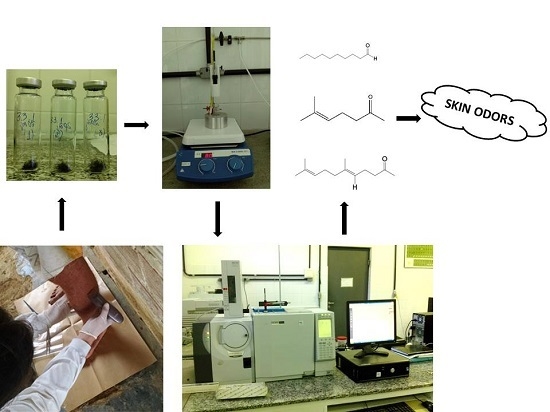
Determination and Profiling of Human Skin Odors Using Hair Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Pilot Test
- (a)
- Sample Collection
- (b)
- SPME-HS/GC-MS Procedures
- (c)
- Data Analysis
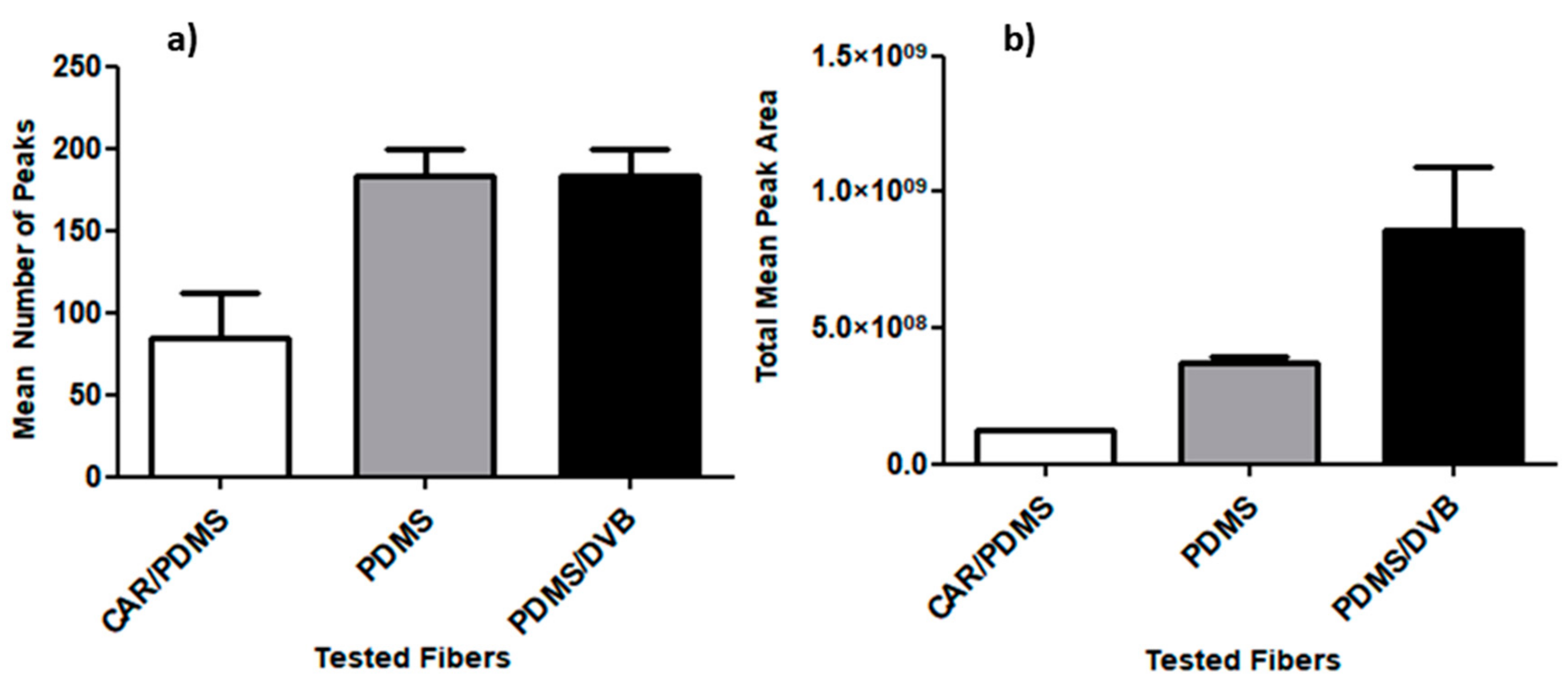
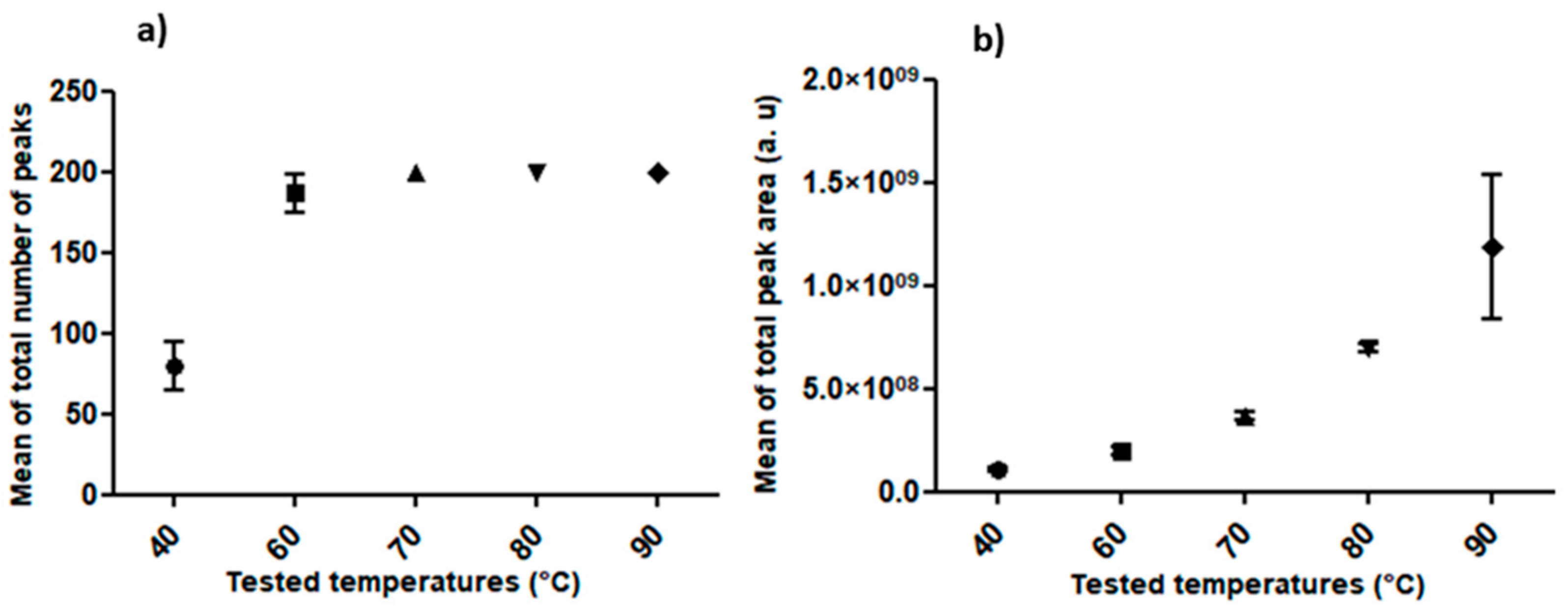
2.2. Standardization of HS-SPME Method Parameters for Human Hair Analysis
- (a)
- SPME Fiber Selection
- (b)
- Extraction Temperature
- (c)
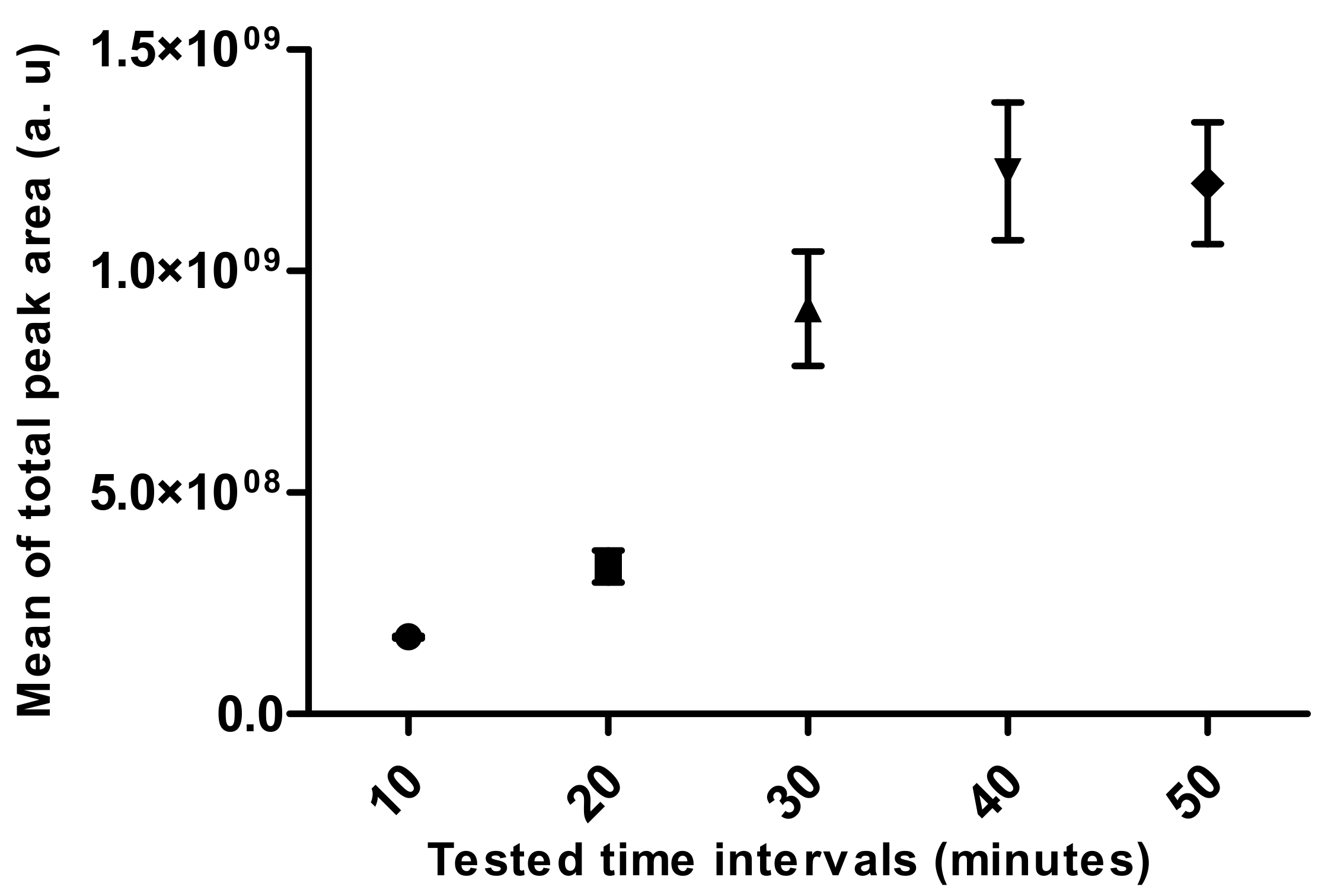
- Extraction Time
- (d)
- Amount of Hair for Sample (mg)
- (e)
- Data Analysis
2.3. Analysis of Human Hair as a Matrix for Skin Odor Profile Determination Using HS-SPME/GC-MS—Laboratory and Field Applicability
- (a)
- Laboratory Sampling and HS-SPME/GC-MS Conditions
- (b)
- Volatile Organic Compound (VOC) Identification
- (c)
- Data Analysis
2.4. Field Applicability of the Standardized Method for Skin Odor Collection and Laboratory Analyses
- (a)
- Field Sampling
- (b)
- Laboratory Analysis
- (c)
- Ethics Statement
- (d)
- Data Analysis
3. Results
3.1. Pilot Test
3.2. Standardization of HS-SPME Method Parameters for Human Hair Analysis
3.3. Analysis of Human Hair as a Matrix for Skin Odor Profile Determination by HS-SPME/GC-MS
3.4. Field Applicability of the Standardized Method for Skin Odor Profile Determination and Laboratory Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dormont, L.; Bessière, J.; Cohuet, A. Human Skin Volatiles: A Review. J. Chem. Ecol. 2013, 39, 569–578. [Google Scholar]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014, 43, 1423–1449. [Google Scholar]
- Logan, J.G.; Birkett, M.A.; Clark, S.J.; Powers, S.; Seal, N.J.; Wadhams, L.J.; Mordue, A.J.; Pickett, J.A. Identification of Human-Derived Volatile Chemicals that Interfere with Attraction of Aedes aegypti Mosquitoes. J. Chem. Ecol. 2008, 34, 308–322. [Google Scholar]
- Smallegange, R.C.; Van Gemert, G.J.; Van De Vegte-Bolmer, M.; Gezan, S.; Takken, W.; Sauerwein, R.W.; Logan, J.G. Malaria Infected Mosquitoes Express Enhanced Attraction to Human Odour. PLoS ONE 2013, 8, 1–3. [Google Scholar]
- Verhulst, N.O.; Weldegergis, B.T.; Menger, D.; Takken, W. Attractiveness of volatiles from different body parts to the malaria mosquito Anopheles coluzzii is affected by deodourant compounds. Sci. Rep. 2016, 1, 1–9. [Google Scholar]
- Pandey, S.K.; Kim, K. Human body-odour components and their determination. Trends Anal. Chem. 2011, 30, 784–796. [Google Scholar]
- Vas, G.; Vekey, K. Solid-phase microextraction: A powerful sample preparation tool prior to mass spectrometric analysis. J. Mass Spectrom. 2004, 33, 233–254. [Google Scholar]
- Gallagher, M.; Wysocki, C.J.; Leyden, J.J.; Spielman, A.I.; Sun, X.; Preti, G. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 2008, 159, 780–791. [Google Scholar]
- Dormont, L.; Bessière, J.M.; McKey, D.; Cohuet, A. New methods for field collection of human skin volatiles and perspectives for their application in the chemical ecology of human–pathogen–vector interactions. J. Exp. Biol. 2013, 216, 2783–2788. [Google Scholar]
- Ara, K.; Hama, M.; Akiba, S.; Koike, K.; Okisaka, K.; Hagura, T.; Kamiya, T.; Tomita, F. Foot odour due to microbial metabolism and its control. Can. J. Microbiol. 2006, 52, 357–364. [Google Scholar]
- Prada, P.A.; Curran, A.M.; Furton, K.G. Comparison of extraction methods for the removal of volatile organic compounds (VOCs) present in sorbents used for human scent evidence. Anal. Methods 2010, 2, 70–78. [Google Scholar]
- Valente, A.L.P.; Augusto, F. Microextração por fase sólida. Quím. Nova 2000, 23, 523–530. [Google Scholar]
- Bernier, U.R.; Booth, M.M.; Yost, R.A. Analysis of Human Skin Emanations by Gas Chromatography/Mass Spectrometry. 1. Thermal Desorption of Attractants for the Yellow Fever Mosquito (Aedes aegypti) from Handled Glass Beads. Anal. Chem. 1999, 71, 1–7. [Google Scholar]
- Penn, D.J.; Oberzaucher, E.; Grammer, K.; Fischer, G.; Soini, H.A.; Wiesler, D.; Novotny, M.V.; Dixon, S.J.; Xu, Y.; Brereton, R.G. Individual and gender fingerprints in human body odour. J. R. Soc. Interface 2007, 4, 331–340. [Google Scholar]
- Verhulst, N.O.; Beijleveld, H.; Qiu, Y.T.; Maliepaard, C.; Verduyn, W.; Haasnoot, G.W.; Claas, F.H.; Mumm, R.; Bouwmeester, H.J.; Takken, W.; et al. Relation between HLA genes, human skin volatiles and attractiveness of humans to malaria mosquitoes. Infect. Genet. Evol. 2013, 18, 87–93. [Google Scholar]
- Strano-Rossi, S.; Chiarotti, M. Solid-Phase Microextraction for Cannabinoids Analysis in Hair and its Possible Application to Other Drugs. J. Anal. Toxicol. 1999, 23, 7–10. [Google Scholar]
- Pragst, F.; Auwaerter, V.; Sporkert, F.; Spiegel, K. Analysis of fatty acid ethyl esters in hair as possible markers of chronically elevated alcohol consumption by headspace solid-phase microextraction (HS-SPME) and gas chromatography-mass scpectometry (GC-MS). Forensic Sci. Int. 2001, 121, 76–88. [Google Scholar]
- De Toledo, F.C.; Yonamine, M.; De Moraes Moreau, R.L.; Silva, O.A. Determination of cocaine, benzoylecgonine and cocaethylene in human hair by solid-phase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. B 2003, 798, 361–365. [Google Scholar]
- Bermejo, A.M.; López, P.; Álvarez, I.; Tabernero, M.J.; Fernández, P. Solid-phase microextraction for the determination of cocaine and cocaethylene in human hair by gas chromatography–mass spectrometry. Forensic Sci. Int. 2006, 156, 2–8. [Google Scholar]
- De Oliveira, L.S.; Rodrigues, F.D.; De Oliveira, F.S.; Mesquita, P.R.; Leal, D.C.; Alcântara, A.C.; Souza, B.M.; Franke, C.R.; Pereira, P.A.; De Andrade, J.B. Headspace solid phase microextraction/gas chromatography–mass spectrometry combined to chemometric analysis for volatile organic compounds determination in canine hair: A new tool to detect dog contamination by visceral leishmaniasis. J. Chromatogr. B 2008, 875, 392–398. [Google Scholar]
- Magalhães-Junior, J.T.; Mesquita, P.R.R.; Oliveira, W.F.S.; Oliveira, F.S.; Franke, C.R.; Rodrigues, F.M.; de Andrade, J.B.; Barrouin-Melo, S.M. Identification of biomarkers in the hair of dogs: new diagnostic possibilities in the study and control of visceral leishmaniasis. Anal. Bioanal. Chem. 2014, 406, 6691–6700. [Google Scholar]
- Zellner, B.A.; Bicchi, C.; Dugo, P.; Rubiolo, P.; Dugo, G.; Mondello, L. Linear retention indices in gas chromatographic analysis: A review Linear retention indices in gas chromatographic analysis. Flavour Fragr. J. 2008, 23, 297–314. [Google Scholar]
- Jiang, R.; Cudjoe, E.; Bojko, B.; Abaffy, T.; Pawliszyn, J. A non-invasive method for in vivo skin volatile compounds sampling. Anal. Chim. Acta 2013, 804, 111–119. [Google Scholar]
- Zhang, Z.; Cai, J.; Ruan, G.; Li, G. The study of fingerprint characteristics of the emanations from human arm skin using the original sampling system by SPME-GC/MS. J. Chromatogr. B 2005, 822, 244–252. [Google Scholar]
- Curran, A.M.; Rabin, S.I.; Prada, P.A.; Furton, K.G. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 2005, 31, 1607–1609. [Google Scholar]
- Meijerink, J.; Braks, M.A.; Brack, A.A.; Adam, W.; Dekker, T.; Posthumus, M.A.; Van Beek, T.A.; Van Loon, J.J. Identification of olfactory stimulants for Anopheles gambiae from human sweat samples. J. Chem. Ecol. 2000, 26, 1367–1382. [Google Scholar]
- Haze, S.; Gozu, Y.; Nakamura, S.; Kohno, Y.; Sawano, K.; Ohta, H.; Yamazaki, K. 2-Nonenal Newly Found in Human Body Odour Tends to Increase with Aging. J. Invest. Dermatol. 2001, 116, 520–524. [Google Scholar]
- Curran, A.M.; Ramirez, C.F.; Schoon, A.A.; Furton, K.G. The frequency of occurrence and discriminatory power of compounds found in human scent across a population determined by SPME-GC/MS. J. Chromatogr. B 2007, 846, 86–97. [Google Scholar]
Sample Availability: Not available. |
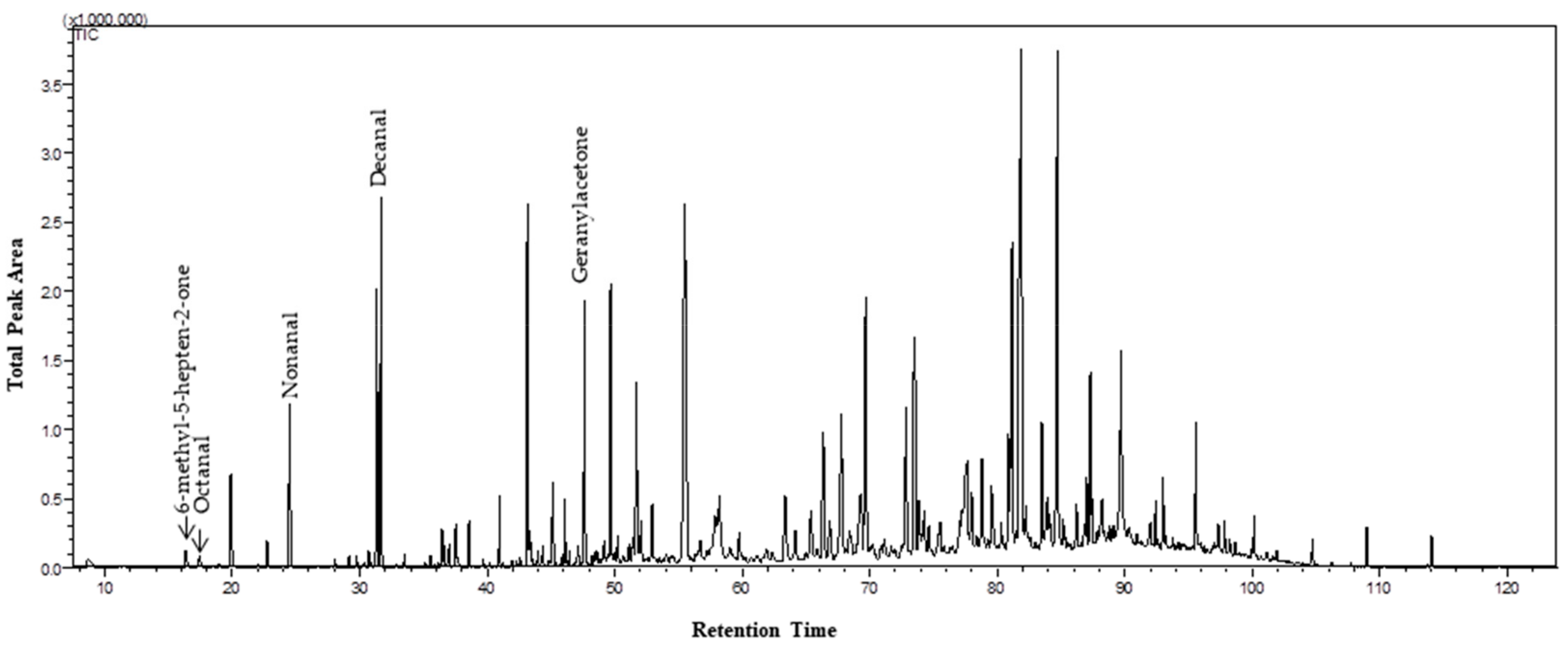
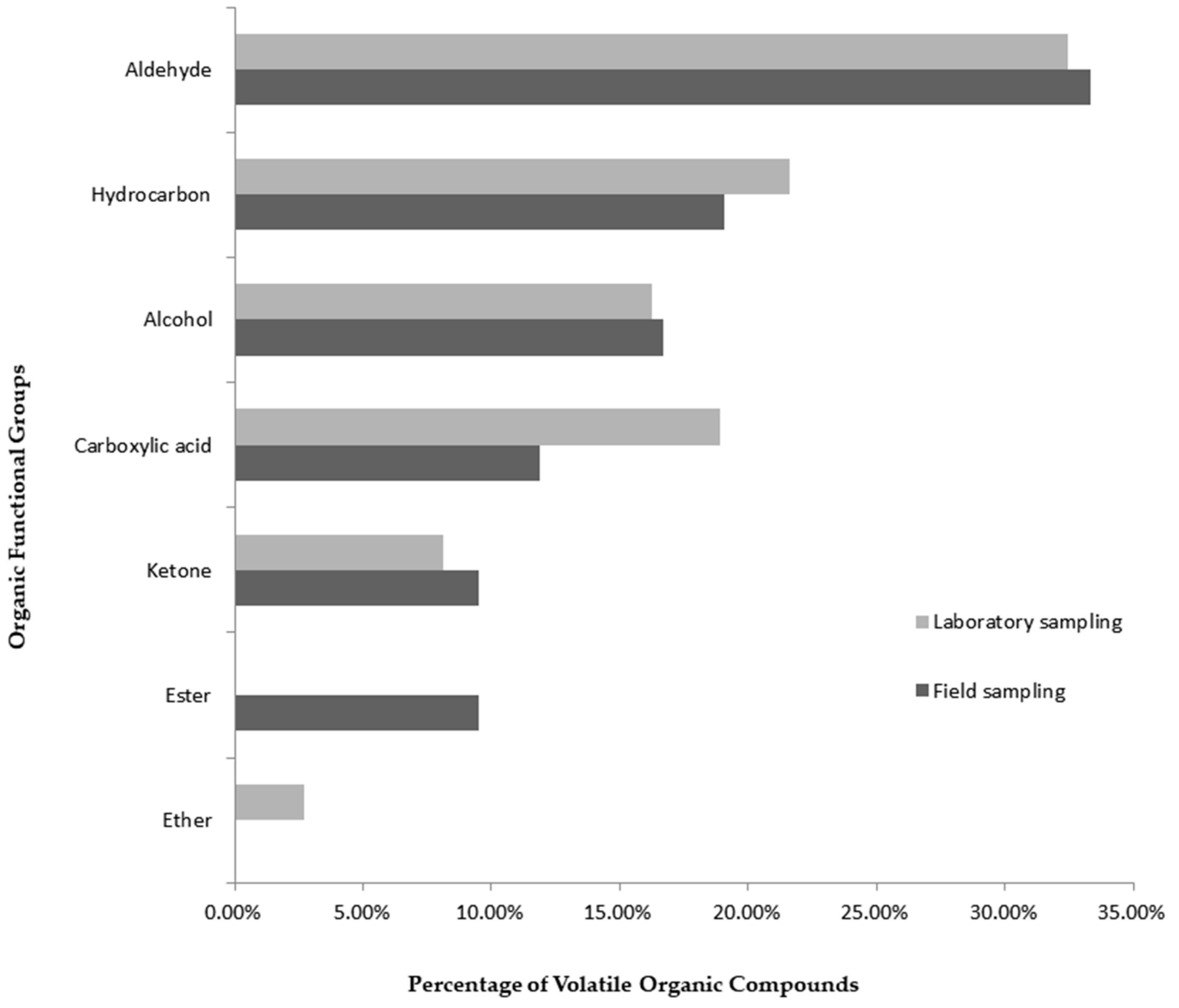
| VOC | Nomenclature (IUPAC) | RT (min) | Functional Group | LRIexp. | LRIlit. | Lab. Samp.–Content (%) | Field Samp.–Content (%) | CSASI | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6-methyl-5-hepten-2-one | 16.10 | Ketone | 962 | 963 | 0.21 | 0.55 | X | [4], [6], [7], [10], [13], [23] |
| 2 | Octanal | 17.15 | Aldehyde | 978 | 978 | 0.27 | 0.51 | X | [4], [6], [7], [9], [10], [13], [24] |
| 3 | 2-phenylacetaldehyde | 18.68 | Aldehyde | 1002 | 1002 | 0.21 | 0.04 | X | [15] |
| 4 | 1-octanol | 22.42 | Alcohol | 1055 | 1055 | 0.03 | 0.06 | X | [4], [9], [13] |
| 5 | 6-methyl-3,5-heptadien-2-one | 23.74 | Ketone | 1074 | 1074 | 0 | 0.05 | [13] | |
| 6 | Nonanal | 24.21 | Aldehyde | 1081 | 1081 | 1.36 | 6.59 | X | [4], [6], [7], [9], [10], [24] |
| 7 | Cis-verbenol | 27.00 | Alcohol | 1121 | 1121 | 0 | 0.02 | - | |
| 8 | 2-nonenal | 27.76 | Aldehyde | 1131 | 1130 | 0.08 | 0.77 | [4], [6], [9] | |
| 9 | 1-nonanol | 29.50 | Alcohol | 1156 | 1157 | 0.12 | 0.27 | X | [4], [10], [13] |
| 10 | Verbenone | 30.42 | Ketone | 1169 | 1176 | 0 | 0.42 | X | - |
| 11 | Decanal | 31.36 | Aldehyde | 1183 | 1183 | 3.87 | 8.90 | X | [4], [6], [9], [10], [13], [24] |
| 12 | 2-decenal | 34.85 | Aldehyde | 1234 | 1236 | 0 | 0.24 | [4], [6] | |
| 13 | Decanol | 36.29 | Alcohol | 1255 | 1255 | 0.20 | 0 | [9] | |
| 14 | Nonanoic acid | 36.91 | Carboxylic acid | 1264 | 1268 | 0.04 | 1.06 | X | [6] |
| 15 | 2-undecanone | 37.43 | Ketone | 1272 | 1272 | 0.23 | 0 | - | |
| 16 | Undecanal | 38.25 | Aldehyde | 1284 | 1286 | 0.41 | 0.99 | X | [4], [10] |
| 17 | 2-undecenal | 41.63 | Aldehyde | 1336 | 1340 | 0.08 | 0.49 | [10] | |
| 18 | Decanoic acid | 43.33 | Carboxylic acid | 1362 | 1359 | 0.27 | 0 | - | |
| 19 | Dodecanal | 44.84 | Aldehyde | 1386 | 1386 | 0.38 | 1.02 | - | |
| 20 | Tetradecane | 45.75 | Hydrocarbon | 1400 | 1400 | 0.28 | 0.34 | X | [2], [4], [6] |
| 21 | Gamma decalactone | 46.94 | Ester | 1418 | 1415 | 0 | 0.08 | - | |
| 22 | Methylparaben | 47.19 | Ester | 1421 | 1420 | 0 | 0.31 | - | |
| 23 | Geranylacetone | 47.30 | Ketone | 1423 | 1423 | 0.99 | 2.92 | [6], [7], [25] | |
| 24 | Dodecen-1-al | 48.10 | Aldehyde | 1435 | 1442 | 0.20 | 0.31 | - | |
| 25 | 1-dodecanol | 49.32 | Alcohol | 1453 | 1456 | 1.87 | 0.66 | X | [6], [10], [13] |
| 26 | Tridecanal | 51.47 | Aldehyde | 1485 | 1488 | 0 | 0.51 | [6] | |
| 27 | Pentadecane | 52.44 | Hydrocarbon | 1500 | 1500 | 0.37 | 1.18 | X | [2], [4], [6], [10] |
| 28 | (E)-2-tridecenal | 55.97 | Aldehyde | 1535 | 1541 | 0.23 | 0.16 | [6] | |
| 29 | Dodecanoic acid | 57.47 | Carboxylic acid | 1550 | 1556 | 1.75 | 1.67 | [2], [4], [7], [10] | |
| 30 | Tetradecanal | 61.17 | Aldehyde | 1587 | 1588 | 0.11 | 0.28 | [4], [10] | |
| 31 | Hexadecane | 62.55 | Hydrocarbon | 1600 | 1600 | 0.94 | 1.07 | X | [4], [6] |
| 32 | Octyl ether | 67.78 | Ether | 1651 | 1657 | 0.63 | 0 | - | |
| 33 | 1-tetradecanol | 69.09 | Alcohol | 1664 | 1664 | 4.97 | 6.25 | X | [2], [10], [13] |
| 34 | Pentadecanal | 72.02 | Aldehyde | 1692 | 1693 | 2.56 | 4.89 | [10] | |
| 35 | Heptadecane | 72.90 | Hydrocarbon | 1701 | 1700 | 1.30 | 1.00 | X | [2], [4], [6] |
| 36 | 6-phenyldodecane | 73.82 | Hydrocarbon | 1713 | 1719 | 0.37 | 0 | - | |
| 37 | Tetradecanoic acid | 77.57 | Carboxylic acid | 1763 | 1762 | 3.12 | 1.33 | X | [2], [7], [10], [24] |
| 38 | 2-ethylhexyl salicylate | 78.32 | Ester | 1773 | 1769 | 0 | 2.26 | X | [7] |
| 39 | Ethyl myristate | 78.88 | Carboxylic acid | 1780 | 1778 | 0.74 | 0 | - | |
| 40 | 2-phenyl dodecane | 79.20 | Hydrocarbon | 1785 | 1794 | 0 | 0.16 | - | |
| 41 | Octadecane | 80.41 | Hydrocarbon | 1801 | 1800 | 1.81 | 1.78 | X | [10] |
| 42 | Pentadecanoic acid | 83.64 | Carboxylic acid | 1853 | 1860 | 0 | 0.80 | [2], [7] | |
| 43 | 1-hexadecanol | 84.31 | Alcohol | 1864 | 1866 | 10.59 | 21.13 | X | [2], [9], [13] |
| 44 | Nonadecane | 86.59 | Hydrocarbon | 1901 | 1900 | 0.38 | 2.17 | X | [10] |
| 45 | Methyl hexadecanoate | 87.08 | Carboxylic acid | 1910 | 1909 | 0.48 | 0 | - | |
| 46 | Hexadecanoic acid | 89.56 | Carboxylic acid | 1955 | 1956 | 2.25 | 2.57 | [2], [7], [10] | |
| 47 | Eicosane | 92.03 | Hydrocarbon | 2001 | 2000 | 0.96 | 2.43 | X | [10] |
| 48 | Isopropyl palmitate | 92.62 | Ester | 2013 | 2017 | 0 | 8.33 | [10] | |
| 49 | 1-octadecanol | 95.26 | Alcohol | 2066 | 2070 | 0 | 13.43 | X | [9] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, D.S.; Mesquita, P.R.R.; Salgado, V.R.; Rodrigues, F.d.M.; Miranda, J.C.; Barral-Netto, M.; de Andrade, J.B.; Barral, A. Determination and Profiling of Human Skin Odors Using Hair Samples. Molecules 2019, 24, 2964. https://doi.org/10.3390/molecules24162964
Tavares DS, Mesquita PRR, Salgado VR, Rodrigues FdM, Miranda JC, Barral-Netto M, de Andrade JB, Barral A. Determination and Profiling of Human Skin Odors Using Hair Samples. Molecules. 2019; 24(16):2964. https://doi.org/10.3390/molecules24162964
Chicago/Turabian StyleTavares, Diva S., Paulo R. R. Mesquita, Vanessa R. Salgado, Frederico de Medeiros Rodrigues, José Carlos Miranda, Manoel Barral-Netto, Jailson B. de Andrade, and Aldina Barral. 2019. "Determination and Profiling of Human Skin Odors Using Hair Samples" Molecules 24, no. 16: 2964. https://doi.org/10.3390/molecules24162964
APA StyleTavares, D. S., Mesquita, P. R. R., Salgado, V. R., Rodrigues, F. d. M., Miranda, J. C., Barral-Netto, M., de Andrade, J. B., & Barral, A. (2019). Determination and Profiling of Human Skin Odors Using Hair Samples. Molecules, 24(16), 2964. https://doi.org/10.3390/molecules24162964