Evaluation of the Antifungal Activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) Essential Oil and Its Synergistic Interaction with Azoles
Abstract
1. Introduction
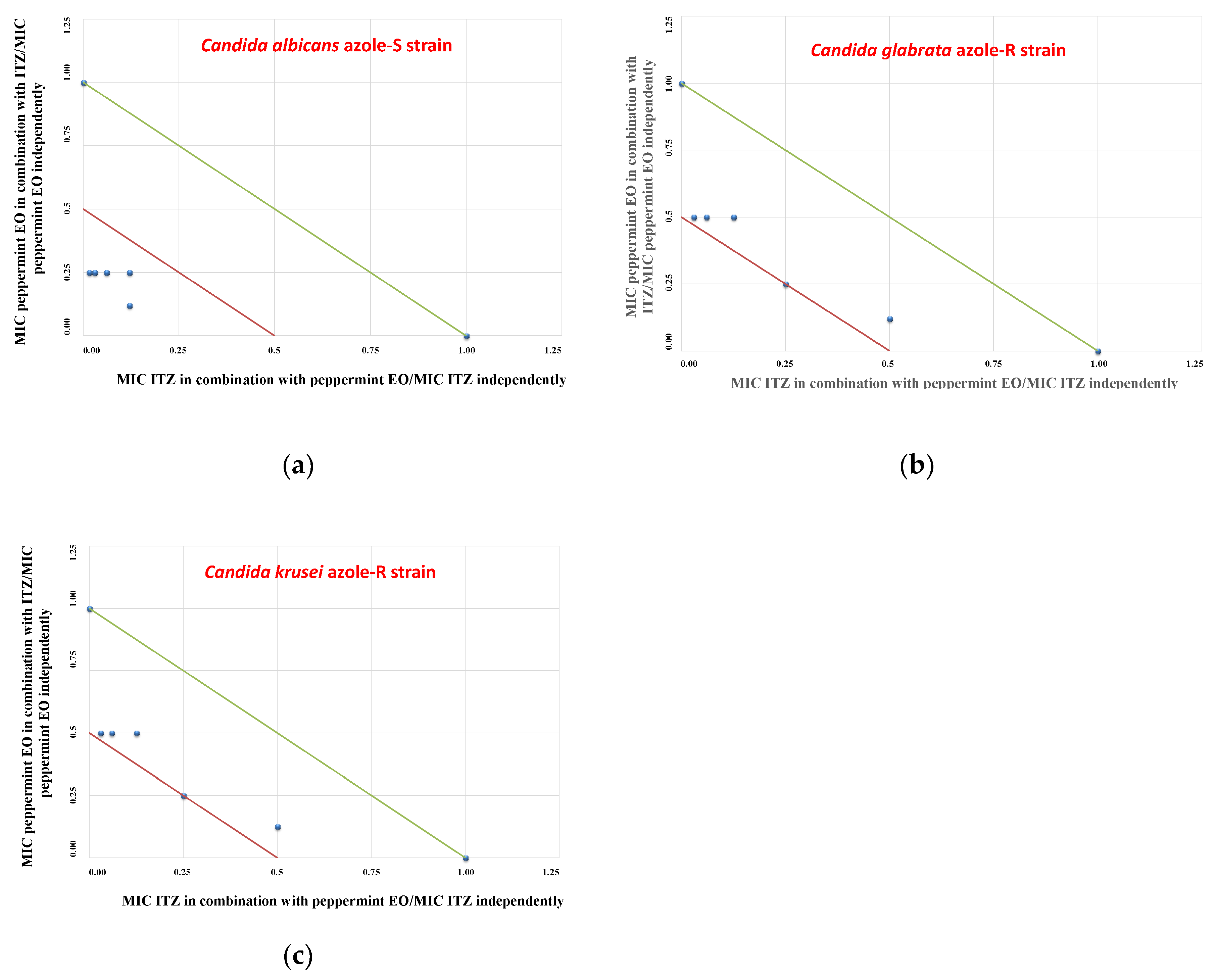
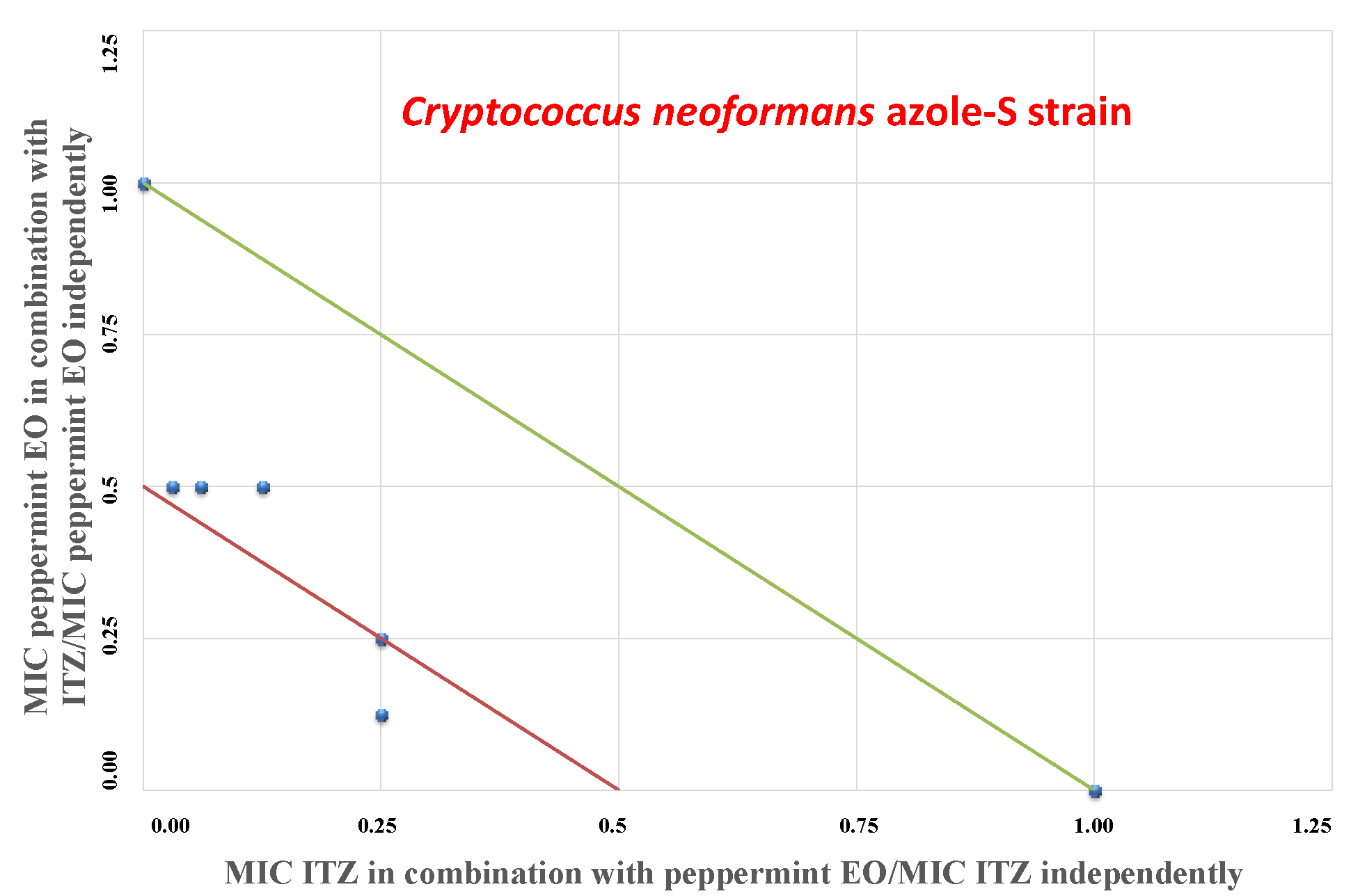
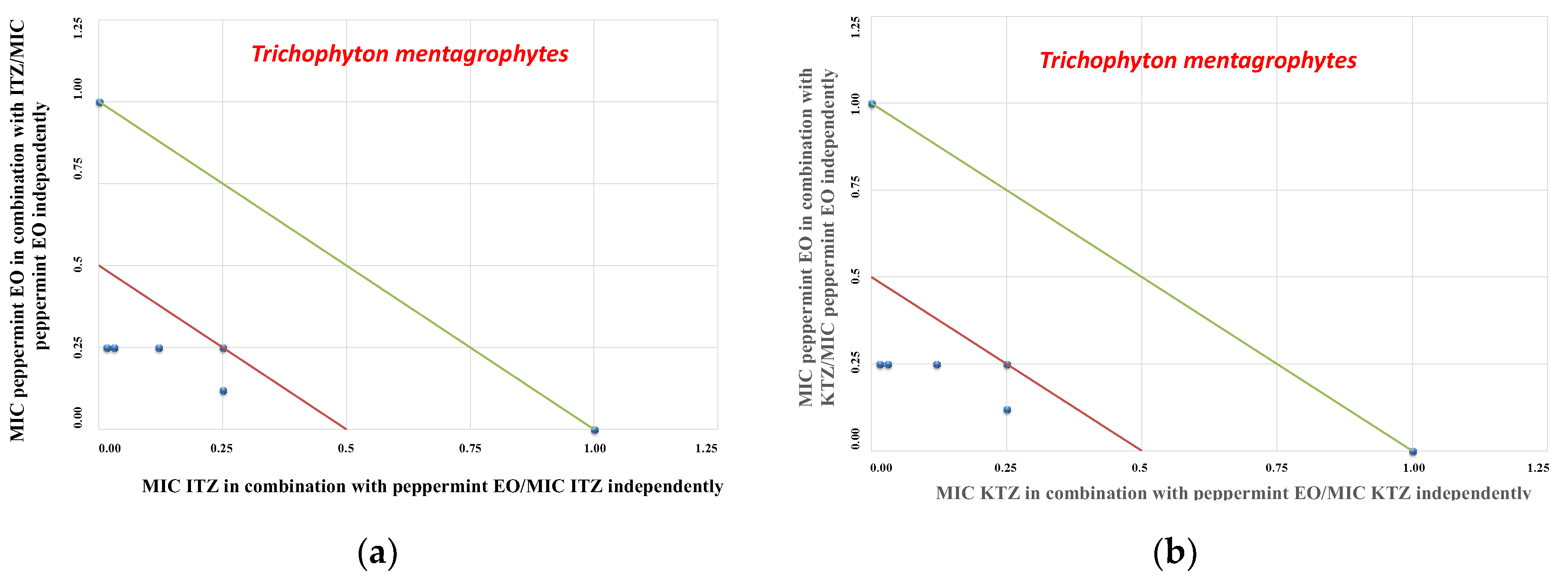
2. Results
3. Discussion
4. Materials and Methods
4.1. Essential Oil
4.2. Antifungal Agents
4.3. Fungal Strains
4.4. In Vitro Antifungal Susceptibility Assays
4.5. Checkerboard Assays and Evaluation of the Fractional Inhibitory Concentration Index
4.6. Isobolograms
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.D.; Vukojevic, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; Van Griensven, L.J.L.D. Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Haran, G.H.; Calisto, V.K.D.S.; Jothi, G.; Quintans, J.D.S.S.; Cuevas, L.E.; Narain, N.; Júnior, L.J.Q.; Cipolotti, R.; et al. Essential oils and its bioactive compounds modulating cytokines: A systematic review on anti-asthmatic and immunomodulatory properties. Phytomedicine 2019, 31, 152854. [Google Scholar] [CrossRef] [PubMed]
- Koulivand, P.H.; Ghadiri, M.K.; Gorji, A. Lavender and the Nervous System. In Corporation Evidence-Based Complementary Alternative Medicine; Article ID681304; Hindawi Publishing: London, UK, 2013; Volume 2013, 10p. [Google Scholar]
- Mittal, R.P.; Rana, A.; Jaitak, V. Essential Oils: An Impending Substitute of Synthetic Antimicrobial Agents to Overcome Antimicrobial Resistance. Curr. Drug Targets 2019, 20, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Ahmad, I.; Khan, M.S.; Ahmad, E.; Tahseen, Q.; Khan, M.S.; Alshabib, N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.Y.; El-Salam, M.M.A. Anti-dermatophyte efficacy and environmental safety of some essential oils commercial and in vitro extracted pure and combined against four keratinophilic pathogenic fungi. Environ. Heal. Prev. Med. 2015, 20, 279–286. [Google Scholar] [CrossRef]
- Marwa, C.; Fikri-Benbrahim, K.; Ou-Yahia, D.; Farah, A. African peppermint (Mentha piperita) from Morocco: chemical composition and antimicrobial properties of essential oil. J. Adv. Pharm. Technol. Res. 2017, 8, 86–90. [Google Scholar] [PubMed]
- Samber, N.; Khan, A.; Varma, A.; Manzoor, N. Synergistic anti-candidal activity and mode of action of Mentha piperita essential oil and its major components. Pharm. Biol. 2015, 53, 1496–1504. [Google Scholar] [CrossRef]
- Heydari, M.; Zanfardino, A.; Taleei, A.; Bushehri, A.A.S.; Hadian, J.; Maresca, V.; Sorbo, S.; Di Napoli, M.; Varcamonti, M.; Basile, A.; et al. Effect of heat stress on yield, monoterpene content and antibacterial activity of essential oils of Mentha x piperita var. Mitcham and Mentha arvensis var. piperascens. Molecules 2018, 23, 1903. [Google Scholar] [CrossRef]
- Fothergill, A.W.; Sutton, D.A.; McCarthy, D.I.; Wiederhold, N.P. Impact of New Antifungal Breakpoints on Antifungal Resistance in Candida Species. J. Clin. Microbiol. 2014, 52, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Woosley, L.N.; Jones, R.N.; Castanheira, M. Echinocandin and Triazole Antifungal Susceptibility Profiles for Clinical Opportunistic Yeast and Mold Isolates Collected from 2010 to 2011: Application of New CLSI Clinical Breakpoints and Epidemiological Cutoff Values for Characterization of Geographic and Temporal Trends of Antifungal Resistance. J. Clin. Microbiol. 2013, 51, 2571–2581. [Google Scholar] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Franchini, C.; Corbo, F.; Carbonara, G.G.; Carrieri, A.; Fracchiolla, G. Elucidation of the synergistic action of Mentha piperita essential oil with common antimicrobials. PLoS ONE 2018, 13, e0200902. [Google Scholar] [CrossRef] [PubMed]
- Carretto, C.F.P.; Almeida, R.B.A.; Furlan, M.R.; Jorge, A.O.C.; Junqueira, J.C. Antimicrobial activity of Mentha piperita L. against Candida spp. Braz. Dent. Sci. 2010, 13, 4–9. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Motamedi, M.; Zomorodian, K.; Pakshir, K.; Miri, R.; Hemyari, K. Chemical Composition, Antifungal and Antibiofilm Activities of the Essential Oil of Mentha piperita L. ISRN Pharm. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sandasi, M.; Leonard, C.; Van Vuuren, S.; Viljoen, A. Peppermint (Mentha piperita) inhibits microbial biofilms in vitro. S. Afr. J. Bot. 2011, 77, 80–85. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.; Qiu, J.-Z.; Wang, J.-F.; Luo, M.-J.; Li, H.-E.; Leng, B.-F.; Ren, W.-Z.; Deng, X.-M. Peppermint Oil Decreases the Production of Virulence-Associated Exoproteins by Staphylococcus aureus. Molecules 2011, 16, 1642–1654. [Google Scholar] [CrossRef]
- da Cruz Almeida, E.T.; de Souza, G.T.; de Sousa Guedes, J.P.; Barbosa, I.M.; de Sousa, C.P.; Castellano, L.R.C.; Magnani, M.; de Souza, E.L. Mentha piperita L. essential oil inactivates spoilage yeasts in fruit juices through the perturbation of different physiological functions in yeast cells. Food Microbiol. 2019, 82, 20–29. [Google Scholar] [CrossRef]
- Scalas, D.; Mandras, N.; Roana, J.; Tardugno, R.; Cuffini, A.M.; Ghisetti, V.; Benvenuti, S.; Tullio, V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Complement. Altern Med. 2018, 18, 143. [Google Scholar] [CrossRef]
- Mazzucotelli, M.; Bicchi, C.; Marengo, A.; Rubiolo, P.; Galli, S.; Anderson, J.L.; Sgorbini, B.; Cagliero, C. Ionic liquids as stationary phases for gas chromatography—Unusual selectivity of ionic liquids with a phosphonium cation and different anions in the flavor, fragrance and essential oil analyses. J. Chromatogr. A 2019, 1583, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.; Cuffini, A.M.; Alonzo, V.; Carlone, N. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Mandras, N.; Nostro, A.; Roana, J.; Scalas, D.; Banche, G.; Ghisetti, V.; Del Re, S.; Fucale, G.; Cuffini, A.M.; Tullio, V. Liquid and vapour-phase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement. Altern. Med. 2016, 16, 330. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts-Third Edition: Approved Standard M27-A3; CLSI: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement; Document M27-S4; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard CLSI Document M38-A2; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1, 8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.D.S.; Rodrigues, A.B.L.; Farias, A.L.F.; Simões, R.C.; Pinheiro, M.T.; Ferreira, R.M.D.A.; Barbosa, L.M.C.; Souto, R.N.P.; Fernandes, J.B.; Santos, L.D.S.; et al. Chemical composition and in vitro antioxidant, cytotoxic, antimicrobial, and larvicidal activities of the essential oil of Mentha piperita L. (Lamiaceae). Sci. World J. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds “Mentha of Pancalieri” EO, fluconazole, itraconazole, ketoconazole, and fungal clinical strains (yeasts and dermatophytes) are available from the authors. |
| Main Components | Mentha of Pancalieri EO (Peak Area %) 1 | Eur. Ph. 8th ed. (Peak Area %) |
|---|---|---|
| Limonene | 1.8 | 1–3.5 |
| 1,8-Cineole | 5.3 | 3.5–8 |
| Menthone | 21.8 2 | 14–32 |
| Isomenthone | 1.5 | 1.5–10 |
| Menthil-acetate | 4.8 | 2.8–10 |
| Isopulegole | 0.16 | max 0.2 |
| Menthol | 41.7 2 | 30–55 |
| Species | Isolates (no.) | MIC Range (%, v/v) | MFC Range (%, v/v) |
|---|---|---|---|
| Candida spp. | |||
| Candida albicans | ATCC 90028 | 0.5 | 1 |
| Candida albicans | 6 | 0.5–1 | 0.5–1 |
| Candida glabrata | ATCC 90030 | 0.5 | 0.5 |
| Candida glabrata | 2 | 0.5 | 0.5–1 |
| Candida krusei | 1 | 0.25 | >1 |
| Candida parapsilosis | 1 | 0.5 | 0.5 |
| Candida tropicalis | 1 | 1 | 1 |
| Candida valida | 2 | 0.25–0.5 | 0.25–0.5 |
| Candida lusitaniae | 1 | 0.5 | 0.5 |
| Candida norvegensis | 2 | 0.25–0.5 | 0.25–0.5 |
| non-Candida spp. | |||
| Cryptococcus neoformans | 7 | 0.06–0.125 | 0.06–0.125 |
| Saccharomyces cerevisiae | 4 | 0.125–0.5 | 0.25–1 |
| Pichia carsonii | 2 | 0.125–0.25 | 0.125–1 |
| Sporobolomyces salmonicolor | 1 | 0.5 | 1 |
| Kloekera japonica | 1 | 0.5 | 0.5 |
| Dermatophytes | |||
| Trichophyton mentagrophytes | 2 | 0.5 | >1 |
| Microsporum canis | 2 | 0.125 | >1 |
| Microsporum gypseum | 1 | 0.125 | >1 |
| Dermatophytes (no.) | Mentha of Pancalieri EO (%, v/v) | Menthol (%, v/v) | Menthone (%, v/v) | |
|---|---|---|---|---|
| Trichophyton mentagrophytes (2) | MIC | 0.5 | 0.06 | 0.5 |
| MFC | >1 | 0.25 | 1 | |
| Microsporum canis (2) | MIC | 0.125 | 0.25 | 0.5–1 |
| MFC | >1 | 1 | >1 | |
| Microsporum gypseum (1) | MIC | 0.125 | 0.25 | 1 |
| MFC | >1 | 1 | 1 |
| Antifungals | C. albicans Azole-S Strain 3 | C. glabrata Azole-S */R ** Strain3 | C. krusei Azole-R Strain 3 | |
|---|---|---|---|---|
| Mentha of Pancalieri EO | MIC (%, v/v) alone | 0.5 | 0.5 | 0.25 |
| Fluconazole | MIC (µg/mL) alone | 0.12 | 4 * | 64 |
| FIC of EO | 0.5 | 0.5 | 1 | |
| FIC of FLC | 0.12 | 0.12 | 1 | |
| FICI | 0.62 | 0.62 | 2 | |
| Interpretation | ADD 4 | ADD | IND | |
| Mentha of Pancalieri EO | MIC (%, v/v) alone | 0.5 | 0.5 | 0.25 |
| Itraconazole | MIC (µg/mL) alone | 0.5 | 2 ** | 2 |
| FIC of EO | 0.12 | 0.25 | 0.25 | |
| FIC of ITZ | 0.12 | 0.25 | 0.25 | |
| FICI | 0.24 | 0.5 | 0.5 | |
| Interpretation | SYN 4 | SYN | SYN |
| Antifungals | C. neoformans Azole-S Strain | C. neoformans Azole-R Strain | |
|---|---|---|---|
| Mentha of Pancalieri EO | MIC (%, v/v) alone | 0.06 | 0.06 |
| Itraconazole | MIC (µg/mL) alone | 0.5 | 2 |
| FIC of EO | 0.12 | 0.5 | |
| FIC of ITZ | 0.25 | 0.12 | |
| FICI | 0.37 | 0.62 | |
| Interpretation | SYN 3 | ADD |
| Antifungals | Trichophyton mentagrophytes | Microsporum canis | Microsporum gypseum | |
|---|---|---|---|---|
| Mentha of Pancalieri EO | MIC (%, v/v) alone | 0.5 | 0.125 | 0.125 |
| Itraconazole | MIC (µg/mL) alone | 0.5 | 1 | 1 |
| FIC of EO | 0.25 | 1 | 1 | |
| FIC of ITZ | 0.12 | 0.03 | 0.125 | |
| FICI | 0.37 | 1.03 | 1.125 | |
| Interpretation | SYN 3 | IND 3 | IND | |
| Mentha of Pancalieri EO | MIC (%, v/v) alone | 0.5 | 0.125 | 0.125 |
| Ketoconazole | MIC (µg/mL) alone | 2 | 4 | 2 |
| FIC of EO | 0.25 | 1 | 1 | |
| FIC of KTZ | 0.12 | 0.015 | 0.015 | |
| FICI | 0.37 | 1.015 | 1.015 | |
| Interpretation | SYN | IND | IND |
| Compounds | Trichophyton mentagrophytes | |
|---|---|---|
| Menthol | MIC (%, v/v) alone | 0.06 |
| Itraconazole | MIC (µg/mL) alone | 0.5 |
| FIC of menthol | 0.5 | |
| FIC of ITZ | 0.5 | |
| FICI | 1 | |
| Interpretation | ADD 3 | |
| 0.125 | ||
| Menthol | MIC (%, v/v) alone | 0.06 |
| Ketoconazole | MIC (µg/mL) alone | 2 |
| FIC of menthol | 0.5 | |
| FIC of KTZ | 0.5 | |
| FICI | 1 | |
| Interpretation | ADD | |
| Menthone | MIC (%, v/v) alone | 0.5 |
| Itraconazole | MIC (µg/mL) alone | 0.5 |
| FIC of menthone | 0.25 | |
| FIC of ITZ | 0.5 | |
| FICI | 0.75 | |
| Interpretation | ADD | |
| 0.125 | ||
| Menthone | MIC (%, v/v) alone | 0.5 |
| Ketoconazole | MIC (µg/mL) alone | 2 |
| FIC of menthone | 0.5 | |
| FIC of KTZ | 0.5 | |
| FICI | 1 | |
| Interpretation | ADD | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Evaluation of the Antifungal Activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) Essential Oil and Its Synergistic Interaction with Azoles. Molecules 2019, 24, 3148. https://doi.org/10.3390/molecules24173148
Tullio V, Roana J, Scalas D, Mandras N. Evaluation of the Antifungal Activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) Essential Oil and Its Synergistic Interaction with Azoles. Molecules. 2019; 24(17):3148. https://doi.org/10.3390/molecules24173148
Chicago/Turabian StyleTullio, Vivian, Janira Roana, Daniela Scalas, and Narcisa Mandras. 2019. "Evaluation of the Antifungal Activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) Essential Oil and Its Synergistic Interaction with Azoles" Molecules 24, no. 17: 3148. https://doi.org/10.3390/molecules24173148
APA StyleTullio, V., Roana, J., Scalas, D., & Mandras, N. (2019). Evaluation of the Antifungal Activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) Essential Oil and Its Synergistic Interaction with Azoles. Molecules, 24(17), 3148. https://doi.org/10.3390/molecules24173148









