Remarkable Effect of [Li(G4)]TFSI Solvate Ionic Liquid (SIL) on the Regio- and Stereoselective Ring Opening of α-Gluco Carbasugar 1,2-Epoxides
Abstract
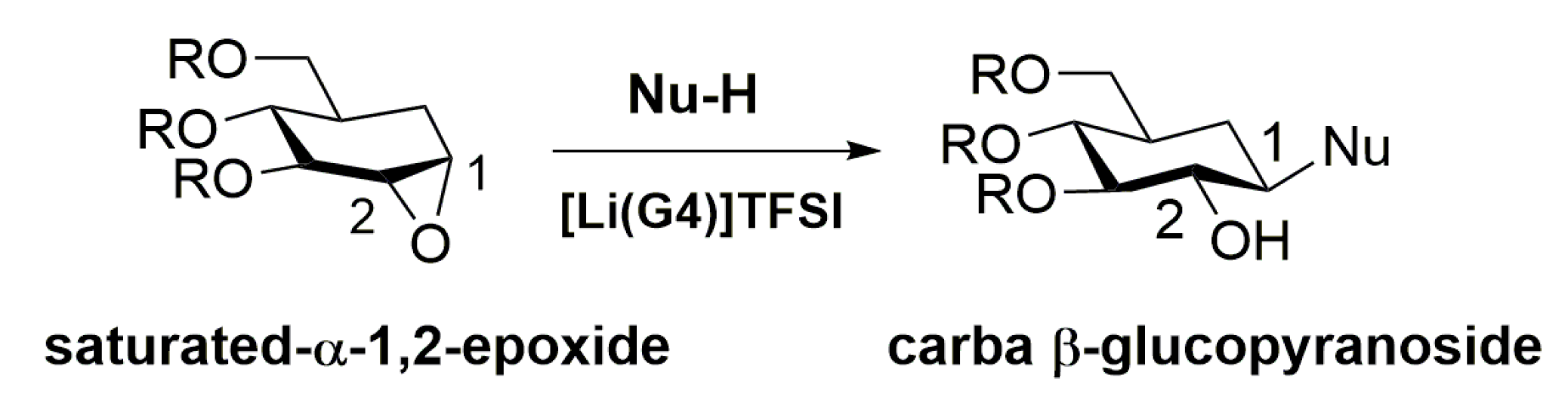
1. Introduction
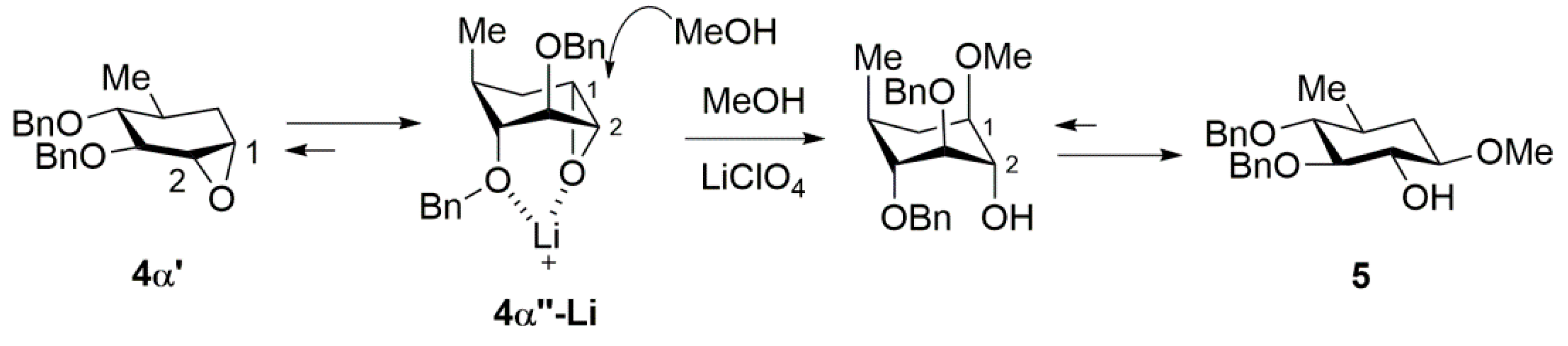
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Welton, T. Ionic liquid: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Armstrong, D.W. Using geminal dicationic ionic liquids as solvents for high-temperature organic reactions. Org. Lett. 2005, 7, 4205–4208. [Google Scholar] [CrossRef] [PubMed]
- Guglielmero, L.; Mezzetta, A.; Guazzelli, L.; Pomelli, C.S.; D’Andrea, F.; Chiappe, C. Systematic synthesis and properties evaluation of dicationic ionic liquids, and a glance into a potential new field. Front. Chem. 2018, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, H.; Hallett, J.P.; Villar-Garcia, I.J.; Hunt, P.A.; Welton, T. Mixtures of ionic liquids. Chem. Soc. Rev. 2012, 41, 7780–7802. [Google Scholar] [CrossRef] [PubMed]
- Mezzetta, A.; Douton, M.J.R.; Guazzelli, L.; Pomelli, C.S.; Chiappe, C. Microheterogeneity in ionic liquids mixtures: Hydrogen bonding, dispersed ions, and dispersed ion cluster. Aust. J. Chem. 2019, 72, 106–111. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Zhang, Y.; Chen, Z.; Watanabe, M.; Deng, Y. Beyond solvents and electrolytes: Ionic liquids-based advanced functional materials. Prog. Mater. Sci. 2016, 77, 80–124. [Google Scholar] [CrossRef]
- Chiappe, C.; Douton, M.J.R.; Mezzetta, A.; Guazzelli, L.; Pomelli, C.S.; Assanelli, G.; De Angelis, A.R. Exploring and exploiting different catalytic systems for the direct conversion of cellulose into levulinic acid. N. J. Chem. 2018, 42, 1845–1852. [Google Scholar] [CrossRef]
- Palazzo, I.; Mezzetta, A.; Guazzelli, L.; Sartini, S.; Pomelli, C.S.; Parker, W.O., Jr.; Chiappe, C. Chiral ionic liquids supported on natural sporopollenin microcapsules. RSC Adv. 2018, 8, 21174–21183. [Google Scholar] [CrossRef]
- Chiappe, C.; Demontis, G.C.; Di Bussolo, V.; Rodriguez Douton, M.J.; Rossella, F.; Pomelli, C.S.; Sartini, S.; Caporali, S. From pollen grains to functionalized microcapsules: A facile chemical route using ioni liquids. Green Chem. 2017, 19, 1028–1033. [Google Scholar] [CrossRef]
- Ghorbanizamani, F.; Timur, S. Ionic liquids from biocompatibility and electrochemical aspects towards applying in biosensing devices. Anal. Chem. 2018, 90, 640–648. [Google Scholar] [CrossRef]
- Longhi, M.; Arnaboldi, S.; Husanu, E.; Grecchi, S.; Buzzi, I.F.; Cirilli, R.; Rizzo, S.; Chiappe, C.; Mussini, P.R.; Guazzelli, L. A family of chiral ionic liquids from the natural pool: Relationships between structure and functional properties and electrochemical enantiodiscrimination tests. Electrochim. Acta 2019, 298, 194–209. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Galan, M.C.; Jones, R.A.; Tran, A.T. Recent developments of ionic liquids in oligosaccharide synthesis: The sweet side of ionic liquids, Carbohydr. Res. 2013, 375, 35–46. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Koschella, A.; Fidale, L.C.; Dorn, S.; Heinze, T. Applications of Ionic Liquids in Carbohydrate Chemistry: A Window of Opportunities. Biomacromolecules 2007, 8, 2629–2647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C. Catalytic transformation of carbohydrates and lignin in ionic liquids. WIREs Energy Environ. 2013, 2, 655–672. [Google Scholar] [CrossRef]
- Ueno, K.; Yoshida, K.; Tsuchiya, M.; Tachikawa, N.; Dokko, K.; Watanabe, M. Glyme−Lithium salt equimolar molten mixtures: Moncentrated solutions or solvate ionic liquids? J. Phys. Chem. B 2012, 116, 11323–11331. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, Y.; Iwata, K.; Imaizumi, S.; Ahn, H.; Kim, S.Y.; Ueno, K.; Park, M.J.; Watanabe, M. Gelation of solvate ionic liquid by self-assembly of block copolymer and characterization as polymer electrolyte. Macromolecules 2014, 47, 6009–6016. [Google Scholar] [CrossRef]
- Mandai, T.; Yoshida, K.; Ueno, K.; Dokko, K.; Watanabe, M. Criteria for solvate ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 8761–8772. [Google Scholar] [CrossRef]
- Moon, H.; Tatara, R.; Mandai, T.; Ueno, K.; Yo-Shida, K.; Tachikawa, N.; Yasuda, T.; Dokko, K.; Watanabe, M. Mechanism of Li Ion Desolvation at the Interface of Graphite Electrode and Glyme–Li Salt Solvate Ionic Liquids. J. Phys. Chem. C 2014, 118, 20246–20256. [Google Scholar] [CrossRef]
- Terada, S.; Mandai, T.; Nozawa, R.; Yoshida, K.; Ueno, K.; Tsuzuki, S.; Dokko, K.; Watanabe, M. Physicochemical properties of pentaglyme–sodium bis (trifluoromethanesulfonyl) amide solvate ionic liquid. Phys. Chem. Chem. Phys. 2014, 16, 11737–11746. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Shinoda, W.; Matsugami, M.; Ume-Bayashi, Y.; Ueno, K.; Mandai, T.; Seki, S.; Dokko, K.; Watanabe, M. Structures of [Li(glyme)]+ complexes and their interactions with anions in equimolar mixtures of glymes and Li[TFSA]: Analysis by molecular dynamics simulations. Phys. Chem. Chem. Phys. 2015, 17, 126–129. [Google Scholar] [CrossRef]
- Ueno, K.; Tatara, R.; Tsuzuki, S.; Saito, S.; Doi, H.; Yoshida, K.; Mandai, T.; Matsugami, M.; Umebayashi, Y.; Dokko, K. Li+ solvation in glyme-Li salt solvate ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 8248–8257. [Google Scholar] [CrossRef]
- Zhang, C.; Ueno, K.; Yamazaki, A.; Yoshida, K.; Moon, H.; Mandai, T.; Umebayashi, Y.; Dokko, K.; Watanabe, M. Chelate effects in glyme/lithium bis(trifluoromethanesulfonyl)amide solvate ionic liquids. I. stability of solvate cations and correlation with electrolyte properties. J. Phys. Chem. B 2014, 118, 5144–5153. [Google Scholar] [CrossRef]
- Zhang, A.C.; Yamazaki, J.; Murai, J.-W.; Park, T.; Mandai, K.; Ueno, K.; Dokko, M. Watanabe, Chelate Effects in Glyme/Lithium Bis (trifluoromethanesulfonyl) amide Solvate Ionic Liquids, Part 2: Importance of Solvate-Structure Stability for Electrolytes of Lithium Batteries. J. Phys. Chem. C 2014, 118, 17362–17373. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Henderson, L.C. A review of solvate ionic liquids: Physical parameters and synthetic applications. Front. Chem. 2019, 7, 263. [Google Scholar] [CrossRef]
- Carbone, L.; Gobet, M.; Peng, J.; Devany, M.; Scrosati, B.; Greenbaum, S.; Hassoun, J. Comparative Study of Ether-Based Electrolytes for Application in Lithium–Sulfur Battery. ACS Appl. Mater. Interfaces 2015, 7, 13859–13865. [Google Scholar] [CrossRef]
- Ueno, K.; Park, J.W.; Yamazaki, A.; Mandai, T.; Tachikawa, N.; Dokko, K.; Watanabe, M. Anionic Effects on Solvate Ionic Liquid Electrolytes in Rechargeable Lithium–Sulfur Batteries. J. Phys. Chem. C 2013, 117, 20509–20516. [Google Scholar] [CrossRef]
- Yoshida, K.; Tsuchiya, M.; Tachikawa, N.; Dokko, K.; Watanabe, M. Correlation between Battery Performance and Lithium Ion Diffusion in Glyme–Lithium Bis (trifluoromethanesulfonyl) amide Equimolar Complexes. J. Electrochem. Soc. 2012, 159, A1005–A1012. [Google Scholar] [CrossRef]
- Black, J.J.; Murphy, T.; Atkin, R.; Dolan, A.; Aldous, L. The thermoelectrochemistry of lithium–glyme solvate ionic liquids: Towards waste heat harvesting. Phys. Chem. Chem. Phys. 2016, 18, 20768–20777. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Champion, M.E.; Fox, B.L.; Yoganantharajah, P.; Gibert, Y.; Welton, T.; Henderson, L.C. Solvate Ionic Liquids as Reaction Media for Electrocyclic Transformations. Eur. J. Org. Chem. 2016, 5, 913–917. [Google Scholar] [CrossRef]
- Obregon-Zuniga, A.; Milàn, M.; Juaristi, E. Improving the catalytic performance of (S)-proline as organocatalyst in asymmetric aldol reactions in the presence of solvate ionic liquids, involvement of a supramolecular aggregate. Org. Lett. 2017, 19, 1108–1111. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Henderson, L.C. Synthesis of α-aminophosphonates using solvate ionic liquids. RSC Adv. 2017, 7, 27900–27904. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Jyothirmai, B.; Murty, M.S.R. Ionic Liquids/H2O Systems for the Reaction of Epoxides with NaN3: A New Protocol for the Synthesis of 2-Azidoalcohols. Tetrahedron Lett. 2005, 46, 6559–6562. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Jin, C.; Zhang, X.; Xie, Y.; Su, W. Highly regioselective ring-opening of epoxides with thiophenols in ionic liquids without the use of any catalyst. Green Chem. 2006, 8, 330–332. [Google Scholar] [CrossRef]
- Yang, M.-H.; Yan, G.-B.; Zheng, Y.-F. Regioselective ring-opening reactions of 1, 2-epoxides with thiols and arylselenols directly promoted by [Bmim] BF4. Tetrahedron Lett. 2008, 49, 6471–6474. [Google Scholar] [CrossRef]
- Rad, M.N.S.; Behrouz, S. The base-free chemoselective ring opening of epoxides with carboxylic acid using [Bmim] Br: A rapid entry into 1, 2-diol mono-esters synthesis. Mol. Divers. 2013, 17, 9–18. [Google Scholar] [CrossRef]
- Terada, S.; Ikeda, K.; Ueno, K.; Dokko, K.; Watanabe, M. Liquid Structures and Transport Properties of Lithium Bis (fluorosulfonyl) amide/Glyme Solvate Ionic Liquids for Lithium Batteries. Aust. J. Chem. 2019, 72, 70–80. [Google Scholar] [CrossRef]
- Arjona, O.; Gómez, A.M.; López, J.C.; Plumet, J. Synthesis and Conformational and Biological Aspects of Carbasugars. Chem. Rev. 2007, 107, 1919–2036. [Google Scholar] [CrossRef]
- Marco-Contelles, J.; Molina, M.T.; Anjum, S. Naturally Occurring Cyclohexane Epoxides: Sources, Biological Activities, and Synthesis. Chem. Rev. 2004, 104, 2857–2900. [Google Scholar] [CrossRef]
- Cumpstey, I. Synthesis of carbasugar-containing non-glycosidically linked pseudodisaccharides and higher pseudooligosaccharides Carbohydr. Res. 2009, 344, 2285–2310. [Google Scholar] [CrossRef]
- Di Bussolo, V.; Frau, I.; Checchia, L.; Favero, L.; Pineschi, M.; Uccello-Barretta, G.; Balzano, F.; Roselli, G.; Renzi, G.; Crotti, P. Synthesis of carba analogs of 6-O-(benzyl)-D-allal-and-D-galactal-derived allyl epoxides and evaluation of the regio-and stereoselective behavior in nucleophilic addition reactions. Tetrahedron 2011, 67, 4696–4709. [Google Scholar] [CrossRef]
- Di Bussolo, V.; Frau, I.; Favero, L.; Bordoni, V.; Crotti, S.; Barretta, G.U.; Balzano, F.; Crotti, P. Carba-D, L-allal-and-D, L-galactal-derived vinyl N-nosyl aziridines as useful tools for the synthesis of 4-deoxy-4-(N-nosylamino)-2, 3-unsaturated-5a-carbasugars. Tetrahedron 2017, 73, 6677–6695. [Google Scholar] [CrossRef]
- Ogawa, S.; Sasaki, S.; Tsunoda, H. Convenient Synthesis of Oxo-Linked 5a-Carba-di- and tri-saccharides of Biological Interests. Chem. Lett. 1993, 9, 1587–1590. [Google Scholar] [CrossRef]
- Frigell, J.; Cumpstey, I. Carbasugar analogues of galactofuranosides: α-O-linked derivatives. Beilstein J. Org. Chem. 2010, 6, 1127–1131. [Google Scholar] [CrossRef]
- Bordoni, V.; Porkolab, V.; Sattin, S.; Thépaut, M.; Frau, I.; Favero, L.; Crotti, P.; Bernardi, A.; Fieschi, F.; Di Bussolo, V. Stereoselective innovative synthesis and biological evaluation of new real carba analogues of minimal epitope Manα (1,2) Man as DC-SIGN inhibitors. RSC Adv. 2016, 6, 89578–89584. [Google Scholar] [CrossRef]
- Roscales, S.; Plumet, J. Biosynthesis and Biological Activity of Carbasugars. Int. J. Carbohydr. Chem 2016, 2016, 4760548. [Google Scholar] [CrossRef]
- Sudha, A.V.R.L.; Nagarajan, M. Biosynthesis and Biological Activity of CarbasugarsCarbohydrates to carbocycles: An expedient synthesis of pseudo-sugars. Chem Comm. 1998, 29, 925–926. [Google Scholar] [CrossRef]
- Frau, I.; Di Bussolo, V.; Favero, L.; Pineschi, M.; Crotti, P. Stereodivergent Synthesis of Diastereoisomeric Carba Analogs of Glycal-Derived Vinyl Epoxides: A New Access to Carbasugars. Chirality 2011, 23, 820–826. [Google Scholar] [CrossRef]
- Bernardi, A.; Arosio, D.; Manzoni, L.; Micheli, F.; Pasquarello, A.; Seneci, P. Stereoselective Synthesis of Conformationally Constrained Cyclohexanediols: A Set of Molecular Scaffolds for the Synthesis of Glycomimetics. J. Org. Chem. 2001, 66, 6209–6216. [Google Scholar] [CrossRef]
- Colombini, M.; Crotti, P.; Di Bussolo, V.; Favero, L.; Gardelli, C.; Macchia, F.; Pineschi, M. Regiochemical control of the ring opening of 1, 2-epoxides by means of chelating processes.9. Synthesis and ring opening reactions of cis- and trans-oxides derived from 3-(benzyloxymethyl) cyclopentene and methyl 2-cyclopenten-1-carboxylate 1. Tetrahedron 1995, 51, 8089–8112. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Demir, B.; Walsh, T.R.; Welton, T.; Henderson, L.C. Determination of Kamlet-Taft parameters for selected solvate ionic liquids. Phys. Chem. Chem. Phys. 2016, 18, 13153–13157. [Google Scholar] [CrossRef]


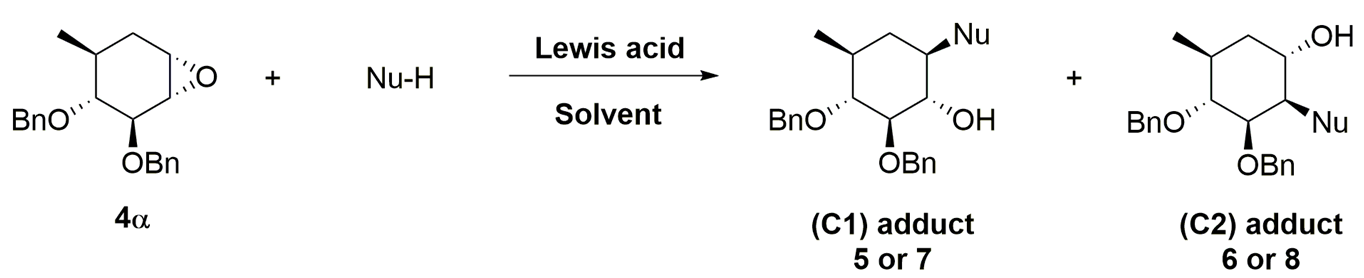
| Entry | Alcohol Nucleophile (Nu-H) | L.A. | Solvent | Time Temp. | Nu | C(1) Adduct1 (%) | C(2) Adduct1 (%) | Isolated Yield2 |
|---|---|---|---|---|---|---|---|---|
| 1 | MeOH | Cu(OTf)2 | CH2Cl2 | 12 h r.t. | OMe | 5 (50) | 6 (50) | C1: 32% C2: 32% |
| 2 | 1,2:3,4-di-O-isopropylidene-d-galactopyranose | Cu(OTf)2 | CH2Cl2 | 12 h r.t. | 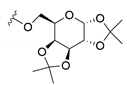 | 7 (55) | 8 (45) | C1: 35% C2: 28% |
| 3 | MeOH | LiClO4 | MeOH | 7 days 80°C | OMe | 5 (>99) | / | C1: 55% |

| Entry | Alcohol Nucleophile (Nu-H) | SIL | Conditions | Nu | C(1) Adduct (%) | C(2) Adduct (%) |
|---|---|---|---|---|---|---|
| 1 | MeOH | 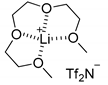 [Li(G3)]TFSI | i) IL dried ii) 70 °C 3 days | OMe | / | / |
| 2 | MeOH | 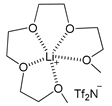 [Li(G4)]TFSI | i) IL dried ii) 70 °C 24 h | OMe | 5 (60)1 | 6 (40)1 |
| 3 | MeOH | [Li(G4)]TFSI | i) IL dried ii) 70 °C LiTf2N excess 3 days | OMe | 5 (60)1 | 6 (40)1 |
| 4 | 1,2:3,4-di-O-isopropylidene-d-galactopyranose | [Li(G4)]TFSI | i) IL dried ii) 70 °C 24 h | 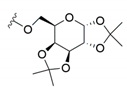 | 7Ac (80)2 | 8Ac (20)2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pietro, S.; Bordoni, V.; Mezzetta, A.; Chiappe, C.; Signore, G.; Guazzelli, L.; Di Bussolo, V. Remarkable Effect of [Li(G4)]TFSI Solvate Ionic Liquid (SIL) on the Regio- and Stereoselective Ring Opening of α-Gluco Carbasugar 1,2-Epoxides. Molecules 2019, 24, 2946. https://doi.org/10.3390/molecules24162946
Di Pietro S, Bordoni V, Mezzetta A, Chiappe C, Signore G, Guazzelli L, Di Bussolo V. Remarkable Effect of [Li(G4)]TFSI Solvate Ionic Liquid (SIL) on the Regio- and Stereoselective Ring Opening of α-Gluco Carbasugar 1,2-Epoxides. Molecules. 2019; 24(16):2946. https://doi.org/10.3390/molecules24162946
Chicago/Turabian StyleDi Pietro, Sebastiano, Vittorio Bordoni, Andrea Mezzetta, Cinzia Chiappe, Giovanni Signore, Lorenzo Guazzelli, and Valeria Di Bussolo. 2019. "Remarkable Effect of [Li(G4)]TFSI Solvate Ionic Liquid (SIL) on the Regio- and Stereoselective Ring Opening of α-Gluco Carbasugar 1,2-Epoxides" Molecules 24, no. 16: 2946. https://doi.org/10.3390/molecules24162946
APA StyleDi Pietro, S., Bordoni, V., Mezzetta, A., Chiappe, C., Signore, G., Guazzelli, L., & Di Bussolo, V. (2019). Remarkable Effect of [Li(G4)]TFSI Solvate Ionic Liquid (SIL) on the Regio- and Stereoselective Ring Opening of α-Gluco Carbasugar 1,2-Epoxides. Molecules, 24(16), 2946. https://doi.org/10.3390/molecules24162946









