The Antioxidant and Anti-Aging Effects of Acetylated Mycelia Polysaccharides from Pleurotus djamor
Abstract
1. Introduction
2. Results
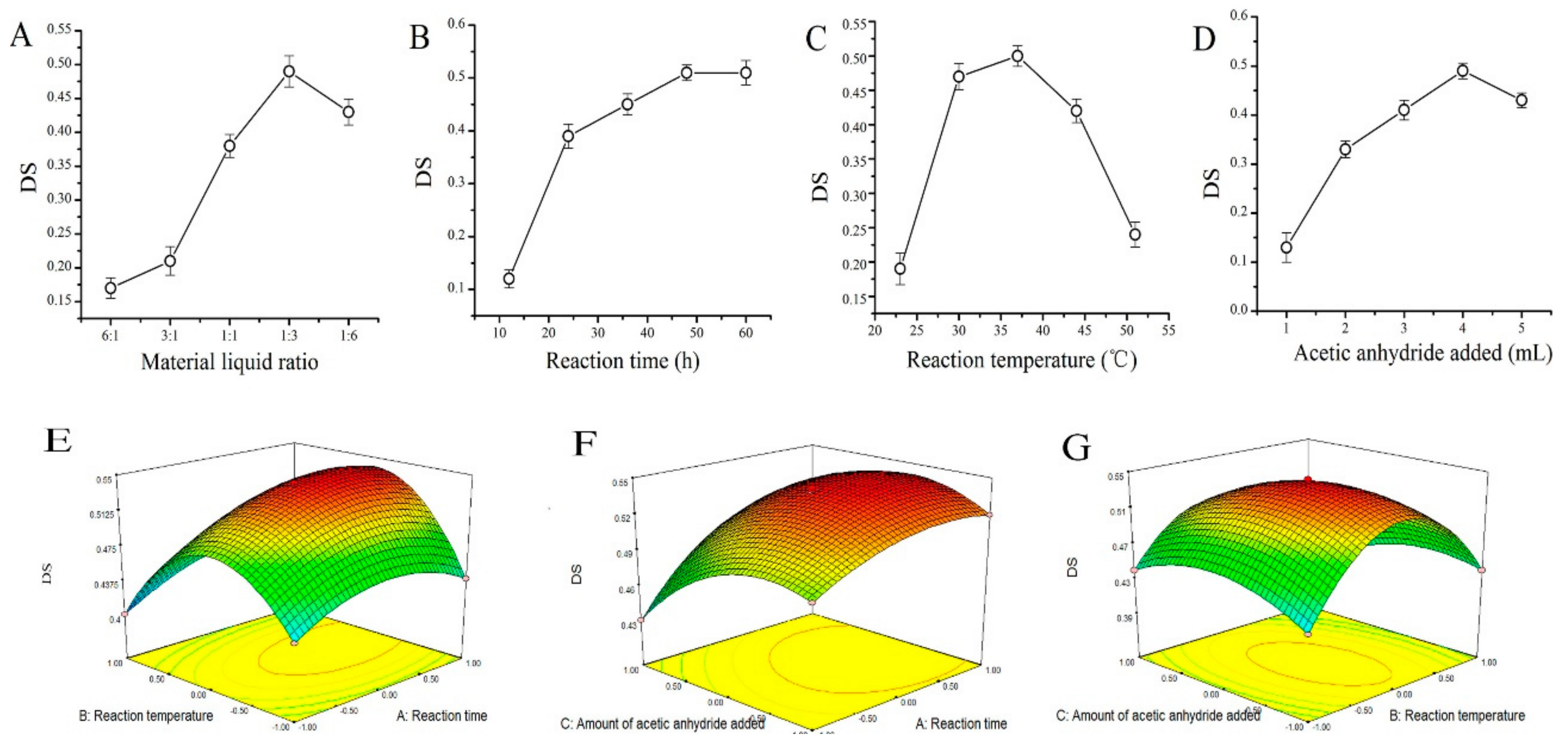
2.1. AMPS Acetyl Substitution Degree
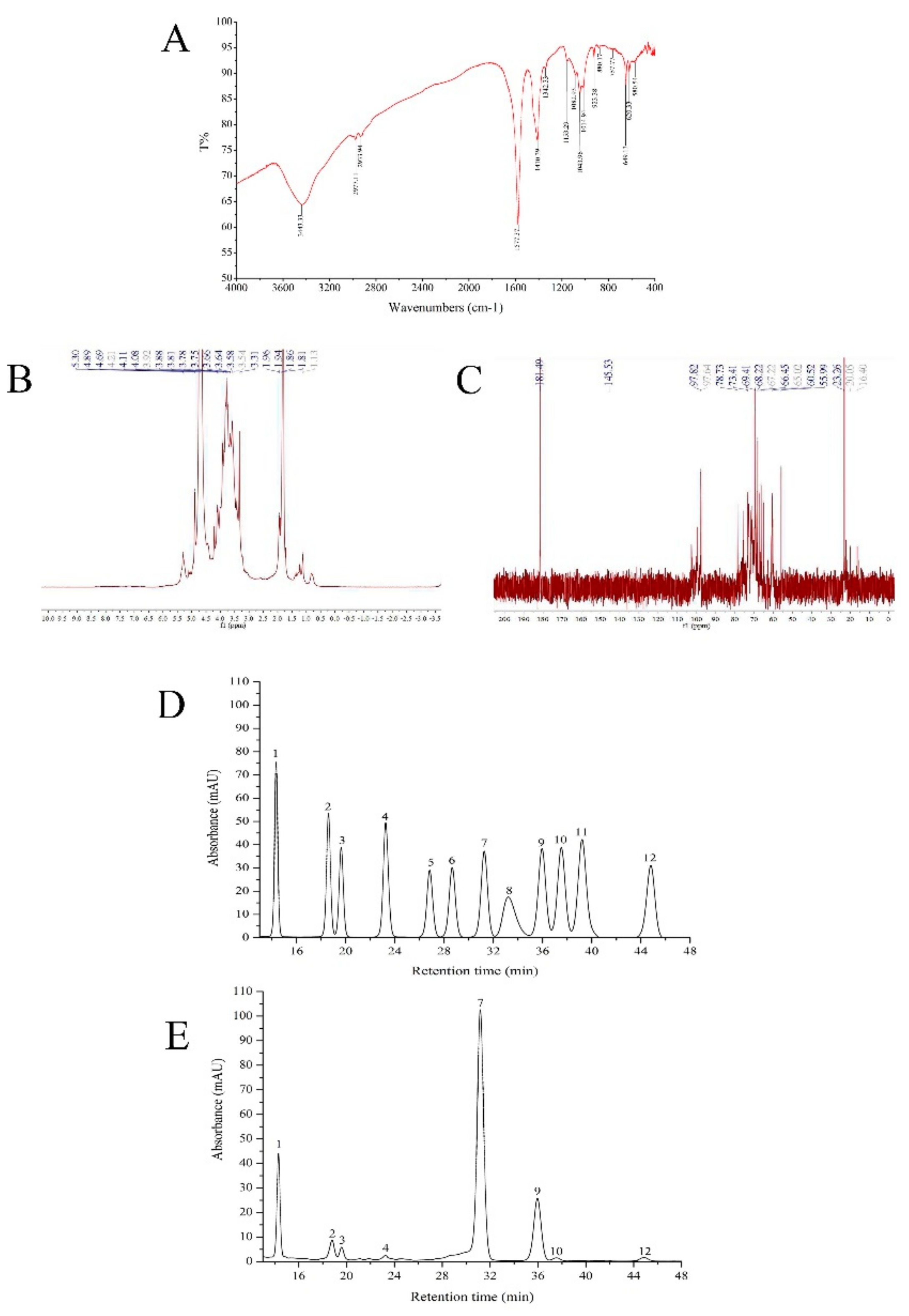
2.2. FT-IR, HPLC, and NMR Analysis
2.3. Antioxidant Properties In Vitro
2.4. Oral Chronic Toxicity Studies
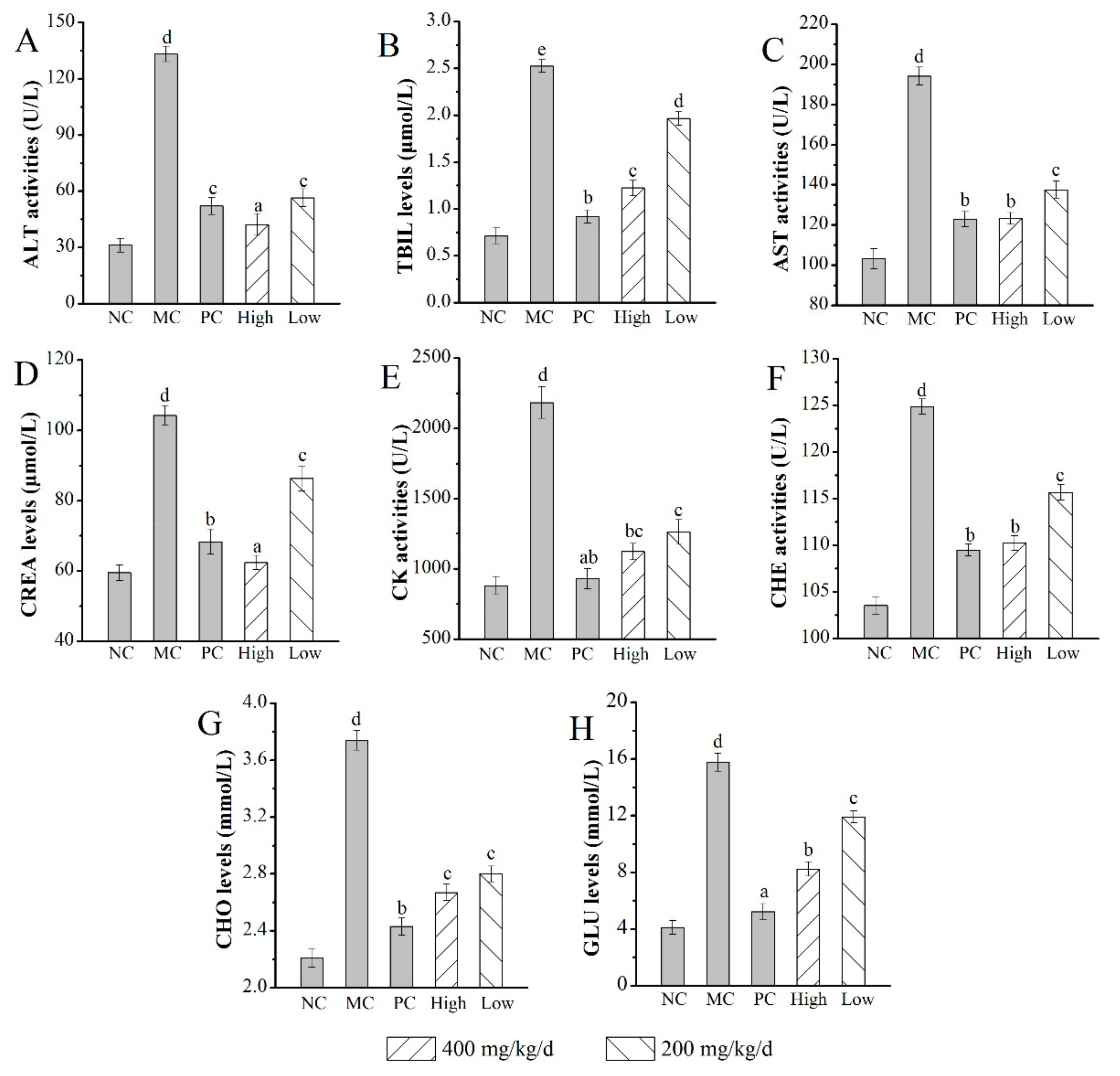
2.5. Effects of AMPS on Body Weight, Liver, and Kidney Indexes
2.6. Anti-Aging Properties In Vivo
2.6.1. Effects of AMPS on SOD, GSH-Px, and CAT Activities
2.6.2. Effects of AMPS on MDA and LPO Contents
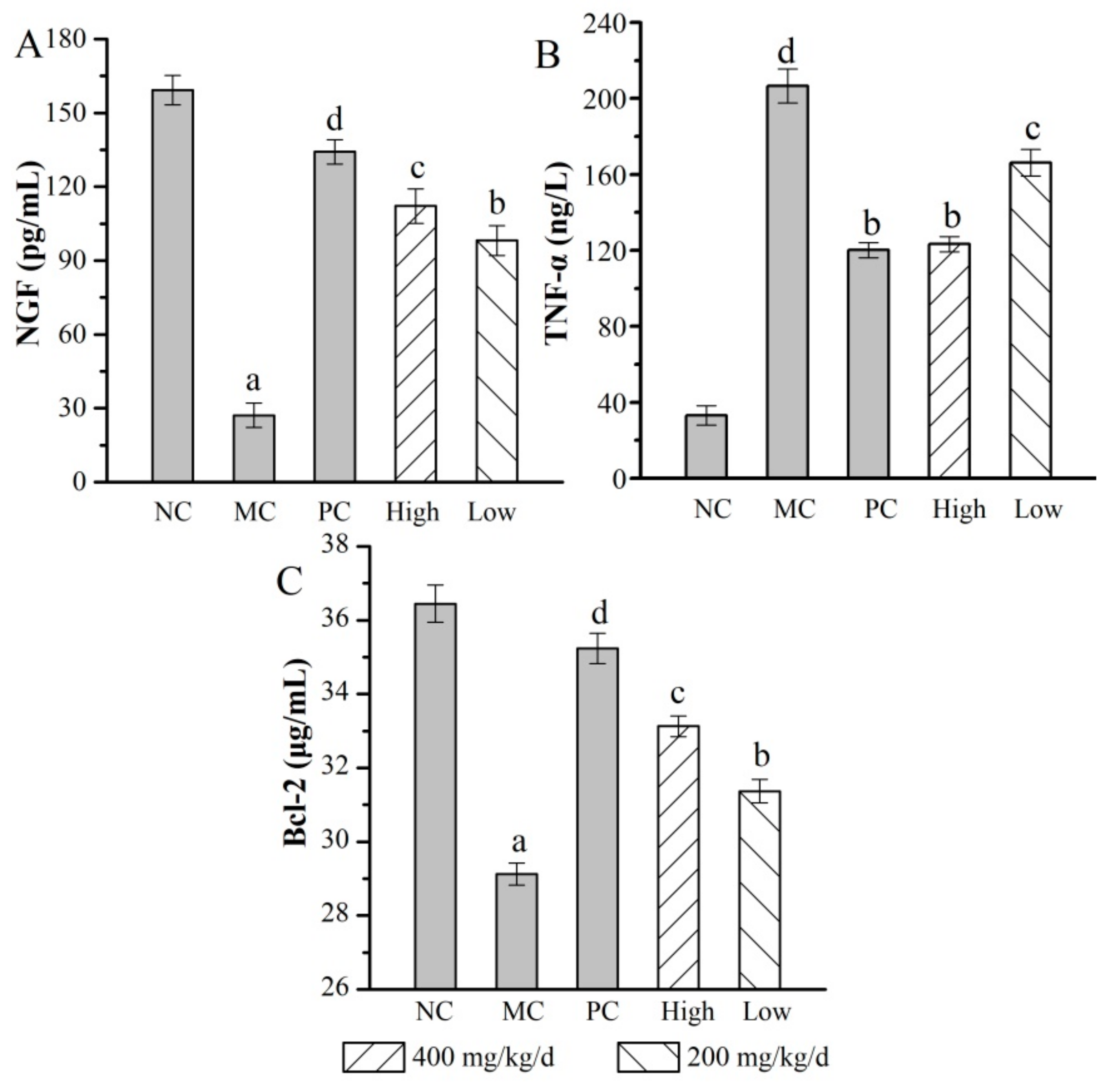
2.6.3. Effects of AMPS on the Serum Biochemical Index
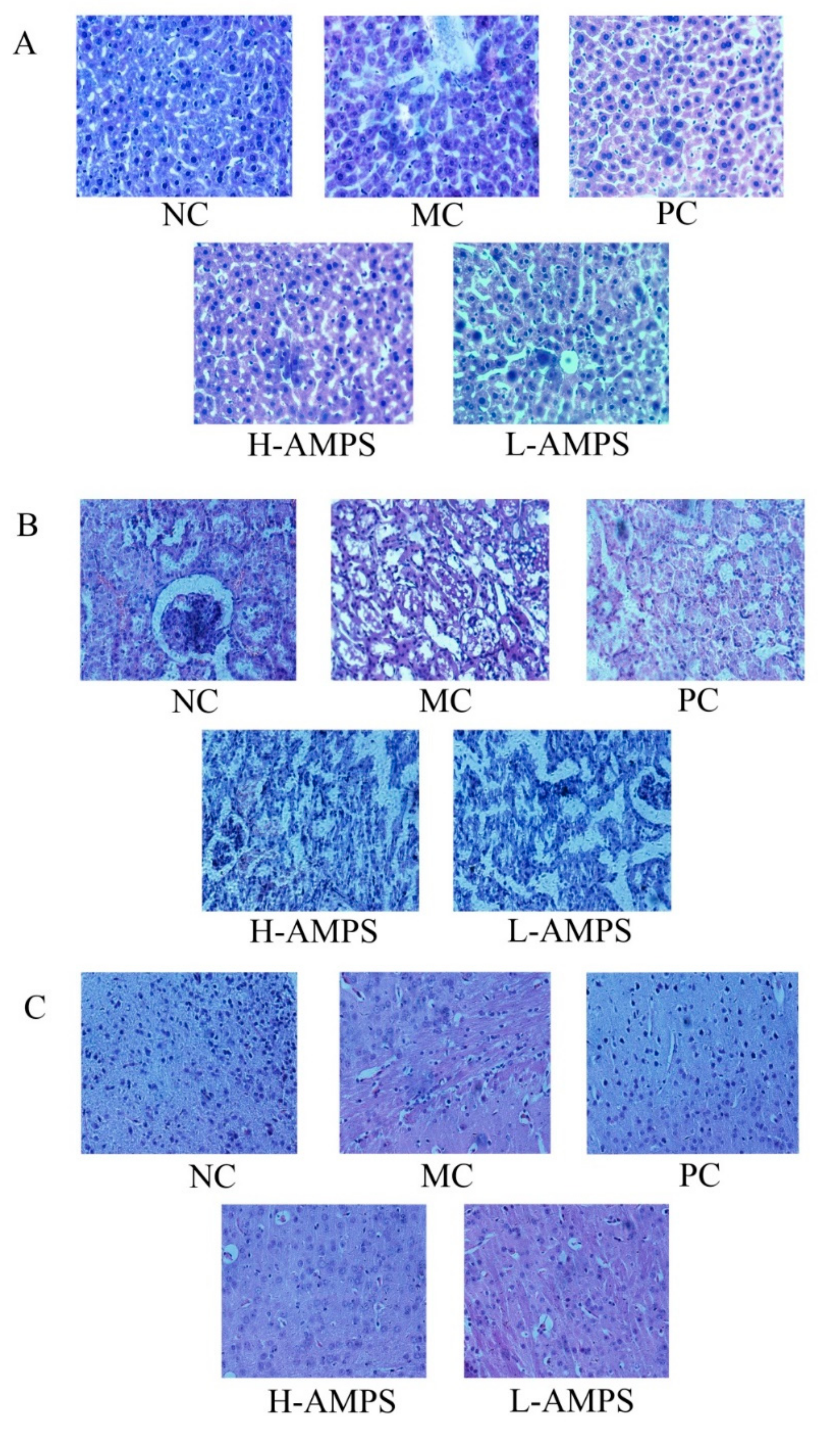
2.6.4. Histopathological Observations
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Preparation of AMPS
4.3. The DS Assay of AMPS
4.3.1. Standard Curve Plotting of Acetyl Content
4.3.2. Calculation of AMPS Acetyl Substitution Degree
4.4. FT-IR, HPLC, and NMR Analysis
4.5. Antioxidant Activity In Vitro
4.5.1. Reducing Power Assay
4.5.2. Scavenging DPPH Radical Assay
4.5.3. Scavenging Hydroxyl Radical Assay
4.5.4. Scavenging Superoxide Radical Assay
4.6. Oral Chronic Toxicity Test
4.7. Anti-Aging Effect In Vivo
4.7.1. Animal Experiments
4.7.2. Biochemical Analysis
4.7.3. Histopathological Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Compliance with Ethical Standards
Abbreviations
References
- Govindan, S.; Johnson, E.E.R.; Christopher, J.; Shanmugam, J.; Thirumalairaj, V.; Gopalan, J. Antioxidant and anti-aging activities of polysaccharides from Calocybe indica var. APK2. Exp. Toxicol. Pathol. 2016, 68, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Cui, F.; Zhang, J.; Gao, X.; Zhou, M.; Xu, N.; Zhao, H.; Liu, M.; Zhang, C.; Jia, L. Antioxidative and renoprotective effects of residue polysaccharides from Flammulina velutipes. Carbohydr. Polym. 2016, 146, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, J.; Liu, J.; Sun, Z.; Wang, M.; Jiang, Y.; Chen, Z.Y.; Chen, F. Anti-aging effects of astaxanthin-rich alga haematococcus pluvialis on fruit flies under oxidative stress. Food Chem. 2013, 61, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhang, Y.; Li, J.; Xia, J.; Chen, X.; Jing, P.; Song, X.; Wang, L.; Wang, Y. Angelica sinensis polysaccharide prevents hematopoietic stem cells senescence in D-galactose-induced aging mouse model. Stem Cells Int. 2017, 3, 3508907. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.Y.; Yang, D.; Zhang, W.N.; Lu, Y.M.; Zhang, M.Z.; Wang, L.M.; Li, X.H.; Zhou, L.Y.; Wu, Q.X.; Pan, W.J.; et al. Antioxidant and anti-aging activities of the polysaccharide TLH-3 from Tricholoma lobayense. Int. J. Biol. Macromol. 2016, 85, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Lv, G.Y.; He, W.Q.; Shi, L.G.; Pan, H.J.; Fan, L.F. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Jin, M.L.; Zhao, K.; Huang, Q.S.; Xu, C.L.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: Areview. Carbohydr. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Li, S.S.; Zhang, J.J.; Che, G.; Zhou, M.; Liu, M.; Zhang, C.; Xu, N.; Lin, L.; Liu, Y.; et al. The antihyperlipidemic activities of enzymatic and acidic intracellular polysaccharides by Termitomyces albuminosus. Carbohydr. Polym. 2016, 151, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Jiang, L.L.; Wu, J.N.; Liu, Z.Y.; Wu, Y.P. Anti-tumor and anti-virus activity of polysaccharides extracted from Sipunculus nudus (SNP) on Hepg 2.2.15. Int. J. Biol. Macromol. 2016, 87, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Liu, M.; Yang, Y.H.; Lin, L.; Xu, N.; Zhao, H.J.; Jia, L. Purification, characterization and hepatoprotective activities of mycelia zinc polysaccharides by Pleurotus djamor. Carbohydr. Polym. 2016, 136, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.Y.; Miao, C.Y.; Zhang, Y.S. Study on the effect of chemical modification on the antitumor activity of polysaccharides from Acrodia obliquus. Chin. J. Pharm. 1996, 10, 37–39. [Google Scholar]
- Song, Y.; Yang, Y.; Zhang, Y.; Duan, L.; Zhou, C.; Ni, Y.; Liao, X.; Li, Q.; Hu, X. Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita pepo, lady godiva). Carbohydr. Polym. 2013, 98, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.B.; Li, Q.; Ou, L.N.; Jiang, L.F.; Zeng, K. GC-MS, FT-IR analysis of black fungus polysaccharides and its inhibition against skin aging in mice. Int. J. Biol. Macromol. 2010, 47, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Kondo, K.; Sanda, S.; Kan, D.; Borges, I.K.; Suzuki, I.; Katahira, M. Enzymatic degradation of β-1, 4-linked N-acetylglucosaminoglucan prepared from Thiothrix nivea. Int. J. Biol. Macromol. 2017, 109, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Pang, F.; Liu, C.Y.; Chen, K.S.; Wang, H. Recent advances in the modification of polysaccharides by acetylation. Aug. Fir. Land Reclam. Univ. 2017, 29, 42–47. [Google Scholar]
- Song, X.; Shen, Q.; Liu, M.; Zhang, C.; Zhang, L.; Ren, Z.; Wang, W.; Dong, Y.; Wang, X.; Zhang, J.; et al. Antioxidant and hepatoprotective effects of intracellular mycelium polysaccharides from Pleurotus geesteranus, against alcoholic liver diseases. Int. J. Biol. Macromol. 2018, 114, 979–988. [Google Scholar] [CrossRef]
- Chang, Y.F.; Gong, W.X.; Zheng, Y.H.; Li, J.W.; Zhou, Y.Z.; Qin, X.M.; Du, G.H. Urinary metabolomics study of the effects of Scutellaria baicalensis Georgi ethanol extract on D-galactose-induced rats. Acta Pharm. Sin. 2016, 51, 86–92. [Google Scholar]
- Mo, R.; Wei, Z.M.; Yang, Y.S. Research progress in anti-aging mechanism. Med. J. Chin. PLA 2017, 42, 743–748. [Google Scholar]
- Fan, Y.L.; Xia, J.Y.; Jia, D.Y. Protective effect of Angelica sinensis polysaccharides on subacute renal damages induced by D-galactose in mice and its mechanism. China J. Chin. Mater. Med. 2015, 40, 4229. [Google Scholar]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014, 2, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, Z.; Hu, C.; Zhang, J.; Sun, X.; Rong, C.; Jia, L. Antioxidant, antibacterial and anti-aging activities of intracellular zinc polysaccharides from Grifola frondosa SH-05. Int. J. Biol. Macromol. 2017, 95, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, W.; Li, X.; Lü, X.; Li, N.; Gao, X.; Song, J. Preparation and in vitro antioxidant activity of kappa-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivatives. Carbohyd. Res. 2005, 340, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.Z.; Zhang, Y.Y.; Zhang, L.N. Chemical modification and antitumor activities of two polysaccharide protein complexes from Pleurotus tuber-regium. Int. J. Biol. Macromol. 2009, 45, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Zhang, Y.; Zhang, N.; Fu, L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohydr. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef]
- Zeng, W.C.; Zhang, Z.; Gao, H.; Jia, L.R.; Chen, W.Y. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr. Polym. 2012, 89, 694–700. [Google Scholar] [CrossRef]
- Lin, R.S.; Liu, H.H.; Wu, S.Q.; Pang, L.F.; Jia, M.S.; Fan, K.M.; Jia, S.H.; Jia, L. Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int. J. Biol. Macromol. 2012, 51, 153–157. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of aging. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, A.S. Dogra Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food Chem. Toxicol. 2010, 48, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Zhang, J.; Wang, W.; Wang, X.; Jing, H.; Ren, Z.; Gao, Z.; Song, X.; Gong, Z.; et al. The Antioxidative, Antiaging, and hepatoprotective effects of alkali-extractable polysaccharides by Agaricus bisporus. Evid. Based Complement. Altern. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Bovera, F.; Moniello, G.; De Riu, N.; Di Meo, C.; Pinna, W.; Nizza, A. Effect of diet on the metabolic profile of ostriches (Struthio camelusvar.domesticus). Trop. Anim. Health Prod. 2007, 39, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Luo, Y.J.; Wang, L.; Cai, K.Y. Effect of ginkgo biloba extract on livers in aged rats. World J. Gastroenterol. 2005, 11, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.K.; Bedrosian, A.S.; Malhotra, A.; Henning, J.R.; Ibrahim, J.; Vera, V.; Cieza-Rubio, N.E.; Hassan, B.U.; Pachter, H.L.; Cohen, S.; et al. In Hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity. J. Immunol. 2010, 185, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, R.; Dai, Z.; Wu, H.; Lin, M.; Tian, F.; Gao, Z.; Zhao, X.; Sun, Y.; Pu, X. Effect of Shenfu injection on lipopolysaccharide (LPS)-indu-ced septic shock in rabbits. J. Ethnopharmacol. 2019, 234, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Spada, T.C.; Silva, J.M.; Francisco, L.S.; Marçal, L.J.; Antonangelo, L.; Zanetta, D.M.; Yu, L.; Burdmann, E.A. High intensity resist-ance training causes muscle damage and increases biomarkers of acute ki-dney injury in healthy individuals. PLoS ONE 2018, 13, e0205791. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Wenfang, L.; Jing, C.; Meng, C.; Chengcheng, D.; Jiqu, X.; Shuang, R. Neuroprotective effects of phytosterol esters against high cholesterol-induced cognitive deficits in aged rat. Food Funct. 2017, 8, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.E.M.; Kosba, A.A. Royal jelly supplementation reduces skeletal muscle lipotoxicity and insulin resistance in aged obese rats. Pathophysiology 2018, 25, 307–315. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Yao, L.; Djang, A.H. Effects of Oncolyn on ATPase activity and expression of nerve growth factor in brain tissue of aging mice. Health Res. 2007, 36, 164–166. [Google Scholar]
- Li, G.; Wang, H.W.; Si, H.L.; Yang, Q.; Jiang, X.F. Action and adjustment of Xinzhi Bimin capsule on the content of IL-13 in blood serum and expression of STAT6 in nasal mucosa of rat model with allergic rhinitis. Chin. J. Immunol. 2010, 26, 901–909. [Google Scholar]
- Pan, C.Y.; Yang, S.S.; Fu, H.L.; Miu, M.Y.; Jiao, B.H. Inhibitory effect of high expression of B-cell lymphoma factor Bcl-6 on apoptosis of rat hepatocytes. Second Mil. Med. Univ. Acad. J. 2013, 32, 349–354. [Google Scholar] [CrossRef]
- Liu, M.; Jing, H.; Zhang, J.; Che, G.; Zhou, M.; Gao, Z.; Li, S.; Ren, Z.; Hao, L.; Liu, Y.; et al. Optimization of mycelia selenium polysaccharide extraction from Agrocybe cylindracea SL-02 and assessment of their antioxidant and anti-ageing activities. PLoS ONE 2016, 11, e0160799. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.S.; Mao, X.H.; Pei, R.; Miao, S.P.; Xiang, C.; Lv, Y.J.; Yang, X.G.; Sun, J.; Jia, S.S.; Liu, Y.P. Antitumor activity of polysaccharides from Lepista sordida against laryngocarcinoma in vitro and in vivo. Int. J. Biol. Macromol. 2013, 60, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.Q.; Liu, W.; Yan, J.; Liang, L.; Zheng, R.F.; Wang, X.J.; Gou, X.J.; He, G. Isolation, purification, acetylation modification and antioxidant activity of polysaccharides from Pleurotus erinatus. Food Ind. Technol. 2018, 39, 39–44. [Google Scholar]
- Yang, C.Y.; Yang, C.L.; Liu, H.L.; Jing, Z.G.; Xu, X.X.; Wang, T.H. Study on the preparation and antioxidant activity of polysaccharides from acetylated Auricularia Japonica. Sci. Technol. Food Ind. 2015, 36, 105–115. [Google Scholar]
- Sunk, T.; Ho, C.T. Antioxidant activities of buckwheat extracts. Food Chem. 2005, 90, 743–749. [Google Scholar]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Ma, P.S.; Wu, W.; Zhou, R.; Hao, Y.J.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Neuroprotective actions of taurine on hypoxic-ischemic brain damage in neonatal rats. Brain Res. Bull. 2016, 124, 295–305. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Groups | Body Weight (g) | Liver Index (g/100g) | Kidney Index (g/100g) | Brain Index (g/100g) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| NC | 24.72 ± 1.21 | 41.44 ± 1.28 | 7.54 ± 0.21 | 3.78 ± 0.05 | 1.05 ± 0.05 |
| MC | 25.11 ± 0.97 | 32.89 ± 1.44 a | 13.38 ± 0.19 d | 6.33 ± 0.09 e | 3.79 ± 0.12 |
| PC | 24.98 ± 1.12 | 39.63 ± 1.57 bc | 8.26 ± 0.14 b | 3.94 ± 0.04 b | 1.12 ± 0.06 |
| AMPS | |||||
| 400 mg/kg/d | 25.02 ± 1.45 | 38.91 ± 1.31 bc | 8.96 ± 0.22 b | 4.25 ± 0.04 c | 1.56 ± 0.04 |
| 200 mg/kg/d | 24.35 ± 1.34 | 37.04 ± 1.19 b | 9.21 ± 0.18 c | 4.98 ± 0.06 d | 2.11 ± 0.09 |
| Groups | Groups MDA Contents (μmol/mg prot) | LPO Contents (nmol/mg prot) | ||||
|---|---|---|---|---|---|---|
| Liver | Kidney | Brain | Liver | Kidney | Brain | |
| NC | 3.13 ± 0.19 | 4.92 ± 0.21 | 2.02 ± 0.09 | 4.21 ± 0.19 | 3.80 ± 0.11 | 1.84 ± 0.08 |
| MC | 13.21 ± 0.45 d | 15.73 ± 0.51 d | 15.09 ± 0.58 e | 17.23 ± 0.59 e | 16.87 ± 0.51 d | 7.44 ± 0.39 d |
| PC | 3.61 ± 0.12 a | 6.24 ± 0.24 b | 8.09 ± 0.31 b | 5.92 ± 0.49 b | 4.71 ± 0.24 a | 2.24 ± 0.12 a |
| AMPS | ||||||
| 400 mg/kg/d | 5.70 ± 0.19 b | 6.12 ± 0.22 b | 10.05 ± 0.38 c | 9.89 ± 0.32 c | 8.81 ± 0.29 b | 4.28 ± 0.23 b |
| 200 mg/kg/d | 9.35 ± 0.34 c | 9.51 ± 0.38 c | 12.03 ± 0.49 d | 12.31 ± 0.39 d | 11.95 ± 0.42 c | 5.67 ± 0.17 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhao, H.; Gao, Z.; Song, X.; Wang, W.; Yuan, F.; Feng, Y.; Zhang, Y.; Zhang, J.; Zhang, S.; et al. The Antioxidant and Anti-Aging Effects of Acetylated Mycelia Polysaccharides from Pleurotus djamor. Molecules 2019, 24, 2698. https://doi.org/10.3390/molecules24152698
Li H, Zhao H, Gao Z, Song X, Wang W, Yuan F, Feng Y, Zhang Y, Zhang J, Zhang S, et al. The Antioxidant and Anti-Aging Effects of Acetylated Mycelia Polysaccharides from Pleurotus djamor. Molecules. 2019; 24(15):2698. https://doi.org/10.3390/molecules24152698
Chicago/Turabian StyleLi, Huaping, Huajie Zhao, Zheng Gao, Xinling Song, Wenshuai Wang, Fangfang Yuan, Yanbo Feng, Yiwen Zhang, Jianjun Zhang, Shuliang Zhang, and et al. 2019. "The Antioxidant and Anti-Aging Effects of Acetylated Mycelia Polysaccharides from Pleurotus djamor" Molecules 24, no. 15: 2698. https://doi.org/10.3390/molecules24152698
APA StyleLi, H., Zhao, H., Gao, Z., Song, X., Wang, W., Yuan, F., Feng, Y., Zhang, Y., Zhang, J., Zhang, S., & Jia, L. (2019). The Antioxidant and Anti-Aging Effects of Acetylated Mycelia Polysaccharides from Pleurotus djamor. Molecules, 24(15), 2698. https://doi.org/10.3390/molecules24152698






