2.2. DPPH Radical Scavenging Capacity
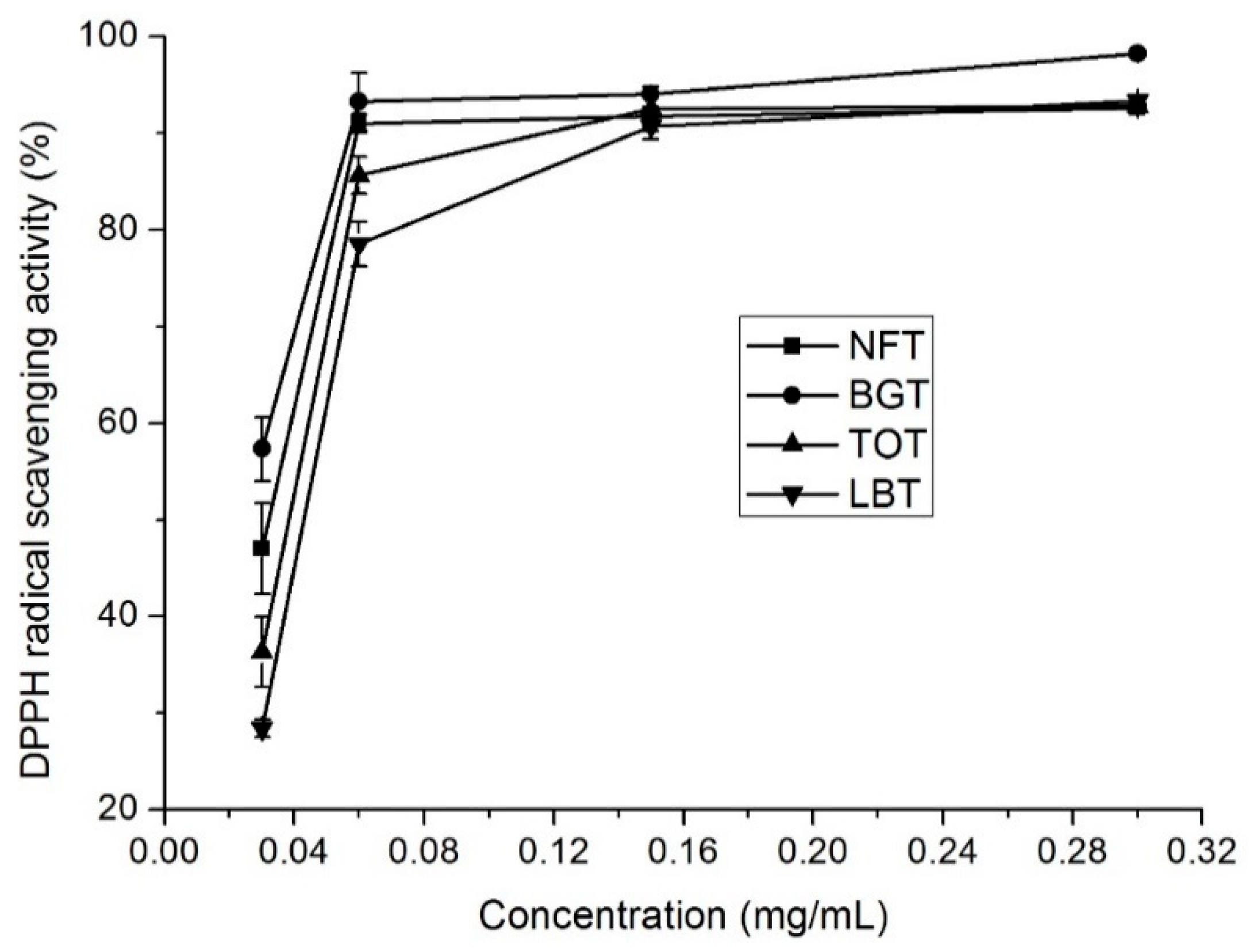
The stable organic free radical, DPPH, has been considered as a useful reagent for determining free radical scavenging capacity of antioxidant materials. In general, the scavenging capacities of NFT, BGT, TOT, and LBT on DPPH radicals were evident at all tested concentrations (
Figure 1). All the tea infusions showed >70% radical scavenging capacities at 0.06 mg/mL concentration. DPPH radical scavenging capacity showed a concentration dependency and increased with increase in concentration. The DPPH scavenging capacity of NFT was medium (46%) at 0.03 mg/mL and, at a concentration of 0.06 mg/mL, reached a plateau of 91% (
Figure 1). EC
50 values (the effective concentration of 50% inhibition) for NFT, BGT, TOT, and LBT infusions were 0.045, 0.029, 0.062, and 0.077 mg/ mL, respectively (
Table 2).
2.6. Catechins, Caffeine, and Theaflavins Contents
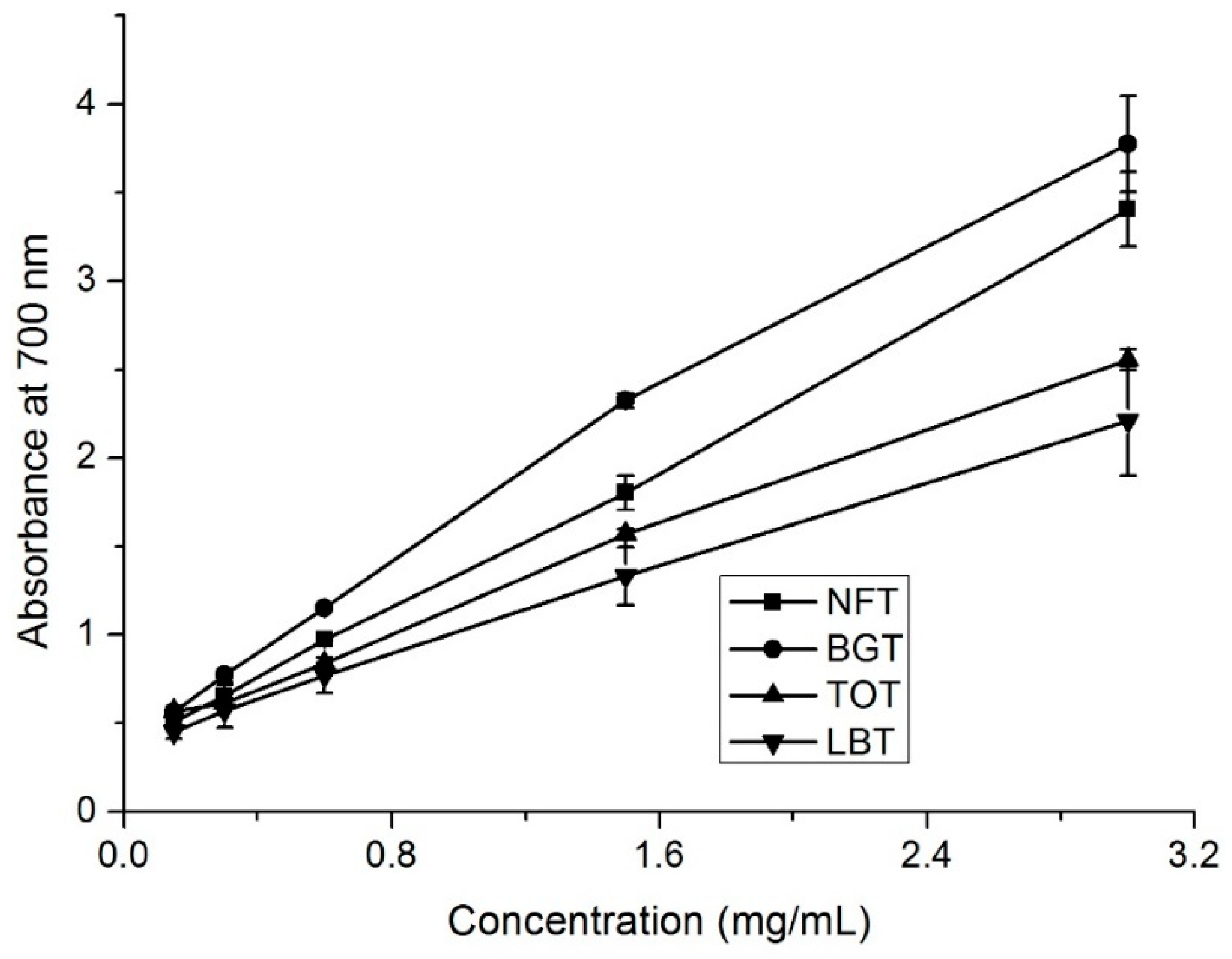
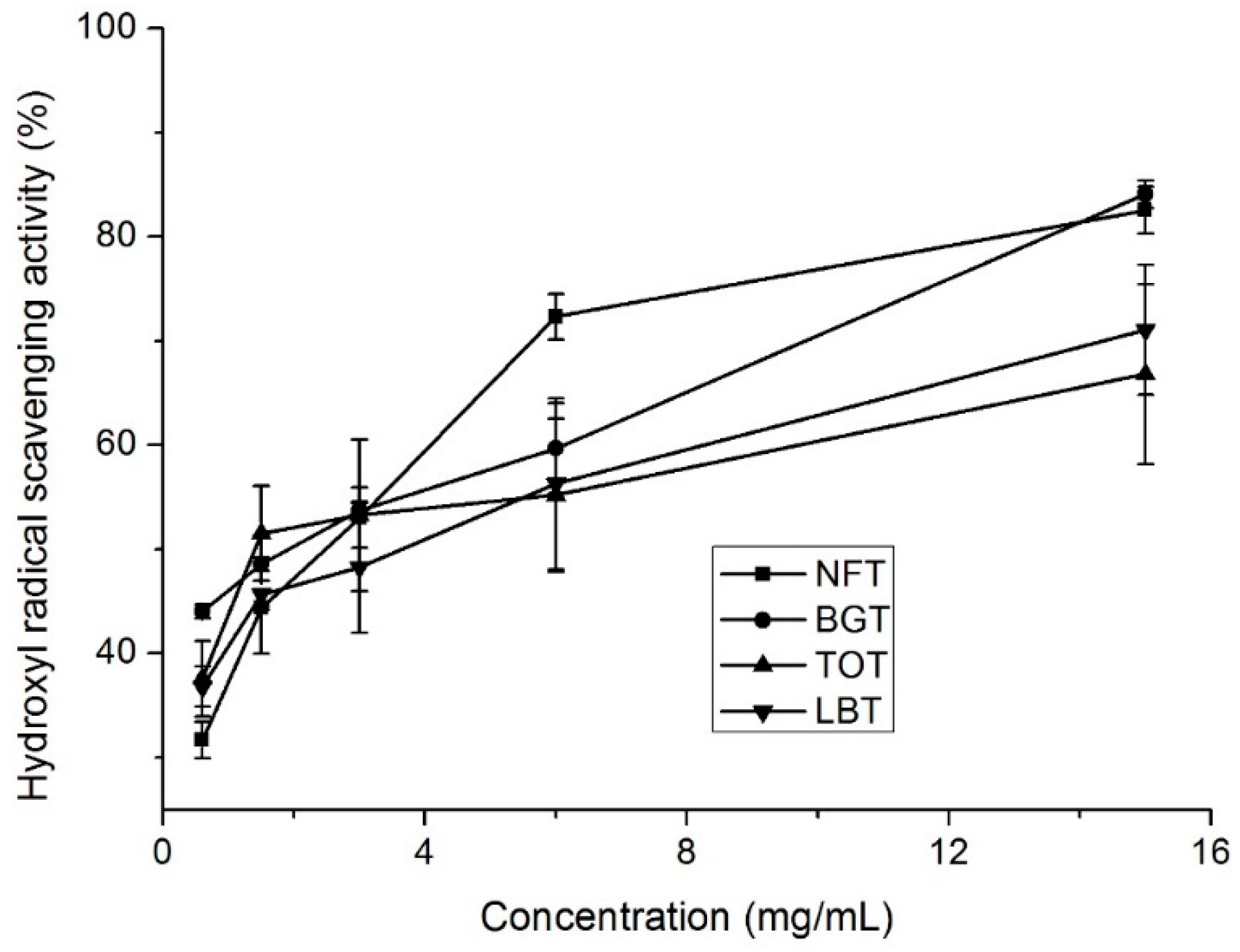
NFT, together with BGT, TOT, and LBT, contained different total phenolic and total flavonoid contents and exhibited favorable antioxidant capacities (
Table 3 and
Figure 1,
Figure 2 and
Figure 3). Next, HPLC analysis of tea samples were conducted to identify and compare major phenolic compounds including catechins, caffeine, and theaflavins.
Catechins (EGC, C, EGCG, EC and ECG) have been regarded as the major phenols in almost all kinds of teas. The contents of five major catechins in tea infusions were determined and the results were shown in
Table 4. Among all catechins tested, EGC and EGCG were the two predominant types of catechin in NFT, BGT, TOT, and LBT infusions accounting for 62, 78, 85, and 70% of the total catechins, respectively (
Table 4). Similarly, Koch and co-workers demonstrated that EGC and EGCG were the two major catechins in several green and black tea samples from different cultivation areas, although they found that the primary catechin in both green and black tea investigated is not EGCG but EGC [
14,
15]. EGC and EGCG have also been reported to be the major catechins in the final Fuzhuan brick teas samples [
16]. The level of EGC in NFT (1013 mg/100 g) was higher than that in LBT (817 mg/100 g), but lower than that in BGT (4020 mg/100 g) and TOT (2604 mg/100 g). The EGCG content of NFT was significantly lower compared to that of BGT, TOT, or LBT. The level of C and ECG in NFT was lower than that of BGT, but higher than that of TOT and LBT (
Table 4). EC ranged from 248 mg/100 g in NFT to 1086 mg/100 g in BGT. The contents of total catechins in NFT was comparable to that of LBT, but lower than that of BGT or TOT. The contents of caffeine are at the same level in NFT and BGT (
Table 4). Previous study demonstrated that the concentration of major phenolic compounds including EC, ECG, EGC, and EGCG in black tea infusion is related to the brewing time and that after 2 min brewing, most phenolics had already been extracted, and extract composition did not significantly change at a prolonged extraction time (4 min) [
17]. Future study aiming to determine the effect of brewing time on the composition and antioxidant properties of NBT is warranted.
During the fermentation process, catechins were degraded into the B ring fission catechins derivatives and polymerize into theaflavin derivatives by polyphenol oxidase or peroxidase. Theaflavin levels are known to be directly related to the taste and quality of the tea [
3]. Therefore, we decided to determine the TFs contents in the tea infusions. We found that total TFs and TF are present in very low concentration in BGT and TOT infusions. In contrast, NFT had the highest contents of total TFs, TF, TF3G, and TF3’G, followed by LBT (
Table 4). Liu et al. reported that with increasing semi-fermentation time, the total catechin concentration decreased, while total theaflavins contents increased significantly [
18].
Although functional studies on TFs have lagged seriously behind those of the catechins, TFs have attracted considerable interest recently, as they are reported to play important physiological roles, such as antioxidant [
19], anticancer [
20], anti-atherosclerotic [
21] activities as well as the prevention of osteoporosis [
22]. Furthermore, these compounds have human health benefits including glucose-lowering [
23] and anti-obesity effects [
24], and are useful in the prevention of lifestyle-related diseases. Takemoto et al. revealed that, the extraction of TFs from black tea leaves at sufficient levels for use in medical studies has been difficult due to the low concentration of TFs present in black tea [
25]. In the present study, we provided a novel tea product containing TFs in high concentration (
Table 4), encouraging future studies to extract theaflavins from NFT for pharmaceutical use or to develop new theaflavin-enriched functional foods.
The composition of bioactive substances in tea infusions is thought to contribute to its antioxidant activities. While numerous reports have indicated that phenolic compounds presented in the tea such as catechins and TFs are the contributor to the antioxidant capacities of tea, it should be noted that the presence of non-phenolic components such as polysaccharides may also contribute to the antioxidant activity. Sun et al. reported that four kinds of green tea polysaccharides, with Mw of 10.88, 8.16, 4.82, and 2.31 kDa, respectively, possessed hydroxyl and ABTS radical scavenging activity and reducing power [
26].
2.7. Free Amino Acids Analysis
Amino acids are important bioactive components of tea and are known to play an important role in the taste of tea. Here, significant differences in total free amino acids were observed among the four kinds of tea samples. NFT had the highest content of total free amino acids (630 mg/100 g), followed by BGT (598 mg/100 g), LBT (150 mg/100 g), and TOT (70 mg/100 g) (
Table 5). According to the chemical structure and biological activity, different amino acids commonly are divided to two groups: essential amino acids and non-essential amino acids. Among essential amino acids, NFT had the highest level of Val, Thr, Ile, Leu, Phe, Lys, and Met compared to BGT, TOT, or LBT. Met was not detected in TOT and LBT but existed in NFT and BGT in a low concentration (
Table 5).
GABA is a non-proteinaceous amino acid that occurs in animals, plants, and bacteria [
27]. It is primarily produced by microorganism and the biosynthesis of GABA is one step reaction of decarboxylating glutamate to GABA, catalyzed by glutamate decarboxylase. GABA generally occurs at a very low level in plants but its content increases substantially after exposure to a range of stresses, especially oxygen-deficiency [
27]. In the present study, NFT had the most abundant GABA (113.8 mg/100 g), followed by BGT (8.59 mg/100 g), LBT (5.82 mg/100 g), and TOT (1.97 mg/100 g) (
Table 5). Compared with previous studies on other types of tea, the level of GABA in fermented tea was much higher than that of pu-erh tea and white tea [
28].
GABA is well known to function as inhibitory neurotransmitters in the central nervous system [
29] and play multiple positive roles, such as reducing hypertension, regulating blood pressure, and ameliorating autism [
30,
31,
32]. Recently, the development of functional foods containing GABA in high concentration has gained popularity in complementary medicine practices as an accessible intervention to reduce the impact of chronic stress-induced autonomic imbalance and increased risk for cardiovascular disease. Several commercially available GABA-enriched foods have been developed such as GABA tea [
33], rice germ [
34], tempeh-like fermented soybean [
35], and black raspberry juice [
36]. Here, we demonstrated that NFT contains high level of GABA (
Table 5), highlighting the potential usefulness of NFT for the prevention or treatment of chronic stress-induced autonomic disorders and increased risk for cardiovascular disease.
2.8. Color Difference Analysis of Tea Infusions and Sensory Quality
Lightness (L*) is the measurement of the white-black color, so that a decrease on the L* values indicates darkening. As can be observed in
Table 6, the L* value of fermented tea infusions was slightly higher than that of LBT infusions, but lower than those of BGT and TOT infusions. Redness (a*) is a parameter that describe the color of samples in the red-green axis. The a* value of NFT infusions was slightly higher than those of BGT and TOT infusions, but lower than that of LBT infusions (
Table 6). Yellowness (b*) is a parameter that measures color changes in the yellow-blue range, becoming more yellowish as the numbers increase. The TOT infusions had the highest b* value, followed by NFT, LBT, and BGT infusions (
Table 6).
It has been established that sensory characteristics are known to be correlated with chemical constituents, which are mostly polyphenols, including catechins, theaflavins, and thearubigins, as well as free amino acids, sugars, organic acids, and caffeine [
37]. In the present study, the NFT had satisfactory color, aroma, taste, and overall acceptability scores (
Table 7). In addition, we found that BGT had highest bitterness and astringency scores compared to NFT, TOT, and LBT (
Table 7). This is consistent with the aforementioned results that the green tea had the highest catechins contents. Tea polyphenols, particularly tea catechins, have been found to activate the human bitter taste receptors hTAS2R14 [
38] and to be responsible for bitterness and astringency [
39,
40].