Floral Scent Variation in the Heterostylous Species Gelsemium sempervirens
Abstract
:1. Introduction
2. Results
2.1. Overall Scent Profile
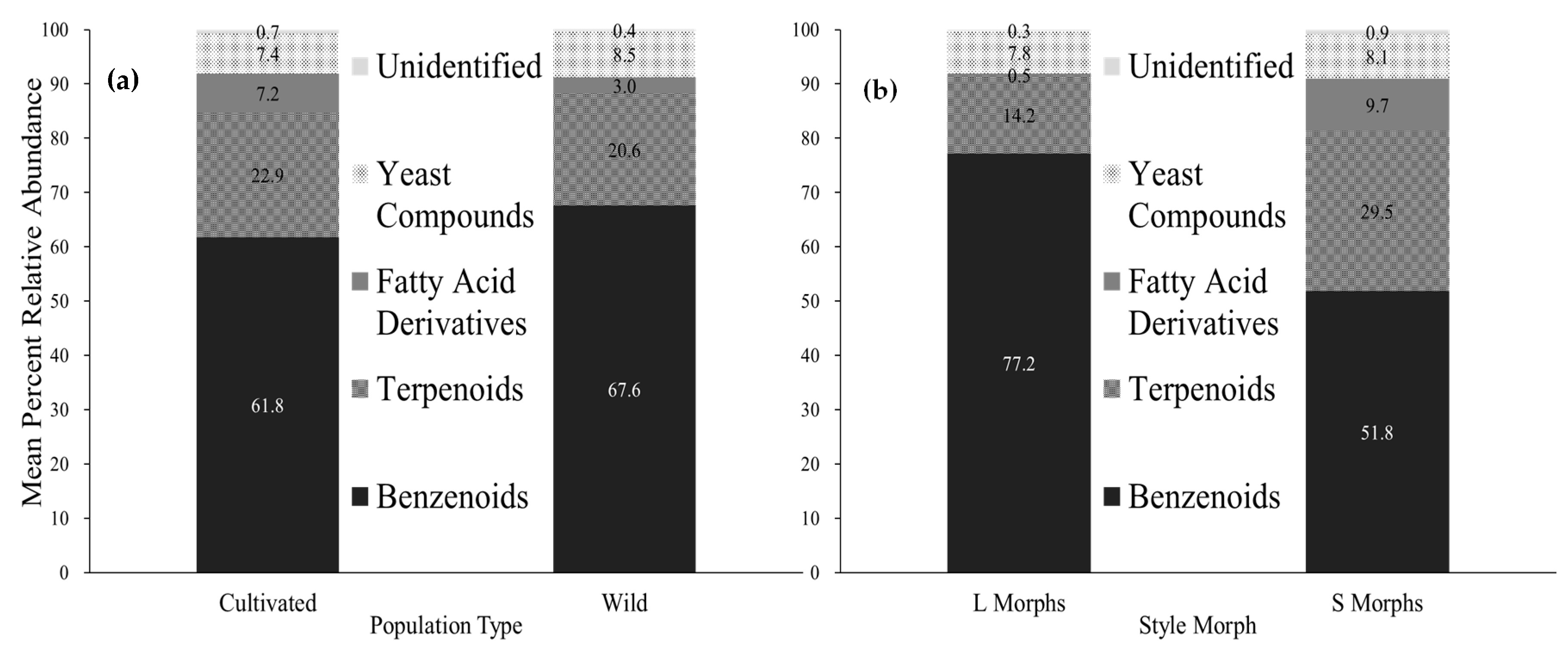
2.2. Variation in Compound Classes
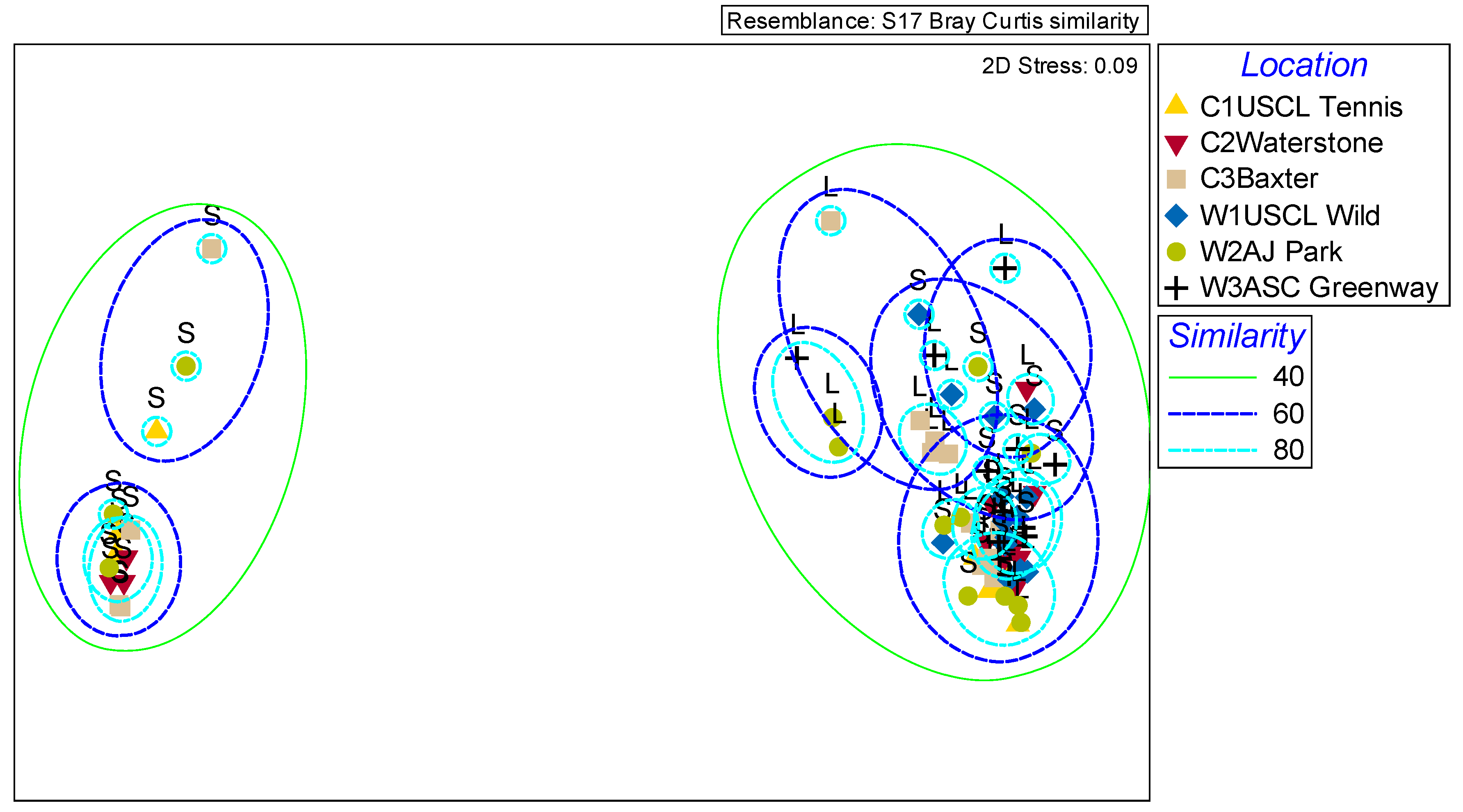
2.3. Variation in Complete Scent Profiles
3. Discussion
4. Materials and Methods
4.1. Study Sites and Flower Collection
4.2. SPME-GC-MS Analysis of Flowers and Standard Compounds
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chittka, L.; Raine, N.E. Recognition of flowers by pollinators. Curr. Opin. Plant Biol. 2006, 9, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Lee, S.H.; Hyun, T.K.; Kim, S.W.; Kim, J.Y. Plant volatiles as method of communication. Plant Biotechnol. Rep. 2013, 7, 9–26. [Google Scholar] [CrossRef]
- Heinrich, B. Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia 1979, 40, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Schiestl, F.P. The evolution of floral scent: The influence of olfactory learning by insect pollinators on the honest signaling of floral rewards. Funct. Ecol. 2009, 23, 841–851. [Google Scholar] [CrossRef]
- Kunze, J.; Gumbert, A. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behav. Ecol. 2001, 12, 447–456. [Google Scholar] [CrossRef]
- Simpson, B.B.; Neff, J.L. Evolution and diversity of floral rewards. In Handbook of Experimental Pollination Biology; Jones, C.E., Little, R.J., Eds.; Scientific and Academic Editions: New York, NY, USA, 1983; pp. 360–372. [Google Scholar]
- Dotterl, S.; Wolfe, L.M.; Jürgens, A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 2005, 66, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Effmert, U.; Buss, D.; Rohrbeck, D.; Piechulla, B. Localization of the synthesis and emission of scent compounds within the flower. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 105–124. [Google Scholar]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Penuelas, J. Floral volatile organic compounds: Between attraction and deterrence of visitors under global change. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 56–67. [Google Scholar] [CrossRef]
- Goodrich, K.R.; Zjhra, M.L.; Ley, C.A.; Raguso, R. When flowers smell fermented: The chemistry and ontogeny of yeasty floral scent in Pawpaw (Asimina triloba: Annonaceae). Int. J. Plant Sci. 2006, 167, 33–46. [Google Scholar] [CrossRef]
- Jetter, R. Examination of the process involved in the emission of scent volatiles from flowers. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 125–144. [Google Scholar]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function, and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Gershenzon, J. The chemical diversity of floral scent. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 27–52. [Google Scholar]
- Dudareva, N.; Pichersky, E. (Eds.) Biology of Floral Scent; CRC Press: Boca Raton, FL, USA, 2006; pp. 55–78. [Google Scholar]
- Struwe, L.; Albert, V.A.; Bremer, B. Cladistics and family level classification of the Gentianales. Cladistics 1994, 10, 175–205. [Google Scholar] [CrossRef]
- Struwe, L.; Soza, V.L.; Manickam, S.; Olmstead, R.G. Gelsemiaceae (Gentianales) expanded to include the enigmatic Asian genus Pteleocarpa. Bot. J. Linn. Soc. 2014, 175, 482–496. [Google Scholar] [CrossRef]
- Ornduff, R. The systematics and breeding system of Gelsemium (Loganiceae). J. Arnold Arbor. 1970, 51, 1–17. [Google Scholar] [CrossRef]
- Adler, L.S.; Irwn, R.E. Ecological costs and benefits of defenses in nectar. Ecology 2005, 86, 2968–2978. [Google Scholar] [CrossRef]
- Adler, L.S.; Irwin, R.E. Nectar alkaloids decrease pollination and female reproduction in a native plant. Oecologia 2012, 168, 1033–1041. [Google Scholar] [CrossRef]
- Lai, C.K.; Chan, Y.W. Confirmation of Gelsemium poisoning by targeted analysis of toxic Gelsemium alkaloids in urine. J. Anal. Toxicol. 2009, 33, 56–61. [Google Scholar] [CrossRef]
- Gaillard, Y.; Pepin, G. Poisoning by plant material: Review of human cases and analytical determination of main toxins by high-performance liquid chromatography (tandem) mass spectrometery. J. Chromatogr. B 1999, 733, 181–229. [Google Scholar] [CrossRef]
- Jin, G.; Su, Y.; Liu, M.; Xu, Y.; Yang, J.; Liao, K.; Yu, C. Medicinal plants of the genus Gelsemium (Gelsemiaceae, Gentianales)—A review of their phytochemistry, pharmacology, toxicology, and traditional use. J. Ethnopharmacol. 2014, 152, 33–52. [Google Scholar] [CrossRef]
- Gegear, R.J.; Manson, J.S.; Thomson, J.D. Ecological context influences pollinator deterrence by alkaloids in floral nectar. Ecol. Lett. 2007, 10, 375–382. [Google Scholar] [CrossRef]
- Irwin, R.E.; Adler, L.S. Correlations among traits associated with herbivore resistance and pollination: Implications for pollination and nectar robbing in a distylous plant. Am. J. Bot. 2006, 93, 64–72. [Google Scholar] [CrossRef]
- Leege, L.M.; Wolfe, L.M. Do floral hervibores respond to variation in flower characteristics in Gelsemium sempervirens (Loganiaceae), a distylous vine? Am. J. Bot. 2002, 89, 1270–1274. [Google Scholar] [CrossRef]
- Irwin, R.E.; Warren, P.S.; Carper, A.L.; Adler, L.S. Plant-animal interactions in suburban environments: Implications for floral evolution. Oecologia 2014, 174, 803–815. [Google Scholar] [CrossRef]
- Gaskett, A.C.; Conti, E.; Schiestl, F.P. Floral odor variation in two heterostylous species of Primula. J. Chem. Ecol. 2005, 31, 1223–1228. [Google Scholar] [CrossRef]
- Ashman, T.L. Sniffing out patterns of sexual dimorphism in floral scent. Funct. Ecol. 2009, 23, 852–862. [Google Scholar] [CrossRef]
- Delle-Vedove, R.; Schatz, B.; Dufay, M. Understanding intraspecific variation of floral scent in light of evolutionary ecology. Ann. Bot. 2017, 120, 2–20. [Google Scholar] [CrossRef]
- Golonka, A.M.; Obi Johnson, B.; Freeman, J.; Hinson, D.W. Impact of nectarivorous yeasts on Silene caroliniana’s scent. East. Biol. 2014, 3, 1–26. [Google Scholar]
- Rering, C.C.; Beck, J.J.; Hall, G.W.; McCartney, M.M.; Vannette, R.L. Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol. 2017, 220, 750–759. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties, and Uses, 6th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016. [Google Scholar]
- Dobson, H.E.M. Relationship between floral fragrance composition and type of pollinator. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 147–198. [Google Scholar]
- Dotterl, S.; Vereecken, N.J. The chemical ecology and evolution of bee-flower interactions: A review and perspectives. Can. J. Zool. 2010, 88, 668–697. [Google Scholar] [CrossRef]
- Irwin, R.E.; Adler, L.S. Nectar secondary compounds affect self-pollen transfer: Implications for female and male reproduction. Ecology 2008, 89, 2207–2217. [Google Scholar] [CrossRef]
- Fu, J.; Hou, D.; Zhang, C.; Bao, Z.; Zhao, H.; Hu, S. The emission of the floral scent of four Osmanthus fragrans cultivars in response to different temperatures. Molecules 2017, 22, 430. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Leng, P.; Zhao, J.; Wang, W.; Wang, S. The emission of floral scent from Lilium ‘Siberia’ in response to light intensity and temperature. Acta Physiol. Plant. 2013, 35, 1691–1700. [Google Scholar] [CrossRef]
- Staudt, M.; Bertin, N.; Hansen, U.L.; Seufert, G.; Cicciolij, P.; Foster, P.; Frenzel, B.; Fugit, J.L. Seasonal and diurnal patterns of monoterpene emissions from Pinus pinea (L.) under field conditions. Atmos. Environ. 1997, 31, 145–156. [Google Scholar] [CrossRef]
- Barták, P.; Bednář, P.; Čáp, L.; Ondráková, L.; Stránský, Z. SPME—A valuable tool for investigation of flower scent. J. Sep. Sci. 2003, 26, 715–721. [Google Scholar] [CrossRef]
- Tholl, D.; Rose, U.S.R. Detection and identification of floral scent compounds. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 3–25. [Google Scholar]
- Raguso, R.A. Floral Scent Analysis: A Primer for the Collection and Characterization of Fragrance. 2004. Available online: https://www.scribd.com/document/237094911/Floral-Scent-Analysis-A-Primer-for-the-Collection-and-Characterization-of-Fragrance (accessed on 15 May 2018).
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E. Ltd.: Plymouth, UK, 2006. [Google Scholar]
- SAS Institute Inc. SAS Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2002–2012. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER_E Ltd., Plymouth Marine Laboratory: Plymoth, UK, 2001. [Google Scholar]
- Majetic, C.J.; Levin, D.A.; Raguso, R.A. Divergence in floral scent profiles among and within cultivated species of Phlox. Sci. Hort. 2014, 172, 285–291. [Google Scholar] [CrossRef]
- Jürgens, A.; Witt, T.; Gottsberger, G. Flower scent composition in night-flowering Silene species (Caryophyllaceae). Biochem. Syst. Ecol. 2002, 30, 383–397. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecology 2001, 26, 32–46. [Google Scholar] [CrossRef]
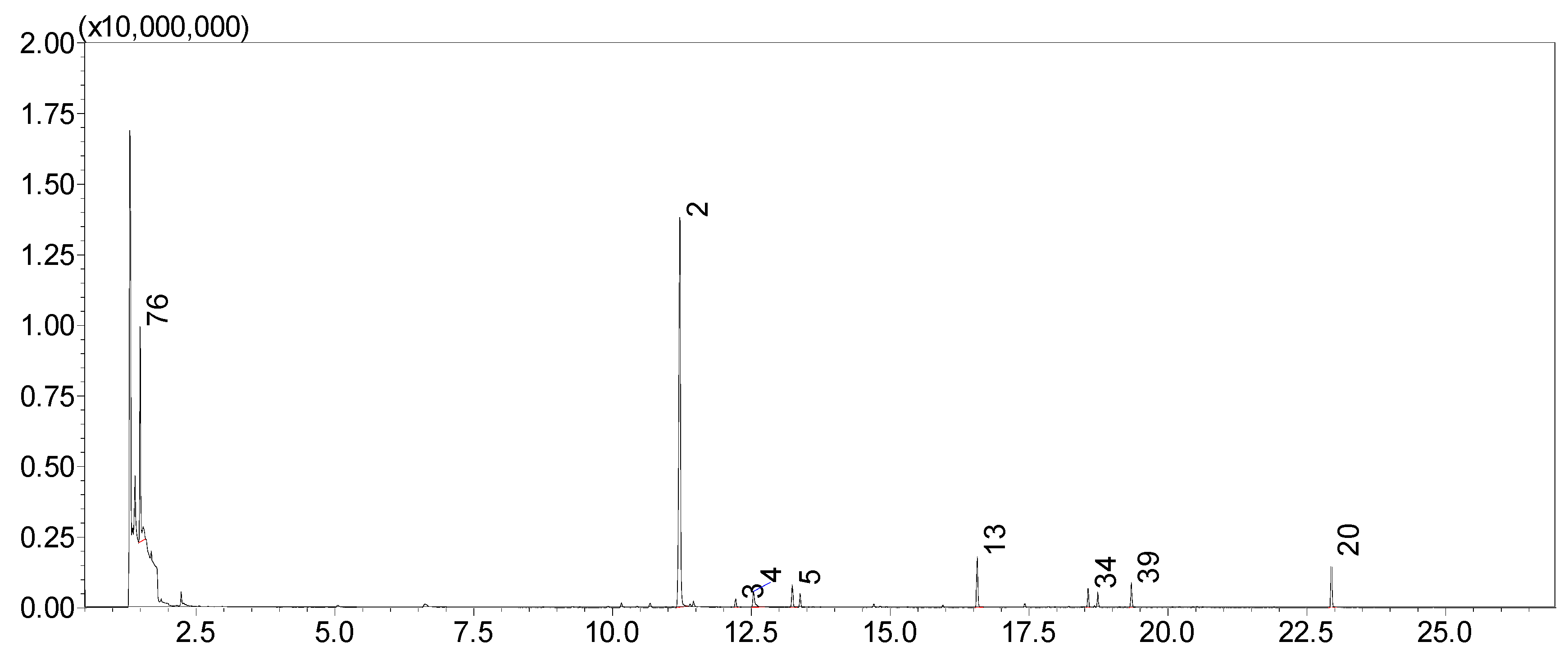
| Compound | R.I. | C1 | C2 | C3 | W1 | W2 | W3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L (N = 3) | S (N = 6) | L (N = 6) | S (N = 6) | L (N = 8) | S (N = 6) | L (N = 8) | S (N = 6) | L (N = 7) | S (N = 6) | L (N = 6) | S (N = 6) | |||
| Benzenoids | 93 (3) | 49 (21) | 79 (5) | 48 (19) | 80 (5) | 31 (17) | 83 (3) | 69 (7) | 72 (7) | 41 (18) | 62 (10) | 73 (4) | ||
| 1 | Benzene | 660 | 0.02 (0.02) | 0.02 (0.02) | 0.011 (0.004) | 0.01 (0.01) | 0.06 (0.02) | 0.02 (0.01) | 0.02 (0.01) | 0.02 (0.02) | - | 0.03 (0.02) | 0.02 (0.01) | 0.03 (0.02) |
| 2 | Benzaldehyde * | 995 | 80 (6) | 37 (16) | 58 (5) | 31 (13) | 46 (7) | 23 (14) | 59 (6) | 48 (7) | 57 (8) | 32 (15) | 44 (9) | 51 (4) |
| 3 | 4-Methylanisole | 1048 | 0.2 (0.1) | 0.7 (0.4) | 0.4 (0.1) | 1.4 (0.7) | 3.4 (0.7) | 0.3 (0.2) | 4 (1) | 3.1 (0.8) | 0.7 (0.1) | 0.5 (0.3) | 1.0 (0.2) | 1.6 (0.4) |
| 4 | Benzyl alcohol * | 1064 | 3.2 (0.8) | 2 (1) | 0.3 (0.1) | 1.0 (0.5) | 3.5 (0.7) | 0.5 (0.3) | 0.9 (0.5) | 1.5 (0.7) | 3.1 (0.9) | 0.3 (0.1) | 0.9 (0.4) | 2 (1) |
| 5 | Acetophenone | 1100 | 0.39 (0.08) | 2.3 (0.6) | 1.6 (0.6) | 2.3 (0.8) | 6 (1) | 2.3 (0.4) | 1.0 (0.5) | 0.8 (0.4) | 1.5 (0.3) | 1.1 (0.3) | 3.6 (0.9) | 6 (1) |
| 6 | Methyl benzoate | 1123 | - | - | 0.01 (0.01) | - | - | - | - | - | 0.01 (0.01) | - | - | 0.03 (0.03) |
| 7 | Benzyl acetate * | 1190 | 0.03 (0.03) | 0.02 (0.01) | 0.3 (0.1) | 0.08 (0.04) | 0.37 (0.07) | 0.04 (0.02) | 0.11 (0.03) | 0.15 (0.07) | 0.25 (0.07) | 0.06 (0.04) | 0.16 (0.08) | 0.09 (0.06) |
| 8 | Ethyl benzoate * | 1197 | - | - | 0.04 (0.03) | - | 0.06 (0.02) | 0.01 (0.01) | - | 0.01 (0.01) | 0.16 (0.09) | 0.02 (0.02) | 0.07 (0.07) | 0.09 (0.09) |
| 9 | 1,4-Dimethoxybenzene * | 1199 | 0.3 (0.1) | 0.01 (0.01) | 0.01 (0.01) | 0.03 (0.02) | 0.17 (0.08) | - | 0.3 (0.1) | 0.01 (0.01) | - | 0.02 (0.02) | 0.08 (0.06) | 0.01 (0.01) |
| 10 | 2`-Hydroxyacetophenone | 1206 | - | 0.2 (0.1) | 0.26 (0.07) | 0.3 (0.2) | 0.7 (0.2) | 0.4 (0.1) | 0.10 (0.04) | 0.18 (0.05) | 0.05 (0.02) | 0.12 (0.04) | 0.17 (0.08) | 0.6 (0.2) |
| 11 | 2-Methoxy-4-methylphenol | 1223 | - | 0.01 (0.01) | - | 0.04 (0.03) | 0.08 (0.02) | - | - | - | - | 0.01 (0.01) | 0.03 (0.03) | 0.01 (0.01) |
| 12 | Estragole | 1230 | 0.09 (0.05) | 0.02 (0.02) | 0.03 (0.01) | 0.10 (0.08) | 0.29 (0.07) | 0.01 (0.01) | 0.10 (0.04) | 0.12 (0.06) | 0.01 (0.01) | 0.03 (0.03) | 0.04 (0.03) | 0.10 (0.05) |
| 13 | 4-Anisaldehyde * | 1313 | 3.8 (0.1) | 4 (2) | 11 (1) | 3 (2) | 5 (1) | 2 (1) | 8 (1) | 5 (1) | 3.1 (0.6) | 3 (2) | 5 (2) | 4.9 (0.8) |
| 14 | Isobutyl benzoate | 1359 | - | - | - | - | - | 0.01 (0.01) | 0.02 (0.01) | - | 0.01 (0.01) | - | 0.04 (0.02) | 0.03 (0.01) |
| 15 | Benzyl butyrate * | 1373 | - | 0.02 (0.01) | 0.24 (0.08) | 0.10 (0.06) | 0.17 (0.04) | 0.07 (0.05) | 0.3 (0.2) | 0.15 (0.03) | 0.20 (0.07) | 0.02 (0.01) | 0.05 (0.02) | 0.11 (0.07) |
| 16 | Butyl benzoate | 1403 | 0.02 (0.01) | 0.03 (0.01) | 0.21 (0.03) | 0.05 (0.03) | 0.02 (0.01) | 0.03 (0.02) | 0.13 (0.05) | 0.17 (0.04) | 0.08 (0.01) | 0.05 (0.03) | 0.04 (0.02) | 0.13 (0.04) |
| 17 | Benzyl tiglate | 1534 | 0.09 (0.05) | 0.04 (0.02) | 0.01 (0.01) | 0.09 (0.06) | 0.10 (0.06) | 0.03 (0.02) | 0.01 (0.01) | 0.02 (0.02) | 0.02 (0.01) | 0.05 (0.04) | 0.01 (0.01) | 0.05 (0.02) |
| 18 | (Z)-3-Hexenyl benzoate | 1605 | 0.04 (0.01) | 0.02 (0.01) | 0.15 (0.04) | 0.2 (0.1) | 0.06 (0.02) | 0.02 (0.01) | 0.09 (0.03) | 0.17 (0.06) | 0.07 (0.04) | 0.05 (0.04) | 0.16 (0.05) | 0.4 (0.2) |
| 19 | Hexyl benzoate | 1612 | - | 0.01 (0.01) | 0.05 (0.01) | 0.06 (0.03) | 0.02 (0.01) | 0.02 (0.01) | 0.05 (0.02) | 0.04 (0.01) | 0.04 (0.01) | 0.03 (0.03) | 0.02 (0.01) | 0.05 (0.03) |
| 20 | Benzyl benzoate * | 1823 | 5 (1) | 3 (2) | 6 (1) | 8 (4) | 15 (2) | 2 (2) | 8 (1) | 9 (2) | 5.7 (0.8) | 3 (2) | 6 (2) | 5.8 (0.7) |
| Terpenoids | 3 (1) | 26 (11) | 20 (5) | 31 (11) | 9 (3) | 43 (13) | 14 (2) | 24 (6) | 10 (5) | 30 (10) | 26 (8) | 24 (4) | ||
| 21 | α-Pinene | 936 | 0.4 (0.2) | 0.25 (0.08) | 0.01 (0.01) | 0.8 (0.2) | 4 (2) | 0.29 (0.09) | 0.15 (0.04) | 0.18 (0.05) | 0.11 (0.04) | 0.06 (0.05) | 0.2 (0.2) | 0.04 (0.03) |
| 22 | [β-Phellandrene] | 980 | - | 0.02 (0.01) | - | - | - | 0.17 (0.09) | - | - | 0.01 (0.01) | 0.01 (0.01) | 0.05 (0.05) | - |
| 23 | β-Pinene | 982 | 0.13 (0.05) | 0.02 (0.01) | - | 0.24 (0.04) | 2 (1) | 0.09 (0.03) | 0.01 (0.01) | - | 0.03 (0.01) | - | 0.05 (0.02) | 0.02 (0.01) |
| 24 | Myrcene | 994 | - | - | - | - | - | 0.05 (0.03) | - | - | - | 0.07 (0.04) | - | - |
| 25 | 6-Methyl-5-hepten-2-one | 1009 | 0.1 (0.1) | 0.05 (0.05) | 0.47 (0.06) | 1.2 (0.4) | 0.21 (0.09) | 0.3 (0.2) | 0.4 (0.2) | 1.0 (0.4) | 0.5 (0.2) | 0.5 (0.2) | 0.35 (0.08) | 0.7 (0.1) |
| 26 | 1,8-Cineole * | 1044 | - | 0.1 (0.1) | 0.01 (0.01) | - | - | 0.5 (0.3) | - | - | - | 0.07 (0.04) | - | 0.01 (0.01) |
| 27 | Limonene * | 1045 | - | - | 0.03 (0.01) | 0.1 (0.1) | - | - | 0.05 (0.02) | - | - | - | 0.02 (0.02) | - |
| 28 | (E)-β-Ocimene * | 1046 | 0.08 (0.08) | 0.13 (0.08) | - | 0.3 (0.1) | - | 0.3 (0.1) | - | - | - | 0.08 (0.05) | - | - |
| 29 | (Z)-α-Ocimene * | 1057 | - | 21 (9) | 0.08 (0.03) | 24 (11) | - | 34 (13) | 0.02 (0.01) | - | - | 18 (9) | 0.02 (0.01) | 0.07 (0.01) |
| 30 | [Ionene] | 1366 | - | - | 0.07 (0.05) | - | - | 0.01 (0.01) | - | 0.7 (0.2) | 0.06 (0.04) | 0.04 (0.04) | 0.2 (0.2) | 0.05 (0.04) |
| 31 | (E)-β-Caryophyllene * | 1449 | - | 0.14 (0.06) | - | 0.4 (0.1) | - | 0.3 (0.2) | - | - | 0.01 (0.01) | 0.14 (0.06) | 0.03 (0.03) | - |
| 32 | Dihydro-α-ionone | 1450 | - | - | 0.8 (0.3) | - | 0.3 (0.1) | 0.05 (0.05) | 0.02 (0.02) | 0.4 (0.2) | 0.08 (0.06) | - | 0.3 (0.3) | 0.6 (0.3) |
| 33 | Geranyl acetone | 1451 | - | - | - | 0.12 (0.05) | - | 0.01 (0.01) | 0.03 (0.01) | 0.03 (0.02) | 0.04 (0.03) | 0.03 (0.03) | 0.3 (0.2) | 0.03 (0.02) |
| 34 | α-Ionone * | 1460 | 0.2 (0.1) | 0.03 (0.02) | 3.0 (0.8) | 1.0 (0.6) | 0.5 (0.3) | 0.6 (0.4) | 2.6 (0.8) | 3.1 (0.8) | 1.4 (0.5) | 2 (1) | 2.5 (0.9) | 1.3 (0.5) |
| 35 | Dihydro-β-ionone * | 1467 | - | 3 (2) | 2 (1) | 0.8 (0.5) | 0.04 (0.03) | 4 (2) | 0.5 (0.3) | 3 (1) | 0.4 (0.2) | 5 (2) | 2 (1) | 1.4 (0.7) |
| 36 | Neryl acetone | 1470 | 1.1 (0.4) | 0.8 (0.5) | 0.09 (0.09) | 1.5 (0.8) | 0.14 (0.08) | - | 1.1 (0.4) | 0.4 (0.2) | 0.8 (0.7) | 1.0 (0.9) | 1.3 (0.7) | 0.5 (0.3) |
| 37 | [(Z)-α-Bergamotene] | 1500 | - | - | 0.08 (0.01) | 0.02 (0.02) | - | 0.01 (0.01) | 0.04 (0.02) | 0.06 (0.05) | 0.04 (0.03) | - | 0.12 (0.05) | 0.15 (0.04) |
| 38 | α-Farnesene * | 1517 | 0.6 (0.2) | 0.3 (0.1) | 4 (2) | 0.4 (0.2) | 1.3 (0.5) | 0.4 (0.2) | 8 (2) | 7 (5) | 5 (4) | 0.1 (0.1) | 11 (4) | 16 (5) |
| 39 | β-Ionone | 1519 | - | - | 9 (4) | - | 0.2 (0.1) | 2 (2) | 1 (1) | 8 (4) | 1.2 (0.7) | 3 (3) | 7 (7) | 3 (3) |
| Fatty Acid Derivatives | 0.37 (0.06) | 14 (6) | 0.19 (0.04) | 17 (7) | 1.0 (0.3) | 9 (4) | 0.5 (0.2) | 0.7 (0.5) | 0.10 (0.06) | 17 (8) | 0.5 (0.1) | 0.5 (0.3) | ||
| 40 | Tert-butyl ethyl ether * | 611 | - | - | - | - | 0.2 (0.1) | - | - | - | - | - | 0.12 (0.07) | - |
| 41 | 1-Butanol | 674 | - | - | - | - | - | - | 0.03 (0.03) | - | - | - | - | - |
| 42 | Heptane | 700 | - | 0.03 (0.03) | - | - | - | - | - | 0.03 (0.03) | - | - | - | - |
| 43 | 2-Pentanone | 706 | - | - | - | 0.05 (0.05) | - | - | - | - | - | - | - | - |
| 44 | 3-Pentanone | 716 | - | - | - | 0.08 (0.05) | - | - | - | - | - | - | - | - |
| 45 | 4-Methyl-2-pentanone | 759 | - | - | - | 0.09 (0.09) | - | - | - | - | - | - | - | - |
| 46 | 2-Methyl-2-butenal | 769 | 0.07 (0.05) | - | - | - | - | 0.04 (0.04) | - | - | 0.01 (0.01) | 0.2 (0.1) | - | - |
| 47 | Octane | 800 | - | 0.01 (0.01) | - | 0.06 (0.04) | - | - | 0.01 (0.01) | 0.1 (0.1) | - | 0.03 (0.03) | - | - |
| 48 | 4-Methyl-3-penten-2-one | 824 | - | - | - | 0.15 (0.08) | - | - | - | 0.01 (0.01) | 0.02 (0.01) | 0.02 (0.02) | - | - |
| 49 | Butyl acetate | 838 | - | - | 0.01 (0.01) | - | - | - | 0.01 (0.01) | - | - | - | - | 0.01 (0.01) |
| 50 | [Ethyl 2-methylbutyrate] | 865 | - | - | - | - | - | - | - | - | - | - | - | 0.01 (0.01) |
| 51 | 3-Hexen-1-ol | 874 | - | - | - | - | - | 0.03 (0.03) | - | 0.03 (0.03) | - | - | 0.04 (0.04) | 0.04 (0.04) |
| 52 | 1-Hexanol | 887 | 0.02 (0.02) | 0.03 (0.03) | - | 0.05 (0.04) | 0.01 (0.01) | - | 0.03 (0.03) | 0.02 (0.02) | 0.01 (0.01) | - | 0.01 (0.01) | 0.01 (0.01) |
| 53 | Nonane | 900 | - | - | - | - | - | - | - | - | - | - | - | - |
| 54 | Pentyl acetate | 930 | - | - | - | - | - | - | - | - | 0.01 (0.01) | - | - | - |
| 55 | Hexyl formate | 957 | - | 0.5 (0.5) | - | - | - | - | 0.1 (0.1) | - | - | - | - | - |
| 56 | Decane | 1000 | - | 0.08 (0.06) | - | - | - | - | - | - | - | - | - | - |
| 57 | (Z)-3-Hexenyl acetate * | 1019 | 0.04 (0.04) | - | 0.11 (0.03) | 0.04 (0.02) | 0.12 (0.04) | - | 0.08 (0.04) | 0.2 (0.2) | 0.01 (0.01) | 0.01 (0.01) | 0.2 (0.1) | 0.3 (0.2) |
| 58 | Hexyl Acetate | 1027 | 0.06 (0.03) | 0.02 (0.02) | 0.04 (0.02) | 0.12 (0.07) | 0.04 (0.02) | - | 0.05 (0.05) | 0.07 (0.04) | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.01) | 0.05 (0.02) |
| 59 | (E)-2-Hexenyl acetate | 1037 | - | - | - | 0.04 (0.04) | - | - | 0.03 (0.03) | 0.09 (0.07) | 0.01 (0.01) | - | - | 0.03 (0.03) |
| 60 | Undecane | 1100 | - | - | - | - | - | - | - | 0.05 (0.04) | - | - | - | - |
| 61 | Nonanal | 1128 | 0.03 (0.03) | 0.01 (0.01) | - | 0.03 (0.02) | - | - | 0.01 (0.01) | - | - | 0.02 (0.02) | 0.01 (0.01) | - |
| 62 | [3-Hexenylbutanoate] | 1197 | - | - | 0.02 (0.01) | - | - | - | - | - | - | - | - | 0.02 (0.02) |
| 63 | Dodecane * | 1200 | - | 0.01 (0.01) | - | 0.09 (0.03) | - | - | - | - | - | 0.01 (0.01) | 0.02 (0.02) | - |
| 64 | [(E)-3-Hexenyl-2-methylbutanoate] | 1242 | - | - | - | - | - | - | - | - | - | - | - | 0.01 (0.01) |
| 65 | Tridecane * | 1300 | - | - | - | 0.13 (0.06) | - | - | - | 0.05 (0.05) | - | - | - | - |
| 66 | 2-Undecanone * | 1314 | - | 0.4 (0.2) | - | 0.2 (0.1) | - | 0.12 (0.04) | - | - | - | 0.15 (0.08) | - | - |
| 67 | [3-Hydroxy-2,2-dimethylhexylbutanoate] | 1397 | - | - | - | 0.09 (0.05) | - | - | - | - | - | - | - | - |
| 68 | Tetradecane | 1400 | - | - | - | - | - | - | - | - | - | 0.03 (0.03) | - | - |
| 69 | Pentadecane * | 1500 | - | 0.13 (0.05) | - | 0.11 (0.07) | - | 0.2 (0.1) | 0.01 (0.01) | 0.02 (0.01) | 0.01 (0.01) | 0.4 (0.3) | 0.02 (0.02) | 0.02 (0.02) |
| 70 | 2-Tridecanone * | 1516 | - | 3 (1) | - | 4 (2) | - | 1.8 (0.8) | - | - | - | 3 (1) | - | - |
| 71 | [Dodecenyl acetate] * | 1692 | - | 0.2 (0.1) | - | 0.2 (0.1) | - | 0.3 (0.1) | - | - | - | 0.4 (0.2) | - | - |
| 72 | Heptadecane | 1700 | - | - | - | - | - | - | - | - | - | - | - | - |
| 73 | 2-Pentadecanone * | 1718 | - | 8 (4) | - | 9 (4) | - | 6 (3) | - | - | - | 12 (6) | - | - |
| 74 | 2-Heptadecanone * | 1922 | 0.16 (0.06) | 1.5 (0.6) | - | 3 (1) | 0.7 (0.3) | 0.7 (0.2) | 0.05 (0.03) | 0.05 (0.03) | - | 1.2 (0.5) | 0.13 (0.07) | 0.07 (0.02) |
| 75 | Heneicosane | 2100 | - | - | - | - | - | - | - | 0.01 (0.01) | - | - | - | - |
| Yeast Associated Compounds | 4 (2) | 9 (6) | 0.9 (0.5) | 3 (1) | 9 (2) | 16 (11) | 2 (1) | 6 (4) | 18 (8) | 12 (7) | 12 (9) | 1.8 (0.9) | ||
| 76 | Ethanol | 426 | 4 (2) | 9 (6) | 0.9 (0.5) | 3 (1) | 9 (2) | 15 (11) | 2 (1) | 6 (3) | 17 (7) | 12 (7) | 12 (9) | 1.8 (0.9) |
| 77 | Ethyl acetate | 615 | 0.01 (0.01) | - | 0.01 (0.01) | - | 0.01 (0.01) | 0.04 (0.03) | 0.08 (0.08) | 0.1 (0.1) | 0.3 (0.2) | 0.01 (0.01) | 0.09 (0.09) | 0.04 (0.03) |
| 78 | 2-Methyl-1-propanol | 624 | - | - | - | 0.1 (0.1) | - | - | - | - | - | - | - | - |
| 79 | Acetic acid | 639 | - | - | - | - | - | - | - | - | - | - | - | - |
| 80 | 3-Hydroxy-2-butanone | 748 | - | - | - | 0.1 (0.1) | - | 0.2 (0.2) | - | - | 0.02 (0.02) | - | - | - |
| 81 | Ethyl butanoate * | 820 | - | - | 0.01 (0.01) | - | - | 0.3 (0.2) | 0.01 (0.01) | - | 0.5 (0.2) | - | - | - |
| Unidentified | 0.2 (0.1) | 1.3 (0.7) | 0.16 (0.02) | 1.2 (0.4) | 0.15 (0.05) | 1.3 (0.4) | 0.7 (0.2) | 0.4 (0.2) | 0.17 (0.08) | 0.5 (0.2) | 0.3 (0.1) | 0.42 (0.06) | ||
| # | Compound | Percent Occurrence (%) | Mean Relative Amount (%) | ||
|---|---|---|---|---|---|
| L Style (N = 38) | S Style (N = 36) | L Style (N = 38) | S Style (N = 36) | ||
| Benzenoids | 77 | 52 | |||
| 2 | Benzaldehyde * | 100 | 100 | 55 | 37 |
| 20 | Benzyl benzoate * | 100 | 75 | 8.4 | 5.3 |
| 13 | 4-Anisaldehyde * | 100 | 64 | 6.1 | 3.6 |
| 5 | Acetophenone | 87 | 89 | 2.6 | 2.5 |
| 3 | 4-Methylanisole | 95 | 64 | 2.0 | 1.3 |
| 4 | Benzyl alcohol * | 84 | 56 | 2.0 | 1.1 |
| Terpenoids | 14 | 30 | |||
| 29 | (Z)-α-Ocimene * | 32 | 58 | 0.02 | 16 |
| 38 | α-Farnesene * | 74 | 44 | 5.4 | 4.0 |
| 39 | β-Ionone | 26 | 22 | 3.1 | 2.6 |
| 32 | Dihydro-β-ionone * | 66 | 81 | 0.8 | 3.1 |
| 34 | α-Ionone * | 97 | 61 | 1.8 | 1.3 |
| 21 | α-Pinene | 68 | 67 | 1.0 | 0.3 |
| Fatty Acid Derivatives | 0.5 | 9.7 | |||
| 73 | 2-Pentadecanone * | 0 | 42 | - | 5.8 |
| 70 | 2-Tridecanone * | 0 | 33 | - | 1.8 |
| 74 | 2-Heptadecanone * | 42 | 75 | 0.2 | 1.1 |
| Yeast Compounds | 7.8 | 8.1 | |||
| 76 | Ethanol | 92 | 94 | 7.7 | 8.0 |
| Total Peak Area (counts) | 49.6 ± 4.9 million | 36.8 ± 4.9 million | |||
| Label | Population Type | Population | Location | GPS Location | # of Flowers and Style Morph |
|---|---|---|---|---|---|
| C1 | Cultivated | USC Lancaster Arbor | Lancaster, SC | 34°44′14.1” N 80°47′01.3” W | 3 L 6 S |
| C2 | Cultivated | Waterstone Neighborhood | Fort Mill, SC | 35°03′04.0” N 80°58′53.2” W | 6 L 6 S |
| C3 | Cultivated | Baxter Village | Fort Mill, SC | 35°01′44.7” N 80°58′00.6” W | 8 L 8 S |
| W1 | Wild | USC Lancaster Nature Trail | Lancaster, SC | 34°44′15.6” N 80°47′00.4” W | 8 L 6 S |
| W2 | Wild | Andrew Jackson State Park | Lancaster, SC | 34°50′37.6” N 80°48′29.1” W | 7 L 6 S |
| W3 | Wild | Anne Springs Close Greenway | Fort Mill, SC | 35°02′07.6” N 80°55′01.1” W | 6 L 8 S |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obi Johnson, B.; Golonka, A.M.; Blackwell, A.; Vazquez, I.; Wolfram, N. Floral Scent Variation in the Heterostylous Species Gelsemium sempervirens. Molecules 2019, 24, 2818. https://doi.org/10.3390/molecules24152818
Obi Johnson B, Golonka AM, Blackwell A, Vazquez I, Wolfram N. Floral Scent Variation in the Heterostylous Species Gelsemium sempervirens. Molecules. 2019; 24(15):2818. https://doi.org/10.3390/molecules24152818
Chicago/Turabian StyleObi Johnson, Bettie, Annette M. Golonka, Austin Blackwell, Iver Vazquez, and Nigel Wolfram. 2019. "Floral Scent Variation in the Heterostylous Species Gelsemium sempervirens" Molecules 24, no. 15: 2818. https://doi.org/10.3390/molecules24152818
APA StyleObi Johnson, B., Golonka, A. M., Blackwell, A., Vazquez, I., & Wolfram, N. (2019). Floral Scent Variation in the Heterostylous Species Gelsemium sempervirens. Molecules, 24(15), 2818. https://doi.org/10.3390/molecules24152818







