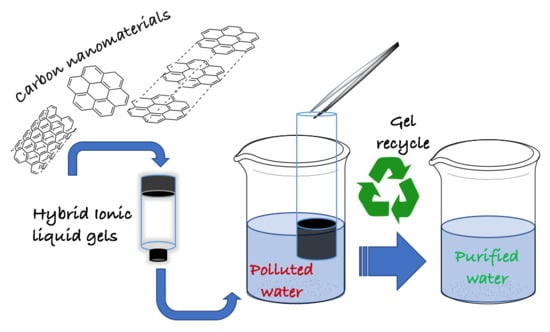
Carbon Nanomaterial Doped Ionic Liquid Gels for the Removal of Pharmaceutically Active Compounds from Water
Abstract
1. Introduction
2. Results and Discussion
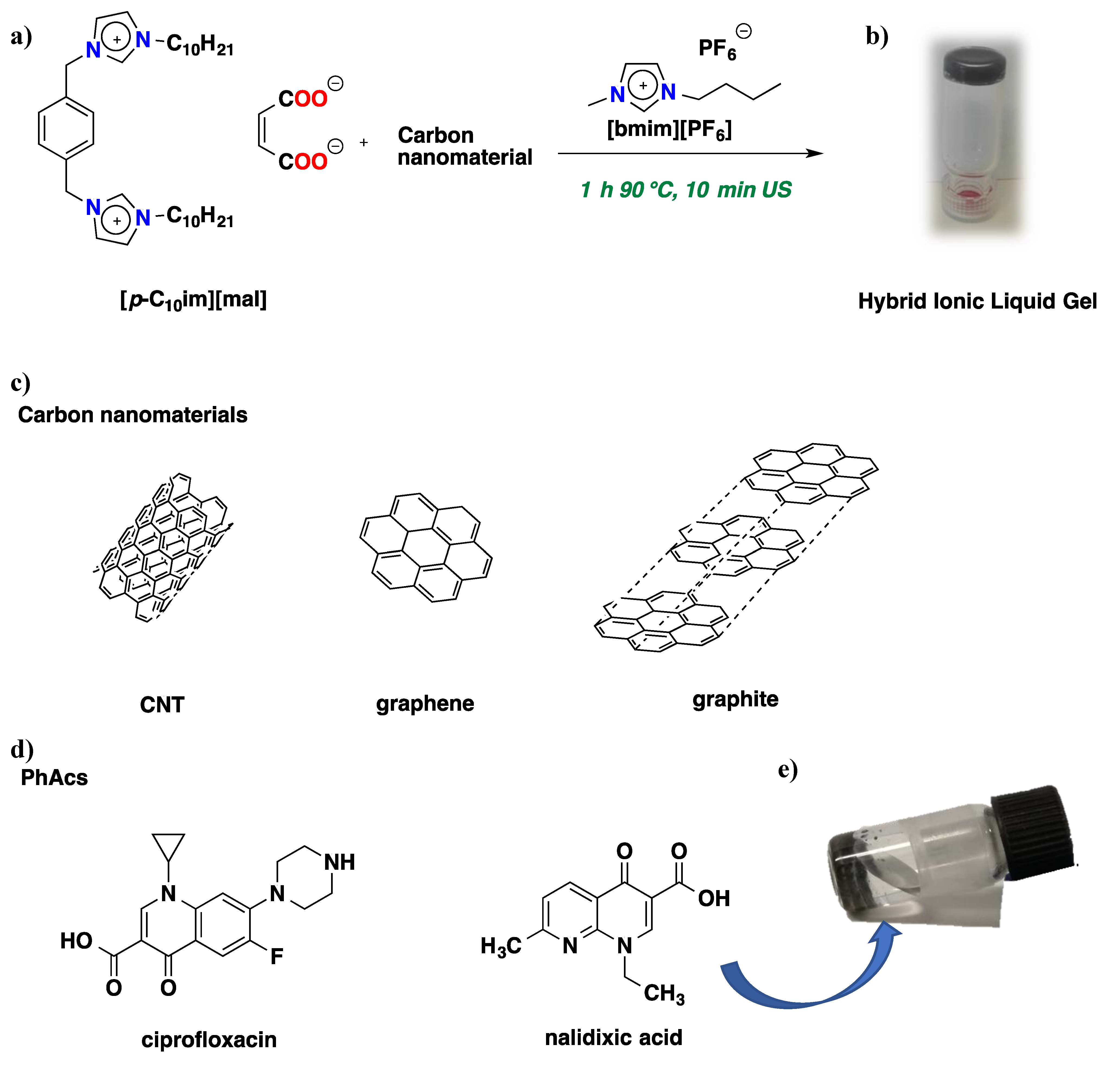
2.1. Gel Preparation and Thermal Behaviour
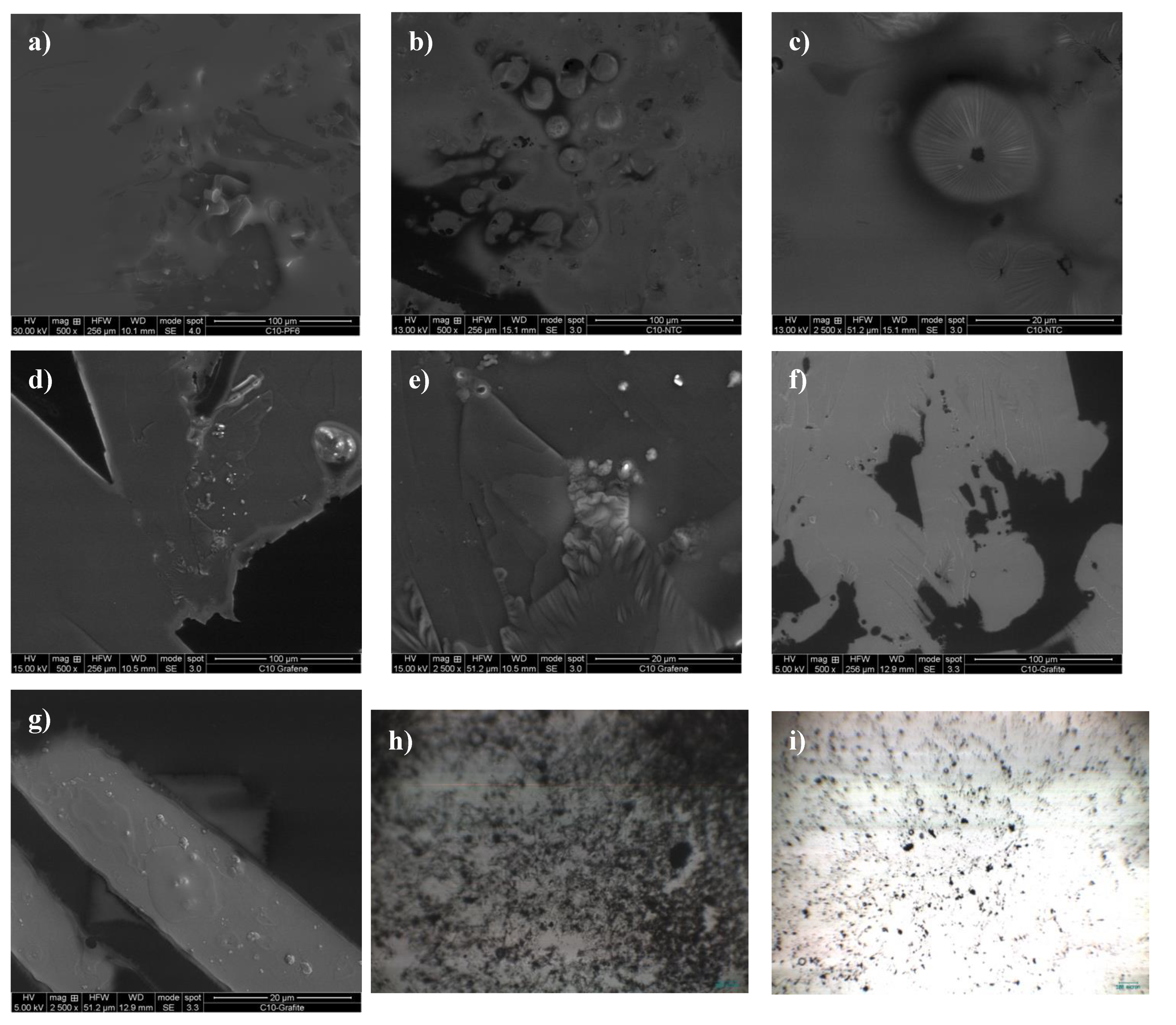
2.2. Morphology of Gels
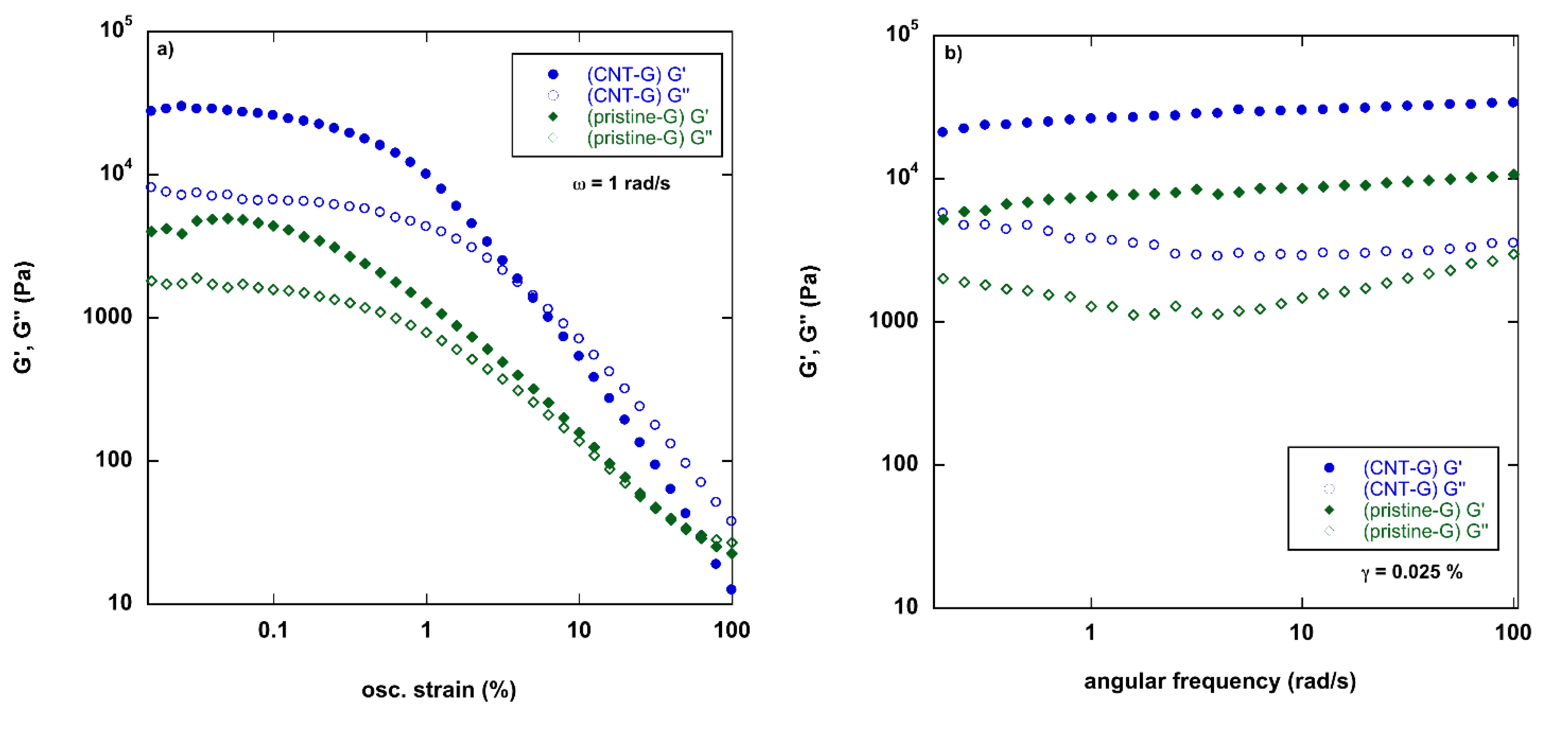
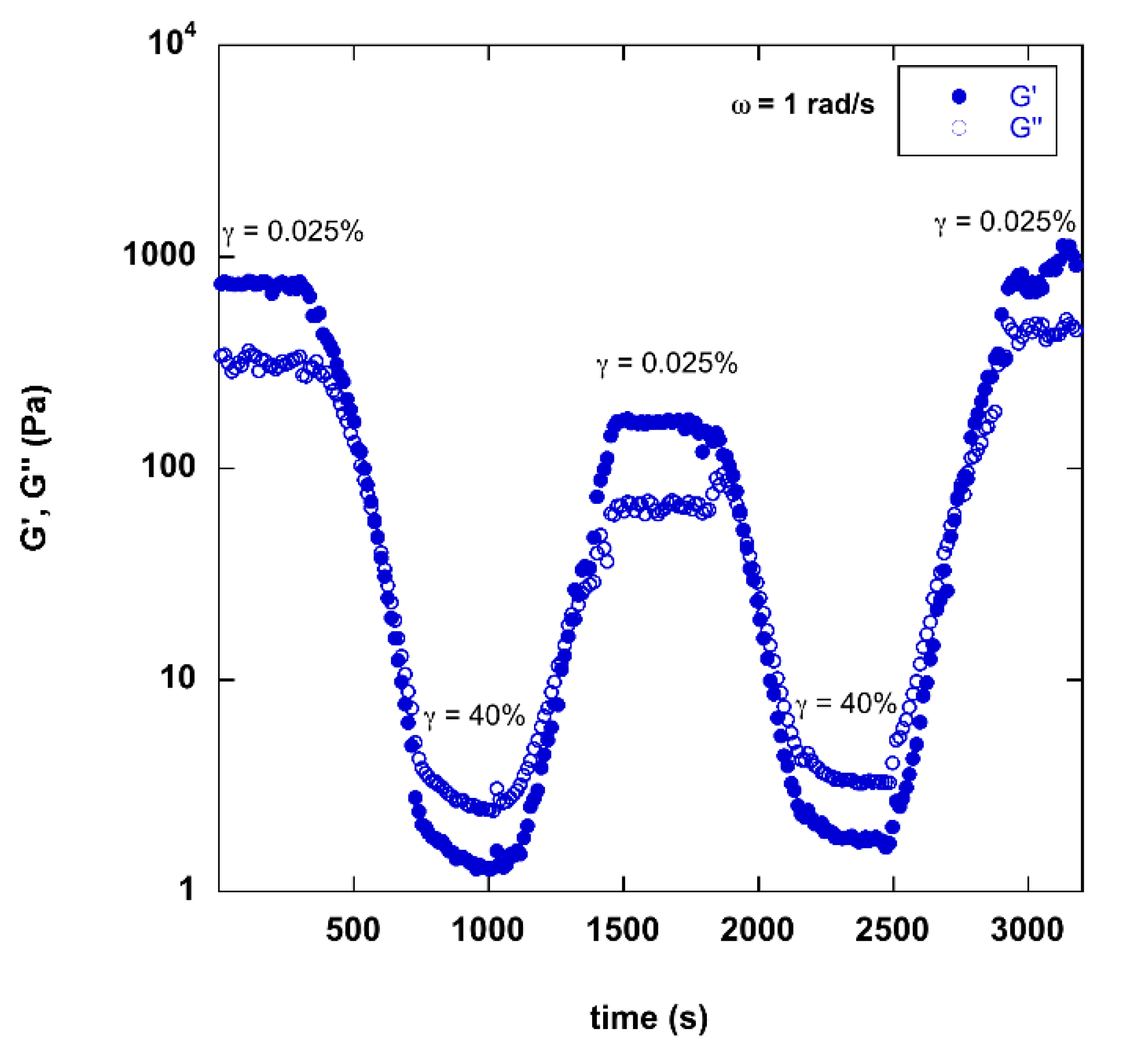
2.3. Rheology of Gels
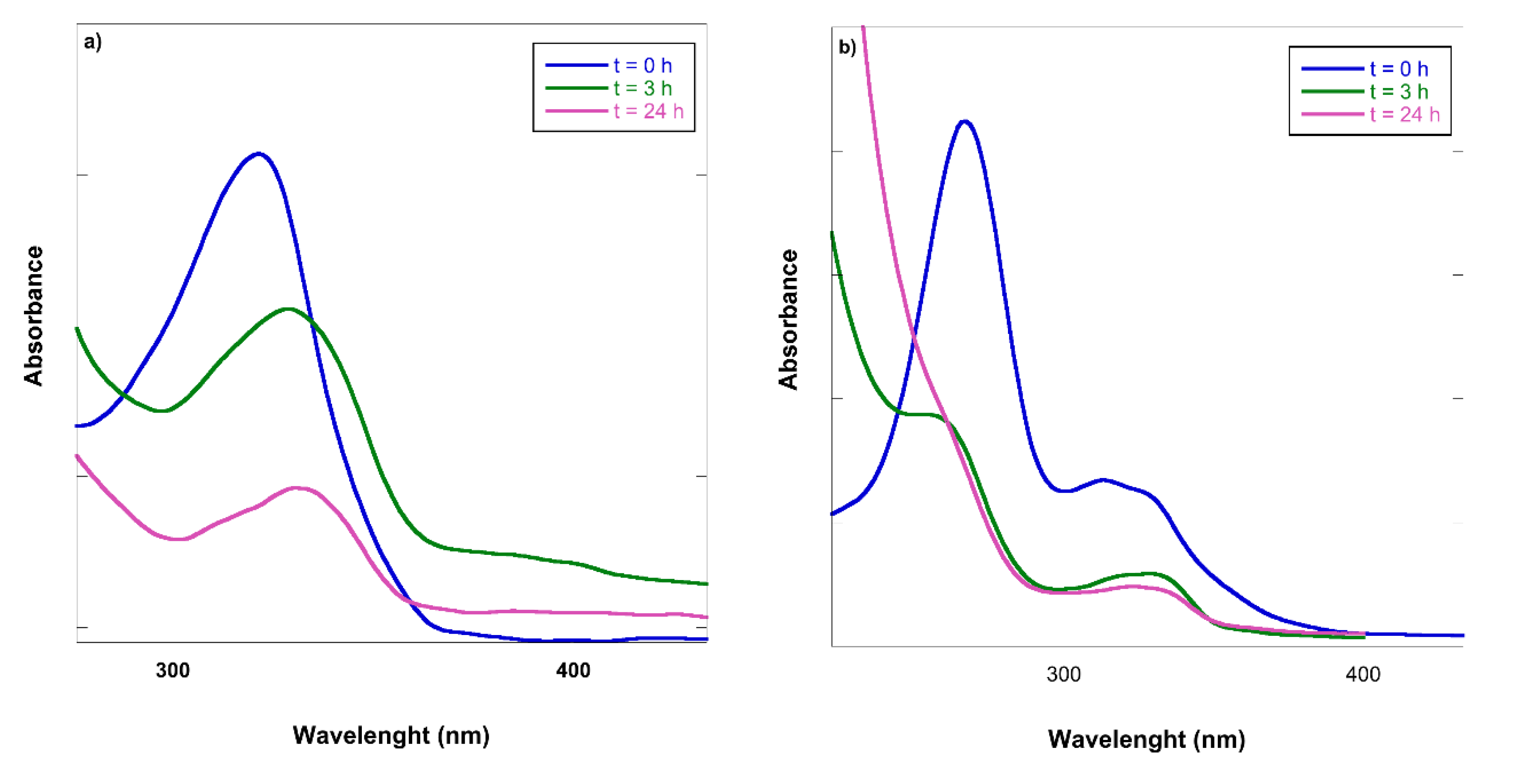
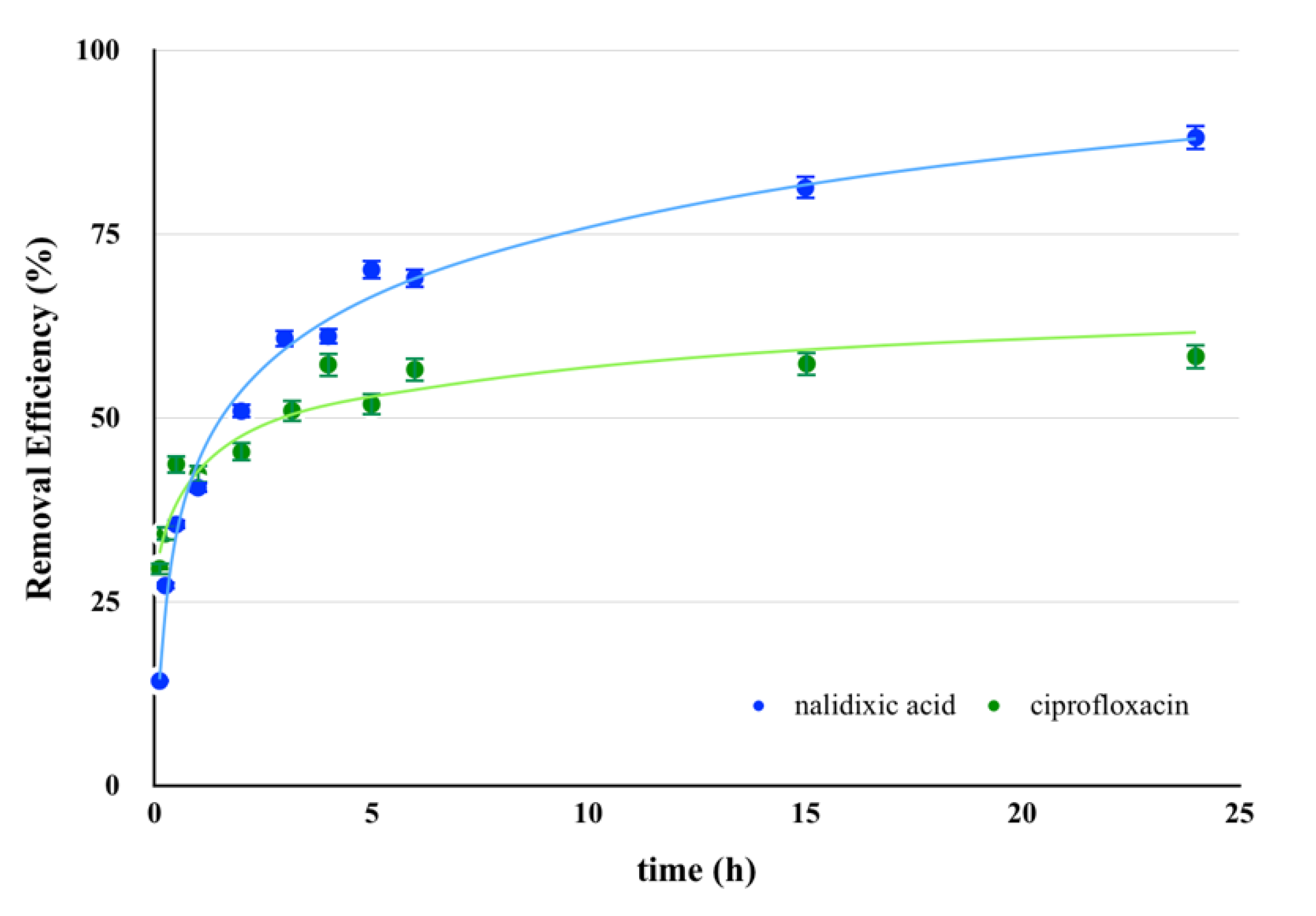
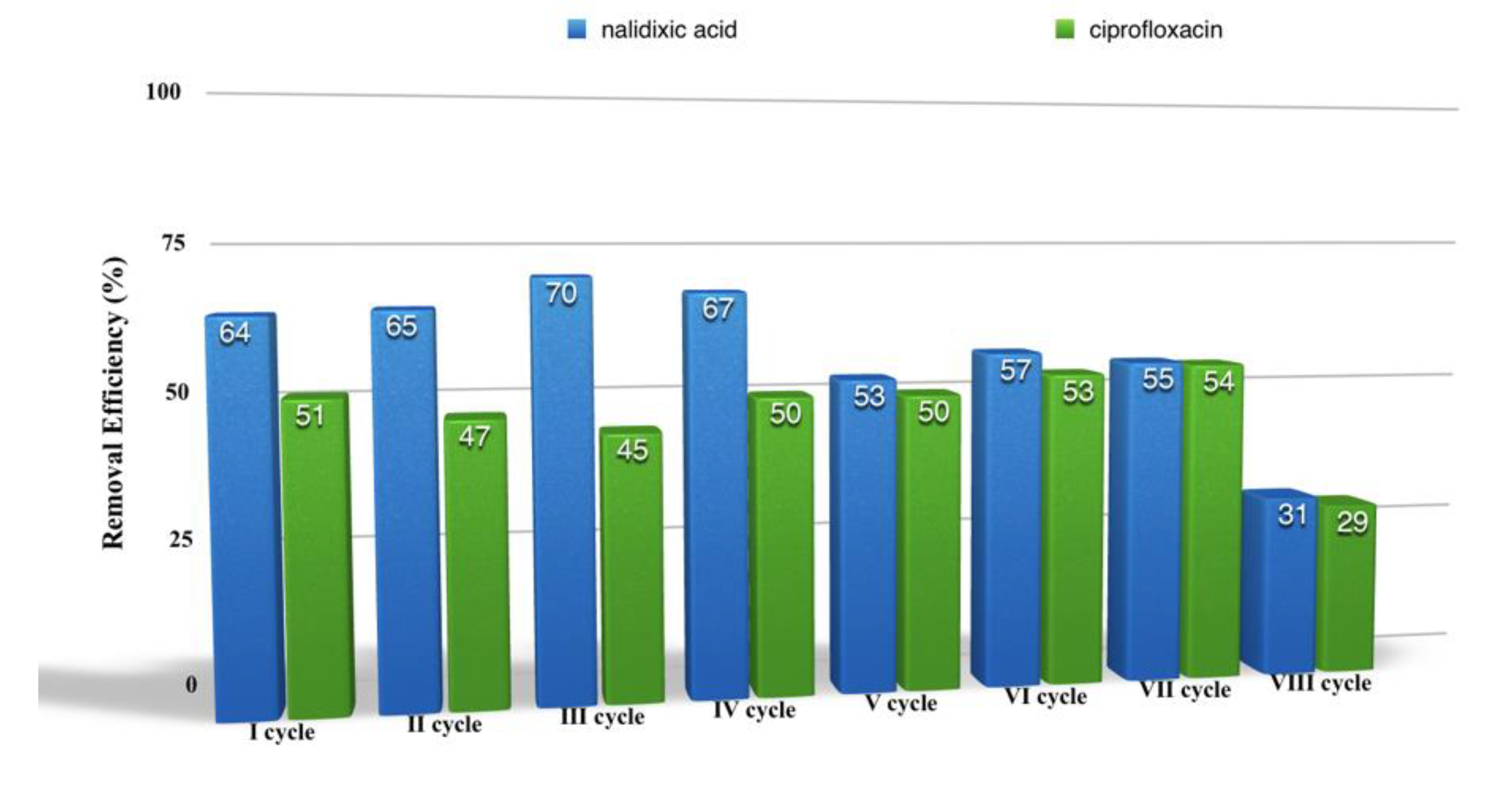
2.4. PhAC Adsorption on Gels
3. Materials and Methods
3.1. Materials
3.2. Gel Preparation
3.3. Tgel Determination
3.4. Morphology of Gels
3.5. Rheological Measurements
3.6. Thixotropic and Sonotropic Behaviors
3.7. PhAC Adsorption
3.8. Kinetics of PhAC Adsorption
3.9. Determination of RE for PhAC at Different Concentrations and Volumes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cai, Z.; Dwivedi, A.D.; Lee, W.-N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.-H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Padhye, L.P.; Yao, H.; Kung’u, F.T.; Huang, C.-H. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res. 2014, 51, 266–276. [Google Scholar] [CrossRef]
- Chelliapan, S.; Wilby, T.; Sallis, P.J. Performance of an up-flow anaerobic stage reactor (UASR) in the treatment of pharmaceutical wastewater containing macrolide antibiotics. Water Res. 2006, 40, 507–516. [Google Scholar] [CrossRef]
- der Beek, T.A.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.M.; Härkki, H.; Tuhkanen, T.; Kronberg, L. Occurrence of Pharmaceuticals in River Water and Their Elimination in a Pilot-Scale Drinking Water Treatment Plant. Environ. Sci. Technol. 2007, 41, 5077–5084. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J.R.; González, T.; Palo, P.; Cuerda-Correa, E.M. Removal of common pharmaceuticals present in surface waters by Amberlite XAD-7 acrylic-ester-resin: Influence of pH and presence of other drugs. Desalination 2011, 269, 231–238. [Google Scholar] [CrossRef]
- Salem Attia, T.M.; Hu, X.L.; Yin, D.Q. Synthesized magnetic nanoparticles coated zeolite for the adsorption of pharmaceutical compounds from aqueous solution using batch and column studies. Chemosphere 2013, 93, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Zhu, W.; Wu, X.; Hou, F.; Xun, S.; Wu, P.; Ji, H.; Xu, H.; Li, H. Application of graphene-like layered molybdenum disulfide and its excellent adsorption behavior for doxycycline antibiotic. Chem. Eng. J. (Lausanne) 2014, 243, 60–67. [Google Scholar] [CrossRef]
- Dong, L.-X.; Huang, X.-C.; Wang, Z.; Yang, Z.; Wang, X.-M.; Tang, C.Y. A thin-film nanocomposite nanofiltration membrane prepared on a support with in situ embedded zeolite nanoparticles. Sep. Purif. Technol. 2016, 166, 230–239. [Google Scholar] [CrossRef]
- Liu, F.-f.; Zhao, J.; Wang, S.; Du, P.; Xing, B. Effects of Solution Chemistry on Adsorption of Selected Pharmaceuticals and Personal Care Products (PPCPs) by Graphenes and Carbon Nanotubes. Environ. Sci. Technol. 2014, 48, 13197–13206. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, M.; Yu, F.; Zheng, J. Water-enhanced Removal of Ciprofloxacin from Water by Porous Graphene Hydrogel. Sci. Rep. 2015, 5, 13578. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, Y.; Zhang, M.; Yang, M.; Gong, X.; Yu, F.; Zheng, J. Comparative Study of Graphene Hydrogels and Aerogels Reveals the Important Role of Buried Water in Pollutant Adsorption. Environ. Sci. Technol. 2017, 51, 12283–12292. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.Z.; Yue, R.; Sun, X.-F.; Song, C.; Wang, S.-G. Enhanced removal of ciprofloxacin using humic acid modified hydrogel beads. J. Colloid Interface Sci. 2019, 543, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. (Wash. Dcu. S.) 1997, 97, 3133–3160. [Google Scholar] [CrossRef]
- Marr, P.C.; Marr, A.C. Ionic liquid gel materials: Applications in green and sustainable chemistry. Green Chem. 2016, 18, 105–128. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting–self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef]
- Marullo, S.; Rizzo, C.; Dintcheva, N.T.; Giannici, F.; D’Anna, F. Ionic liquids gels: Soft materials for environmental remediation. J. Colloid Interface Sci. 2018, 517, 182–193. [Google Scholar] [CrossRef]
- Billeci, F.; D’Anna, F.; Gunaratne, H.Q.N.; Plechkova, N.V.; Seddon, K.R. “Sweet” Ionic Liquid Gels: Materials for Sweetening of Fuels. Green Chem. 2018, 20, 4260–4276. [Google Scholar] [CrossRef]
- Rizzo, C.; D’Anna, F.; Noto, R.; Zhang, M.; Weiss, R.G. Insights into the Formation and Structures of Molecular Gels by Diimidazolium Salt Gelators in Ionic Liquids or “Normal” Solvents. Chem. Eur. J. 2016, 22, 11269–11282. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Samanta, S.K. Soft-Nanocomposites of Nanoparticles and Nanocarbons with Supramolecular and Polymer Gels and Their Applications. Chem. Rev. (Wash. Dcu. S.) 2016, 116, 11967–12028. [Google Scholar] [CrossRef]
- Samanta, S.K.; Pal, A.; Bhattacharya, S.; Rao, C.N.R. Carbon nanotube reinforced supramolecular gels with electrically conducting, viscoelastic and near-infrared sensitive properties. J. Mater. Chem. 2010, 20, 6881–6890. [Google Scholar] [CrossRef]
- Rizzo, C.; Arcudi, F.; Đorđević, L.; Dintcheva, N.T.; Noto, R.; D’Anna, F.; Prato, M. Nitrogen-Doped Carbon Nanodots-Ionogels: Preparation, Characterization, and Radical Scavenging Activity. ACS Nano 2018, 12, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Andrews, J.L.; Steed, J.W.; D’Anna, F. Carbohydrate-supramolecular gels: Adsorbents for chromium(VI) removal from wastewater. J. Colloid Interface Sci. 2019, 548, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tao, S.; Xing, B. Sorption and Competition of Aromatic Compounds and Humic Acid on Multiwalled Carbon Nanotubes. Environ. Sci. Technol. 2009, 43, 6214–6219. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Adsorption of Organic Compounds by Carbon Nanomaterials in Aqueous Phase: Polanyi Theory and Its Application. Chem. Rev. (Wash. Dcu. S.) 2010, 110, 5989–6008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-S.; Zheng, T.; Wang, P.; Jiang, J.-P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. (Lausanne) 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ma, J.; Bi, D. Enhanced adsorptive removal of selected pharmaceutical antibiotics from aqueous solution by activated graphene. Environ. Sci. Pollut. Res. 2015, 22, 4715–4724. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. J. Colloid Interface Sci. 2016, 484, 196–204. [Google Scholar] [CrossRef]
- Yu, L.; Liu, X.; Yuan, W.; Brown, L.J.; Wang, D. Confined Flocculation of Ionic Pollutants by Poly(l-dopa)-Based Polyelectrolyte Complexes in Hydrogel Beads for Three-Dimensional, Quantitative, Efficient Water Decontamination. Langmuir 2015, 31, 6351–6366. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, C.; Lin, Z. EDTA-Induced Self-Assembly of 3D Graphene and Its Superior Adsorption Ability for Paraquat Using a Teabag. ACS Appl. Mater. Interfaces 2014, 6, 19766–19773. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Marullo, S.; Campodonico, P.R.; Pibiri, I.; Dintcheva, N.T.; Noto, R.; Millan, D.; D’Anna, F. Self-Sustaining Supramolecular Ionic Liquid Gels for Dye Adsorption. ACS Sustain. Chem. Eng. 2018, 6, 12453–12462. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Entry | Gel | C (wt%) | Appearance b | Tgel (°C) c |
|---|---|---|---|---|
| 1 | pristine-G [18] | 2.8 a | G | SM |
| 2 | 4.0 | G | 20 | |
| 3 | 6.25 | G | 37 | |
| 4 | graphite-G * | 3.25 a | G | 45 |
| 5 | 4.0 | G | 42 | |
| 6 | 6.25 | G | 58 | |
| 7 | CNT-G * | 3.25 a | G | 41 |
| 8 | 4.0 | G | 42 | |
| 9 | 6.25 | G | 55 | |
| 10 | graphene-G * | 3.25 a | G | 42 |
| 11 | 4.0 | G | 42 | |
| 12 | 6.25 | G | 54 |
| Gel | G′ (Pa) | G′′ (Pa) | tan δ |
|---|---|---|---|
| pristine-G | 3800 ± 10 | 1800 ± 150 | 0.4 ± 0.05 |
| CNT-G | 28,200 ± 2600 | 6000 ± 2000 | 0.23 ± 0.08 |
| graphite-G | 1600 ± 900 | 730 ± 70 | 0.4 ± 0.02 |
| graphene-G | 600 ± 100 | 340 ± 20 | 0.5 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, C.; Marullo, S.; Dintcheva, N.T.; D’Anna, F. Carbon Nanomaterial Doped Ionic Liquid Gels for the Removal of Pharmaceutically Active Compounds from Water. Molecules 2019, 24, 2788. https://doi.org/10.3390/molecules24152788
Rizzo C, Marullo S, Dintcheva NT, D’Anna F. Carbon Nanomaterial Doped Ionic Liquid Gels for the Removal of Pharmaceutically Active Compounds from Water. Molecules. 2019; 24(15):2788. https://doi.org/10.3390/molecules24152788
Chicago/Turabian StyleRizzo, Carla, Salvatore Marullo, Nadka Tz. Dintcheva, and Francesca D’Anna. 2019. "Carbon Nanomaterial Doped Ionic Liquid Gels for the Removal of Pharmaceutically Active Compounds from Water" Molecules 24, no. 15: 2788. https://doi.org/10.3390/molecules24152788
APA StyleRizzo, C., Marullo, S., Dintcheva, N. T., & D’Anna, F. (2019). Carbon Nanomaterial Doped Ionic Liquid Gels for the Removal of Pharmaceutically Active Compounds from Water. Molecules, 24(15), 2788. https://doi.org/10.3390/molecules24152788