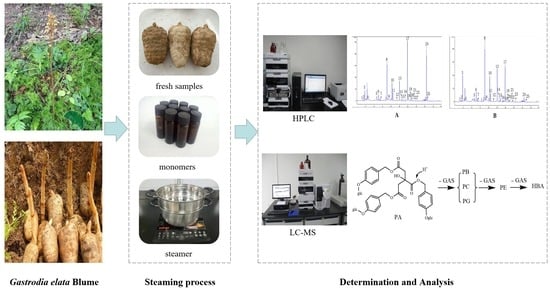
Transformation Mechanisms of Chemical Ingredients in Steaming Process of Gastrodia elata Blume
Abstract
:1. Introduction
2. Results
2.1. Optimization of UHPLC/Q-TOF-MS/MS Conditions
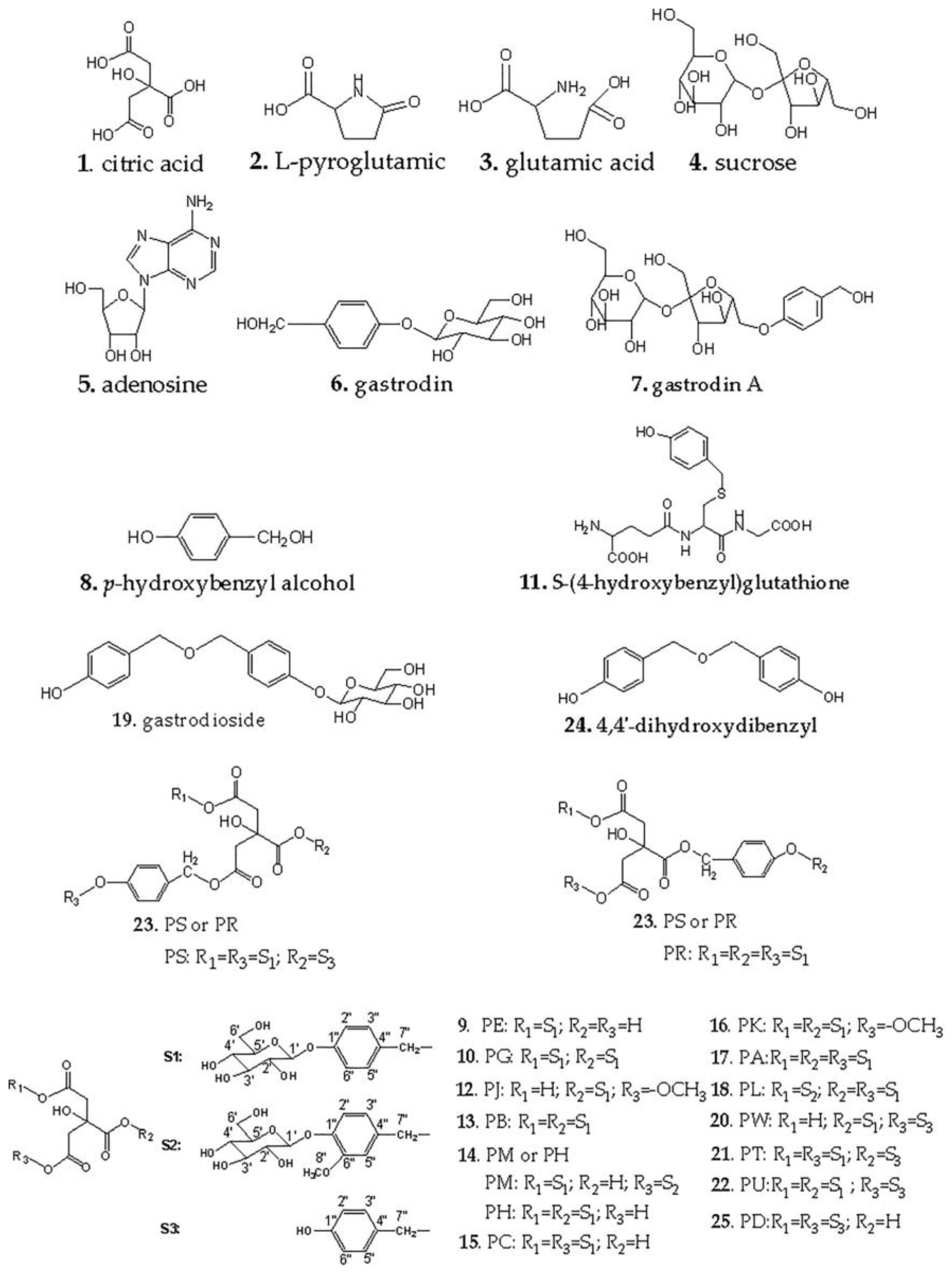
2.2. Structural Elucidation of Parishins by LC/MS
2.3. Effects of Steaming Process on 25 Compounds in RGE
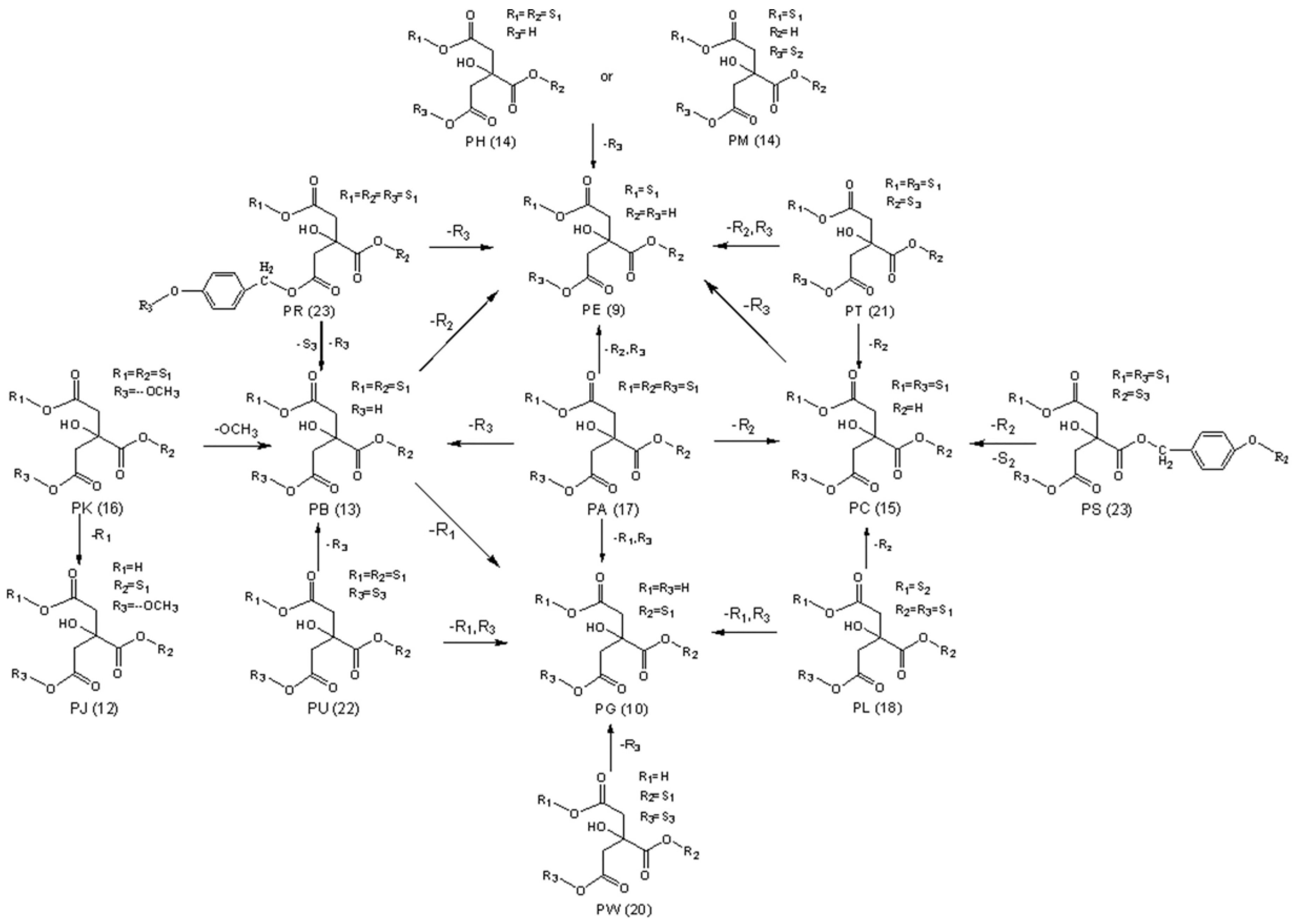
2.4. Summary of Steaming-induced Parishins Conversion in RGE
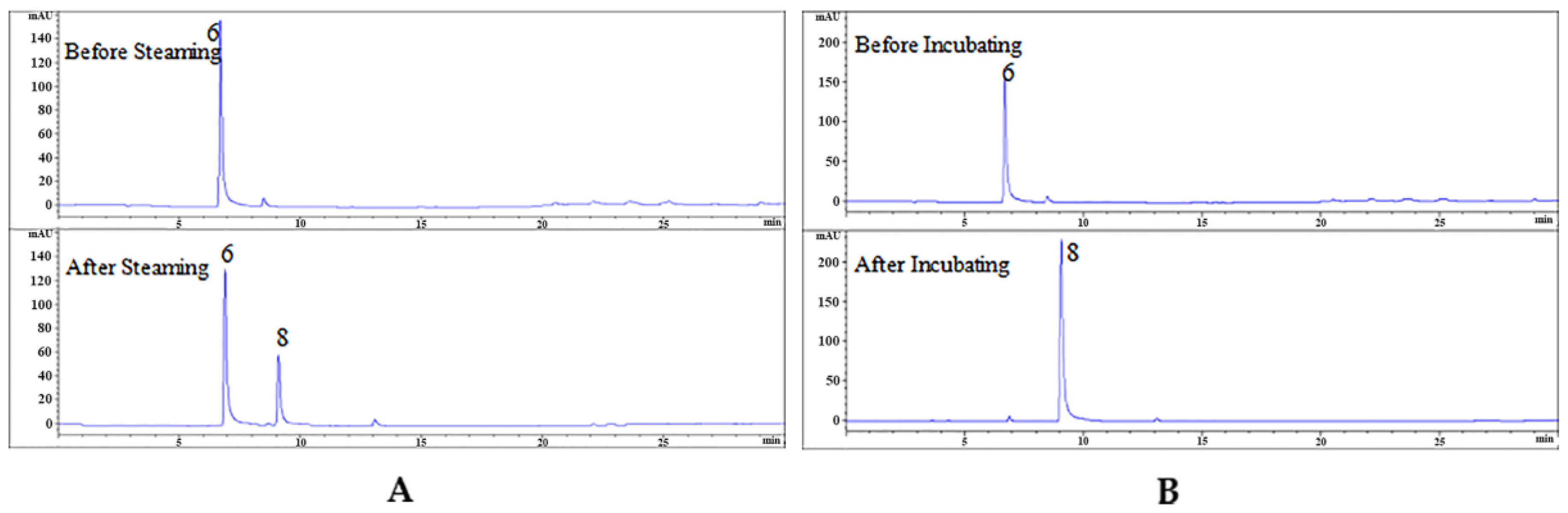
2.5. Experimental Verification of Hydrolysis of Monomeric Compounds
2.5.1. Structural Elucidation of Parishin A after Hydrolysis by LC/MS
2.5.2. Results of 10 Compounds after Hydrolysis
2.6. Enzymatic Hydrolysis of GAS
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Instrumentation
3.4. Sample Preparation
3.4.1. Fresh Products
3.4.2. Steaming Products
3.5. Chromatographic Procedures
3.6. Acid Hydrolysis Experiment
3.7. The β-d-glucosidase Enzymatic Hydrolysis Experiment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Pharmacopoeia Commission of PRC. Pharmacopoeia of the People’s Republic of China (Part 1); China Medical Science and Technology Press: Beijing, China, 2015; pp. 58–59. [Google Scholar]
- Zhan, H.D.; Zhou, H.Y.; Sui, Y.P.; Du, X.L.; Wang, W.H.; Dai, L.; Sui, F.; Huo, H.R.; Jiang, T.L. The rhizome of Gastrodia elata Blume–An ethnopharmacological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.B.; Zhao, T.; Muna, S.S.; Jin, H.M.; Park, J.I.; Jo, K.S.; Lee, B.H.; Chae, S.W.; Kim, S.Y.; Park, S.H.; et al. Therapeutic potential of Gastrodia elata Blume for the treatment of Alzheimer’s disease. Neural Regen. Res. 2013, 8, 1061–1070. [Google Scholar]
- Kumar, H.; Kim, I.S.; More, S.V.; Kim, B.W.; Bahk, Y.Y.; Choi, D.K. Gastrodin protects apoptotic dopaminergic neurons in a toxin-induced Parkinson’s disease model. Evid.-Based Complment. Altern. 2013, 2013, 514095. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H.; Li, C.Y.; Shen, W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer’s disease. Neuropathol. 2014, 34, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Chung, H.; Shim, E.; Jeong, J.K.; Han, B.K.; Choi, H.J.; Hwang, J. Gastrodia elata Blume extract modulates antioxidant activity and ultraviolet A-irradiated skin aging in human dermal fibroblast cells. J. Med. Food. 2016, 19, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.E.; Lin, S.H.; Chen, W.C.; Ho, C.T.; Lai, Y.S.; Panyod, S.; Lai, Y.S.; Ho, C.T.; Sheen, L.Y. Antidepressant-like effects of water extract of Gastrodia elata blume in rats exposed to unpredictable chronic mild stress via modulation of monoamine regulatory pathways. J. Ethnopharmacol. 2016, 187, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Yoon, B.H.; Oh, H.R.; Ahn, J.H.; Kim, S.Y.; Park, S.Y.; Ryu, J.F. Anxiolytic-Like effects of Gastrodia elata and its phenolic constituents in mice. Biol. Pharm. Bull. 2006, 29, 261–265. [Google Scholar] [CrossRef]
- Ahn, E.K.; Jeon, H.J.; Lim, E.J.; Jung, H.J.; Park, E.H. Anti-inflammatory and anti-angiogenic activities of Gastrodia elata Blume. J. Ethnopharmacol. 2007, 110, 476–482. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Kang, R.X.; Shi, J.G.; Liu, G.T.; Zhang, J.J. NHBA isolated from Gastrodia elata exerts sedative and hypnotic effects in sodium pentobarbital-treated mice. Pharmacol. Biochem. Behav. 2012, 102, 450–457. [Google Scholar] [CrossRef]
- Chen, W.C.; Lai, Y.S.; Lin, S.H.; Lu, K.H.; Lin, Y.E.; Panyod, S.; Ho, C.T.; Sheen, L.Y. Anti-depressant effects of Gastrodia elata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling. J. Ethnopharmacol. 2016, 182, 190–199. [Google Scholar] [CrossRef]
- Wang, X.L.; Xing, G.H.; Hong, B.; Li, X.M.; Zou, Y.; Zhang, X.J.; Dong, M.X. Gastrodin prevents motor deficits and oxidative stress in the MPTP mouse model of Parkinson’s disease: Involvement of ERK1/2–Nrf2 signaling pathway. Life Sci. 2014, 114, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, R.; Poursina, M.; Zeraati, F.; Nadi, F. Gastrodin microinjection suppresses 6-OHDA-induced motor impairments in parkinsonian rats: Insights into oxidative balance and microglial activation in SNc. Inflammopharmacology 2018, 26, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hao, X.W.; Yu, L.; Zhang, P.; Cao, W.; Chen, H.Y.; Zhu, D.L. Gastrodin causes vasodilation by activating KATP channels in vascular smooth muscles via PKA-dependent signaling pathway. J. Recept. Signal Transduct. Res. 2017, 37, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Kim, S.W.; Lee, H.K.; Kim, I.D.; Lee, H.; Lee, J.K. Anti-oxidative effects of 4-hydroxybenzyl alcohol in astrocytes confer protective effects in autocrine and paracrine manners. PloS ONE 2017, 12, e0177322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Wang, W.P.; Feng, N.; Wang, L.; Shi, J.G.; Wang, X.L. Parishin C’s prevention of A β (1-42)-induced inhibition of long-term potentiation is related to NMDA receptors. Acta Pharm. Sin. B 2016, 6, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Whang, W.K.; Kim, S.; Bach, J.H.; Kim, J.M.; Nguyen, X.K.T.; Nguyen, T.T.L.; Jung, B.D.; Yamada, K.; Nabeshima, T. Parishin C attenuates phencyclidine-induced schizophrenia-like psychosis in mice: Involvements of 5-HT1A receptor. J. Pharmacol. Sci. 2010, 113, 404–408. [Google Scholar] [CrossRef]
- Lin, Y.F.; Sun, Y.J.; Weng, Y.F.; Matsuura, A.; Xiang, L.; Qi, J.H. Parishin from Gastrodia elata extends the lifespan of yeast via regulation of Sir2/Uth1/TOR signaling pathway. Oxid. Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar]
- Jang, Y.W.; Lee, J.Y.; Kim, C.J. Anti-asthmatic activity of phenolic compounds from the roots of Gastrodia elata Bl. Int. Immunopharmacol. 2010, 10, 147–154. [Google Scholar] [CrossRef]
- Hu, M.H.; Yan, H.; Fu, Y.Y.; Jiang, Y.L.; Yao, W.F.; Yu, S.; Zhang, L.; Wu, Q.N.; Ding, A.W.; Shan, M.Q. Optimal Extraction Study of Gastrodin-Type Components from Gastrodia Elata Tubers by Response Surface Design with Integrated Phytochemical and Bioactivity Evaluation. Molecules 2019, 24, 547. [Google Scholar] [CrossRef]
- Shan, M.Q.; Zhang, L.; Yu, S.; Qian, Y.; Wang, J.Y.; Ding, A.W. Simultaneous determination of eight active components in Gastrodiae Rhizoma by HPLC-MS. Chin. Tradit. Herb. Drugs 2015, 46, 2087–2091. [Google Scholar]
- Li, Y.H.; Zhang, Y.M.; Zhang, Z.J.; Hu, Y.P.; Cui, X.M.; Xiong, Y. Quality Evaluation of Gastrodia Elata Tubers Based on HPLC Fingerprint Analyses and Quantitative Analysis of Multi-Components by Single Marker. Molecules 2019, 24, 1521. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.H.; Wang, D.; Zhang, X.G. Study on the influence factors of gastrodin content in G. elata. Acta Botanica Yunnanica. 2008, 30, 110–114. [Google Scholar]
- Xu, W.; Li, J.; Song, F.; Li, Z.; Zhang, Z. Research Process of the Drying Technology of Chinese Herbal Medicine. Med. Plant 2014, 5, 8–11. [Google Scholar]
- Chen, Y.N.; Dong, H.J.; Li, J.K.; Guo, L.P.; Wang, X. Evaluation of a Nondestructive NMR and MRI Method for Monitoring the Drying Process of Gastrodia elata Blume. Molecules 2019, 24, 236. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Qian, Y.; Yu, S.; Zhang, L.; Ding, A. Study on integrative technology of primary processing for Gastrodiae Rhizoma based on response surface methodology. Chin. Tradit. Herb. Drugs 2016, 47, 420–424. [Google Scholar]
- Tang, C.L.; Wu, B.C.; Wu, J.Y.; Zhang, Z.; Yu, B.C. Novel Strategies Using Total Gastrodin and Gastrodigenin, or Total Gastrodigenin for Quality Control of Gastrodia elata. Molecules 2018, 23, 270. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Wang, Y.W.; Ouyanga, H.; Lu, Y.; Qiu, Y.; Feng, Y.L.; Jiang, H.L.; Zhou, X.; Yang, S.L. A novel dereplication strategy for the identification of two new trace compounds in the extract of Gastrodia elata using UHPLC/Q-TOF-MS/MS. J. Chromatogr. B 2015, 988, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.P.; Xiao, H.; Feng, S.H.; Chen, S. Analysis of Degradation Mechanism of Parishin in Gastrodiae Rhizoma. Chin. J.Exp. Tradit. Med Formulae 2017, 23, 18–21. [Google Scholar]
- Tang, C.L.; Wang, L.; Li, J.J.; Liu, X.X.; Cheng, M.C.; Xiao, H.B. Analysis of the metabolic profile of parishin by ultra-performance liquid chromatography/quadrupole-time of flight mass spectrometry. Biomed. Chromatogr. 2015, 29, 1913–1920. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, Y.; Liu, D.H.; Mu, Y.; Dong, H.J.; Zhou, H.L.; Guo, L.P.; Wang, X. Four new phenolic constituents from the rhizomes of Gastrodia elata Blume. Nat. Prod. Res. 2019, 33, 1140–1146. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, Y.; Liu, D.H.; Mu, Y.; Dong, H.J.; Zhou, H.L.; Wang, X. Chemical constituents from the rhizomes of Gastrodia elata f. glauca and their potential neuroprotective effects. Phytochem. Lett. 2018, 24, 167–171. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 6, 8, 11, 13, 15, 17, 19, 24 are available from the authors. |
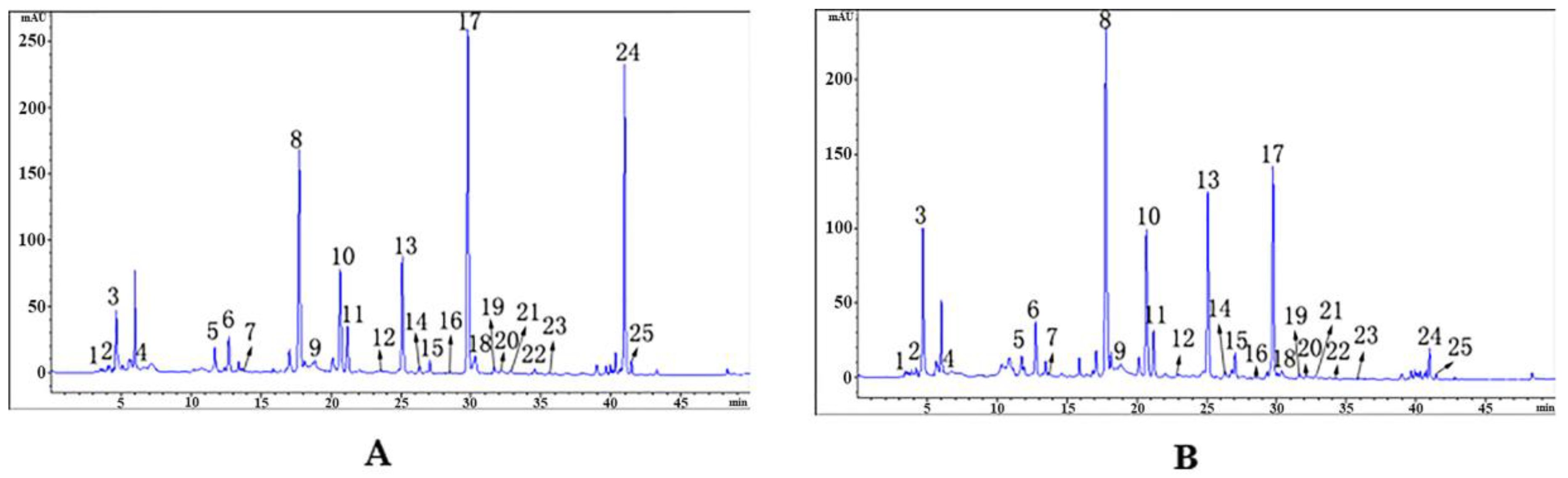
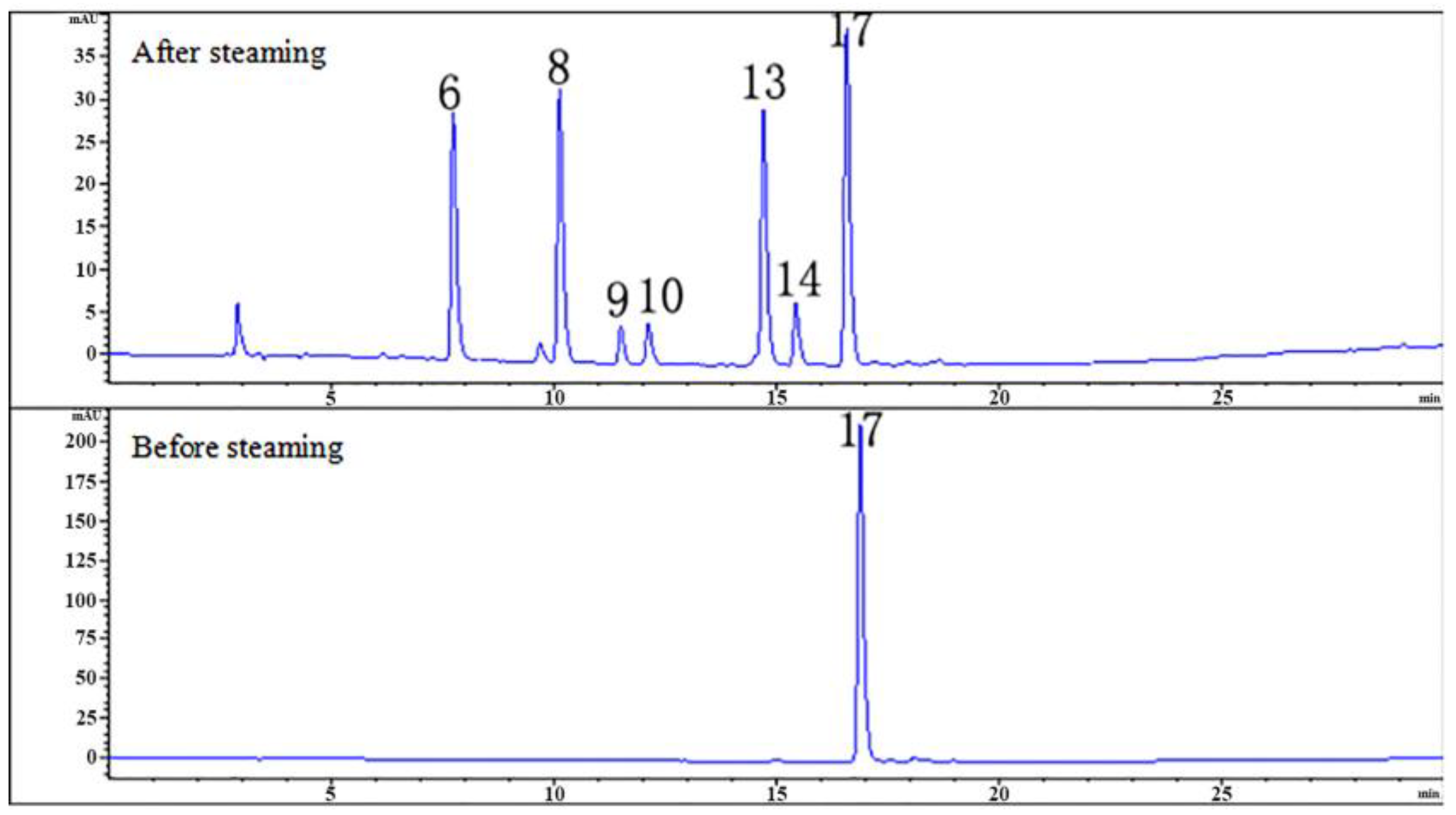
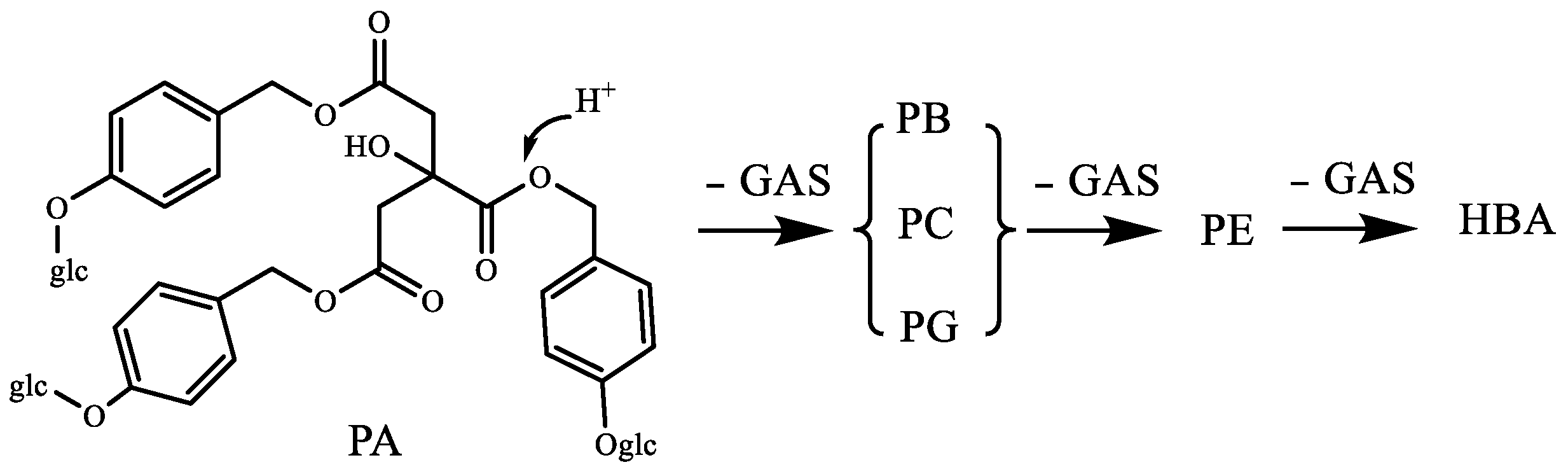
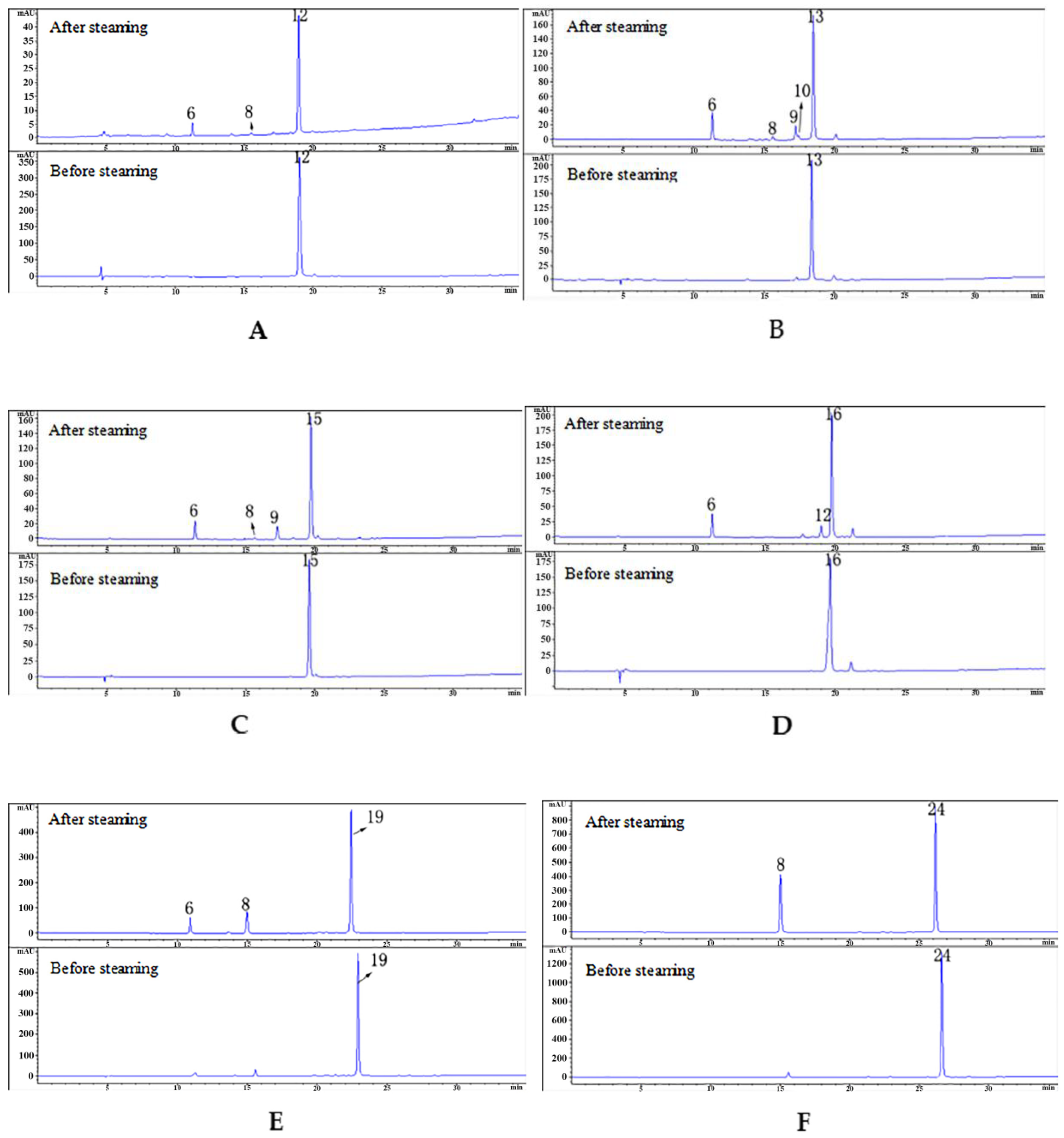
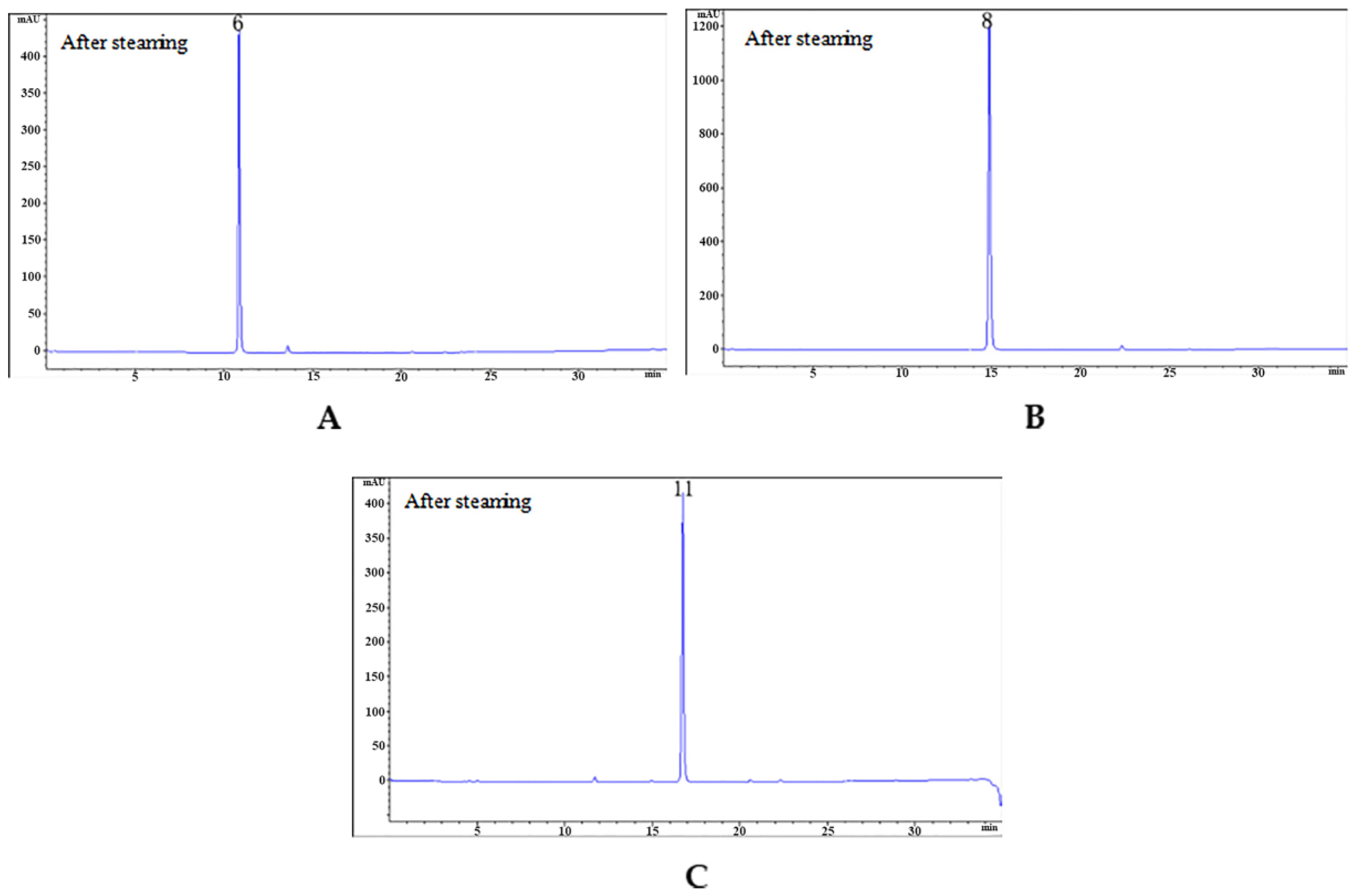
| Peak No. | Retention Time/min | Compound | Molecular Formula | M | Fragment Peaks |
|---|---|---|---|---|---|
| 1 | 3.53 | citric acid | C6H8O7 | 192.0270 | 214.9171 [M + Na]+ |
| 2 | 4.06 | l-pyroglutamic acid | C5H7NO3 | 129.0426 | 130.0490 [M + H]+ |
| 3 | 4.64 | glutamic acid | C5H9NO4 | 147.0532 | 148.0608 [M + H]+ |
| 4 | 5.12 | sucrose | C12H22O11 | 342.1162 | 365.1074 [M + Na]+ |
| 5 | 11.71 | adenosine | C10H13N5O4 | 267.2413 | 268.1044 [M + H]+ |
| 6 | 12.69 | gastrodin | C13H18O7 | 286.1052 | 309.0922 [M + Na]+ |
| 7 | 13.41 | gastrodin A | C19H28O12 | 448.1581 | 471.1473 [M + Na]+ |
| 8 | 17.72 | p-hydroxybenzyl alcohol | C7H8O2 | 124.0524 | 125.0507 [M + H]+ |
| 9 | 18.85 | parishin E | C19H24O13 | 460.1217 | 483.1125 [M + Na]+ |
| 10 | 20.65 | parishin G | C19H24O13 | 460.1217 | 483.1139 [M + Na]+ |
| 11 | 21.17 | S-(4-hydroxybenzyl)glutathion | C17H23N3O7S | 413.4454 | 414.1410 [M + H]+ |
| 12 | 23.54 | parishin J | C20H26O13 | 474.4126 | 497.1252 [M + Na]+ |
| 13 | 25.10 | parishin B | C32H40O19 | 728.2164 | 751.2087 [M + Na]+ |
| 14 | 26.79 | parishin H/M | C33H42O20 | 758.2269 | 757.2201 [M − H]− |
| 15 | 27.05 | parishin C | C32H40O19 | 728.2164 | 751.2060 [M + Na]+ |
| 16 | 28.46 | parishin K | C33H42O19 | 742.6752 | 765.2213 [M + Na]+ |
| 17 | 29.71 | parishin A | C45H56O25 | 996.9111 | 1019.3028 [M + Na]+ |
| 18 | 30.29 | parishin L | C46H58O26 | 1026.3216 | 1049.3098 [M + Na]+ |
| 19 | 31.46 | gastrodioside | C20H24O8 | 392.3998 | 391.1414 [M − H]− |
| 20 | 32.25 | parishin W | C26H30O14 | 566.1636 | 589.1519 [M + Na]+ |
| 21 | 32.82 | parishinT/U | C39H46O20 | 834.2583 | 833.2524 [M − H]− |
| 22 | 34.54 | parishin T/U | C39H46O20 | 834.2583 | 833.2522 [M − H]− |
| 23 | 35.56 | parishin R/S | C52H62O26 | 1102.3529 | 1101.3611 [M − H]− |
| 24 | 40.80 | 4,4’-dihydroxydibenzyl ether | C14H14O3 | 230.0937 | 229.0873 [M − H]− |
| 25 | 41.47 | parishin D | C20H20O9 | 404.1107 | 427.0998 [M + Na]+ |
| Peak No. | Retention Time/min | Compound | Fresh Product (A) | Steamed Product (B) |
|---|---|---|---|---|
| 1 | 3.53 | citric acid | 1 | 1.2599 |
| 2 | 4.06 | l-pyroglutamic acid | 1 | 0.9366 |
| 3 | 4.64 | glutamic acid | 1 | 1.9929 |
| 4 | 5.12 | sucrose | 1 | - |
| 5 | 11.71 | adenosine | 1 | 0.7680 |
| 6 | 12.69 | gastrodin | 1 | 1.3630 |
| 7 | 13.41 | gastrodin A | 1 | 0.5475 |
| 8 | 17.72 | p-hydroxybenzyl alcohol | 1 | 1.3769 |
| 9 | 18.85 | parishin E | 1 | 0.8431 |
| 10 | 20.65 | parishin G | 1 | 1.2545 |
| 11 | 21.17 | S-(4-hydroxybenzyl)glutathione | 1 | 0.8722 |
| 12 | 23.54 | parishin J | 1 | 0.3504 |
| 13 | 25.10 | parishin B | 1 | 1.4318 |
| 14 | 26.79 | parishin H/M | 1 | 0.9091 |
| 15 | 27.05 | parishin C | 1 | 1.7347 |
| 16 | 28.46 | parishinK | 1 | 0.4400 |
| 17 | 29.71 | parishin A | 1 | 0.5532 |
| 18 | 30.29 | parishin L | 1 | 0.2824 |
| 19 | 31.46 | gastrodioside | 1 | 1.0220 |
| 20 | 32.25 | parishin W | 1 | 0.7028 |
| 21 | 32.82 | parishin T/U | 1 | 0.6596 |
| 22 | 34.54 | parishin T/U | 1 | 0.6336 |
| 23 | 35.56 | parishin R/S | 1 | 0.6495 |
| 24 | 40.80 | 4,4’-dihydroxydibenzyl ether | 1 | 0.0844 |
| 25 | 41.47 | parishin D | 1 | 0.3535 |
| Peak No. | Retention Time)/min | Compound | Molecule Formula | M | Fragment Peaks |
|---|---|---|---|---|---|
| 6 | 7.73 | gastrodin | C13H18O7 | 286.1052 | 309.0970 [M + Na]+ |
| 8 | 10.12 | p-hydroxybenzyl alcohol | C7H8O2 | 124.0524 | 125.0507 [M + H]+ |
| 9 | 11.49 | parishin E | C19H24O13 | 460.1269 | 483.1145 [M + Na]+ |
| 10 | 12.11 | parishin G | C19H24O13 | 460.1269 | 483.1141 [M + Na]+ |
| 13 | 14.70 | parishin B | C32H40O19 | 728.2163 | 751.2170 [M + Na]+ |
| 14 | 15.43 | parishin C | C32H40O19 | 728.2163 | 751.2176 [M + Na]+ |
| 17 | 16.57 | parishin A | C45H56O25 | 996.3110 | 997.3108 [M + H]+ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, X.-Q.; Liu, S.-S.; Liu, D.-h.; Wang, X.; Wang, Z.-M. Transformation Mechanisms of Chemical Ingredients in Steaming Process of Gastrodia elata Blume. Molecules 2019, 24, 3159. https://doi.org/10.3390/molecules24173159
Li Y, Liu X-Q, Liu S-S, Liu D-h, Wang X, Wang Z-M. Transformation Mechanisms of Chemical Ingredients in Steaming Process of Gastrodia elata Blume. Molecules. 2019; 24(17):3159. https://doi.org/10.3390/molecules24173159
Chicago/Turabian StyleLi, Yun, Xiao-Qian Liu, Shan-Shan Liu, Da-hui Liu, Xiao Wang, and Zhi-Min Wang. 2019. "Transformation Mechanisms of Chemical Ingredients in Steaming Process of Gastrodia elata Blume" Molecules 24, no. 17: 3159. https://doi.org/10.3390/molecules24173159
APA StyleLi, Y., Liu, X.-Q., Liu, S.-S., Liu, D.-h., Wang, X., & Wang, Z.-M. (2019). Transformation Mechanisms of Chemical Ingredients in Steaming Process of Gastrodia elata Blume. Molecules, 24(17), 3159. https://doi.org/10.3390/molecules24173159