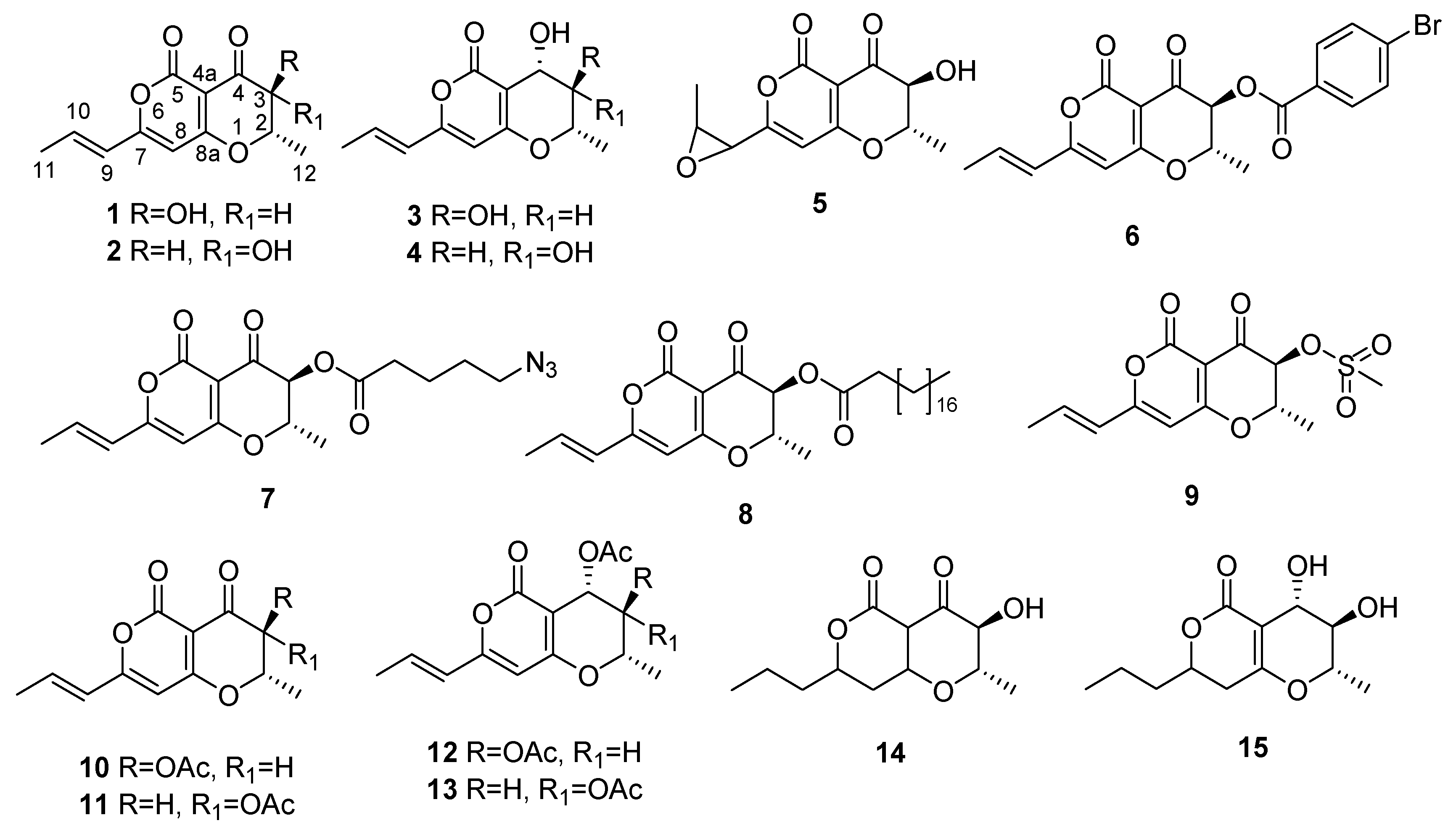
1. Introduction
Buffelgrass (
Cenchrus ciliaris or
Pennisetum ciliare) is an important pasture grass in many semi-arid regions of the world but it is also an invasive weed in some areas of North America [
1]. In the Sonoran Desert of southern Arizona it has infested thousands of acres of public and private lands, including Saguaro National Park and the Coronado and Tonto National Forests [
2,
3,
4]. The increased fire frequency and intensity in the infested areas is negatively affecting the native species including the iconic saguaro cactus [
5]. The main products currently used to manage buffelgrass are glyphosate and imazapyr. However, these broad-spectrum herbicides cause heavy damage to the non-target plants and have a negative environmental and ecological impact [
6]. In the past decades the biological control has become an effective alternative to combat many weeds that invade natural systems [
7,
8]. In particular, the phytotoxins produced by weed pathogenic fungi are an efficient tool to design natural and safe bioherbicides [
9]. Thus,
Cochliobolus australiensis (recently classified as
Curvularia tsudae) and
Pyricularia grisea, two foliar pathogens that commonly occur on buffelgrass in the invaded North American range, were studied to evaluate their ability to produce phytotoxic metabolites that can potentially be used as natural herbicides against this weed. A total of 14 secondary metabolites belonging to different classes of natural compounds were purified from the in vitro cultures of these two pathogens [
10,
11,
12]. When tested by leaf puncture assay on host plant at different concentrations, radicinin (
1,
Figure 1) and (10
S,11
S)-
epi-pyriculol resulted to be the most promising compounds. Thus, their phytotoxic activity was also evaluated on non-host indigenous plants. Radicinin demonstrated high target-specific toxicity on buffelgrass, low toxicity to native plants and no teratogenic, sublethal, or lethal effects on zebrafish (
Brachydanio rerio) embryos [
13].
1 is now under consideration for the development of target-specific bioherbicide to be used against buffelgrass, and a rapid and sensitive HPLC method for its quantification in complex mixtures was recently optimized in order to evaluate its production by different fungal strains and in different cultural conditions [
14]. This manuscript reports the semisynthesis of some radicinin derivatives and the evaluation of their phytotoxic activity against buffelgrass. Furthermore, their phytotoxicity was also compared with that of the natural analogues 3-
epi-radicinin, radicinol, 3-
epi-radicinol, and cochliotoxin (
2–
5,
Figure 1) and some of their derivatives (
6–
15,
Figure 1) in order to obtain clues about the structure–activity relationship (SAR) of these compounds.
2. Results and Discussion
The buffelgrass pathogenic fungus
C. australiensis was grown by fermentation and the natural compounds
1–
5 were isolated from potato dextrose broth (PDB) cultures according to the procedures previously published [
10,
11]. The purity of
1–
5 was >98%, as checked by
1H-NMR and LC–MS.
To investigate the structure activity relationship for radicinin as a target-specific phytotoxin against buffelgrass, seven derivatives (
6–
10 and
14–
15,
Figure 1) were prepared as reported in detail in the experimental part. The acetyl derivatives of 3-
epi-radicinin, radicinol, and 3-
epi-radicinol
11–
13 were also prepared. All the derivatives were characterized as described in Materials and Methods in detail, and their
1H-NMR data are reported in
Table 1 and
Table 2.
Radicinin (
1) by reaction with 4-bromobenzoyl chloride yielded its corresponding
p-bromobenzoyl ester (
6). Its
1H-NMR spectrum differed from that of
1 for the presence of the typical signals pattern of the aromatic
para-disubstituted residue, appearing as two doublets at δ 7.94 and 7.65 (
J = 8.6 Hz), and for the downfield shift (Δδ 1.52) at δ 5.52 of the signal of H-3. These data were very similar to those already reported by Robeson et al. [
15]. As a stronger evidence of the derivatization, the ESI-MS spectrum showed the typical signals because of the presence of
79Br and
81Br isotopic peaks, at
m/
z 419 [M + H]
+ and 421 [M + 2 + H]
+, respectively.
Radicinin by esterification with 5-azidopentanoic acid was converted into the corresponding 5-azidopentanoyl derivative (7). Its 1H-NMR spectrum differed from that of radicinin for the downfield shift (Δδ = 1.25) of H-3 at δ 5.25 and for the presence of the signals pattern typical of 5-azidopentanoyl residue resonating as two triplets at δ 3.35 (J = 6.5 Hz) and 2.53 (J = 7.2 Hz) due to CH2-5′ and CH2-2′, and two multiplets at δ 1.80−1.72 and 1.68−1.71 due to CH2-3′ and CH2-4′. Further confirmation was obtained by the stretching of the –N3 bond in the IR spectrum, with a signal at 2097 cm−1 and by the ESI-MS spectrum, which showed both the dimeric sodiated [2M + Na]+ and protonated [M + H]+ forms at m/z 745 and 362, respectively.
1 by reaction with stearoyl chloride afforded the corresponding acyl derivative (8). Its 1H-NMR spectrum, compared with that of radicinin, differed for the downfield shift (Δδ = 1.27) of H-3 at δ 5.27 and showed typical signals of the stearoil residue appearing as two triplets at δ 2.44 (J = 7.5 Hz) and 0.90 (J = 6.7 Hz) due to CH2-2′ and Me-18′ and the presence at δ 2.0–1.0 of the complex multiplet due to the protons of the residual 15 CH2 groups. The ESI-MS spectrum showed the protonated [M + H]+ form at m/z 517.
Radicinin by reaction with mesyl chloride in pyridine, afforded the corresponding mesyl ester (9). Its 1H-NMR spectrum, compared to that of 1 showed the downfield shift of H-3 (Δδ = 1.01) appearing as a doublet (J = 12.4 Hz) at δ 5.01 and the singlet of the mesyl group at δ 3.40. A further confirmation was obtained by the ESI-MS spectrum, which showed the dimeric sodiated [2M + Na]+ and the protonated [M + H]+ forms at m/z 651 and 315, respectively.
Radicinin (
1) was acetylated by usual reaction with Ac
2O and pyridine to yield the corresponding 3-
O-acetylderivative (
10). Its
1H-NMR differed from that of
1 essentially for the downfield shift of H-3 (Δδ = 1.28) appearing as singlet at δ 5.28 and the singlet of the acetyl group at δ 2.23. These data were very similar to those previously reported [
16]. Furthermore, its ESI-MS spectrum showed the dimeric sodiated [2M + Na]
+ and protonated [M + H]
+ forms at
m/
z 579 and 279, respectively.
To investigate the role of the ethenyl side chain as well as those of the dihydropyranopyran-4,5-dione moiety, a catalytic hydrogenation of radicinin was carried out and two hexahydro derivatives (14 and 15) were obtained as main products. The 1H-NMR of both derivatives showed the absence of the olefinic pyron proton (H-8) and that (H-9) of the side chain at C-7. In fact 1H-NMR spectrum of 14 showed a triplet (J = 7.1 Hz) at δ 0.95 of the terminal methyl group (H3-11) of the propyl side chain at C-7, which in the COSY (Correlated spectroscopy) spectrum coupled with the adjacent methylene group (CH2-10) and this in turn with those of the other one (CH2-9) appearing as overlapped complex multiplet in the region of δ 1.62–1.43. The presence of the proton H-7 of the α-lactone ring appearing as dd (J = 12.2 and 4.7) at δ 4.42 was also significant, which in the COSY spectrum coupled with the broad double doublet (J = 13.2 and 4.7 Hz) and the double doublet (J = 13.2 and 12.2 Hz) at δ 12.19 and 1.63 of the proton of the adjacent methylene group (H2C-8). Another relevant difference was the upfield shift (Δδ 0.72) of the double quartet (J = 11.2 and 6.4 Hz) of H-2 resonating at δ 3.69 probably due to the saturation of the alpha α-pyrone ring that also generated the protons of the two headbridge carbons of the junction between the two rings appearing as multiplets at δ 4.33 and 1.62–1.43 (H-8a and H-4a, respectively). Furthermore, its ESI-MS spectrum showed the dimeric sodiated [2M + Na]+, the sodiated [M + Na]+ and protonated [M + H]+ forms at m/z 507, 265 and 243. Similarly, the 1H-NMR spectrum of 15 showed the signal of the propyl residue of C-7 with a triplet (J = 6.7 Hz) and three multiplets appearing at δ 0.96 (H3-11), 1.56 (H2-10), and 1.78 and 1.65 (H2-9). The proton assigned to H-7 resonated at δ 4.42 which in the COSY spectrum coupled with the two double doublets (J = 17.1 and 14.2 and J = 17.1 and 4.3 Hz) at δ 2.46 and 2.34 assigned to the proton of the adjacent methylene group of CH2-8. Moreover, the significant presence of the doublet (J = 9.2 Hz) of H-4, which coupled in the COSY spectrum with H-3, resonating as triplet (J = 9.2 Hz) δ 3.63 also coupled with H-2 appearing as a double quartet (J = 9.2 and 6.2 Hz) at δ 4.04. These coupling constants suggested the stereoselective reduction of the carbonyl group at C-4. H-4 appeared to be pseudoaxial located with respect to H-3. Furthermore, its ESI-MS spectrum showed the dimeric sodiated [2M + Na]+, the sodiated [M + Na]+ and protonated [M + H]+ forms at m/z 507, 265 and 243. The same sectrum also showed the ion at m/z 225 generated from the pseudomolecular ion by los of H2O.
3-epi-Radicinin (2) by reaction with Ac2O in pyridine, yielded the corresponding 3-O-acetyl derivative (11). Its 1H-NMR spectrum, differed from that of 3-epi-radicinin for the downfield shift of H-3 (Δδ = 1.0) resonating as a doublet (J = 3.8 Hz) at δ 5.51 and the singlet of the acetyl group at δ 2.19. Its ESI-MS spectrum showed both the protonated [M + H]+ and the dimeric sodiated [2M + Na]+ forms at m/z 279 and 579, respectively.
Radicinol (3) by acetylation, as above described, afforded the corresponding 3,4-O-O’-diacetyl derivative (12). Its 1H-NMR spectrum, compared with the spectrum of radicinol showed the typical downfield shifts of H-3 (Δδ = 1.40) and H-4 (Δδ = 1.0) appearing as two broad singlets at δ 5.12 and 5.78 respectively. The spectrum also showed the singlets of the overlapped signals of the two acetyl groups at δ 2.10. Its ESI-MS spectrum showed the protonated form [M + H]+ at m/z 323.
Similarly, 3-epi-radicinol was converted into its corresponding 3,4-O-O’-diacetyl derivative (13). The 1H-NMR spectrum of 13 differed from that of 3-epi-radicinol, for the downfield shifts of H-3 (Δδ = 1.31) and H-4 (Δδ = 1.00) appearing as a double doublet (J = 2.8 and 1.2 Hz) and a doublet (J = 2.8 Hz) at δ 5.16 and 5.72, respectively. The spectrum also showed the singlet at δ 2.11 due to the overlapped signals of the two acetyl groups. Its ESI-MS spectrum showed the protonated form [M + H]+ at m/z 323.
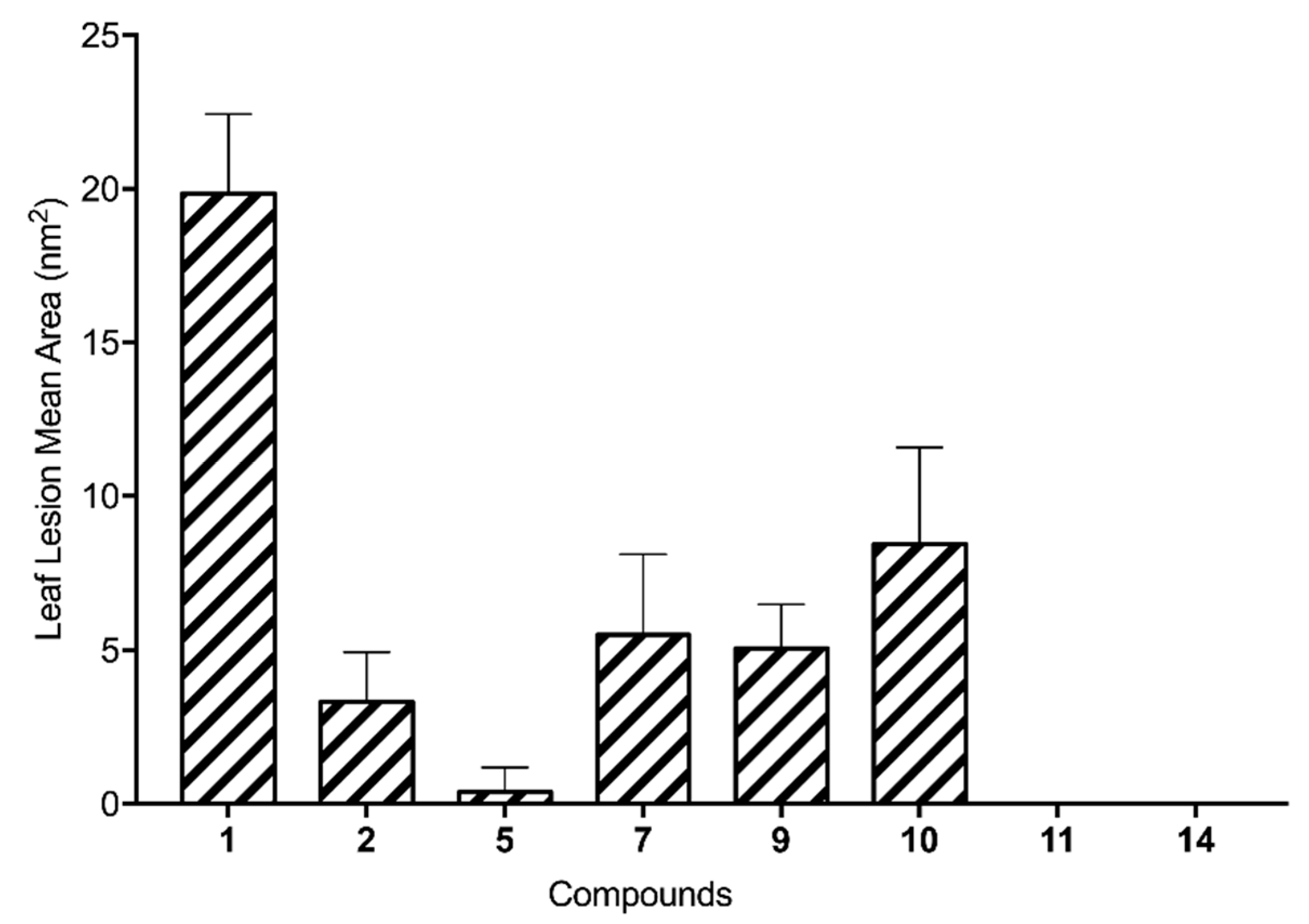
The ten hemisynthetic derivatives (
6–
15) were tested by the buffelgrass leaf puncture assay at 2.5 × 10
−3 M as reported in the Materials and Methods section in detail. Their activity was evaluated in comparison with that showed by the natural metabolites (
1–
5) previously reported by Masi et al. [
13]. Overall, five derivatives (
6,
8,
12,
13, and
15) produced no necrosis and were thus completely nontoxic to buffelgrass, while the other five compounds (
7,
9,
10,
11, and
14) were moderately toxic (
Figure 2).
A very important factor for the activity appear to be the carbonyl group of the dihydro γ-pyrone as the phytotoxicity was lost in 3. This was also confirmed by the expected lacking of activity of radicinol diacetyl derivative (12). The stereochemistry at C-3 also plays a significant role to impart activity as was with the strong reduction observed by testing 3-epi-radicinin (2). This was confirmed by the very low activity of its acetyl derivative 11 and the total loss of activity observed by testing the diacetyl derivatives (12 and 13) of radicinol and 3-epi-radicinol. The hydrogenation of 1 generated 14 which showed the absence of α,β unsaturated carbonyl group explaining the strong loss of activity. The double bond of the side chain at C-7 also plays a role to impart activity as demonstrated by the reduction of phytotoxicity observed by testing 5 and confirmed by the decrease of the activity of 14. The acyl derivatives of 1 showed a strong or total loss of activity, which was only in part retained by the acetyl and the mesyl derivatives (9 and 10). These compounds are probably more easily hydrolysable in comparison to the other compounds (6, 7, and 8) as their conjugated bases are more stabilized for resonance.
The five hemisynthetic derivatives (
7,
9,
10,
11, and
14) that showed toxic activity on buffelgrass leaves at 2.5 × 10
−3 M were also tested at a lower concentration of 10
−3 M (
Figure 3), confirming the results obtained at higher concentration.
3. Materials and Methods
3.1. General Experimental Procedures
IR spectra were recorded as deposit glass film on a Perkin Elmer Thermo Nicolet 5700 FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA). UV spectra were measured in MeOH on a Jasco V-530 spectrophotometer (Jasco, Tokyo, Japan). 1H-NMR spectra were recorded at 400 or 500 MHz in CDCl3 on Bruker (Bruker, Karlsruhe, Germany) and Varian (Varian, Palo Alto, CA, USA) instruments. ESIMS (Electrospray ionization mass spectrometry) were recorded using LC/MS ESIMS-TOF (Electrospray ionization mass spectrometry –Time of flight) system (Agilent 6230B, HPLC 1260 Infinity) (Agilent Technologies, Milan, Italy). Analytical, preparative and reverse phase TLCs (Thin layer chromatography) were carried out on silica gel (Kieselgel 60, F254, 0.25, 0.5 mm, and RP-18 F254s respectively) plates (Merck, Darmstadt, Germany). The spots were visualized by exposure to UV radiation, or by spraying first with 10% H2SO4 in MeOH, and then with 5% phosphomolybdic acid in EtOH, followed by heating at 110 °C for 10 min. Column chromatography was performed using silica gel (Merck, Kieselgel 60, 0.063–0.200 mm).
3.2. Fungal Strains
Cochliobolus australiensis (LJ-4B) strains used in this study were isolated from diseased buffelgrass tissue collected in Saguaro National Monument, Arizona, AZ, USA, in autumn 2014 and near La Joya, Hidalgo County in south Texas, USA, in September 2014, respectively.
3.3. Isolation of Fungal Metabolites
The fungal metabolites
1–
5 were isolated from in vitro PDB (potato dextrose broth) cultures of
C. australiensis according to procedures previously reported [
10,
11].
3.4. Preparation of Semisynthetic Derivatives
3.4.1. p-Bromobenzoyl Ester of Radicinin (6)
To radicinin (
1, 5.0 mg), dissolved in anhydrous MeCN (300 µL), DMAP (5.0 mg) and
p-bromobenzoylchloride (5.0 mg) were added. The reaction mixture was left under stirring for 5 h. It was then quenched with a 1 N NaHCO
3 and extracted with CH
2Cl
2. The residue obtained by evaporation was then purified by preparative TLC eluted with CHCl
3:
i-PrOH (9:1) affording the
p-bromobenzoyl ester of radicinin (
6, 4.17 mg). Derivative
6 had: IR
νmax 1763, 1730, 1602, 1532, 1433 cm
−1; UV λ
max nm (log ε) 345 (4.23), 247 (4.40), 224 (4.23). These data were very similar to those already reported by Robeson et al. [
15];
1H-NMR, see
Table 1; MS (ESI-TOFMS)
m/
z 421 [M + 2 + H]
+, 419 [M + H]
+.
3.4.2. 5-Azidopentanoyl Ester of Radicinin (7)
DCC (5.6 mg) and 5-azidopentanoic acid (20 µL) were added to a solution of radicinin (
1) in pyridine (5.0 mg in 300 µL). The mixture was kept under stirring at room temperature for 4 days and then dried under N
2 stream by evaporation of the azeotope obtained by adding MeOH and C
6H
6. The residual oil was then purified by TLC eluted with CHCl
3-
i-PrOH (97:3) affording 5-azidopentanoyl ester of radicinin (
7, 4.38 mg). Derivative
7 had: IR ν
max 2097, 1754, 1601, 1523, 1433 cm
−1; UV λ
max nm (log ε) 341 (4.15), 221 (4.14);
1H-NMR, see
Table 1; MS (ESI-TOFMS)
m/
z 745 [2M + Na]
+, 362 [M + H]
+.
3.4.3. Stearoyl Ester of Radicinin (8)
Stearoyl chloride (12.8 mg), DMAP (1.6 mg), and DCC (9.0 mg) were added to radicinin (
1, 5.0 mg) in CH
2Cl
2 (300 µL) together with 9 mg of DCC. The reaction mixture was kept under stirring for 3 days and was then dried under N
2 stream. The residual oil was purified by preparative TLC eluted with
n-hexane-EtOAc (55:45) yielding the stearoyl ester of radicinin (
8, 1.81 mg). Derivative
8 had: IR ν
max 1750, 1532, 1433 cm
−1; UV λ
max nm (log ε) 341 (4.15), 221 (4.14);
1H-NMR, see
Table 1; MS (ESI-TOFMS)
m/
z 517 [M + H]
+.
3.4.4. Mesyl Ester of Radicinin (9)
Mesyl chloride (23 µL) was added to radicinin (
1, 5.0 mg) dissolved in CH
2Cl
2 (300 µL) and pyridine (40 µL). The reaction mixture was kept overnight and then quenched with a 1 N solution of NaHCO
3. The mixture was then extracted with EtOAc and the organic extract purified by TLC eluted with CHCl
3-
i-PrOH (97:3) affording the mesyl ester derivative of radicinin (
9, 3.34 mg). Derivative
9 had: IR ν
max 1757, 1602, 1532, 1434 cm
−1; UV λ
max nm (log ε) 342 (4.10), 224 (4.12);
1H-NMR, see
Table 1; MS (ESI-TOFMS)
m/
z 651 [2M + Na]
+, 315 [M + H]
+.
3.4.5. Acetyl Ester of Radicinin (10)
Radicinin (
1, 4 mg), dissolved in pyridine (100 µL), was acetylated with Ac
2O (5 µL) to obtain derivative
10. The reaction was carried out under stirring for 3 h at room temperature. It was stopped with MeOH, and the azeotrope formed by addition of C
6H
6 was evaporated under nitrogen stream. The residue was then purified by TLC eluted with CHCl
3-
i-PrOH (95:5) yielding
10 (2.3 mg) as an amorphous solid. Derivative
10 had: IR ν
max 1754, 1602, 1531, 1433 cm
−1; UV λ
max nm (log ε) 345 (4.12), 227 (4.15);
1H-NMR data (see
Table 1) were very similar to those previously reported by Aldrich et al. [
16]; MS (ESI-TOFMS)
m/
z 579 [2M + Na]
+, 279 [M + H]
+.
3.4.6. Acetyl Ester of 3-epi-Radicinin (11)
3-
epi-radicinin (
2, 3 mg), dissolved in pyridine (100 µL), was acetylated with Ac
2O (3 µL). The reaction was carried out following the same procedure used for conversion of
1 into
10 yielding the corresponding acetyl ester of 3-
epi-radicinin (
11, 1.76 mg). Derivative
11 had: IR ν
max 1750, 1602, 1532, 1433 cm
−1; UV λ
max nm (log ε) 348 (4.10), 228 (4.12);
1H-NMR, see
Table 2; MS (ESI-TOFMS)
m/
z 579 [2M + Na]
+, 279 [M + H]
+.
3.4.7. Diacetyl Ester of Radicinol (12)
To radicinol (
3, 5 mg), dissolved in pyridine (100 µL), Ac
2O (10 µL) was added. The reaction was kept under stirring at room temperature for 4 h and was then dried as previously reported for the preparation of
10. The residue was then purified by TLC eluted with CHCl
3-
i-PrOH (98:2) to afford the derivative
12 (3.46 mg). Derivative
12 had: IR ν
max 1680, 1650, 1610, 1560 cm
−1; UV λ
max nm (log ε) 319 (4.04), 271 (3.40), 261 (3.38) 225 (4.51);
1H-NMR, see
Table 2; MS (ESI-TOFMS)
m/
z 323 [M + H]
+.
3.4.8. Diacetyl Ester of 3-epi-Radicinol (13)
To
epi-radicinol (
4, 5 mg), dissolved in pyridine (100 µL), Ac
2O (10 µL) was added. The reaction was carried out following the same procedure used for the conversion of
2 into
11 yielding the corresponding acetyl ester of 3-
epi-radicinol (
13, 4.36 mg). Derivative
13 had: IR ν
max 1682, 1649, 1611, 1560 cm
−1; UV λ
max nm (log ε) 318 (4.05), 271 (3.41), 260 (3.36) 225 (4.50);
1H-NMR, see
Table 2; MS (ESI-TOFMS)
m/
z 323 [M + H]
+.
3.4.9. Hydrogenation of Radicinin: Derivatives 14 and 15
Radicinin (
1, 6 mg), dissolved in MeOH (1 mL), was added to a presaturated suspension of 10% Pd/C in MeOH (1 mL). Hydrogenation was carried out at room temperature and atmospheric pressure with continuous stirring. After 3h, the reaction was stopped by filtration on short silica gel column, eluted with CHCl
3-
i-PrOH (9:1) and the clear solution was evaporated under reduced pressure. The crude residue was purified by preparative TLC eluted with CHCl
3-
i-PrOH (95:5) affording two different hydrogenated derivatives as main products (
14 and
15, 2.12 and 1.40 mg respectively). Derivative
14 had: IR ν
max 3452, 1726, 1673, 1212 cm
−1; UV λ
max nm (log ε) <220 nm;
1H-NMR, see
Table 2; MS (ESI-TOFMS)
m/
z 507 [2M + Na]
+, 265 [M + Na]
+ and 243 [M + H]
+. Derivative
15 had: IR ν
max 3450, 1681, 1643 cm
−1; UV λ
max nm (log ε) <220 nm;
1H-NMR, see
Table 2; MS (ESI-TOFMS)
m/
z 507 [2M + Na]
+, 265 [M + Na]
+, 243 [M + H]
+ and 225 [M − H
2O + H]
+.
3.5. Leaf Puncture Bioassays
The ten hemisynthetic derivatives (6–15) were first assayed at 2.5 × 10−3 M for phytotoxicity on the leaves of buffelgrass (Cenchrus ciliaris) and those active at this concentration were then bioassayed at 10−3 M. Compounds were first dissolved in MeOH (final concentration 4%) and stock solutions at the two concentrations using sterile distilled water were then prepared. An incision of ca. 3 mm was made on the adaxial surface of each leaf section of 3 cm with an insulin needle. The leaf sections were placed in groups of six on the surface of a water-saturated filter paper in each of the four petri dishes. A total of five leaf sections in each petri dish were tested with the solution containing the compound, while one leaf section was used as a negative control (4% MeOH only). A droplet (10 μL) of the appropriate solution was applied over each needle incision using a micropipette. The dishes were sealed with parafilm and incubated at 24 °C for 3 days in a temperature-regulated chamber under a photoperiod of 14–10 h (light/dark). After 3 days of treatment, necrotic lesion development was evaluated by removing the petri dish cover, placing a glass disc on the leaf sections to flatten them into a single plane, and photographing each dish with its leaf sections. Each acquired image was then analyzed with the software ImageJ to measure the necrotic area caused by the solution.
3.6. Statistical Analyses
Statistical analyses were carried out using the GraphPad Prism 8 software (GraphPadSoftware, San Diego, CA, USA). Data were represented as the mean ± standard deviation and analyzed for statistical significance using ordinary one-way or two-ways analysis of variance and multiple comparisons. For all test, p < 0.5 was considered to indicate a statistically significant difference.