Abstract
In this review, we discuss the nature of the different physicochemical factors affecting the valence isomerism between 2H-pyrans (2HPs) and 1-oxatrienes, and we describe the most versatile synthetic methods reported in recent literature to access to 2HPs, with the only exception of 2HPs fused to aromatic rings (i.e., 2H-chromenes), which are not included in this review.
1. Introduction
The 2H-pyran (2HP) ring constitutes a structural motif present in many natural products (Figure 1) [1] and is a strategic key intermediate in the construction of many of these structures [2,3]. In spite of their importance, the literature of 2HPs is relatively scarce [4,5,6,7,8,9], mainly due to the instability associated with the heterocyclic ring, which makes these heterocycles establish an equilibrium with their opened isomeric forms (Scheme 1). Fusion of a 2HP to an aromatic ring confers stability to these heterocycles. Thus, while simple 2HPs are difficult to synthesize as pure and isolated compounds, many of their benzo derivatives (i.e., 2H-chromenes) constitute stable molecules, with a broad spectrum of biological activities and a widespread representation in the higher plants (Figure 1). Because the chemistry and reactivity of 2H-chromenes have been already previously revised [1,10,11,12,13,14,15], they will not be included in this review. Instead, we will focus on the recent advances on accessing 2HPs, either as simple and stable monocyclic structures or as part of fused polycyclic structures, excluding the 2H-chromene system.
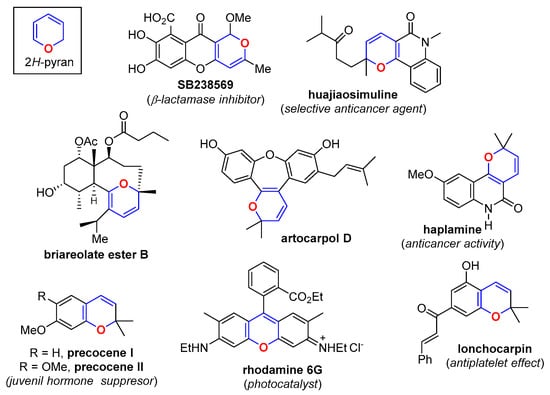
Figure 1.
Examples of natural products containing the 2HP motif.
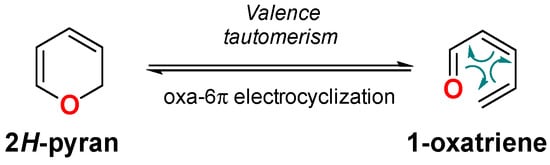
Scheme 1.
Valence tautomerism of 2H-pyrans (2HPs).
2. Dienone/2HP Equilibrium
2HPs are prone to undergo spontaneous valence isomerization [16] to the corresponding 1-oxatrienes through a reversible pericyclic oxa-6π-electrocyclization process (Scheme 1) [17]. This valence tautomerism determines the chemistry of these heterocycles, which commonly exist as a mixture of valence tautomers (isomers) [1]. In this sense, it is important to take into account that because this interconversion is fast in the majority of cases, the method of synthesis used to access these structures does not determine the valence tautomer obtained.
Although this valence isomerization was invoked to explain some enigmatic results found in earlier examples with these molecules, the first clear-cut example of it came from the irradiation of trans-β-ionone (1) (Scheme 2) [18]. Authors found that the irradiation afforded a mixture of cis-β-ionone (2) and 2HP 3, with a value for the equilibrium constant K = 4.61 at 327 °K (K = 1.52 at 386 °K), and values for k1 = 1.4 × 10−3·s−1 and k−1 = 1.3 × 10−4·s−1. In addition, measurements at different temperatures gave activation energies (Ea) of 20 Kcal/mol for the cis-dienone to 2HP reaction, and 27 Kcal/mol for the reverse process [19].
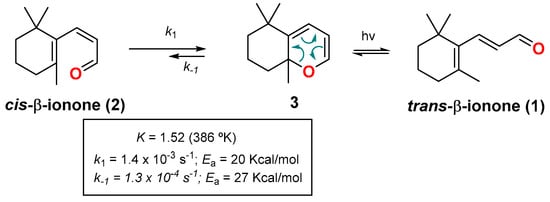
Scheme 2.
Valence isomerism of cis-β-ionone (2) and 2HP 3.
Further studies on the conformation of conjugated dienones allowed the establishment of some general patterns for these dienone/2HP equilibria (Scheme 3) [20]. It was observed that steric destabilization of the dienones shifted the equilibria toward the 2HPs. This was the case for tetrasubstituted dienones 4 and 5, which fully isomerized to the corresponding 2HPs. On the other hand, simpler dienones 8–12, which could adopt a stable planar conformation, existed in the opened form. Likewise, trisubstituted dienones 6–7, which are representative examples of dienones featuring non-stable planar conformations, preferred their closed forms. Along with these results, the authors also observed that the substitution of a δ-alkyl substituent (R5 or R6) by a substituent able to extend the conjugation of the π-system (e.g, vinyl group) favored the dienone form (Scheme 3). A main conclusion from these and other studies [20,21] is that the existence of the 2HP form depends primarily on the steric destabilization of the dienone rather than on its specific substitution pattern. Thus, the design of stable 2HPs must include, among other structural/electronic criteria, enough steric destabilization on the dienone to penalize the valence isomerization.
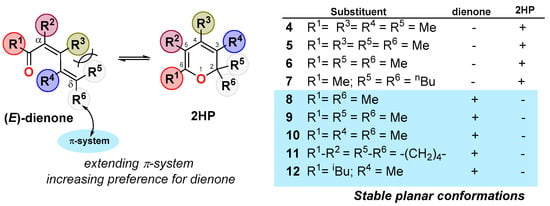
Scheme 3.
Implications of the cis-dienone conformation on the valence isomerism.
More recently, Krasnaya et al. carried out a systematic investigation on the influence of substituents and solvents on the valence isomerization of trisubstituted α-acyl-dienones 13 (Scheme 4). In this study, the authors quantified the equilibrium compositions of 26 differently substituted α-acyl-dienones 13 (Table 1), and determined the thermodynamic and activation parameters for some of these equilibria (Scheme 5) [22].
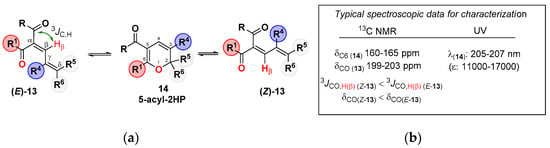
Scheme 4.
(a) Valence tautomerism of 5-acyl-2HPs 14. (b) Spectroscopic characteristic of tautomers.

Table 1.
α-Acyl-dienones 13 and their equilibrium isomeric compositions.a
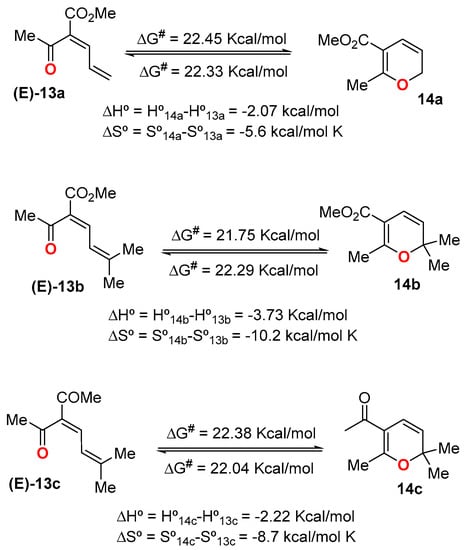
Scheme 5.
Thermodynamic data for 13a–c/14a–c valence isomerization.
Table 1 summarizes the earlier observed importance of structural effects on the valence equilibrium, and it confirms some general patterns:
- The successive substitution at position C2 in the ring (Cδ on dienone) leads to an increase in the content of 2HP (steric strain on the dienone) (compare entries 1–3 and 7–8).
- The elongation of the conjugated system results in an increase in the content of dienone (resonance delocalization) (compare entries 15, 25 and 17, 26).
- Substitution at the C2-position of the ring (Cδ on dienone) with two methyl groups strongly shifts the equilibrium toward the 2HP (entries 17, 19). In this case, it is possible to observe only the 2HP (compare entries 7, 17 with 11, 19).
- Aprotic polar solvent shifts the equilibrium toward the formation of the dienone.
- Although the electronic effect of the acyl group at the C5-position of the ring (Cα in the dienone) is masked in Table 1, other studies have shown that the presence of an electron-withdrawing substituent(s) at the ring, preferentially at this C5-position, favors the 2HP [23,24,25]. Table 1 shows that although this effect could be operative in α-acyl-dienones 13, it can be completely surpassed by other structural/electronic effects (Table 1, entries 11–14).
With regard to thermodynamic parameters of some of these equilibria (Scheme 5), Krasnaya et al. found that, in all cases, the enthalpies of the α-acyl-dienones 13 were appreciably higher than those of 5-acyl-2HPs 14, which is in full agreement with the observed increase in the dienone content with the increase in temperature. As should be expected, the entropy contents were also higher for the closed structures. In all the investigated cases, ΔG# values were on the order of 21.88 Kcal/mol to 22.86 Kcal/mol.
Finally, other structural factors, such as annulation, also favor the 2HP form. It has been well established that annulation favors the closed form by restricting conformational freedom (entropic trap), and it has been used as a design element in the synthesis of stable 2HPs [26,27]. Scheme 6 graphically summarizes the main conclusions of these studies. Structures 3, 15, 16 represent prototypical examples of room temperature stable 2HPs.
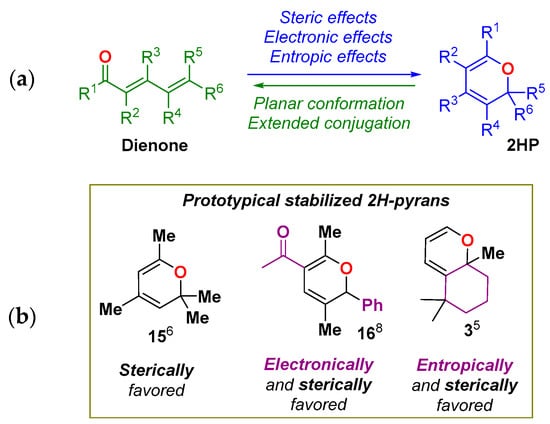
Scheme 6.
(a) Summary of parameters affecting the valence isomerization. (b) Prototypical examples of stable 2HPs.
3. Synthesis of the 2HP Core
The most common route for synthesizing these heterocycles relies on the oxa-6π-electrocyclization of dienones, the so-called 1-oxatriene pathway [28]. As already discussed in the previous section, this methodology requires endowing the 1-oxatriene unit with structural or electronic information, or both, to shift the valence equilibrium toward the 2HP form (Scheme 7). Thus, different synthetic pathways to the 1-oxatriene core have been successfully explored, involving, among others, the classic Knoevenagel condensation between active methylene compounds and α, β-unsaturated aldehydes (enals), Claisen rearrangements of propargyl vinyl ethers, Stille coupling of vinyl stannanes and vinyl iodides, and cycloisomerization of dienols (Scheme 7).
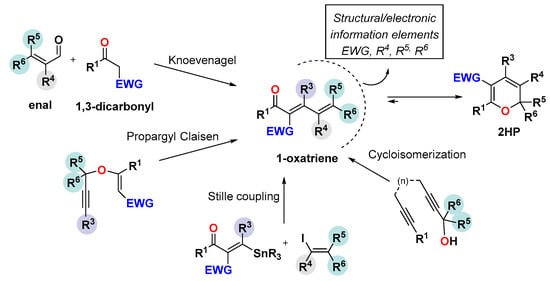
Scheme 7.
Synthesis of 1-oxatriene core incorporating structural/electronic information.
3.1. The Knoevenagel/Electrocyclization Protocol
The Knoevenagel condensation constitutes the most common access to 1-oxatrienes, and most generally involves the reaction of an enal with a 1,3-dicarbonyl compound [29]. The sequential performance of the Knoevenagel condensation and the electrocyclization reaction generates 2HPs (Scheme 8). From a synthetic point of view, the whole tandem process can be considered a formal [3 + 3] cycloaddition [2,28]. There is a plethora of examples of this strategy in the literature, mainly in the field of total synthesis of natural products. In this review, we will pay attention exclusively to established synthetic methods that allow or have allowed general access to these heterocycles. Specific cases utilized to access a particular structure or a specific natural product will not be covered. We refer the reader to the excellent published reviews covering this issue [2,3].
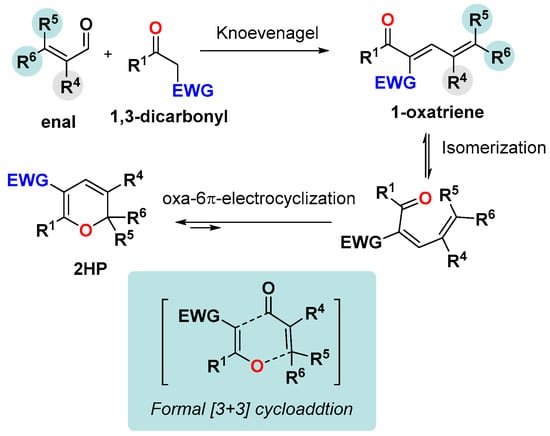
Scheme 8.
Knoevenagel/electrocyclization strategy.
Fusion to a ring favors the electrocyclization of the 1-oxatriene intermediate, and it has been used as a steering element to synthesize stable 2HPs. As an earlier example, the pyridine-mediated condensation of different cyclic 1,3-dicarbonyl compounds 17 and functionalized enals 18 generated the stable bicyclic 2HPs 19 in good yields (Scheme 9a) [30]. Therefore, the double substitution at the terminal position of the enal also contributed to the global stability of 2HPs 19. Using this methodology, the same authors synthesized the alkaloid flindersine (21) in 86% yield and in just one synthetic step (Scheme 9b).
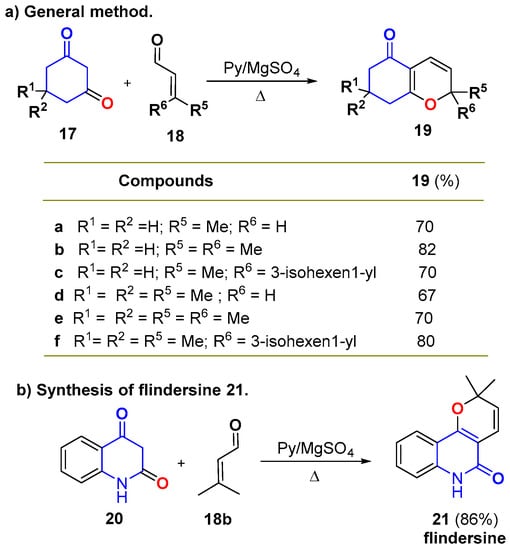
Scheme 9.
Knoevenagel/electrocyclization: (a) synthesis of annellated 2HPs 19 and (b) synthesis of flindersine (21).
The iminium-mediated Knoevenagel condensation (IMKC) [31,32] has been currently used to condense 1,3-dicarbonyl (active methylene) compounds with 2-alkenyliminiums (activated enals), and it constitutes a very versatile route to 1-oxatrienes [2,33]. The chemical outcome of the reaction is that of a formal [3 + 3] cycloaddition between enols and enals (see Scheme 8). The reaction is productive when functionalized cyclohexane-1,3-diones (e.g., 21) (Scheme 10) or 4-hydroxypyrones 25 (Scheme 11) are used as the active methylene compounds in the process. In this manner, 2HPs 23a–g were synthesized from the functionalized cyclohexa-1,3-dione 21 and different functionalized enals 22 (Scheme 10) [34]. These 2HPs were used as key intermediates in the total synthesis of (−)-daurichromenic acid and analogues. The use of Lewis [35] or Brønsted [36] acids, In3+ [37], or iodine [38] as catalysts resulted complementary to the iminium formation and afforded similar reaction outcomes.
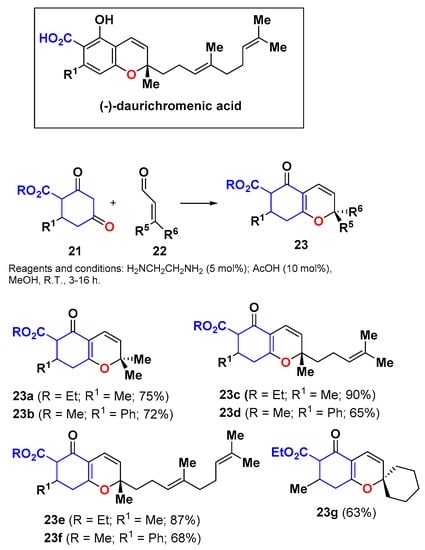
Scheme 10.
Synthesis of 2HPs 23 by iminium-mediated Knoevenagel condensation (IMKC) of cyclohexa-1,3-dione 21 and enals 22.
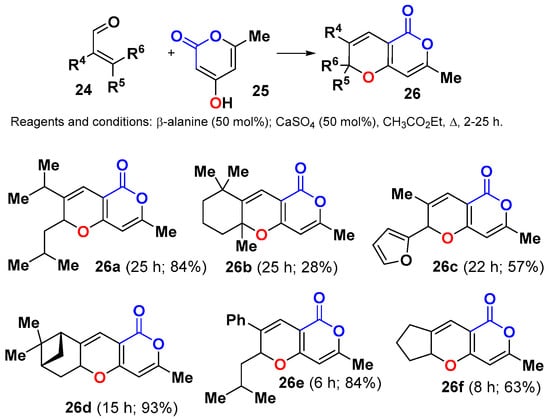
Scheme 11.
Construction of a small library of annulated 2HPs 26 by the IMKC of 4-hydroxypyranone 25 and functionalized enals 24. A selection of library members is shown.
This methodology is well suited for use in diversity oriented synthesis programs [39], as long as the structural control elements are incorporated into the library design. Thus, a small and structurally varied library of 2HPs 26 was constructed using the β-alanine-mediated IMKC between 4-hydroxypyranone 25 and different enals 24 (Scheme 11) [40]. In vitro studies of antiproliferative/cytotoxic activity with human SH-SY5Y neuroblastoma cells showed IC50 values ranging from 6.7 to >200 μM. 2HP 26a exhibited the highest cytotoxicity to the neuroblastome cells and necrotic effects on the human IPC melanoma cells.
Although the use of cyclic 1,3-dienones has been beneficial for the synthesis and stability of the resulting 2HPs, it is not a mandatory requirement, and acyclic active methylene compounds, such as methyl acetoacetate 27, have been successfully condensed with 2-alkyl-2-enals 28 to deliver the corresponding stable 2,3,6-trisubstituted 2HPs 29 (Scheme 12) [41].
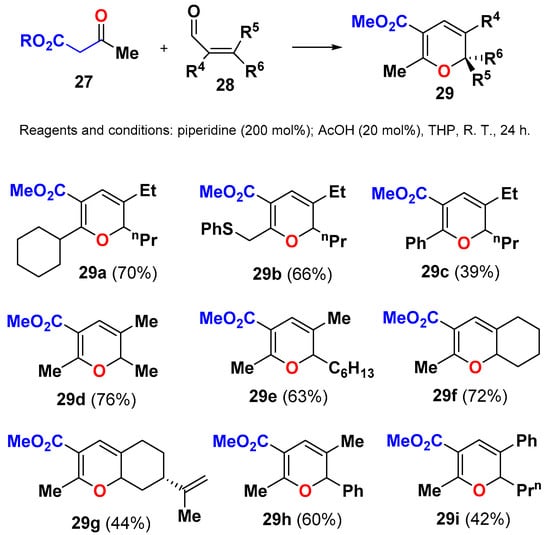
Scheme 12.
Synthesis of 2,3,6-trisubstituted 2HPs 29 by IMKC of methyl acetoacetate 27 and enals 28.
Pyrano[3,2-c]quinolone is a core structural motif in alkaloids and is endowed with important pharmacological and therapeutic activities. As part of a research program aimed at developing efficient synthesis of natural product-like small molecules, a small 23-membered library focused on carbohydrate fused pyrano[3,2-c]quinolone structures 32 was synthesized and subjected to antiproliferative activity studies (Scheme 13) [42]. The library was synthesized using the microwave assisted pyrrolidine-AcOH catalyzed IMKC of formyl galactal (30-Gal) and formyl glucal (30-Glu) with 4-hydroxyquinolones 31, and although the electron donating or electron withdrawing character of groups R1, R2, R3, and R4 of 4-hydroxyquinolones significantly affected neither the yield nor the reaction completion time, the best yields were obtained when unsubstituted 31 was used (70% with 30-Gal and 71% with 30-Glu). The other combinations gave yields ranging from 62 to 69%.
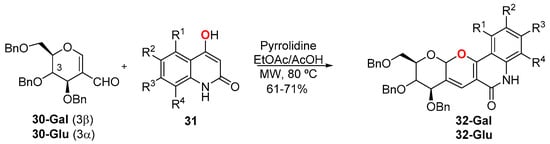
Scheme 13.
Carbohydrate fused pyrano[3,2-c]quinolone library.
The Knoevenagel/electrocyclization strategy is suitable to be performed in water (Scheme 14) [43]. This methodology was applied to the synthesis of biologically interesting 2HPs of general structure 39, comprising pyranocoumarin, pyranoquinolinone, and pyranonaphthoquinone derivatives along with selected natural and non-natural products (X = CH2, O, NH). The reactions were performed by mixing the 1,3-dicarbonyl compound 33–37 with enal 38 in water at 80 °C for 4–6 h. Although authors did not specify the physical conditions of these reactions, the high hydrophobicity of the reactants suggested that these reactions were carried out as aqueous suspensions (the so-called “on water” conditions [44], rather than as homogeneous solutions. Solvent-free protocols have been also described for the IMKC reaction [45,46].
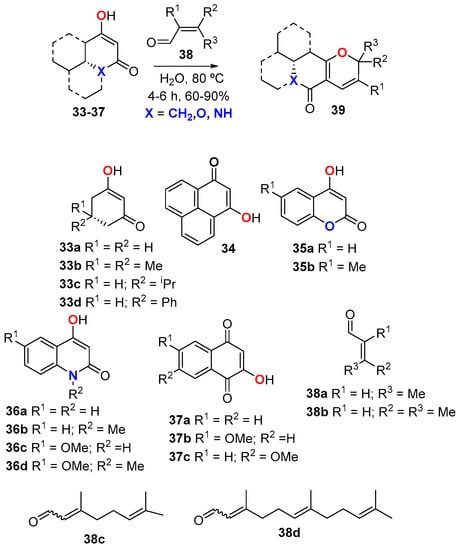
Scheme 14.
Knoevenagel/electrocyclization in water.
3.2. From Other Heterocycles
The condensation of methyl coumalate (40) with a wide range of active methylene compounds 41 has been implemented to access an extensive series of 2,3,5,6-tetrasubstituted 2HPs 42 (Scheme 15) [47]. The reaction involved a domino 1,6-Michael/6π-electrocyclic ring opening/[1,5]-H transfer/(decarboxylation)/6π-electrocyclization reaction. The methyl substituent allocated at C2 position of the 2HP ring corresponds to the α-methine group alpha to the lactone in the coumalate ring (highlighted as CH in Scheme 15).
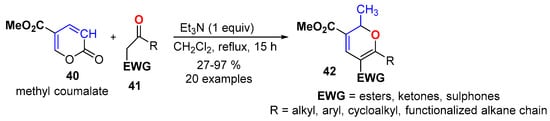
Scheme 15.
Domino synthesis of tetrasubstituted 2HPs 42 from methyl coumalate 40.
A one pot synthesis of 2,2,4,6-tetrasubstituted 2HPs 46 has been developed using Bayllis–Hillman carbonates 43 and β,γ-unsaturated α-oxo-esters 44 (Scheme 16) [48]. The one-pot reaction involved a phosphine-catalyzed condensation of 43 and 44 to give intermediate 4,5-dihydrofuran 45, which, in the presence of pyrrolidine and heat, rearranged to the 2HP 46. Authors gave a tentative mechanism for this pyrrolidine-catalyzed rearrangement. All the examples incorporated an aromatic (heterocyclic) substituent at R1, but the authors do not explain if this was a mandatory property of this substituent.
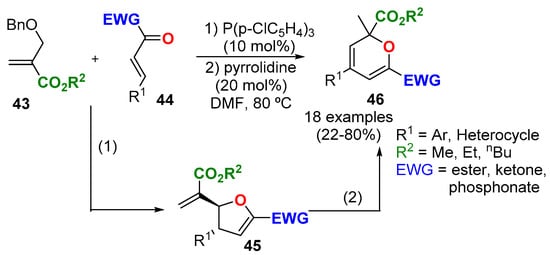
Scheme 16.
Synthesis of tetrasubstituted 2HPs 46 from 4,5-dihydrofurane 45.
3.3. From Allenolates
Stable 2,4,5,6-tetrasubstituted 2HPs 49 have been synthesized by the phosphine-catalyzed [3 + 3] annulation of ethyl 5-acetoxypenta-2,3-dienoate 47 and 1,3-dicarbonyl compounds 48 (Scheme 17) [49]. The scope of the reaction was wide, tolerating a good variety of the substituents. The presence of the ester group at the C5 position of the ring was fundamental for the stability of the 2HP 49.
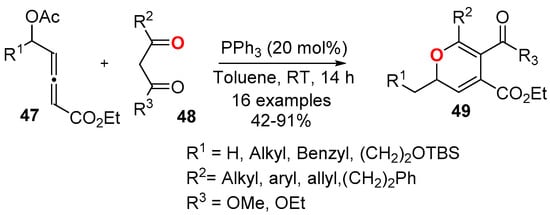
Scheme 17.
PPh3-catalyzed synthesis of 2,4,5,6-tetrasubstituted 2HPs 49 from ethyl 5-acetoxypent-2,3-dienoate 47 and 1,3-dicarbonyl compounds 48.
3.4. From Alkynes
3.4.1. From Propargyl Vinyl Ethers
Propargyl vinyl ethers 50 have been successfully rearranged into stable 2,4,5,6-tetrasubstituted 2HPs 51 through a one pot procedure involving a Ag(I)-catalyzed propargyl Claisen rearrangement followed by a tandem DBU-catalyzed isomerization/6π-oxa-electrocyclization reaction (Scheme 18) [50]. The protocol used secondary propargyl vinyl ethers (they bear only one substituent at the propargylic position; R3), and it required the installation of an ester group at the C5 position of the ring and substitution at the C6 position to give stability to the monocyclic 2HP 51.
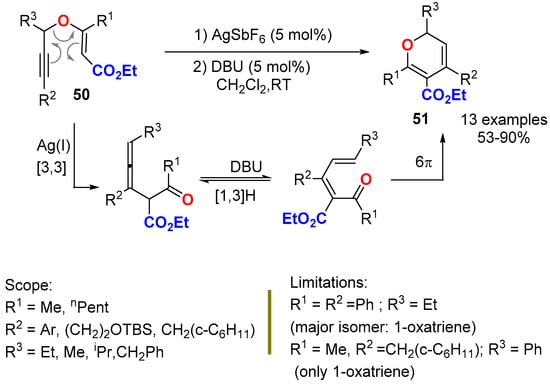
Scheme 18.
One pot synthesis of 2,3,4,6-tetrasubstituted 2HPs 51 from propargyl vinyl ethers 50.
More recently, a metal-free domino strategy has been developed for the synthesis of 2,2,4,5-tetrasubstituted 2HPs 53 from propargyl vinyl esters 52 (Scheme 19) [51,52,53]. The strategy made use of an imidazole-catalyzed all-pericyclic domino manifold entailing a sequential propargyl Claisen rearrangement/[1,3]-H shift/oxa-6π-electrocyclization set of reactions. Again, the presence of an ester functionality at the position C5 of the ring was mandatory to stabilize the final 2HP 53. The double substitution at C2 favored the 2HP formation (steric information) and offered a wide range of optional substitution patterns at the ring (Alk/Alk, Ar/Alk, Ar/Ar). The protocol delivered 2HP structures endowed with different topologies, including monocycles (53-mc), 2,2-spiro-bicycles (53-sbc), and 2,2-spiro-macrobicycles (53-smbc). The main limitation arose from the presence of a tBu substituent at the alkyne position (R1): In this case, the reaction followed a different pathway through a sequential [1,7]-H shift/6π-electrocyclization/MeOH elimination set of reactions [54].
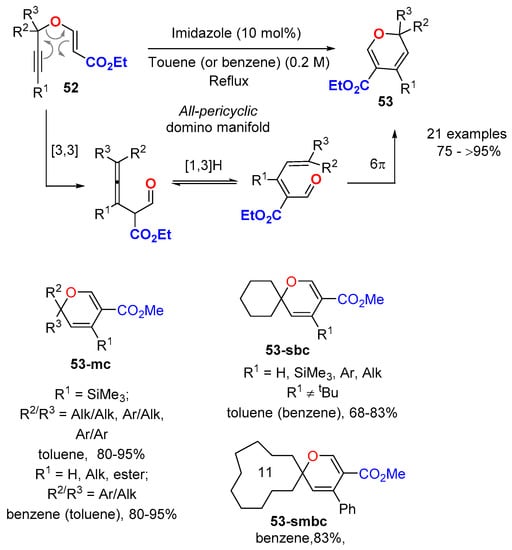
Scheme 19.
All pericyclic domino synthesis of 2,2,4,5-tetrasubstituted 2HPs 53 from propargyl vinyl ethers 52.
An alternative protocol using propargyl alcohols 54 and alkyl ethylendicarboxylates 55 has been developed (Scheme 20) [24,55]. The protocol generated 2,3,4,5,6-pentasubstituted 2HPs 56, incorporating two identical ester functionalities at C5 and C6, and a halogen atom at C3. The protocol used DABCO as the catalyst and N-iodosuccinimide (NIS) or N-bromosuccinimide (NBS) as the halogenation agent to generate 2HPs 56 in moderate to good yields. In all the conditions explored in Scheme 20, the substituents at the propargyl alcohol were aromatics (R1/R2 = Ar). The authors did not specify if this was a limitation to the procedure, or was just an inconvenient choice of starting materials.
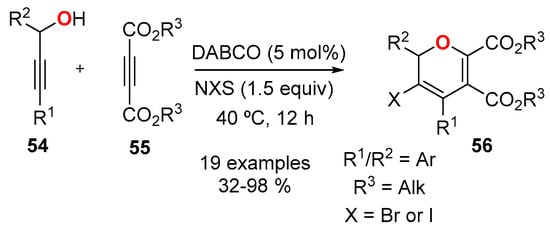
Scheme 20.
Synthesis of 2,3,4,5,6-pentasubstituted 2HP 56 from propargyl alcohols 54 and dialkyl acetelynedicarboxylates 55.
3.4.2. From Diynes
The Ni(0)-catalyzed cycloaddition of diynes 57 and aldehydes 58 has been explored in the construction of bicyclic 2HPs 59 (Scheme 21) [26,56]. Authors found that the structure of the diyne 57, mainly the substitution at the terminal positions (R1 ≠ H), and the length of the chain connecting the alkyne units, exerted a great influence on the bicyclic 2HP formation reaction. The worst yield was observed when acetaldehyde was used as the aldehyde (28%), whereas the best was observed with n-butanal (90%).
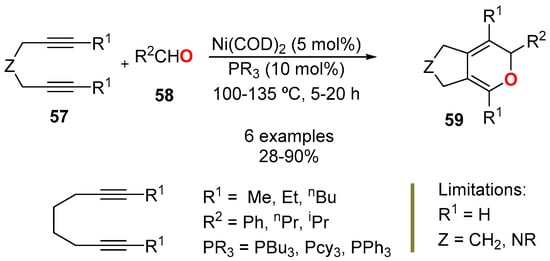
Scheme 21.
Ni(0)-catalyzed synthesis of bicyclic 2HP 59 from diynes 57 aldehydes 58.
A transition metal-free, cycloisomerization of diynols 60 to generate bicyclic 2HPs 61 has been reported (Scheme 22) [26]. The reaction was catalyzed by a cooperative catalytic system entailing Ca2+ catalyst (5 mol%) and camphorsulphonic acid (10 mol%), in the presence of benzaldehyde as a mild Lewis basic electron donor. The reaction was carried out without exclusion of air and moisture, and it tolerated a wide range of functionalities on the electron rich 2HP ring. The main limitations arose from substituents R2/R3 at the propargylic terminal position, which only allowed the alkyl/alkyl combination, and from R1, which had to be aromatic. The only limitation for the aromatic substituent at R1 was the presence of a free hydroxyl group at the ortho position of the ring. As long these restrictions were kept, excellent yields of 2HPs were obtained. The mechanistic proposal involved the formation of a propargylic tertiary cation 61, which afforded the cyclic 1-oxa-2,4,5-triene intermediate 62, which isomerized to 63 and rearranged into 2HP 64.
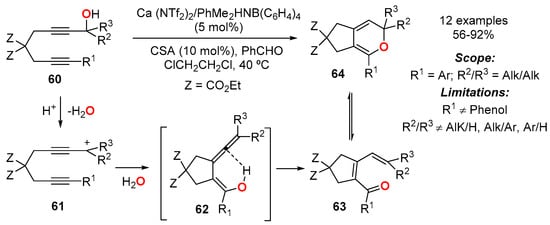
Scheme 22.
Ca2+/H+-catalyzed synthesis of bicyclic 2HP 64 from diynols 60.
3.4.3. From Alkenes: Tandem Stille-Oxa-Electrocyclization Reaction
Highly substituted bicyclic 2HPs 67 have been synthesized by a palladium-catalyzed tandem Stille-oxa-electrocyclization reaction between vinyl stannanes 65 and vinyl iodides 66 (Scheme 23) [57,58]. The strategy was a convergent alternative to the known methods for constructing similar bicyclic 2HPs, and it has been used in the total synthesis of natural products [59,60,61]. Although it required the prior stereoselective construction of both vinyl derivatives, the strategy had several advantages: It was convergent, highly diastereoselective, and required mild reaction conditions with low catalyst loadings (5 mol%). In the pattern of construction depicted in Scheme 23, the main restriction came from the nature of the vinyl iodide 66, which had to have substituents at the vinyl (R3 ≠ H) and allylic positions (R/R’ ≠ H) to stabilize the 2HP ring form by steric destabilization of the 1-oxatriene form.
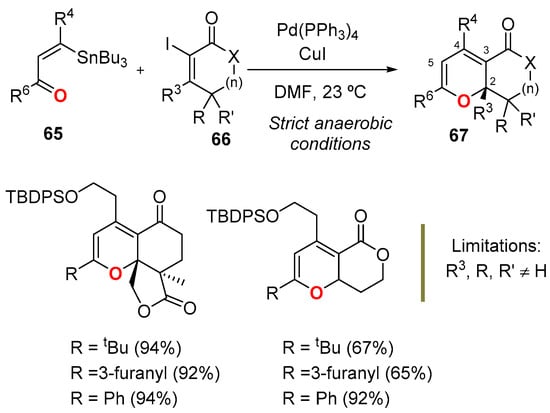
Scheme 23.
Stille-oxa-electrocyclization strategy to access bicyclic 2HP 67.
4. Summary and Conclusions
We have discussed the structural and electronic factors controlling the valence isomerism of 2HPs and how they can be harnessed to design effective synthesis of 2HPs. The most common routes to access these heterocycles relies on the 6π-electrocyclization of the corresponding 1-oxatriene isomers; thus, the synthetic challenge translates into the synthesis of the 1-oxatriene precursor. We have gathered the most transited routes to these species, including the proper Knoevenagel reaction, the tandem propargyl Claisen rearrangement/[1,3]-H shift reactions hosted by propargyl vinyl ethers, the cycloisomerization of diynes, and the Stille coupling of vinyl iodides and vinyl stannanes. From the large number of methods reported in the literature to access these heterocycles, we have selected only those able to generate stable rings with a convenient amount of structural/functional diversity. We hope that this review has filled the existing gap in literature regarding the reactivity and synthesis of these heterocycles, and that it finds use in future applications of these heterocycles.
Acknowledgments
The authors thank the Spanish Ministries of Economy and Competitiveness (MINECO) and Science, Innovation and Universities (MICINN), and the European Regional Development Funds (ERDF) for financial support (CTQ2015-63894-P and PGC2018-094503-B-C21). S.D.H. thanks La Laguna University and Cajasiete for a pre-doctoral contract.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Fravel, B.W. Pyrans and their Benzo Derivatives: Applications. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsdem, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 7, pp. 701–726. [Google Scholar]
- Hsung, R.P.; Kurdyumov, A.V.; Sydorenko, N. A formal [3 + 3] cycloaddition approach to Natural-Product synthesis. Eur. J. Org. Chem. 2005, 2005, 23–44. [Google Scholar] [CrossRef]
- Beaudry, C.M.; Malerich, J.P.; Trauner, D. Biosynthetic and biomimetic electrocyclizations. Chem. Rev. 2005, 105, 4757–4778. [Google Scholar] [CrossRef]
- Drygina, O.V.; Garnovskii, A.D.; Kazantsev, A.V. 2H-pyrans (review). Chem. Heterocycl. Compd. 1985, 21, 239–253. [Google Scholar] [CrossRef]
- Brimble, M.A.; Gibson, J.S.; Sperry, J. Pyrans and their Benzo Derivatives: Synthesis. In Comprehensive Heterocycles Chemistry III; Katritzky, A.R., Ramsdem, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 5, pp. 419–699. [Google Scholar]
- Hepworth, J.D.; Gabbutt, C.D.; Heron, B.M. Pyrans and their Benzo Derivatives: Synthesis. In Comprehensive Heterocycles Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, R.J.K., Eds.; Elsevier: Oxford, UK, 1996; Volume 7, pp. 301–468. [Google Scholar]
- Hepworth, J.D. Pyrans and Fused Pyrans: (iii) Synthesis and Applications. In Comprehensive Heterocycles Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Elsevier: Oxford, UK, 1984; Volume 3, pp. 737–883. [Google Scholar]
- Hepworth, J.D.; Heron, B.M. Six-membered ring systems: With O and/or S atoms. In Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Oxford, UK, 2009; Volume 20, pp. 365–398. [Google Scholar]
- Kuthan, J.; Šebek, P.; Böhm, S. New Developments in the Chemistry of Pyrans. Adv. Heterocyclic. Chem. 1995, 62, 19–135. [Google Scholar]
- Pratap, R.; Ram, V.J. Natural and Synthetic Chromenes, Fused Chromenes, and Versatility of Dihydrobenzo[h]chromenes in Organic Synthesis. Chem. Rev. 2014, 114, 10476–10526. [Google Scholar] [CrossRef]
- Majumdar, N.; Paul, N.P.; Mandal, S.; de Bruin, B.; Wulff, W.D. Catalytic Synthesis of 2H-Chromenes. ACS Catal. 2015, 5, 2329–2366. [Google Scholar] [CrossRef]
- Ellis, G.P.; Lockhart, I.M. The Chemistry of Heterocyclic Compounds: Chromenes, Chromanones, and Chromones; Ellis, G.P., Ed.; Wiley-VCH: New York, NY, USA, 2009; Volume 31, pp. 1–1196. [Google Scholar]
- Ellis, G.P. Pyrans and fused pyrans: (ii) reactivity. In Comprehensive Heterocycles Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Elsevier: Oxford, UK, 1984; Volume 3, pp. 647–736. [Google Scholar]
- Schweizer, E.E.; Meeder-Nycz, O. Chromenes, Chromanes, Chromones; Ellis, G.P., Ed.; Wiley-Interscience: New York, NY, USA, 1977; Volume 31, pp. 11–139. [Google Scholar]
- Hepworth, J.D.; Heron, B.M. Synthesis and photochromic properties of naphthopyrans. In Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Oxford, UK, 2005; Volume 17, pp. 33–62. [Google Scholar]
- Vogel, E. Valence Isomerizations in compounds with strained rings. Angew. Chem. Int. Ed. Engl. 1963, 2, 1–11. [Google Scholar] [CrossRef]
- Rodriguez-Otero, J. Study of the electrocyclization of (Z)-hexa-1,3,5-triene and its heterosubstituted analogues based on ab initio and DFT calculations. J. Org. Chem. 1999, 64, 6842–6848. [Google Scholar] [CrossRef]
- Marvell, E.N.; Caple, G.; Gosink, T.A.; Zimmer, G. Valence isomerization of a cis-dienone to an α-pyran. J. Am. Chem. Soc. 1966, 88, 619–620. [Google Scholar] [CrossRef]
- Marvell, E.N.; Gosink, T.A. Valence isomerization of 2,4,6-trimethyl-2H-pyran. J. Org. Chem. 1972, 37, 3036–3037. [Google Scholar] [CrossRef]
- Lillya, C.P.; Kluge, A.F. Molecular spectra and conformations of conjugated dienones. J. Org. Chem. 1971, 36, 1977–1988. [Google Scholar] [CrossRef]
- Gosink, T.A. Valence isomers. Substituent effects on the equilibrium between 2H-pyrans and cis dienones. J. Org. Chem. 1974, 39, 1942–1943. [Google Scholar] [CrossRef]
- Krasnaya, Z.A. Dienone ⇄ 2H-pyran valence isomerization. Chem. Heterocycl. Comp. 1999, 35, 1255–1271. [Google Scholar] [CrossRef]
- Adams, R.D.; Chen, L. Coupling of alkynes to carbon monoxide at a dimanganese center. A new route to carboxylate-functionalized pyrans. J. Am. Chem. Soc. 1994, 116, 4467–4468. [Google Scholar] [CrossRef]
- Fan, M.; Yan, Z.; Liu, W.; Liang, Y. DABCO-catalyzed reaction of α-halo carbonyl compounds with dimethyl acetylenedicarboxylate: A novel method for the preparation of polysubstituted furans and highly functionalized 2H-pyrans. J. Org. Chem. 2005, 70, 8204–8207. [Google Scholar] [CrossRef] [PubMed]
- Qing, F.L.; Gao, W.Z. The first synthesis of 4-trifluoromethyl-2H-pyrans by palladium-catalyzed cyclization of (E)-3-alkynyl-3-trifluoromethyl allylic alcohols. Tetrahedron Lett. 2000, 41, 7727–7730. [Google Scholar] [CrossRef]
- Rauser, M.; Schroeder, S.; Niggemann, M. Cooperative catalysis: Calcium and camphorsulfonic acid catalyzed cycloisomerization of diynols. Chem. Eur. J. 2015, 21, 15929–15933. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Kiyoi, T.; Miyane, T.; Saegusa, T. Nickel(0)-catalyzed reaction of diynes with aldehydes. J. Am. Chem. Soc. 1988, 110, 8570–8572. [Google Scholar] [CrossRef]
- Voskressensky, L.G.; Festa, A.A.; Varlamov, A.V. Domino reactions based on Knoevenagel condensation in the synthesis of heterocyclic compounds. Recent advances. Tetrahedron 2014, 70, 551–572. [Google Scholar] [CrossRef]
- Tietze, L.F.; Beifuss, U. The Knoevenagel reaction. In Comprehensive Organic Synthesis; Trost, B.M., Ed.; Pergamon Press: Oxford, UK, 1992; Volume 2, pp. 341–394. [Google Scholar]
- de Groot, A.; Jansen, B.J.M. A simple synthesis of 2H-pyrans; a one-step synthesis of flindersine. Tetrahedron Lett. 1975, 39, 3407–3410. [Google Scholar] [CrossRef]
- Lelais, G.; MacMillan, D.W.C. Modern Strategies in organic catalysis: The advent and development of iminium activation. Aldrichimica Acta 2006, 39, 79–87. [Google Scholar]
- Erkkila, A.; Majander, I.; Pihko, P.M. Iminium catalysis. Chem. Rev. 2007, 107, 5416–5470. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.C.; Wang, J.; Cole, K.P.; McLaughlin, M.J.; Morgan, C.D.; Douglas, C.J.; Hsung, R.P.; Coverdale, H.A.; Gerasyuto, A.I.; Hahn, J.M.; et al. A formal [3 + 3] cycloaddition reaction. improved reactivity using α,β-unsaturated iminium salts and evidence for reversibility of 6π-electron electrocyclic ring closure of 1-oxatrienes. J. Org. Chem. 2003, 68, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Harrison, T.J.; Wilson, P.D. A modular and concise total synthesis of (±)-daurichromenic acid and analogues. J. Org. Chem. 2004, 69, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Kurdyumov, A.V.; Lin, N.; Hsung, R.P.; Gullickson, G.C.; Cole, K.P.; Sydorenko, N.; Swidorski, J.J. A Lewis acid-catalyzed formal [3 + 3] cycloaddition of α,β-unsaturated aldehydes with 4-hydroxy-2-pyrone, diketones, and vinylogous esters. Org. Lett. 2006, 8, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Hubert, C.; Moreau, J.; Batany, J.; Duboc, A.; Hurvois, J.P.; Renaud, J.L. Brønsted acid-catalyzed synthesis of pyrans via a formal [3 + 3] cycloaddition. Adv. Synth. Catal. 2008, 350, 40–42. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, D.H.; Shim, J.J.; Kim, S.K.; Park, J.H.; Cha, J.S.; Lee, C.S. One-pot synthesis of 2H-pyrans by indium(iii) chloride-catalyzed reactions. Efficient synthesis of pyranocoumarins, pyranophenalenones, and pyranoquinolinones. Bull. Korean Chem. Soc. 2002, 23, 998–1002. [Google Scholar] [CrossRef]
- Jung, E.J.; Lee, Y.R.; Lee, H.J. Iodine-catalyzed one-pot synthesis of 2H-pyrans by domino Knoevenagel/6π-electrocylization. Bull. Korean Chem. Soc. 2009, 30, 2833–2836. [Google Scholar]
- Burke, M.D.; Schreiber, S.L. A planning strategy for diversity-oriented synthesis. Angew. Chem. Int. Ed. 2004, 43, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Leutbecher, H.; Williams, L.A.D.; Rösner, H.; Beifuss, U. Efficient synthesis of substituted 7-methyl-2H,5H-pyrano[4,3-b]pyran-5-ones and evaluation of their in vitro antiproliferative/cytotoxic activities. Bioorg. Med. Chem. Lett. 2007, 17, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Hirabaru, T.; Kawafuchi, H.; Inokuchi, T. Substituent-controlled electrocyclization of 2,4-dienones: Synthesis of 2,3,6-trisubstituted 2H-pyran-5-carboxylates and their transformations. Eur. J. Org. Chem. 2011, 2011, 5469–5474. [Google Scholar] [CrossRef]
- Kumari, P.; Narayana, C.; Dubey, S.; Gupta, A.; Sagar, R. Stereoselective synthesis of natural product inspired carbohydrate fused pyrano[3,2-c]quinolones as antiproliferative agents. Org. Biomol. Chem. 2018, 16, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Park, B.H.; Lee, Y.R. Environmentally benign, one-pot synthesis of pyrans by domino Knoevenagel/6π-electrocyclization in water and application to natural products. Green Chem. 2010, 12, 2003–2011. [Google Scholar] [CrossRef]
- Narayan, S.; Muldoon, J.; Finn, M.G.; Fokin, V.V.; Kolb, H.C.; Sharpless, K.B. “On water”: Unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 2005, 44, 3275–3279. [Google Scholar] [CrossRef] [PubMed]
- Riviera, M.J.; Mischine, M.P. Green one-pot synthesis of 2H-pyrans under solvent-free conditions catalyzed by ethylenediammonium diacetate. Synth. Commun. 2013, 43, 208–220. [Google Scholar] [CrossRef]
- Peña, J.; Moro, R.F.; Basabe, P.; Marcos, I.S.; Díez, D. Solvent free L-proline-catalysed domino Knoevenagel/6π-electrocyclization for the synthesis of highly functionalised 2H-pyrans. RSC Adv. 2012, 2, 8041–8049. [Google Scholar] [CrossRef]
- Chang, L.; Plevová, K.; Thorimbert, S.; Dechoux, L. Preparation of substituted 2H-pyrans via a cascade reaction from methyl coumalate and activated methylene nucleophiles. J. Org. Chem. 2017, 82, 5499–5505. [Google Scholar] [CrossRef]
- Xie, P.; Yang, J.; Zheng, J.; Huang, Y. Sequential catalyst phosphine/secondary amine promoted [1 + 4]/rearrangement domino reaction for the construction of (2H)-pyrans and 2-oxabicyclo[2,2,2]oct-5-ene skeletons. Eur. J. Org. Chem. 2014, 2014, 1189–1194. [Google Scholar] [CrossRef]
- Hu, J.; Dong, W.; Wu, X.Y.; Tong, X. PPh3-catalyzed [3 + 3] annulations of 5-acetoxypenta-2,3-dienoate with 1C,3O-bisnucleophiles: Facile entry to stable monocyclic 2H-pyrans. Org. Lett. 2012, 14, 5530–5533. [Google Scholar] [CrossRef]
- Menz, H.; Kirsch, S.F. Synthesis of stable 2H-pyran-5-carboxylates via a catalyzed propargyl-Claisen rearrangement/oxa-6π electrocyclization strategy. Org. Lett. 2006, 8, 4795–4797. [Google Scholar] [CrossRef]
- Tejedor, D.; Delgado-Hernández, S.; Diana-Rivero, R.; Díaz-Díaz, A.; García-Tellado, F. A domino strategy for the synthesis of 2H-pyrans from propargyl vinyl ethers. Eur. J. Org. Chem. 2019, 2019, 1784–1790. [Google Scholar] [CrossRef]
- Tejedor, D.; Díaz-Díaz, A.; Diana-Rivero, R.; Delgado-Hernández, S.; García-Tellado, F. Synthesis and utility of 2,2-dimethyl-2H-pyrans: Dienes for sequential Diels−Alder/Retro-Diels−Alder reactions. Org. Lett. 2018, 20, 7987–7990. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, D.; Delgado-Hernández, S.; Peyrac, J.; González-Platas, J.; García-Tellado, F. Integrative pericyclic cascade: An atom economic, multi C-C bond-forming strategy for the construction of molecular complexity. Chem. Eur. J. 2017, 23, 10048–10052. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, D.; Cotos, L.; Márquez-Arce, D.; Odriozola-Gimeno, M.; Torrent-Sucarrat, M.; Cossío, F.P.; García-Tellado, F. Microwave-assisted organocatalyzed rearrangement of propargyl vinyl ethers to salicylaldehyde derivatives: An experimental and theoretical study. Chem. Eur. J. 2015, 21, 18280–18289. [Google Scholar] [CrossRef] [PubMed]
- Chong, Q.; Wang, C.; Wang, D.; Wang, H.; Wu, F.; Xin, X.; Wan, B. DABCO-catalyzed synthesis of 3-bromo-/3-iodo-2H-pyrans from propargyl alcohols, dialkyl acetylene dicarboxylates, and N-bromo-/N-iodosuccinimides. Tetrahedron Lett. 2015, 56, 401–403. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Okude, Y.; Mori, S.; Shibuya, M. Combined experimental and computational study on ruthenium(II)-catalyzed reactions of diynes with aldehydes and N,N-dimethylformamide. J. Org. Chem. 2017, 82, 7964–7973. [Google Scholar] [CrossRef]
- Tambar, U.K.; Kano, V.; Zepernick, J.F.; Stoltz, B.M. The development and scope of a versatile tandem Stille-oxa-electrocyclization reaction. Tetrahedron Lett. 2007, 48, 345–350. [Google Scholar] [CrossRef]
- Tambar, U.K.; Kano, T.; Stoltz, B.M. Progress toward the total synthesis of saudin: Development of a tandem Stille-oxa-electrocyclization reaction. Org. Lett. 2005, 7, 2413–2416. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Johnson, R.P.; Porco, J.A. Total synthesis of the quinone epoxide dimer (+)-torreyanic acid: Application of a biomimetic oxidation/electrocyclization/Diels-Alder dimerization cascade. J. Am. Chem. Soc. 2003, 125, 5095–5106. [Google Scholar] [CrossRef]
- Shoji, M.; Yamaguchi, J.; Kakeya, H.; Osada, H.; Hayashi, Y. Total synthesis of (+)-epoxyquinols A and B. Angew. Chem. Int. Ed. 2002, 41, 3192–3194. [Google Scholar] [CrossRef]
- Li, C.; Lobkovsky, E.; Porco, J.A. Total synthesis of (±)-torreyanic acid. J. Am. Chem. Soc. 2000, 122, 10484–10485. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).