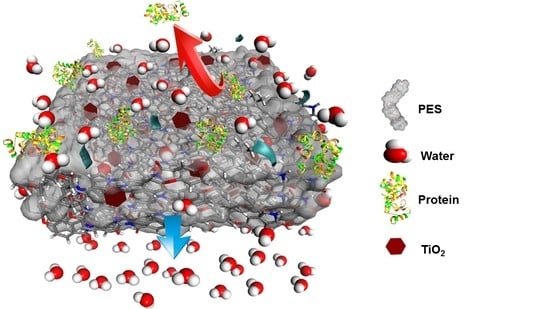
ANOVA Design for the Optimization of TiO2 Coating on Polyether Sulfone Membranes
Abstract
:1. Introduction
2. Experimental Section
2.1. Material
2.2. Fractional Factorial Design
2.3. Membrane Preparation
2.4. Effect of Template Agent and TTIP Concentration
2.5. Membrane Characterization
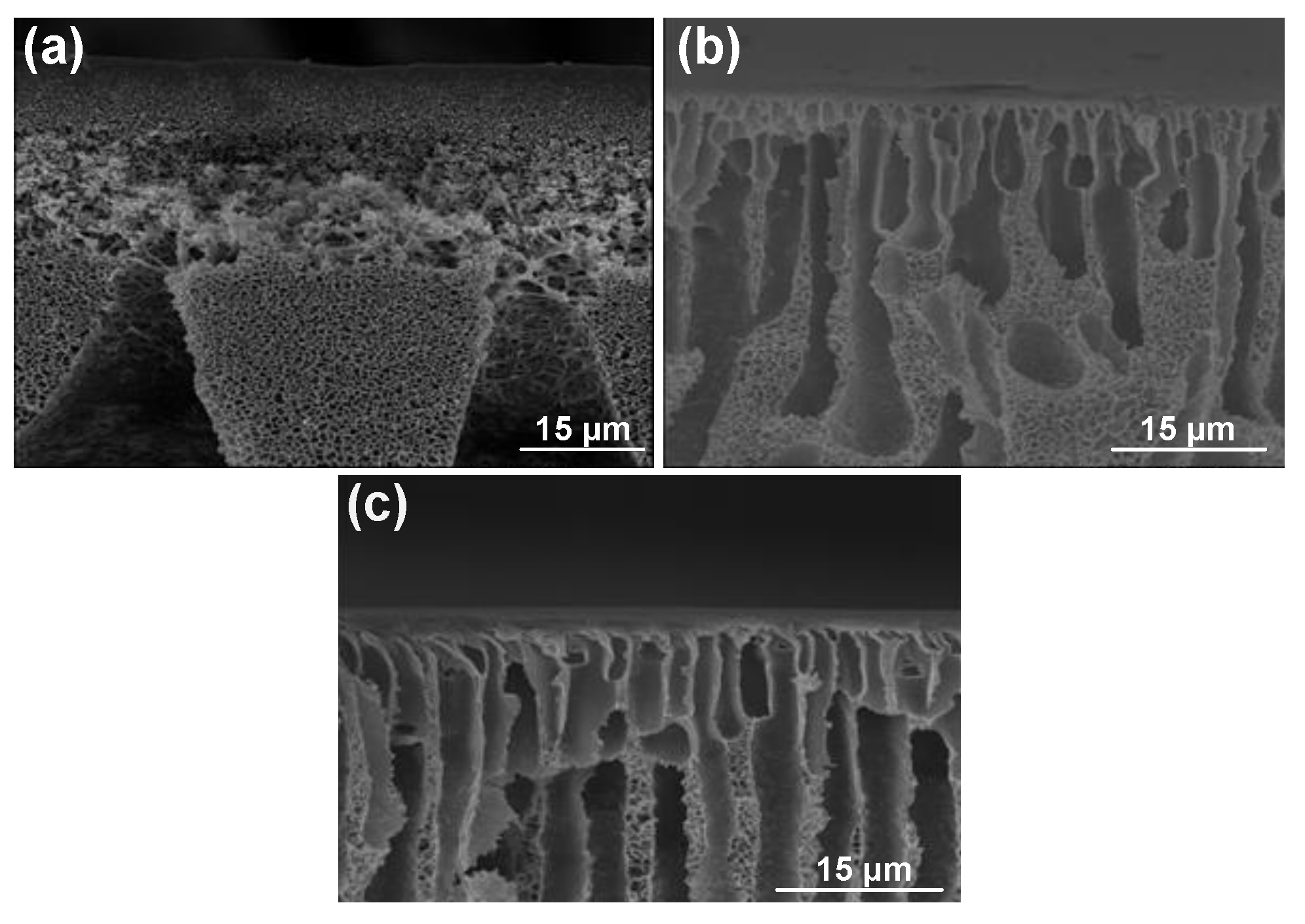
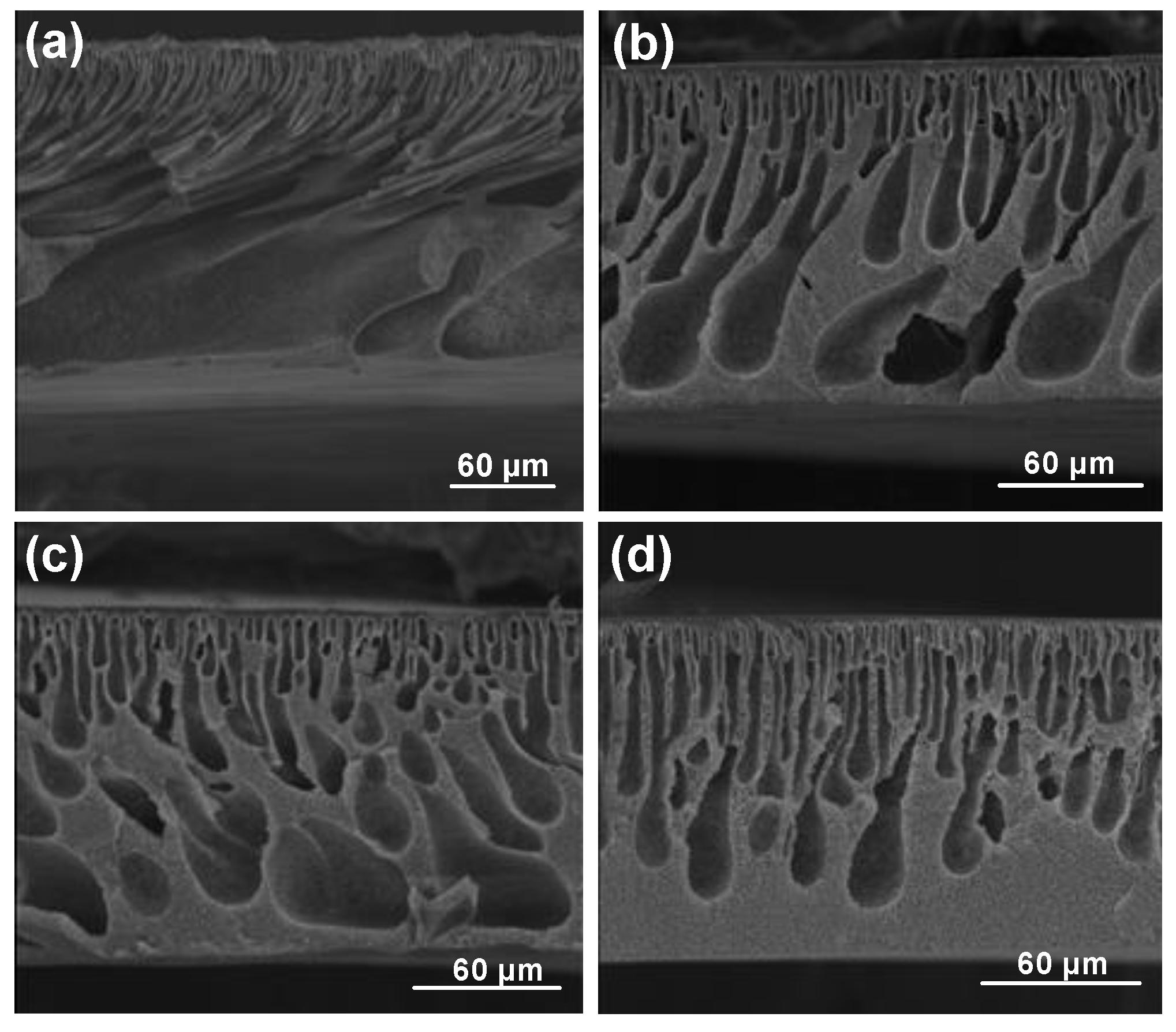
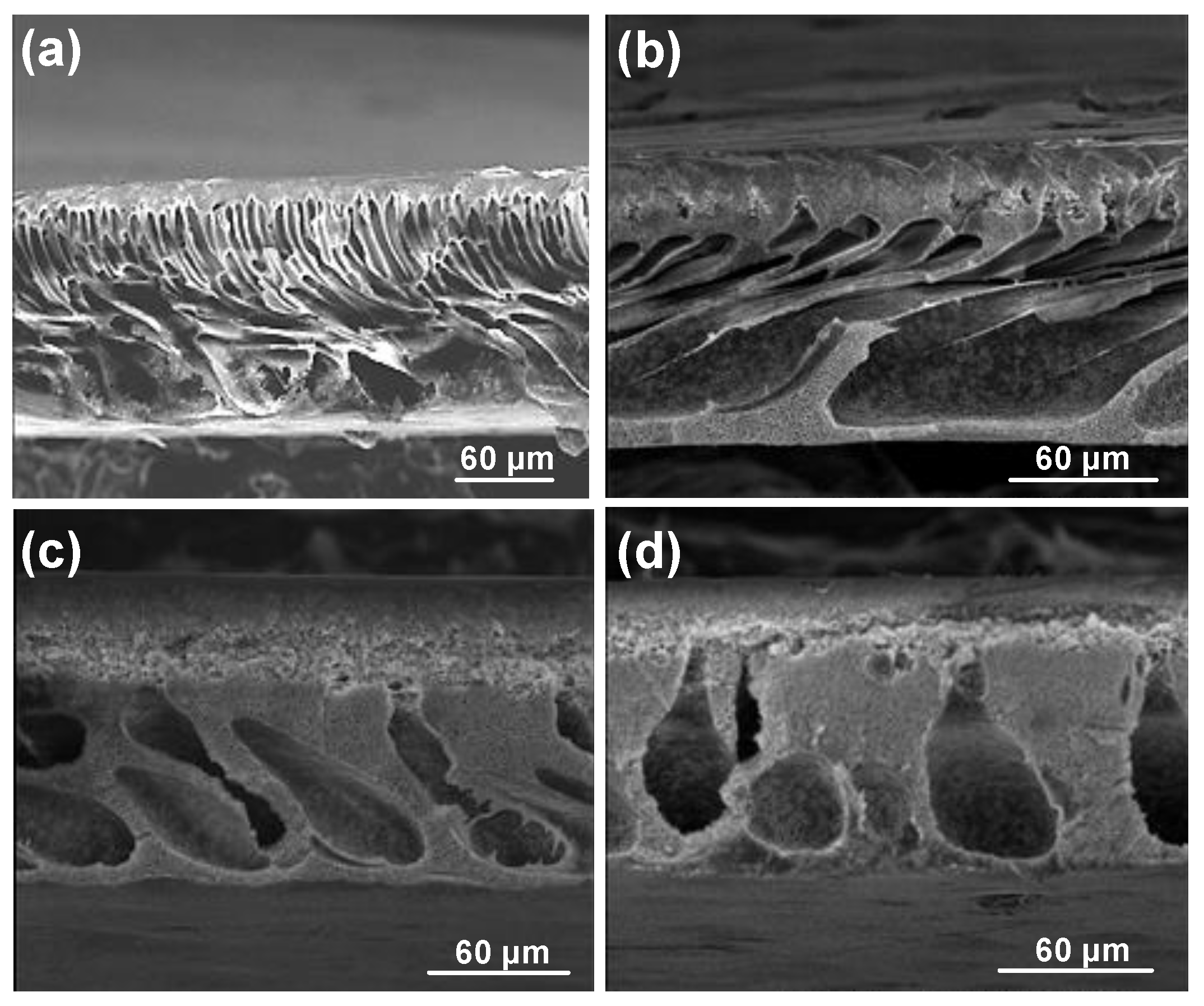
3. Results and Discussions
3.1. One-Half Fractional Factorial Design
3.2. Two-Way ANOVA
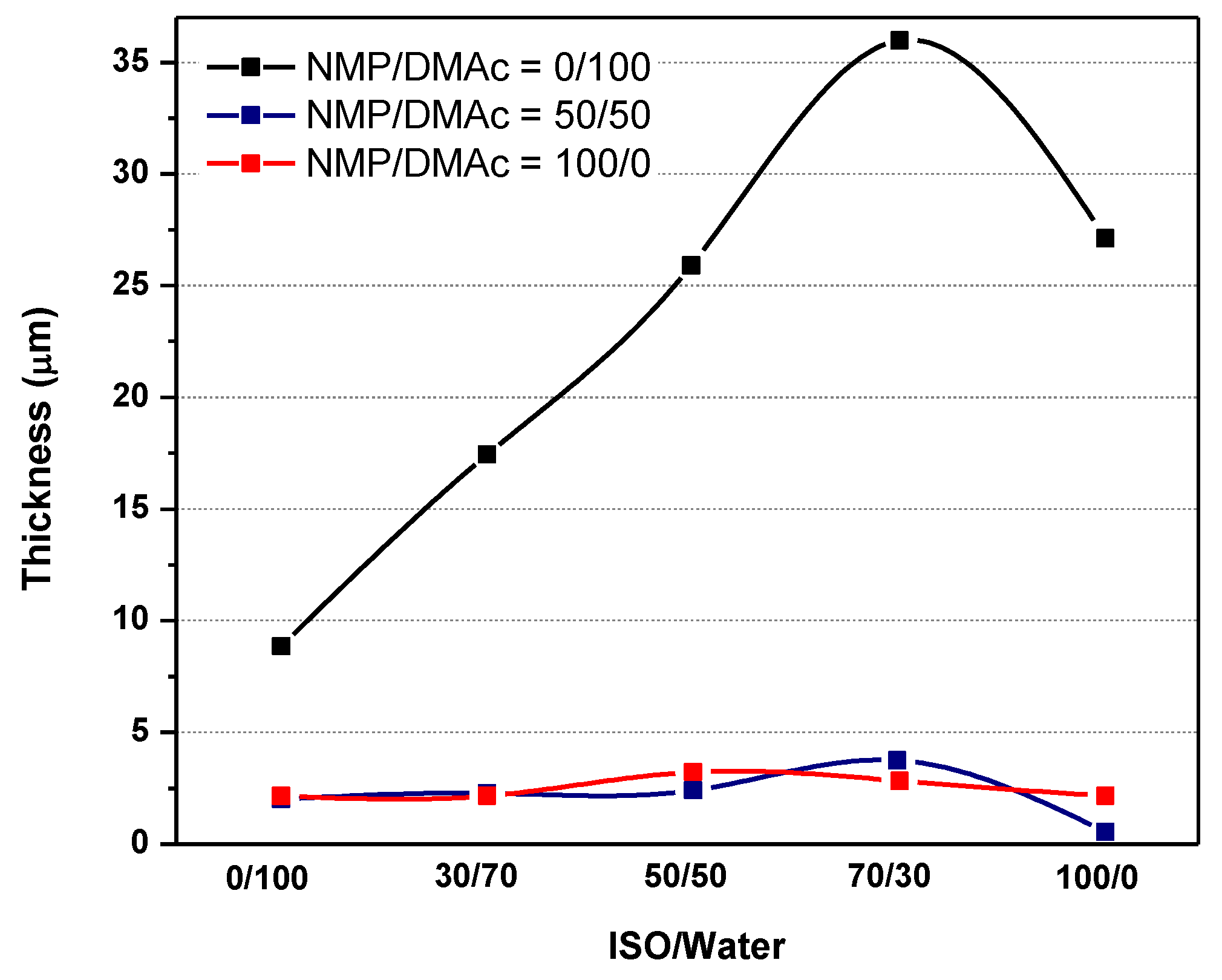
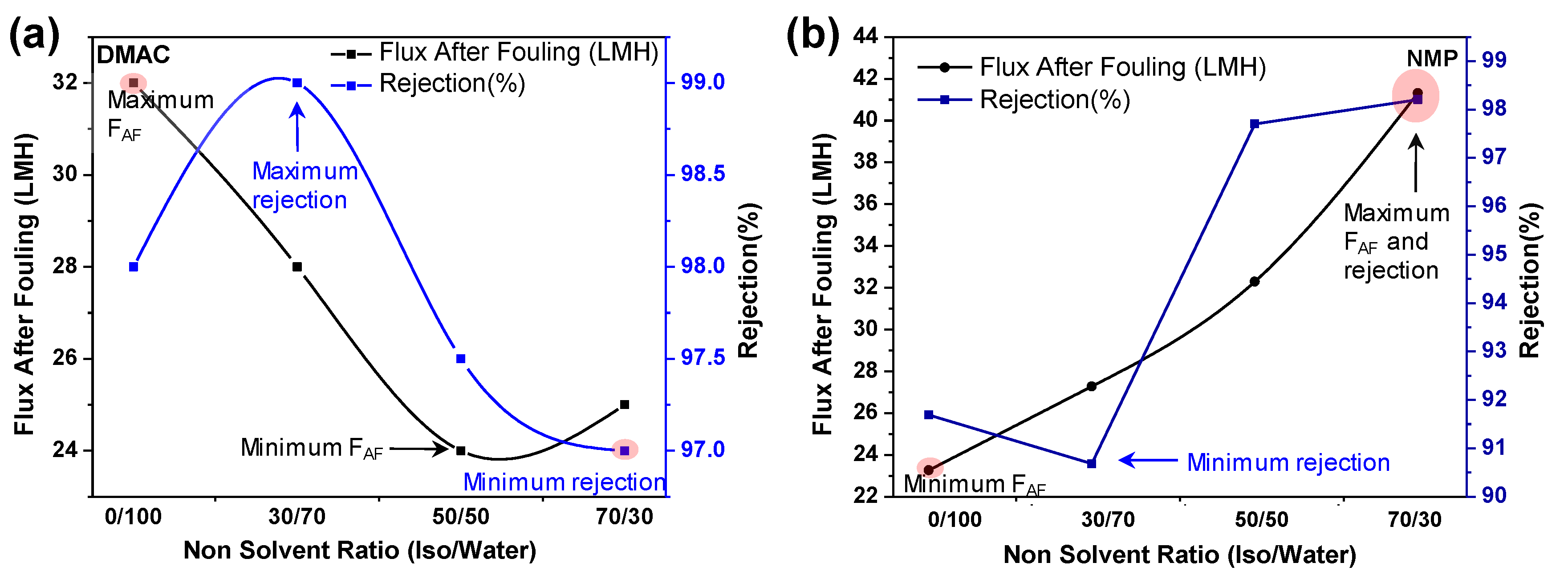
3.2.1. Effect of Nonsolvent (Factor E)
3.2.2. BSA Rejection
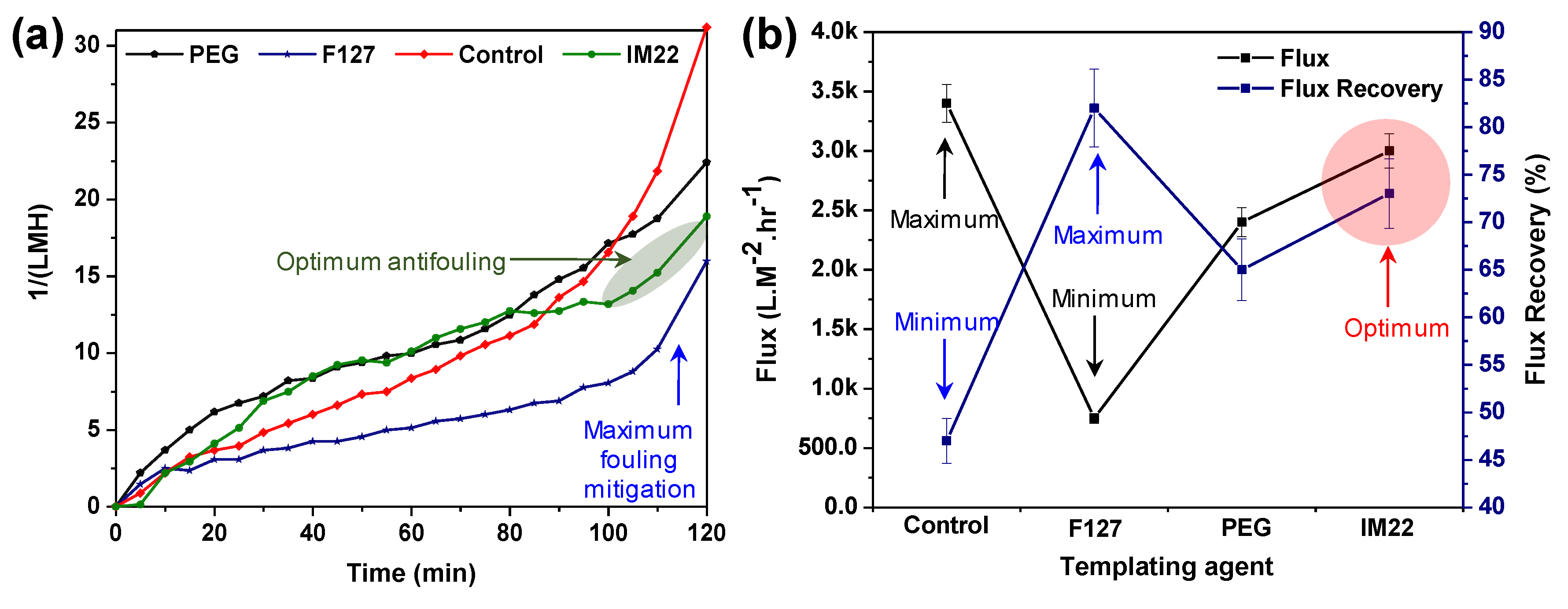
3.3. Effect of Template Agents
BSA Rejection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhai, Y.; Wang, J.; Wang, H.; Song, T.; Hu, W.; Li, S. Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes. Molecules 2018, 23, 2073. [Google Scholar] [CrossRef]
- He, K.; He, K.Q.; Chen, C.l.; Liao, C.Z.; Xu, Y.; Tang, J.N.; Li, R.K.Y. Polyethylene oxide/garnet-type Li6.4La3Zr1.4Nb0.6O12 composite electrolytes with improved electrochemical performance for solid state lithium rechargeable batteries. Compos. Sci. Technol. 2019, 175, 28–34. [Google Scholar] [CrossRef]
- Iulianelli, A.; Alavi, M.; Bagnato, G.; Liguori, S.; Wilcox, J.; Rahimpour, M.R.; Eslamlouyan, R.; Anzelmo, B.; Basile, A. Supported Pd-Au Membrane Reactor for Hydrogen Production: Membrane Preparation, Characterization and Testing. Molecules 2016, 21, 581. [Google Scholar] [Green Version]
- Arefi-Oskoui, S.; Khataee, A.; Safarpour, M.; Orooji, Y.; Vatanpour, V. A review on the applications of ultrasonic technology in membrane bioreactors. Ultrasonics Sonochemistry 2019, 58, 104633. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Reed, L.; Alex, M.; Alshammari, N.; Hestekin, J.L.; Servoss, S. PEG-mimetic peptoid reduces protein fouling of polysulfone hollow fibers. Colloids Surf. B Biointerfaces 2017, 149, 23–29. [Google Scholar]
- Razmjou, A.; Eshaghi, G.; Orooji, Y.; Hossein, I.; Korayem, A.H.; Mohagheghian, F. Lithium ion-selective membrane with 2D subnanometer channels. Water Res. 2019, 159, 313–323. [Google Scholar] [CrossRef]
- Orooji, Y.; Faghih, M.; Razmjou, A.; Hou, J.W.; Moazzam, P.; Emami, N.; Aghababaie, M.; Nourrisfa, F.; Chen, V.; Jin, W.Q. Nanostructured Mesoporous Carbon Polyethersulfone Composite Ultrafiltration Membrane with Significantly Low Protein Adsorption and Bacterial Adhesion. Carbon 2017, 111, 689–704. [Google Scholar] [CrossRef]
- Maiti, J.; Kakati, N.; Woo, S.P.; Yoon, Y.S. Nafion based hybrid composite membrane containing GO and dihydrogen phosphate functionalized ionic liquid for high temperature polymer electrolyte membrane fuel cell. Compos. Sci. Technol. 2018, 155, 189–196. [Google Scholar] [CrossRef]
- Orooji, Y.; Liang, F.; Razmjou, A.; Li, S.; Mofid, M.R.; Liu, Q.; Guan, K.; Liu, Z.; Jin, W. Excellent Biofouling Alleviation of Thermoexfoliated Vermiculite Blended Poly(ether sulfone) Ultrafiltration Membrane. ACS Appl. Mater. Interfaces 2017, 9, 30024–30034. [Google Scholar] [CrossRef]
- Orooji, Y.; Liang, F.; Razmjou, A.; Liu, G.; Jin, W.Q. Preparation of anti-adhesion and bacterial destructive polymeric ultrafiltration membranes using modified mesoporous carbon. Sep. Purif. Technol. 2018, 205, 273–283. [Google Scholar] [CrossRef]
- Razmjou, C. The Effect of TiO2 Nanoparticles on the Surface Chemistry, Structure and Fouling Performance of Polymeric Membranes. Ph.D. Thesis, University of New South Wales, Kensington, Australia, 2012. [Google Scholar]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Razmjou, A.; Resosudarmo, A.; Holmes, R.L.; Li, H.Y.; Mansouri, J.; Chen, V. The effect of modified TiO2 nanoparticles on the polyethersulfone ultrafiltration hollow fiber membranes. Desalination 2012, 287, 271–280. [Google Scholar] [CrossRef]
- Leong, S.W.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.W.; Wang, H.T. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Yadav, M.; Mishra, D.K.; Hwang, J.S. Catalytic hydrogenation of xylose to xylitol using ruthenium catalyst on NiO modified TiO2 support. Appl. Catal. A Gen. 2012, 425–426, 110–116. [Google Scholar] [CrossRef]
- Meng, S.; Mansouri, J.; Ye, Y.; Chen, V. Effect of templating agents on the properties and membrane distillation performance of TiO2-coated PVDF membranes. J. Membr. Sci. 2014, 450, 48–59. [Google Scholar] [CrossRef]
- Emami, N.; Razmjou, A.; Noorisafa, F.; Korayem, A.H.; Zarrabi, A.; Ji, C. Fabrication of smart magnetic nanocomposite asymmetric membrane capsules for the controlled release of nitrate. Environ. Nanotechnol. Monit. Manag. 2017, 8, 233–243. [Google Scholar] [CrossRef]
- Barth, C.; Goncalves, M.C.; Piers, A.T.M.; Wolf, B.A. Asymmetric polysulfone and polyethersulfone membranes: effects of thermodynamic conditions during formation on their performance. J. Membr. Sc. 2000, 169, 287–299. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Wang, Q. Poly(vinylidene fluoride)/polyethersulfone blend membranes: Effects of solvent sort, polyethersulfone and polyvinylpyrrolidone concentration on their properties and morphology. J. Membr. Sci. 2006, 285, 290–298. [Google Scholar] [CrossRef]
- See-Toh, Y.H.; Ferreira, F.C.; Livingston, A.G. The influence of membrane formation parameters on the functional performance of organic solvent nanofiltration membranes. J. Membr. Sci. 2007, 299, 236–250. [Google Scholar] [CrossRef]
- Young, T.H.; Huang, Y.H.; Chen, L.Y. Effect of solvent evaporation on the formation of asymmetric and symmetric membranes with crystallizable EVAL polymer. J. Membr. Sci. 2000, 164, 111–120. [Google Scholar] [CrossRef]
- Cheng, L.P. Effect of temperature on the formation of microporous PVDF membranes by precipitation from 1-octanol/DMF/PVDF and water/DMF/PVDF systems. Macromolecules 1999, 32, 6668–6674. [Google Scholar] [CrossRef]
- Yeow, M.; Liu, Y.; Li, K. Morphological study of poly (vinylidene fluoride) asymmetric membranes: Effects of the solvent, additive, and dope temperature. J. Appl. Polym. Sci. 2004, 92, 1782–1789. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Huang, Y. Facile and simple fabrication of strong, transparent and flexible aramid nanofibers/bacterial cellulose nanocomposite membranes. Compos. Sci. Technol. 2018, 159, 70–76. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Wang, Z.X.; Cheng, G.J.; Dong, S.J. Functionalized inorganic–organic composite material derivated by sol–gel for construction of mediated amperometric hydrogen peroxide biosensor. Anal. Chim. Acta 1999, 388, 71–78. [Google Scholar] [CrossRef]
- Ping, L.; Liu , P.; Lin, H.; Fu, X.; Meng, C. Preparation of the doped TiO2 film photocatalyst and its bactericidal mechanism. Chin. J. Catal. 1995, 20, 327–328. [Google Scholar]
- Mansouri, J.; Harrisson, S.; Chen, V. Strategies for controlling biofouling in membrane filtration systems: challenges and opportunities. J. Mater. Chem. 2010, 20, 4567–4586. [Google Scholar] [CrossRef]
- Razmjou, A.A.; Mansouri, J.; Chen, V.; Lim, M.; Amal, R. Titania nanocomposite polyethersulfone ultrafiltration membranes fabricated using a low temperature hydrothermal coating process. J. Membr. Sci. 2011, 380, 98–113. [Google Scholar] [CrossRef]
- Mahmoudi, N. Design of Peptoid-Based Coating to Reduce Biofouling in Gas Exchange Devices. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2018. [Google Scholar]
- Deshmukh, S.P.; Li, K. Effect of ethanol composition in water coagulation bath on morphology of PVDF hollow fibre membranes. J. Membr. Sci. 1998, 150, 75–85. [Google Scholar] [CrossRef]
- Tarrahi, R.; Movafeghi, A.; Khataee, A.; Rezanejad, F.; Gohari, G. Evaluating the Toxic Impacts of Cadmium Selenide Nanoparticles on the Aquatic Plant Lemna minor. Molecules 2019, 24, 410. [Google Scholar] [CrossRef]
- Khaledian, H.R.; Zolfaghari, P.; Elhami, V.; Aghbolaghy, M.; Khorram, S.; Karimi, A.; Khataee, A. Modification of Immobilized Titanium Dioxide Nanostructures by Argon Plasma for Photocatalytic Removal of Organic Dyes. Molecules 2019, 24, 383. [Google Scholar] [CrossRef]
| Run | Treatment Combination | Level of Factors (High Level:+; Low Level: −) | Response: Flux (LMH) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicates | Average | |||||||||||||
| Aa | Bb | Cc | Dd | Ee | Aa | Bb | Cc | Dd | Ee | i | ii | iii | ||
| 1 | 100 | 0 | 1 | 0 | 0 | + | − | − | − | − | 0 | 0 | 0 | 0 |
| 2 | 100 | 0 | 1 | 4 | 100 | + | − | − | + | + | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 16 | 0 | 0 | − | − | + | − | − | 4 | 3.86 | 4.5 | 4.12 |
| 4 | 0 | 60 | 16 | 0 | 100 | − | + | + | − | + | 180 | 225 | 160 | 188 |
| 5 | 0 | 0 | 16 | 4 | 100 | − | − | + | + | + | 540 | 520 | 510 | 523 |
| 6 | 0 | 0 | 1 | 4 | 0 | − | − | − | + | − | 580 | 556 | 568 | 568 |
| 7 | 0 | 60 | 1 | 4 | 100 | − | + | − | + | + | 580 | 640 | 610 | 610 |
| 8 | 100 | 60 | 16 | 4 | 100 | + | + | + | + | + | 9 | 8.5 | 8 | 8.5 |
| 9 | 100 | 60 | 16 | 0 | 0 | + | + | + | − | − | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 1 | 0 | 100 | − | − | − | − | + | 72 | 82.5 | 76 | 76.8 |
| 11 | 0 | 60 | 1 | 0 | 0 | − | + | − | − | − | 0 | 0 | 0 | 0 |
| 12 | 0 | 60 | 16 | 4 | 0 | − | + | + | + | − | 740 | 686 | 680 | 702 |
| 13 | 50 | 30 | 8.5 | 2 | 50 | 0 | 0 | 0 | 0 | 0 | 343 | 390 | 367 | 367 |
| 14 | 100 | 0 | 16 | 0 | 100 | + | − | + | − | + | 0 | 0 | 0 | 0 |
| 15 | 100 | 60 | 1 | 0 | 100 | + | + | − | − | + | 0 | 0 | 0 | 0 |
| 16 | 100 | 60 | 1 | 4 | 0 | + | + | − | + | − | 455 | 461 | 450 | 455 |
| 17 | 100 | 0 | 16 | 4 | 0 | + | − | + | + | − | 480 | 509 | 501 | 497 |
| Run | Treatment Combination | Level of Factors (High Level: +; Low Level: −) | Average of Membrane Top Layer Thickness (µm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aa | Bb | Cc | Dd | Ee | Aa | Bb | Cc | Dd | Ee | ||
| 1 | 100 | 0 | 1 | 0 | 0 | + | − | − | − | − | 2.65 |
| 2 | 100 | 0 | 1 | 4 | 100 | + | − | − | + | + | 0 |
| 3 | 0 | 0 | 16 | 0 | 0 | − | − | + | − | − | 4.3 |
| 4 | 0 | 60 | 16 | 0 | 100 | − | + | + | − | + | 2 |
| 5 | 0 | 0 | 16 | 4 | 100 | − | − | + | + | + | 1.12 |
| 6 | 0 | 0 | 1 | 4 | 0 | − | − | − | + | − | 8 |
| 7 | 0 | 60 | 1 | 4 | 100 | − | + | − | + | + | 2 |
| 8 | 100 | 60 | 16 | 4 | 100 | + | + | + | + | + | 0.75 |
| 9 | 100 | 60 | 16 | 0 | 0 | + | + | + | − | − | 1.2 |
| 10 | 0 | 0 | 1 | 0 | 100 | − | − | − | − | + | 2 |
| 11 | 0 | 60 | 1 | 0 | 0 | − | + | − | − | − | 3.2 |
| 12 | 0 | 60 | 16 | 4 | 0 | − | + | + | + | − | 9 |
| 13 | 50 | 30 | 8.5 | 2 | 50 | 0 | 0 | 0 | 0 | 0 | 2.5 |
| 14 | 100 | 0 | 16 | 0 | 100 | + | − | + | − | + | 1.5 |
| 15 | 100 | 60 | 1 | 0 | 100 | + | + | − | − | + | 0.18 |
| 16 | 100 | 60 | 1 | 4 | 0 | + | + | − | + | − | 1.8 |
| 17 | 100 | 0 | 16 | 4 | 0 | + | − | + | + | − | 2.15 |
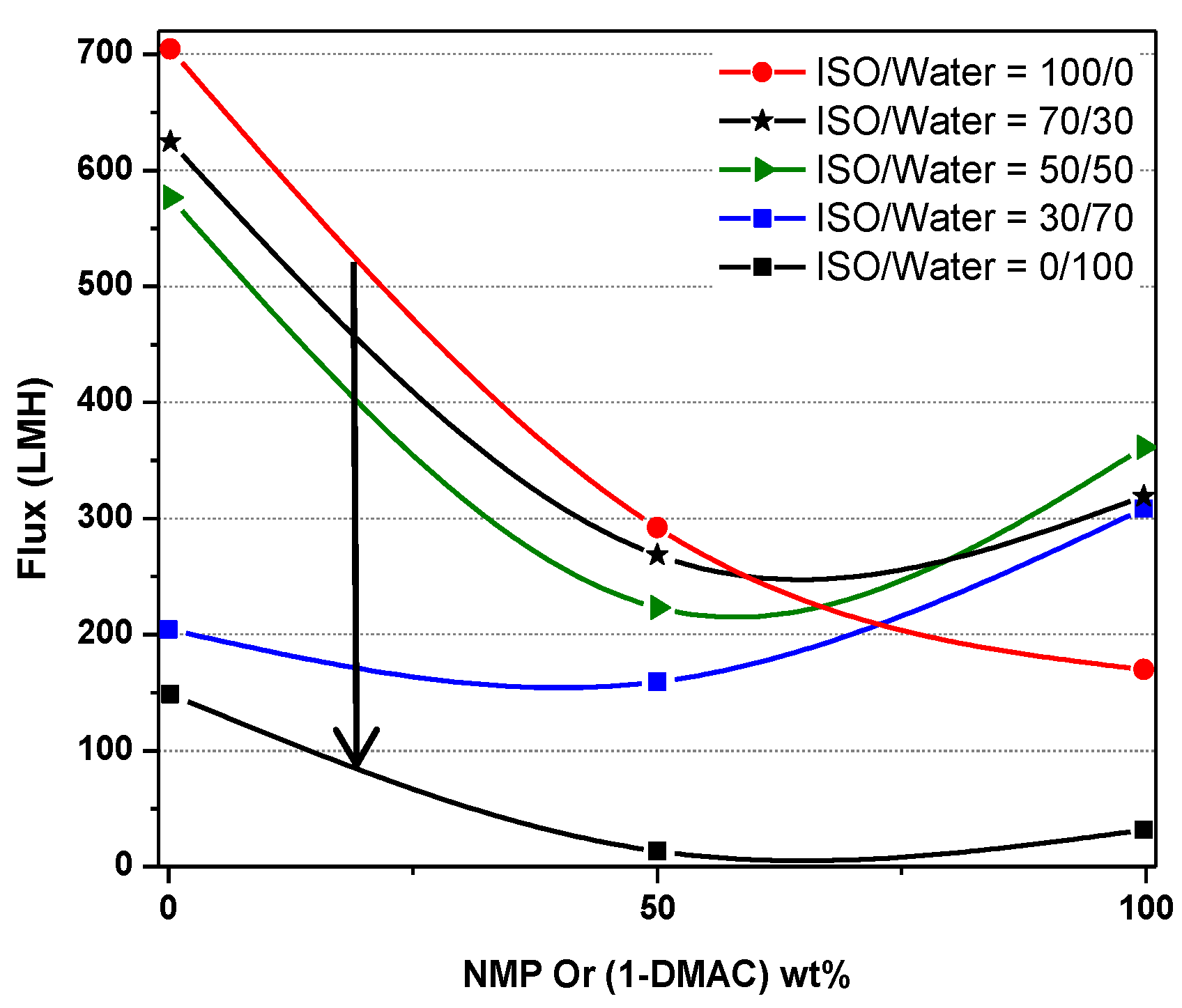
| NMP/DMAc (A) | ISO/Water (E) | ||||
|---|---|---|---|---|---|
| 0/100 | 30/70 | 50/50 | 70/30 | 100/0 | |
| 0/100 | 702 | 622 | 582 | 204 | 146.5 |
| 50/50 | 290 | 265 | 224 | 158 | 8 |
| 100/0 | 268 | 319 | 360 | 310 | 27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orooji, Y.; Ghasali, E.; Emami, N.; Noorisafa, F.; Razmjou, A. ANOVA Design for the Optimization of TiO2 Coating on Polyether Sulfone Membranes. Molecules 2019, 24, 2924. https://doi.org/10.3390/molecules24162924
Orooji Y, Ghasali E, Emami N, Noorisafa F, Razmjou A. ANOVA Design for the Optimization of TiO2 Coating on Polyether Sulfone Membranes. Molecules. 2019; 24(16):2924. https://doi.org/10.3390/molecules24162924
Chicago/Turabian StyleOrooji, Yasin, Ehsan Ghasali, Nahid Emami, Fatemeh Noorisafa, and Amir Razmjou. 2019. "ANOVA Design for the Optimization of TiO2 Coating on Polyether Sulfone Membranes" Molecules 24, no. 16: 2924. https://doi.org/10.3390/molecules24162924
APA StyleOrooji, Y., Ghasali, E., Emami, N., Noorisafa, F., & Razmjou, A. (2019). ANOVA Design for the Optimization of TiO2 Coating on Polyether Sulfone Membranes. Molecules, 24(16), 2924. https://doi.org/10.3390/molecules24162924