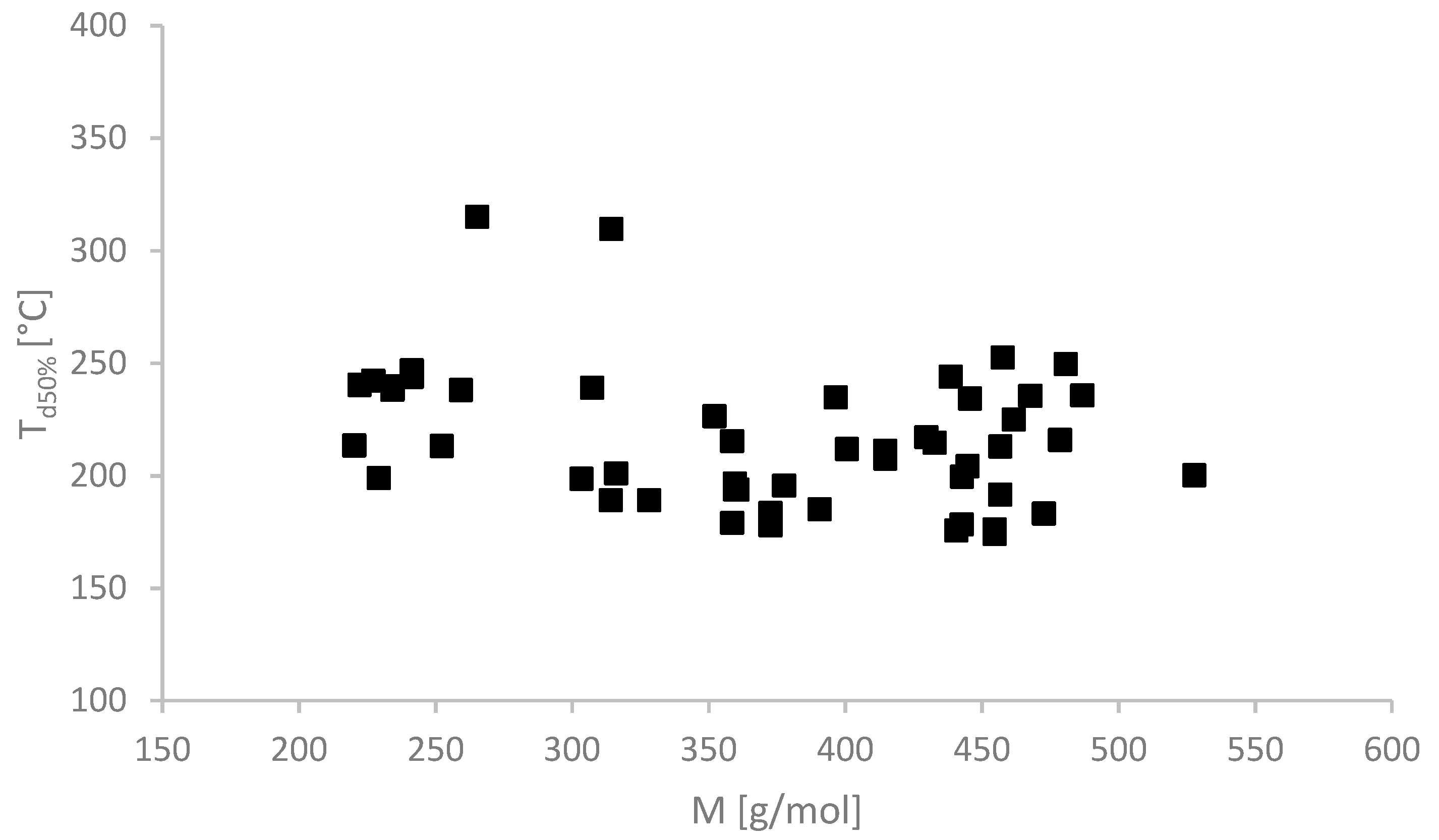
2.2. Physicochemical Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of Amino Acid Ionic Liquids
3.3. General Analytical Procedures
3.3.1. Structural Characterization of Obtained Amino Acid Ionic Liquids
3.3.2. Water Content
3.3.3. Specific Rotation
3.3.4. Viscosity
3.3.5. Thermogravimetric Analysis
3.3.6. Phase Transformations (DSC)
3.3.7. Measurement of Surface Tension
- Γmax—surface excess concentration at the saturated interface, mol·m−2,
- R—gas constant, 8.3144598(48) J·mol−1·K−1,
- T—absolute temperature, K,
- c—concentration of the salt, mol·dm−3.
- NA—the Avogadro number.
3.3.8. Cellulose Solubility
- Ccel—cellulose concentration [%],
- mcel—weight of cellulose dissolved in the ionic liquid [g],
- mcj—weight of ionic liquids [g].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef]
- Earle, M.; Seddon, K. Ionic liquids: Green solvents for the future. In Clean Solvents Alternative Media for Chemical Reactions and Processing; Abraham, M.A., Moens, L., Eds.; American Chemical Society: Washington, DC, USA, 2013; pp. 10–25. [Google Scholar]
- Kirk-Othmer. Chemical Technology and the Environment, 1st ed.; Wiley-Interscience: New York, NY, USA, 2007; Volume 1. [Google Scholar]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Wiley: Weinheim, Germany, 2008; Volume 1. [Google Scholar]
- Wasserscheid, P.; Keim, W. Ionic Liquids-New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Payagala, T.; Armstrong, D.W. Chiral ionic liquids: a compendium of syntheses and applications (2005-2012). Chirality 2012, 24, 17–53. [Google Scholar] [CrossRef]
- Marra, A.; Chiappe, C.; Mele, A. Sugar-Derived Ionic Liquids. CHIMIA Int. J. Chem. 2011, 65, 76–80. [Google Scholar] [CrossRef]
- Van Buu, O.N.; Aupoix, A.; Vo-Thanh, G. Synthesis of novel chiral imidazolium-based ionic liquids derived from isosorbide and their applications in asymmetric aza Diels–Alder reaction. Tetrahedron 2009, 65, 2260–2265. [Google Scholar] [CrossRef]
- Feder-Kubis, J.; Kubicki, M.; Pernak, J. 3-Alkoxymethyl-1-(1R,2S,5R)-(-)-menthoxymethylimidazolium salts-based chiral ionic liquids. Tetrahedron: Asymmetry 2010, 21, 2709–2718. [Google Scholar] [CrossRef]
- Baudequin, C.; Brégeon, D.; Levillain, J.; Guillen, F.; Plaquevent, J.C.; Gaumont, A.C. Chiral ionic liquids, a renewal for the chemistry of chiral solvents? Design, synthesis and applications for chiral recognition and asymmetric synthesis. Tetrahedron: Asymmetry 2005, 16, 3921–3945. [Google Scholar] [CrossRef]
- Nageshwar, D.; Rao, D.M.; Acharyulu, P.V.R. Terpenes to Ionic Liquids: Synthesis and Characterization of Citronellal-Based Chiral Ionic Liquids. Synth. Commun. 2009, 39, 3357–3368. [Google Scholar] [CrossRef]
- Van Buu, O.N.; Aupoix, A.; Hong, N.D.T.; Vo-Thanh, G. Chiral ionic liquids derived from isosorbide: synthesis, properties and applications in asymmetric synthesis. New J. Chem. 2009, 33, 2060–2072. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, T.; Zhang, Z.; Zhu, A.; Han, B.; Song, J.; Xie, Y.; Li, W. Functional ionic liquid from biorenewable materials: synthesis and application as a catalyst in direct aldol reactions. Tetrahedron Lett. 2007, 48, 5613–5617. [Google Scholar] [CrossRef]
- Zhao, D.; Liao, Y.; Hang, Z. Toxicity of Ionic Liquids. Clean 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.B.; AzmiBustam, M.; Abdul Mutalib, M.I.; Wilfred, C.D.; Ra, S. Synthesis and Thermophysical Properties of Low Viscosity Amino Acid-Based Ionic Liquids. J. Chem. Eng. Data 2011, 56, 3157–3162. [Google Scholar] [CrossRef]
- Rohman, N.; Dass, N.; Mahiuddin, S. Isentropic Compressibility of Aqueous and Methanolic Sodium Thiocyanate Solutions. J. Chem. Eng. Data 1999, 44, 465–472. [Google Scholar] [CrossRef]
- Abraham, R.; Abdulkhadar, M.; Asokan, C. Ultrasonic investigation of molecular interaction in binary mixtures of nitriles with methanol/toluene. J. Chem. Termodyn. 2000, 32, 1–16. [Google Scholar] [CrossRef]
- Jiang, Y.-Y.; Wang, G.-N.; Zhou, Z.; Wu, Y.-T.; Geng, J.; Zhang, Z.-B. Tetraalkylammoniumamino acids as functionalized ionic liquids of low viscosity. Chem. Commun. 2008, 4, 505–507. [Google Scholar] [CrossRef]
- Siyutkin, D.; Kucherenko, A.; Zlotin, S. Hydroxy-α-amino acids modified by ionic liquid moieties: recoverable organocatalysts for asymmetric aldol reactions in the presence of water. Tetrahedron 2009, 65, 1366–1372. [Google Scholar] [CrossRef]
- Tao, G.; He, L.; Liu, W.; Xu, L.; Xiong, W.; Wang, T.; Kou, Y. Preparation, characterization and application of amino acid-based green ionic liquids. Green Chem. 2006, 6, 639–646. [Google Scholar] [CrossRef]
- Qian, Y.; Xiao, S.; Liu, L.; Wang, Y. A mild and efficient procedure for asymmetric Michael additions of cyclohexanone to chalcones catalyzed by an amino acid ionic liquid. Tetrahedron: Asymmetry 2008, 19, 1515–1518. [Google Scholar] [CrossRef]
- Cybulski, J.; Wiśniewska, A.; Kulig-Adamiak, A.; Dąbrowski, Z.; Praczyk, T.; Michalczyk, A.; Walkiewicz, F.; Materna, K.; Pernak, J. Mandelate and prolinate ionic liquids: synthesis, characterization, catalytic and biological activity. Tetrahedron Lett. 2011, 52, 1325–1328. [Google Scholar] [CrossRef]
- Kagimoto, J.; Fukumoto, K.; Ohno, H. Effect of tetrabutylphosphonium cation on the physico-chemical properties of amino-acid ionic liquids. Chem. Commun. 2006, 2254–2256. [Google Scholar] [CrossRef]
- Song, Z.; Liang, Y.; Fan, M.; Zhou, F.; Liu, W. Ionic liquids from amino acids: fully green fluid lubricants for various surface contacts. RSC Adv. 2014, 4, 19396–19402. [Google Scholar] [CrossRef]
- Liu, Q.-P.; Hou, X.-D.; Li, N.; Zong, M.-H. Ionic liquids from renewable biomaterials: synthesis, characterization and application in the pretreatment of biomass. Green Chem. 2012, 14, 304–307. [Google Scholar] [CrossRef]
- Tong, J.; Hong, M.; Chen, Y.; Wang, H.; Yang, J.-Z. Surface properties of aqueous solutions of amino acid ionic liquids: [C3mim][Gly] and [C4mim][Gly]. J. Chem. Eng. Data 2012, 57, 2265–2270. [Google Scholar] [CrossRef]
- Dagade, D.H.; Shinde, S.P.; Madkar, K.R.; Barge, S.S. Density and sound speed study of hydration of 1-butyl-3-methylimidazolium based amino acid ionic liquids in aqueous solutions. J. Chem. Thermodyn. 2014, 79, 192–204. [Google Scholar] [CrossRef]
- Ossowicz, P.; Janus, E.; Schroeder, G.; Rozwadowski, Z. Spectroscopic Studies of Amino Acid Ionic Liquid-Supported Schiff Bases. Molecules 2013, 18, 4986–5004. [Google Scholar] [CrossRef]
- Ohno, H.; Fukumoto, K. Amino Acid ionic liquids. Acc. Chem. Res. 2007, 40, 1122–1129. [Google Scholar] [CrossRef]
- Ossowicz, P.; Janus, E.; Rozwadowski, Z.; Pilawka, R. Synthesis and properties of didecyldimethylammonium salts of amino acids. Przem. Chem. 2013, 92, 1000–1003. [Google Scholar]
- Plaquevent, J.-C.; Levillain, J.; Guillen, F.; Malhiac, C.; Gaumont, A.C. Ionic liquids: new targets and media for alpha-amino acid and peptide chemistry. Chem Rev. 2008, 108, 5035–5060. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Jing, H.; Yao, W.; Yan, P. Chiral ionic liquids improved the asymmetric cycloaddition of CO2 to epoxides. Green Chem. 2009, 11, 935–938. [Google Scholar] [CrossRef]
- Allen, C.R.; Richard, P.L.; Ward, A.J.; van de Water, L.G.A.; Masters, A.F.; Maschmeyer, T. Facile Synthesis of Ionic Liquids Possessing Chiral Carboxylates. Tetrahedron Lett. 2006, 47, 7367–7370. [Google Scholar] [CrossRef]
- Ossowicz, P.; Janus, E.; Błaszak, M.; Zatoń, K.; Rozwadowski, Z. Benzalkonium salts of amino acids-physicochemical properties and anti-microbial activity. Tenside Surf. Det. 2017, 54, 500–509. [Google Scholar] [CrossRef]
- Przybylski, P.; Pyta, K.; Ratajczak-Sitarz, M.; Katrusiak, A.; Brzezinski, B. X-ray, FT-IR, ESI MS and PM5 studies of Schiff base of gossypol with allylamine and its complexes with alkali metal cations and perchlorate anion. Struct. Chem. 2008, 19, 983–995. [Google Scholar] [CrossRef]
- Rozwadowski, Z. Deuterium isotope effects on 13C chemical shifts of lithium salts of Schiff bases amino acids. J. Mol. Struct. 2006, 753, 127–131. [Google Scholar] [CrossRef]
- Breitmaier, E.; Voelter, W. 13C NMR Spectroscopy, Methods and Applications Monographs. In Monographs in Modern Chemistry; Ebel, H.E., Ed.; Verlag Chemie: Weinheim, Germany, 1974; Volume 5. [Google Scholar] [CrossRef]
- Ossowicz, P.; Janus, E.; Szady-Chełmniecka, A.; Rozwadowski, Z. Influence of modification of the amino acids ionic liquids on their physicochemical properties: ionic liquids versus ionic liquids-supported Schiff bases. J. Mol. Liq. 2016, 224, 211–218. [Google Scholar] [CrossRef]
- Rubčić, M.; Užarević, K.; Halasz, I.; Bregović, N.; Mališ, M.; Dilović, I.; Kokan, Z.; Stein, R.S.; Dinnebier, R.E.; Tomišić, V. Desmotropy, Polymorphism, and Solid-State Proton Transfer: Four Solid Forms of an Aromatic O–Hydroxy Schiff Base. Chem. Eur. J. 2012, 18, 5620–5631. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, D.; Wen, L.; Yang, S.; Chen, X. Viscosity of ionic liquids: Database, observation, and quantitative structure-property relationship analysis. AIChE J. 2012, 58, 2885–2899. [Google Scholar] [CrossRef]
- Harris, K.R.; Kanakubo, M.; Woolf, L.A. Temperature and pressure dependence of the viscosity of the ionic liquids 1-methyl-3-octylimida-zolium hexafluorophosphate and 1-methyl-3-octylimidazolium tetra-fluoroborate. J. Chem. Eng. Data 2006, 51, 1161–1167. [Google Scholar] [CrossRef]
- Harris, K.R.; Woolf, L.A. Temperature and pressure dependence of the viscosity of the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J. Chem. Eng. Data 2005, 50, 1777–1782. [Google Scholar] [CrossRef]
- Schreiner, C.; Zugmann, S.; Hartl, R.; Gores, H.J. Temperature dependence of viscosity and specific conductivity of fluoroborate-based ionic liquids in light of the fractional Walden rule and Angell’s fragility concept. J. Chem. Eng. Data. 2010, 55, 4372–4377. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Zare, M.; Zolghadr, A.R.; Moosavi, F. Temperature dependence of viscosity and relation with the surface tension of ionic liquids. Fluid Phase Equilib. 2010, 291, 188–194. [Google Scholar] [CrossRef]
- Wen-Li, Y.; Xiao, Y.; Ling, H.; Ying, X.; Song, Q.; GuO-Hong, T. Viscosity, Conductivity, and Electrochemical Property of Dicyanamide Ionic Liquids. Front. Chem. 2018, 6, 59. [Google Scholar] [CrossRef]
- Kowsari, M.H.; Fakhraee, M.; Alavi, S.; Najafi, B. Molecular dynamics and ab initio studies of the effects of substituent groups on the thermodynamic properties and structure of four selected imidazolium-based [Tf2N–] ionic liquids. J. Chem. Eng. Data. 2014, 59, 2834–2849. [Google Scholar] [CrossRef]
- Yu, H.; Wu, Y.-T.; Jiang, Y.-Y.; Zhou, Z.; Zhang, Z.-B. Low viscosity amino acid ionic liquids with asymmetric tetraalkylammonium cations for fast absorption of CO2. New J. Chem. 2009, 33, 2385–2390. [Google Scholar] [CrossRef]
- Snyder, L.R. Classification of the solvent properties of common liquids. J. Chromatogr. 1974, 92, 223–230. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–52 are available from the authors. |



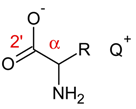 | |||
| Ionic Liquid | 1H-NMR (δ/ppm) (α) | 13C-NMR (δ/ppm) (α) | C=O (2′) |
| [DDTMA][Gly] | 3.09 | 44.7 | 181.7 |
| [HDTMA][Gly] | 3.25 | 43.9 | 179.1 |
| [TBA][l-Val]a) | 2.59 | 62.0 | 176.8 |
| [tBMA][l-Val] | 2.56 | 78.7 | 176.3 |
| [EMIM][l-Val] | 2.63 | 62.0 | 177.2 |
| [DDA][l-Val]b) | 2.55 | 62.6 | 176.3 |
| [BA][l-Val]c) | 3.38 | 63.8 | 184.2 |
| [Chol][l-Val] | 2.66 | 61.5 | 177.4 |
| [HDTMA][l-Val] | 3.00 | 61.6 | 180.4 |
| [ODTMA][l-Val] | 3.00 | 61.7 | 180.6 |
| [TBA][l-Leu]a) | 2.77 | 56.1 | 177.8 |
| [tBMA][l-Leu] | 2.66 | 71.1 | 177.7 |
| [EMIM][l-Leu] | 2.75 | 56.3 | 178.8 |
| [DDA][l-Leu]b) | 2.78 | 62.6 | 177.7 |
| [BA][l-Leu]c) | 3.22 | 64.8 | 185.7 |
| [Chol][l-Leu] | 2.77 | 54.5 | 178.7 |
| [DDTMA][l-Leu] | 3.25 | 54.4 | 181.4 |
| [HDTMA][l-Leu] | 3.25 | 54.2 | 180.7 |
| [TBA][l-Ile]a) | 2.63 | 61.8 | 176.5 |
| [tBMA][l-Ile] | 2.64 | 78.1 | 176.4 |
| [EMIM][l-Ile] | 2.71 | 61.7 | 177.6 |
| [DDA][l-Ile]b) | 2.62 | 61.3 | 176.4 |
| [BA][l-Ile]c) | 3.14 | 64.1 | 185.7 |
| [Chol][l-Ile] | 2.72 | 61.0 | 177.2 |
| [DDTMA][l-Ile] | 3.05 | 60.9 | 180.6 |
| [HDTMA][l-Ile] | 3.09 | 61.2 | 181.8 |
| [TBA][l-Thr]a) | 2.86 | 59.1 | 176.3 |
| [tBMA][l-Thr] | 2.92 | 68.0 | 175.8 |
| [EMIM][l-Thr] | 2.86 | 59.2 | 176.6 |
| [DDA][l-Thr]b) | 2.80 | 62.6 | 175.8 |
| [BA][l-Thr]c) | 3.16 | 64.2 | 183.3 |
| [Chol][l-Thr] | 2.85 | 58.4 | 176.5 |
| [TBA][l-His]a) | 3.02 | 56.8 | 178.9 |
| [tBMA][l-His] | 3.93 | 47.0 | 176.6 |
| [EMIM][l-His] | 3.05 | 56.9 | 177.1 |
| [DDA][l-His]b) | 3.43 | 62.7 | 176.6 |
| [BA][l-His]c) | 3.57 | 58.1 | 183.3 |
| [HDTMA][l-His] | 3.41 | 52.8 | 180.4 |
| [ODTMA][l-His] | 3.43 | 52.8 | 180.1 |
| [TBA][l-Met]a) | 2.79 | 56.0 | 176.6 |
| [EMIM][l-Met] | 2.86 | 55.5 | 176.7 |
| [BA][l-Met]c) | 2.69 | 58.4 | 185.8 |
| [Chol][l-Met] | 2.88 | 55.30 | 177.2 |
| [DDTMA][l-Met] | 3.36 | 55.4 | 182.2 |
| [HDTMA][l-Met] | 3.36 | 55.4 | 181.8 |
| [ODTMA][l-Met] | 3.20 | 55.2 | 181.1 |
| [TBA][l-Trp]a) | 3.40 | 58.0 | 176.9 |
| [EMIM][l-Trp] | 3.44 | 56.0 | 172.2 |
| [BA][l-Trp]c) | 3.40 | 56.5 | 181.2 |
| [Chol][l-Trp] | 2.65 | 57.0 | 177.4 |
| [HDTMA][l-Arg] | 3.27 | 55.5 | 183.2 |
| [ODTMA][l-Arg] | 3.15 | 55.5 | 183.1 |
| [TBA][l-Tyr] | 2.19 | 58.8 | 176.8 |
| [BA][l-Tyr] | 3.45 | 58.8 | 176.8 |
| Ionic Liquid | v(XH) | v(CH3-)as. | v(CH3-) | v(COO−)as. | v(COO−)sym. |
|---|---|---|---|---|---|
| [DDTMA][Gly] | 3600–3000 br. | 2918 | 2850 | 1578 | 1396 |
| [HDTMA][Gly] | 3600–3000 br. | 2918 | 2850 | 1585 | 1407 |
| [TBA][l-Val]a) | 3600–3100 br. | 2959 | 2874 | 1570 | 1397 |
| [DDA][l-Val]b) | 3500–3100 br. | 2956 | 2854 | 1573 | 1397 |
| [BA][l-Val]c) | 3600–3000 br. | 2957 | 2854 | 1583 | 1396 |
| [Chol][l-Val]d) | 3362 | 2961 | 2874 | 1571 | 1399 |
| [HDTMA][l-Val] | 3600–3000 br. | 2920 | 2851 | 1584 | 1396 |
| [ODTMA][l-Val] | 3600–3000 br. | 2918 | 2849 | 1569 | 1408 |
| [TBA][l-Leu]a) | 3500–3100br. | 2961 | 2875 | 1573 | 1383 |
| [DDA][l-Leu]b) | 3500–3100 br. | 2925 | 2855 | 1576 | 1398 |
| [BA][l-Leu]c) | 3600–3000 br. | 2954 | 2854 | 1578 | 1396 |
| [Chol][l-Leu]d) | 3377 | 2956 | 2871 | 1578 | 1401 |
| [DDTMA][l-Leu] | 3600–3000 br. | 2918 | 2850 | 1580 | 1395 |
| [HDTMA][l-Leu] | 3600–3000 br. | 2918 | 2850 | 1580 | 1395 |
| [TBA][l-Ile]a) | 3500–3100br. | 2960 | 2875 | 1574 | 1384 |
| [DDA][l-Ile]b) | 3500–3100 br. | 2957 | 2855 | 1577 | 1396 |
| [BA][l-Ile]c) | 3600–3000 br. | 2957 | 2854 | 1572 | 1396 |
| [Chol][l-Ile]d) | 3378 | 2963 | 2876 | 1575 | 1402 |
| [DDTMA][l-Ile] | 3600–3000 br. | 2918 | 2851 | 1579 | 1395 |
| [HDTMA][l-Ile] | 3600–3000 br. | 2920 | 2851 | 1576 | 1395 |
| [TBA][l-Thr]a) | 3600–3100br. | 2961 | 2874 | 1577 | 1396 |
| [DDA][l-Thr]b) | 3500–3100 br. | 2924 | 2854 | 1578 | 1378 |
| [BA][l-Thr]c) | 3600–3100 br. | 2956 | 2854 | 1575 | 1353 |
| [Chol][l-Thr]d) | 3366 | 2973 | 2933 | 1577 | 1402 |
| [TBA][l-His]a) | 3600–3100 br. | 2959 | 2875 | 1574 | 1398 |
| [DDA][l-His]b) | 3500–3100 br. | 2905 | 2854 | 1587 | 1398 |
| [BA][l-His]c) | 3600–3000 br. | 2956 | 2854 | 1574 | 1342 |
| [HDTMA][l-His] | 3650–3000 br. | 2917 | 2848 | 1570 | 1417 |
| [ODTMA][l-His] | 3650–3000 br. | 2918 | 2850 | 1578 | 1342 |
| [TBA][l-Met]a) | 3600–3100 br. | 2958 | 2874 | 1580 | 1380 |
| [BA][l-Met]c) | 3600–3100 br. | 2954 | 2853 | 1574 | 1406 |
| [Chol][l-Met]d) | 3366 | 2970 | 2840 | 1579 | 1406 |
| [DDTMA][l-Met] | 3600–3000 br. | 2918 | 2851 | 1581 | 1395 |
| [HDTMA][l-Met] | 3700–3000 br. | 2920 | 2851 | 1581 | 1391 |
| [ODTMA][l-Met] | 3700–3000 br. | 2919 | 2850 | 1582 | 1407 |
| [TBA][l-Trp]a) | 3600–3100 br. | 2962 | 2875 | 1587 | 1354 |
| [BA][l-Trp]c) | 3600–3100 br. | 2955 | 2854 | 1575 | 1355 |
| [Chol][l-Trp]d) | 3252 | 2921 | 2874 | 1573 | 1404 |
| [HDTMA][l-Arg] | 3600–3000 br. | 2917 | 2850 | 1570 | 1407 |
| [ODTMA][l-Arg] | 3600–3000 br. | 2918 | 2849 | 1569 | 1408 |
| [TBA][l-Tyr] | 3600–3000 br. | 2960 | 2850 | 1573 | 1383 |
| [BA][l-Tyr] | 3600–3000 br. | 2925 | 2854 | 1578 | 1378 |
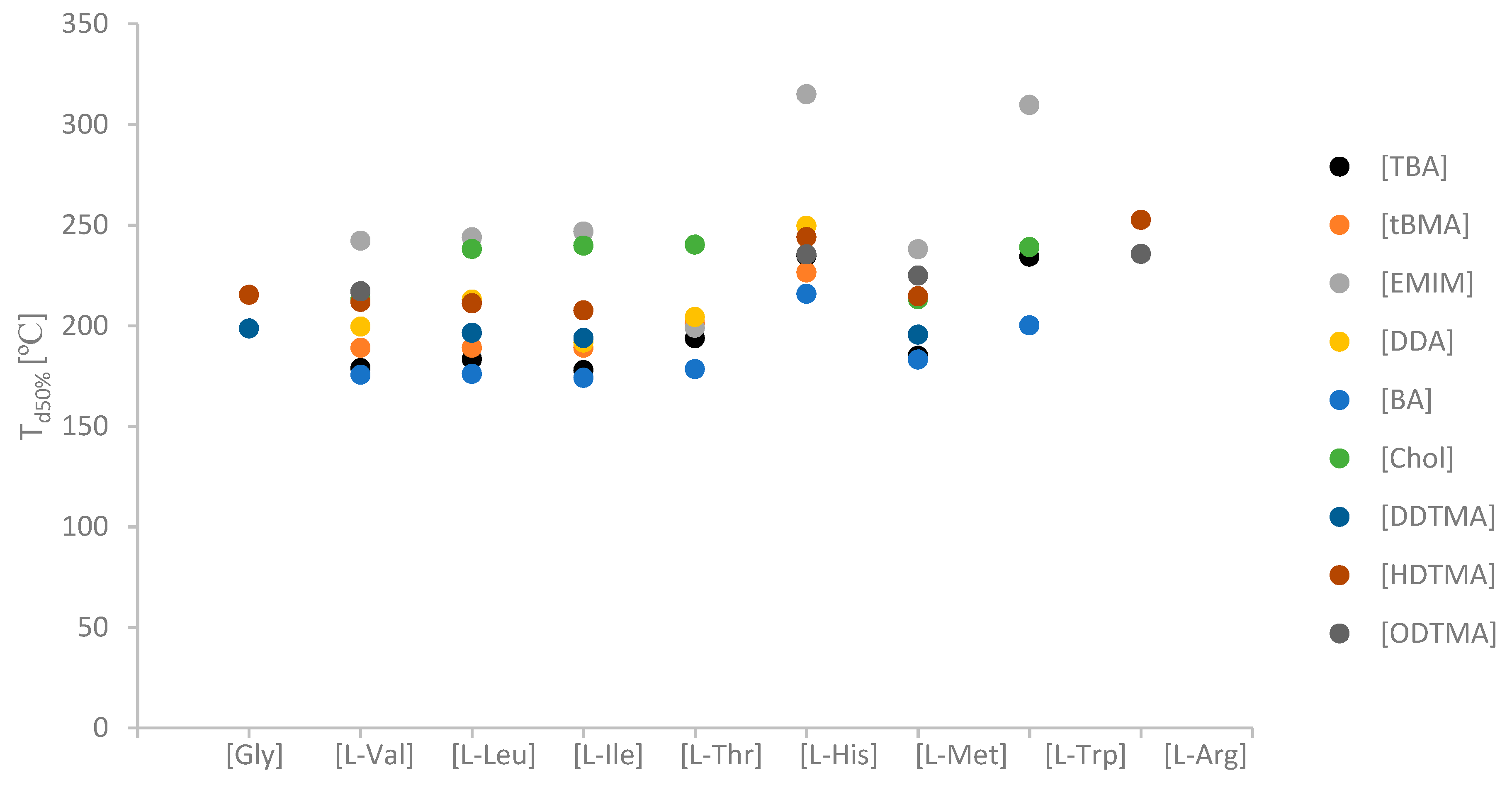
| No. | Ionic Liquids | State at RT (Tm (°C)) | Colour | M (g mol−1) | Tg/°C | Td5%/°C | Td50%/°C | η/Pa·s 65 °C | [α]λT | [M]λT | Solubility of Cellulose |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [DDTMA][Gly] | W | white | 303.50 | na | 160.2 ± 1.1 | 198.6 ± 1.3 | na | - | - | N |
| 2 | [HDTMA][Gly] | W | white | 358.61 | na | 177.4 ± 1.9 | 215.3 ± 1.8 | na | - | - | N |
| 3 | [TBA][l-Val] | L | colorless | 358.61 | −53.4 ± 2.1 | 150.0 ± 1.7 | 178.9 ± 1.9 | 0.148±0.031 | +4.10 | +14.7 | N |
| 4 | [tBMA][l-Val] | L | colorless | 314.20 | −55.8 ± 1.9 | 156.0 ± 2.0 | 189.0 ± 2.1 | 1.019±0.056 | +4.07 | +12.8 | N |
| 5 | [EMIM][l-Val] | L | colorless | 227.32 | −73.7 ± 1.2 | 194.6 ± 2.2 | 242.2 ± 2.0 | 1.420±0.073 | +6.16 | +14.0 | Y |
| 6 | [DDA][l-Val] | L | colorless | 442.78 | −23.4 ± 1.6 | 143.0 ± 1.8 | 199.5 ± 2.1 | 0.417±0.009 | +2.60 | +11.5 | N |
| 7 | [BA][l-Val] | L | colorless | 440.70 | −59.6 ± 1.7 | 149.5 ± 2.1 | 175.6 ± 1.8 | na | +2.69 | +11.9 | N |
| 8 | [Chol][l-Val] | L | colorless | 220.32 | −74.0c) | 168.9 ± 1.2 | 213.3 ± 1.4 | 372c) | +6.81 | +15.0 | Y |
| 9 | [HDTMA][l-Val] | W | white | 400.69 | na | 183.4 ± 1.7 | 211.8 ± 1.6 | na | +3.49 | +14.0 | N |
| 10 | [ODTMA][l-Val] | W | white | 429.75 | na | 177.1 ± 1.6 | 217.0 ± 1.8 | na | +2.48 | +10.7 | N |
| 11 | [TBA][l-Leu] | L | colorless | 372.64 | −51.8 ± 2.4 | 153.0 ± 2.1 | 183.3 ± 1.9 | 0.149 ± 0.025 | +2.44 | +9.1 | N |
| 12 | [tBMA][l-Leu] | L | colorless | 328.23 | −55.9 ± 2.1 | 158.8 ± 1.5 | 189.0 ± 1.7 | 0.207 ± 0.011 | +1.47 | +4.8 | N |
| 13 | [EMIM][l-Leu] | L | colorless | 241.35 | −60.3 ± 2.5 | 199.3 ± 1.8 | 243.8 ± 2.1 | 4.659 ± 0.073 | +2.08 | +5.0 | Y |
| 14 | [DDA][l-Leu] | L | colorless | 456.81 | −24.7 ± 1.9 | 134.2 ± 1.1 | 212.9 ± 0.8 | 0.596 ± 0.027 | +1.78 | +8.1 | N |
| 15 | [BA][l-Leu] | L | colorless | 454.73 | −55.5 ± 1.6 | 149.1 ± 0.9 | 176.0 ± 1.2 | na | +1.47 | +6.7 | N |
| 16 | [Chol][l-Leu] | L | colorless | 234.35 | −47.0c) | 183.8 ± 0.7 | 238.1 ± 1.5 | 476c) | +2.80 | +6.6 | Y |
| 17 | [DDTMA][l-Leu] | W | white | 359.61 | na | 182.3 ± 2.0 | 196.4 ± 2.4 | na | +0.96 | +3.5 | N |
| 18 | [HDTMA][l-Leu] | W | white | 414.71 | na | 175.7 ± 1.8 | 211.0 ± 1.4 | na | +2.96 | +12.3 | N |
| 19 | [TBA][l-Ile] | L | colorless | 372.64 | −53.2 ± 1.3 | 146.5 ± 1.6 | 177.8 ± 1.8 | 0.176 ± 0.032 | +3.91 | +14.6 | N |
| 20 | [tBMA][l-Ile] | L | colorless | 328.23 | −55.7 ± 2.1 | 157.4 ± 1.8 | 189.0 ± 1.4 | 3.880 ± 0.041 | +3.75 | +12.3 | N |
| 21 | [EMIM][l-Ile] | L | colorless | 241.35 | −61.8 ± 2.4 | 198.2 ± 2.2 | 246.7 ± 2.3 | 3.499 ± 0104 | +5.78 | +14.0 | Y |
| 22 | [DDA][l-Ile] | L | colorless | 456.81 | −23.3 ± 1.8 | 137.3 ± 2.1 | 191.5 ± 1.6 | 0.655 ± 0.028 | +2.80 | +12.8 | N |
| 23 | [BA][l-Ile] | L | colorless | 454.73 | −63.9 ± 1.7 | 147.3 ± 1.9 | 174.0 ± 1.5 | na | +2.54 | +11.6 | N |
| 24 | [Chol][l-Ile] | L | colorless | 234.35 | −47.0c) | 190.8 ± 1.3 | 239.7 ± 1.0 | 480c) | +6.07 | +14.2 | Y |
| 25 | [DDTMA][l-Ile] | W | white | 359.61 | na | 166.4 ± 1.9 | 193.8 ± 1.3 | na | +1.29 | +4.6 | N |
| 26 | [HDTMA][l-Ile] | W | white | 414.71 | na | 173.6 ± 1.7 | 207.5 ± 2.5 | na | +5.27 | +21.9 | N |
| 27 | [TBA][l-Thr] | L | colorless | 360.58 | −40.0 ± 2.1 | 163.0 ± 1.5 | 193.7 ± 2.1 | 0.316 ± 0.002 | -2.46 | -8.9 | N |
| 28 | [tBMA][l-Thr] | L | colorless | 316.17 | −47.5 ± 2.3 | 163.5 ± 1.4 | 200.9 ± 2.2 | 0.626 ± 0.038 | -1.47 | -4.6 | N |
| 29 | [EMIM][l-Thr] | L | colorless | 229.29 | −55.1 ± 1.4 | 169.1 ± 1.5 | 198.9 ± 1.2 | 0.129 ± 0.004 | -3.7 | -8.9 | Y |
| 30 | [DDA][l-Thr] | L | colorless | 444.75 | −58.7 ± 1.7 | 158.8 ± 2.1 | 204.2 ± 1.7 | 0.548 ± 0.007 | −1.78 | −7.9 | N |
| 31 | [BA][l-Thr] | L | colorless | 442.67 | −55.2 ± 1.3 | 153.6 ± 2.2 | 178.3 ± 1.6 | na | −2.57 | −11.4 | N |
| 32 | [Chol][l-Thr] | L | colorless | 222.29 | −39.0c) | 194.9 ± 1.6 | 240.2 ± 1.9 | 454c) | −3.05 | −6.8 | Y |
| 33 | [TBA][l-His] | S (128.5 ± 2.1) | yellow | 396.62 | −17.1 ± 1.2 | 181.6 ± 1.4 | 234.7 ± 1.5 | - | −3.90 | −15.5 | N |
| 34 | [tBMA][l-His] | S (58.9 ± 1.2) | yellow | 352.21 | −42.6 ± 1.5 | 173.4 ± 2.2 | 226.4 ± 2.6 | - | −3.33 | −11.7 | N |
| 35 | [EMIM][l-His] | W | yellow | 265.33 | −33.4 ± 2.2 | 216.4 ± 1.7 | 315.0 ± 1.2 | 0.497 ± 0.071 | −5.07 | −13.5 | Y |
| 36 | [DDA][l-His] | L | yellow | 480.79 | −39.0 ± 0.4 | 170.5 ± 1.4 | 249.6 ± 1.9 | 1.604 ± 0.008 | −3.62 | −17.4 | N |
| 37 | [BA][l-His] | L | yellow | 478.71 | −39.8 ± 0.3 | 140.3 ± 0.5 | 215.8 ± 1.3 | na | −3.89 | −18.6 | N |
| 38 | [HDTMA][l-His] | W | white | 438.70 | na | 171.9 ± 1.0 | 243.9 ± 1.6 | na | −6.00 | −26.3 | N |
| 39 | [ODTMA][l-His] | W | white | 467.76 | na | 212.2 ± 1.3 | 235.4 ± 2.1 | na | −4.92 | −23.0 | N |
| 40 | [TBA][l-Met] | L | pale yellow | 390.67 | −57.9 ± 1.1 | 155.3 ± 1.8 | 185.0 ± 2.1 | na | +1.20 | +4.7 | N |
| 41 | [EMIM][l-Met] | L | yellow | 259.38 | −57.0 ± 1.7 | 194.0 ± 2.1 | 238.0 ± 1.6 | na | +1.41 | +3.7 | Y |
| 42 | [BA][l-Met] | L | colorless | 472.76 | −61.0 ± 1.5 | 148.5 ± 1.9 | 183.1 ± 1.3 | na | +2.01 | +9.5 | N |
| 43 | [Chol][l-Met] | W | pale yellow | 252.38 | −61.0c) | 179.9 ± 1.3 | 213.1 ± 1.6 | 330c) | +2.30 | +5.8 | Y |
| 44 | [DDTMA][l-Met] | W | white | 377.64 | na | 170.3 ± 2.4 | 195.5 ± 1.9 | na | +4.00 | +15.1 | N |
| 45 | [HDTMA][l-Met] | W | white | 432.76 | na | 173.6 ± 1.7 | 214.6 ± 1.5 | na | +1.28 | +5.6 | N |
| 46 | [ODTMA][l-Met] | W | white | 461.81 | na | 176.7 ± 0.4 | 224.9 ± 1.3 | na | +0.65 | +3.0 | N |
| 47 | [TBA][l-Trp] | S (129.6 ± 2.3) | creamy beige | 445.68 | - | 181.7 ± 1.8 | 234.2 ± 0.9 | - | ch−13.1a) | -−58.4a) | N |
| 48 | [EMIM][l-Trp] | L | brown | 314.40 | −31.0 ± 2.1 | 191.5 ± 2.5 | 310.4 ± 2.1 | na | −1.65 | −5.2 | Y |
| 49 | [BA][l-Trp] | W | brown | 527.78 | −28.3 ± 1.9 | 145.0 ± 2.3 | 206.3 ± 1.7 | na | −2.54 | −13.4 | N |
| 50 | [Chol][l-Trp] | L | dark brown | 307.40 | −12.0c) | 187.6 ± 1.2 | 239.2 ± 1.8 | 5640c) | −2.55 | −7.8 | Y |
| 51 | [HDTMA][l-Arg] | W | white | 457.74 | na | 184.4 ± 1.4 | 252.5 ± 1.6 | na | +6.34 | +29.0 | N |
| 52 | [ODTMA][l-Arg] | W | white | 486.80 | na | 186.9 ± 2.1 | 235.6 ± 2.0 | na | +4.34 | +21.1 | N |
| Ionic Liquids | Water (9.0) | Acetone (5.4) | Ethanol (5.2) | Chloroform (4.4) | Ethyl Acetate (4.3) | Benzene (3.0) | Diethyl Ether (2.9) | N–Hexane (0.0) |
|---|---|---|---|---|---|---|---|---|
| [DDTMA][Gly] | m | p | p | i | i | i | i | i |
| [HDTMA][Gly] | m | p | p | i | i | i | i | i |
| [TBA][l-Val] | m | m | m | m | m | i | i | i |
| [tBMA][l-Val] | m | m | m | m | m | i | i | i |
| [EMIM][l-Val] | m | m | m | m | m | i | i | i |
| [DDA][l-Val] | m | m | m | m | m | i | i | i |
| [BA][l-Val] | m | m | m | m | m | i | i | i |
| [Chol][l-Val] | m | m | m | m | m | i | i | i |
| [HDTMA][l-Val] | m | p | p | i | i | i | i | i |
| [ODTMA][l-Val] | m | m | m | i | i | i | i | i |
| [TBA][l-Leu] | m | m | m | m | m | i | i | i |
| [tBMA][l-Leu] | m | m | m | m | m | i | i | i |
| [EMIM][l-Leu] | m | m | m | m | m | i | i | i |
| [DDA][l-Leu] | m | m | m | m | m | i | i | i |
| [BA][l-Leu] | m | m | m | m | m | i | i | i |
| [Chol][l-Leu] | m | m | m | m | m | i | i | i |
| [DDTMA][l-Leu] | m | m | m | m | i | i | i | i |
| [HDTMA][l-Leu] | m | m | m | m | i | i | i | i |
| [TBA][l-Ile] | m | m | m | m | m | i | i | i |
| [tBMA][l-Ile] | m | m | m | m | m | i | i | i |
| [EMIM][l-Ile] | m | m | m | m | m | i | i | i |
| [DDA][l-Ile] | m | m | m | m | m | i | i | i |
| [BA][l-Ile] | m | m | m | m | m | i | i | i |
| [Chol][l-Ile] | m | m | m | m | m | i | i | i |
| [DDTMA][l-Ile] | m | m | m | m | i | i | i | i |
| [HDTMA][l-Ile] | m | m | m | m | i | i | i | i |
| [TBA][l-Thr] | m | m | m | p | m | i | i | i |
| [tBMA][l-Thr] | m | m | m | m | m | i | i | i |
| [EMIM][l-Thr] | m | m | m | m | m | i | i | i |
| [DDA][l-Thr] | m | m | m | m | m | i | i | i |
| [BA][l-Thr] | m | m | m | m | m | i | i | i |
| [Chol][l-Thr] | m | m | m | m | m | i | i | i |
| [TBA][l-His] | m | m | m | m | p | i | i | i |
| [tBMA][l-His] | m | m | m | p | p | i | i | i |
| [EMIM][l-His] | m | m | m | m | m | i | i | i |
| [DDA][l-His] | m | m | m | m | p | i | i | i |
| [BA][l-His] | m | m | m | m | m | i | i | i |
| [HDTMA][l-His] | m | m | p | i | i | i | i | i |
| [ODTMA][l-His] | m | m | m | i | i | i | i | i |
| [TBA][l-Met] | m | m | m | m | m | i | i | i |
| [EMIM][l-Met] | m | m | m | m | m | i | i | i |
| [BA][l-Met] | m | m | m | m | m | i | i | i |
| [Chol][l-Met] | m | m | m | m | m | i | i | i |
| [DDTMA][l-Met] | m | m | m | m | i | i | i | i |
| [HDTMA][l-Met] | m | m | m | m | i | i | i | i |
| [ODTMA][l-Met] | m | m | m | i | i | i | i | i |
| [TBA][l-Trp] | m | m | m | p | p | i | i | i |
| [EMIM][l-Trp] | m | m | m | m | m | i | i | i |
| [BA][l-Trp] | m | m | m | m | m | i | i | i |
| [Chol][l-Trp] | m | m | m | p | p | i | i | i |
| [TBA][l-Tyr] | m | m | m | m | m | i | i | i |
| [BA][l-Tyr] | m | m | m | m | m | i | i | i |
| [HDTMA][l-Arg] | m | p | m | i | i | i | i | i |
| [ODTMA][l-Arg] | m | m | m | i | i | i | i | i |
| IL | CMC (mmol·L−1) | ϒCMC (mN·m−1) | Γmax·106 (mol·m−2) | Amin·10−19 (m2) | pC20 (mmol·L−1) |
|---|---|---|---|---|---|
| [DDA][l-Val]a) | 0.64 | 28.3 | 3.302 | 5.186 | 0.029 |
| [DDA][l-Leu]a) | 0.61 | 27.9 | 3.364 | 4.936 | 0.021 |
| [DDA][l-Ile]a) | 0.60 | 28.0 | 3.373 | 4.932 | 0.014 |
| [DDA][l-Thr]a) | 0.87 | 28.2 | 2.922 | 5.683 | 0.018 |
| [DDA][l-His]a) | 0.82 | 29.4 | 3.071 | 5.407 | 0.025 |
| [BA][l-Val]b) | 0.28 | 32.3 | 4.853 | 3.423 | 0.056 |
| [BA][l-Leu]b) | 0.25 | 32.9 | 5.645 | 2.943 | 0.063 |
| [BA][l-Ile]b) | 0.26 | 32.8 | 5.654 | 2.938 | 0.062 |
| [BA][l-Thr]b) | 0.31 | 32.8 | 7.151 | 2.322 | 0.048 |
| [BA][l-His]b) | 0.28 | 34.1 | 5.604 | 2.964 | 0.061 |
| [BA][l-Met]b) | 0.25 | 34.8 | 11.751 | 1.414 | 0.055 |
| [BA][l-Trp]b) | 0.34 | 33.7 | 7.463 | 2.226 | 0.068 |
| [DDTMA][Gly] | 12.26 | 40.3 | 5.471 | 3.035 | 6.386 |
| [DDTMA][l-Leu] | 11.81 | 40.3 | 5.273 | 3.103 | 3.621 |
| [DDTMA][l-Ile] | 12.17 | 40.2 | 5.283 | 3.143 | 3.599 |
| [DDTMA][l-Met] | 12.09 | 40.4 | 7.561 | 2.196 | 2.645 |
| [HDTMA][Gly] | 0.78 | 41.3 | 5.273 | 3.149 | 0.356 |
| [HDTMA][l-Val] | 0.78 | 41.7 | 8.157 | 2.036 | 0.316 |
| [HDTMA][l-Leu] | 0.76 | 40.8 | 5.663 | 2.892 | 0.271 |
| [HDTMA][l-Ile] | 0.74 | 40.7 | 5.753 | 2.887 | 0.264 |
| [HDTMA][l-Met] | 0.86 | 41.8 | 4.179 | 3.974 | 0.187 |
| [HDTMA][l-His] | 0.96 | 41.7 | 5.870 | 2.829 | 0.372 |
| [HDTMA][l-Arg] | 1.09 | 41.7 | 8.040 | 2.065 | 0.165 |
| [ODTMA][l-Val] | 0.53 | 40.2 | 3.281 | 5.062 | 0.015 |
| [ODTMA][l-Met] | 0.52 | 40.3 | 2.503 | 6.635 | 0.007 |
| [ODTMA][l-His] | 0.50 | 42.0 | 2.903 | 5.720 | 0.011 |
| [ODTMA][l-Arg] | 0.61 | 40.1 | 3.492 | 4.755 | 0.008 |
| Ionic Liquid | Cellulose (mg g−1) |
|---|---|
| [EMIM][l-Val] | 21.6 (60 °C) |
| [EMIM][l-Leu] | 34.4 (60 °C) |
| [EMIM][l-Ile] | 13.7 (60 °C) |
| [EMIM][l-Thr] | 43.2 (60 °C) |
| [EMIM][Cl] | 31.4 (85 °C) |
| [Chol][l-Val]a) | <5 (90 °C) |
| [Chol][l-Leu]a) | <5 (90 °C) |
| [Chol][l-Ile]a) | <5 (90 °C) |
| [Chol][l-Thr]a) | <5 (90 °C) |
| [Chol][l-His]a) | <5 (90 °C) |
| [Chol][l-Met]a) | <5 (90 °C) |
| [Chol][l-Trp]a) | <5 (90 °C) |
| [Chol][l-Tyr]a) | <5 (90 °C) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossowicz, P.; Klebeko, J.; Roman, B.; Janus, E.; Rozwadowski, Z. The Relationship between the Structure and Properties of Amino Acid Ionic Liquids. Molecules 2019, 24, 3252. https://doi.org/10.3390/molecules24183252
Ossowicz P, Klebeko J, Roman B, Janus E, Rozwadowski Z. The Relationship between the Structure and Properties of Amino Acid Ionic Liquids. Molecules. 2019; 24(18):3252. https://doi.org/10.3390/molecules24183252
Chicago/Turabian StyleOssowicz, Paula, Joanna Klebeko, Barbara Roman, Ewa Janus, and Zbigniew Rozwadowski. 2019. "The Relationship between the Structure and Properties of Amino Acid Ionic Liquids" Molecules 24, no. 18: 3252. https://doi.org/10.3390/molecules24183252
APA StyleOssowicz, P., Klebeko, J., Roman, B., Janus, E., & Rozwadowski, Z. (2019). The Relationship between the Structure and Properties of Amino Acid Ionic Liquids. Molecules, 24(18), 3252. https://doi.org/10.3390/molecules24183252