Catalytic Production of Levulinic Acid (LA) from Actual Biomass
Abstract
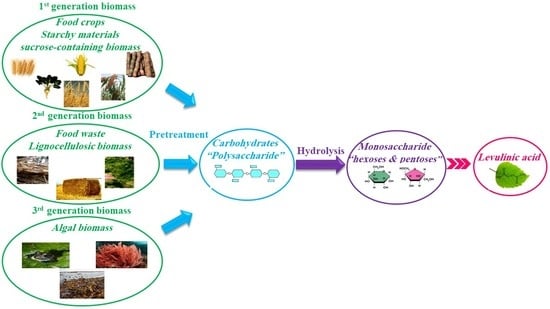
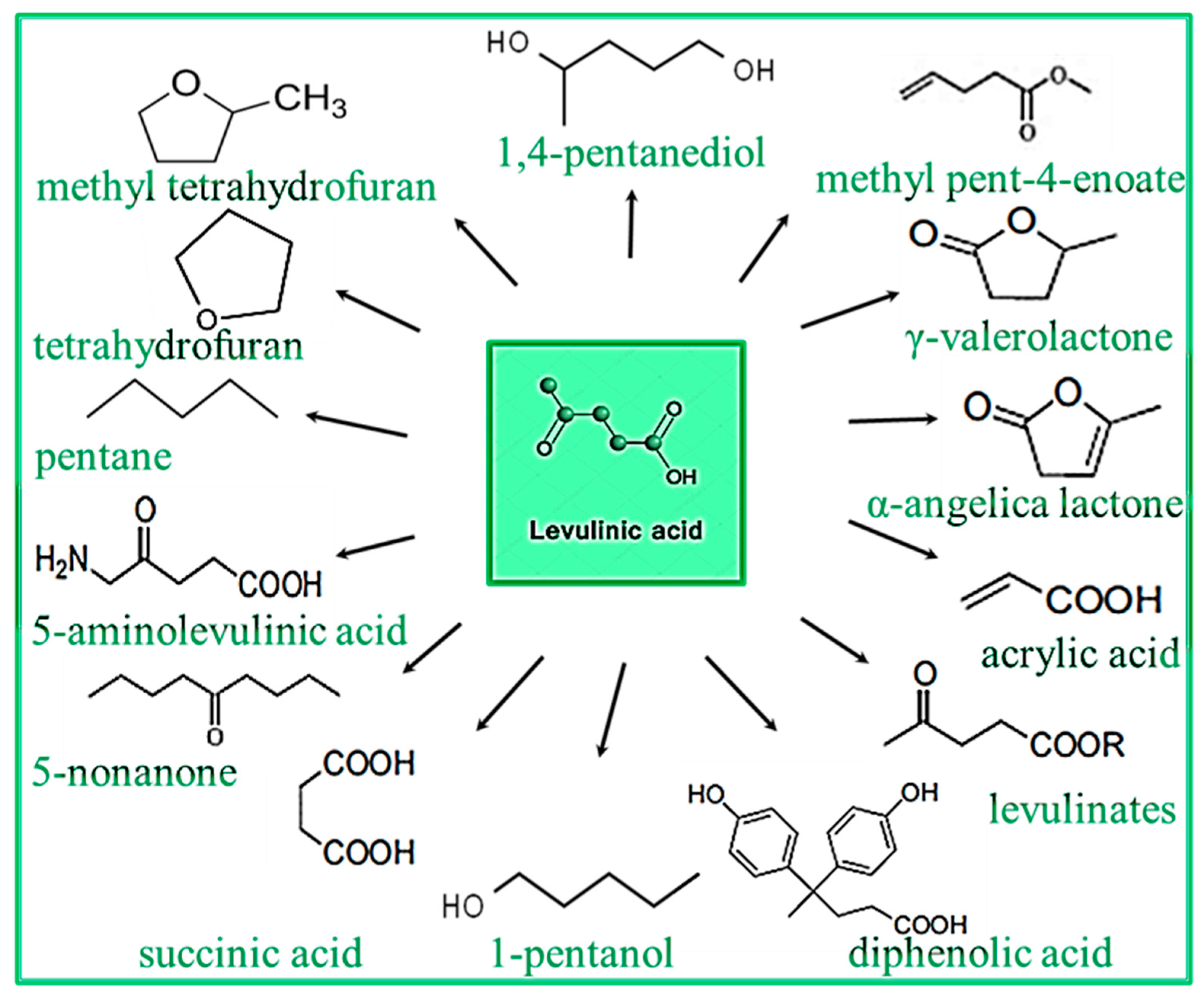
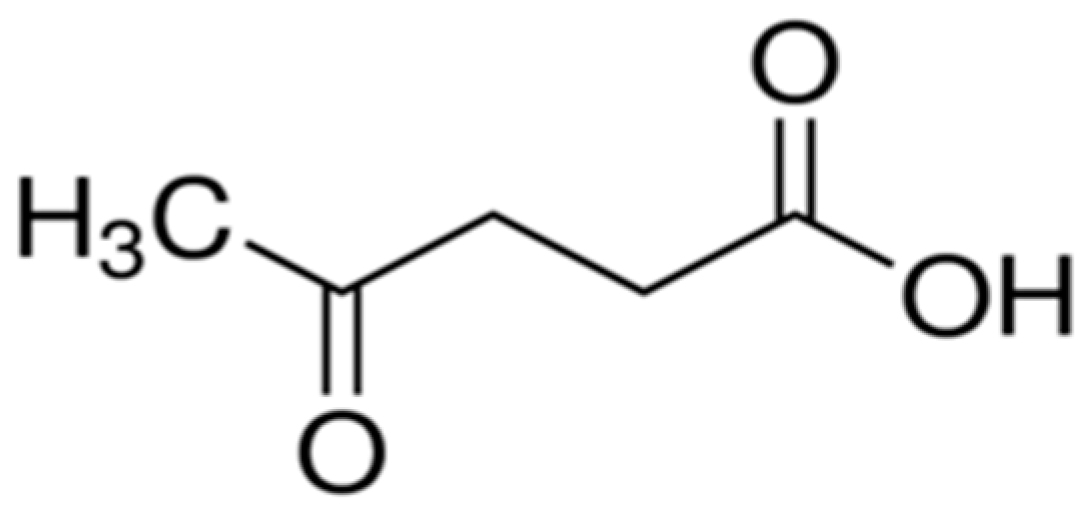
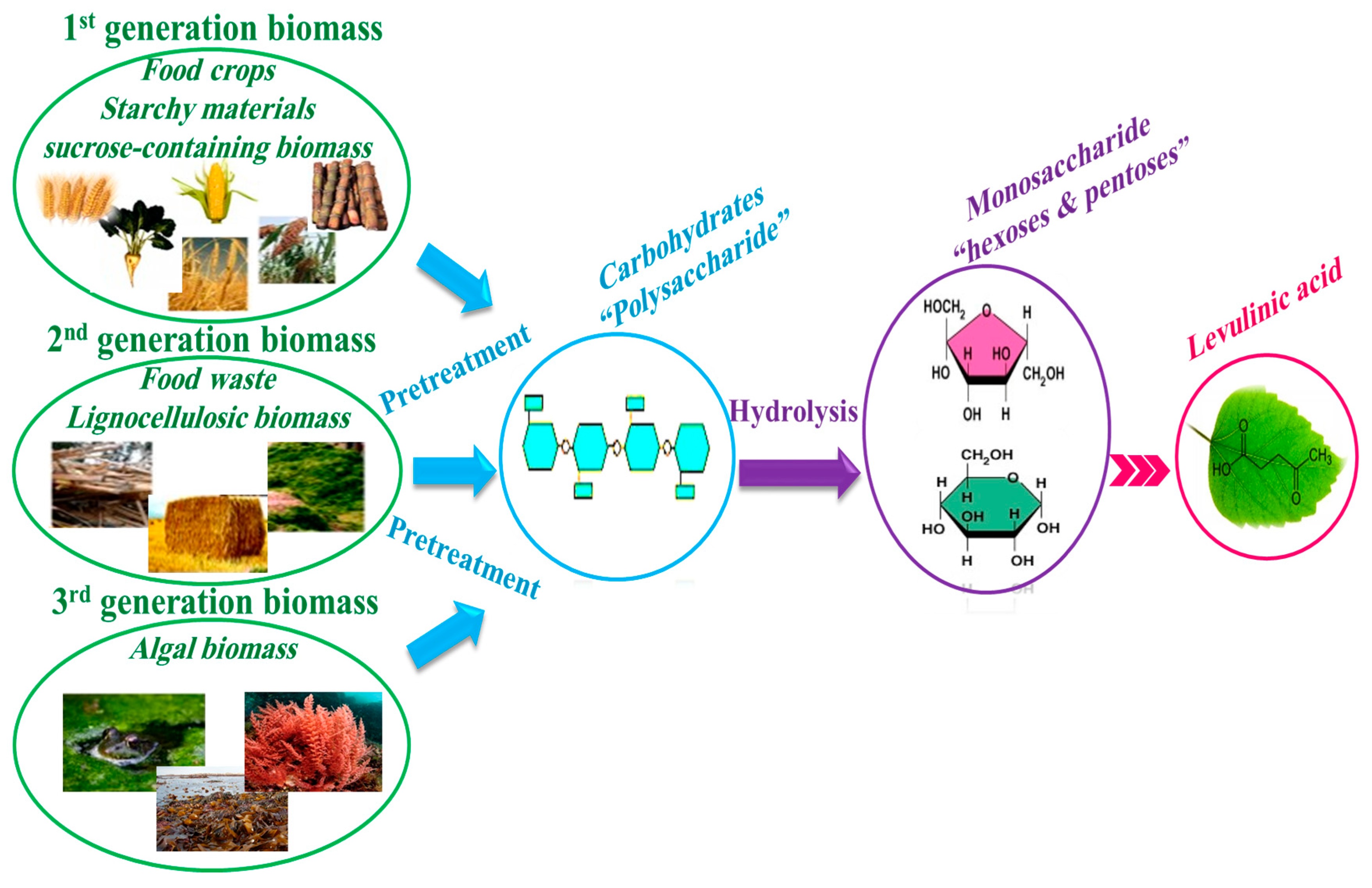
1. Introduction
- (i)
- First generation of biomass comes from food crops such as sugar, starchy crops, vegetable oil, or animal fat.
- (ii)
- Second generation of biomass is non-food crops such as wood, organic waste, food crop waste, and specific biomass crops. Most of biomasses in this generation are considered as the lignocellulosic biomass.
- (iii)
- Third generation of biomass comes from algae.
- (i)
- pretreatment of biomass to extract polysaccharides,
- (ii)
- hydrolysis of polysaccharides into monosaccharides such as hexoses and pentoses, and pentoses,
- (iii)
- conversion of monosaccharides to LA during several steps [31].
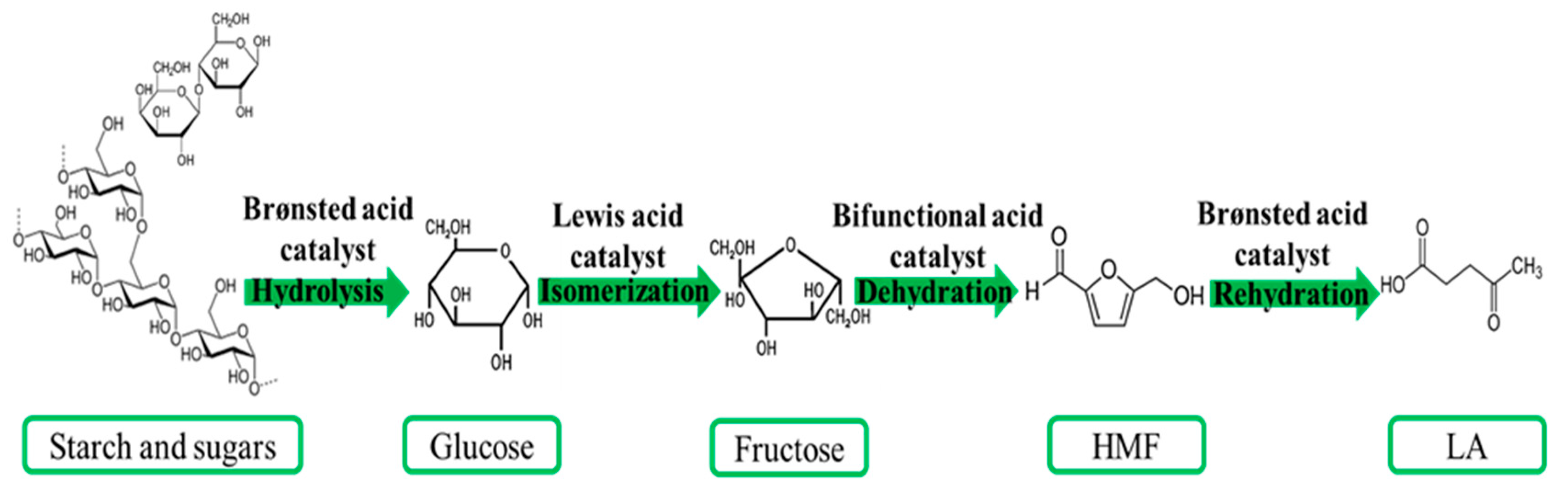
2. LA Production from First Generation of Biomass: Starchy and Sugary Biomass
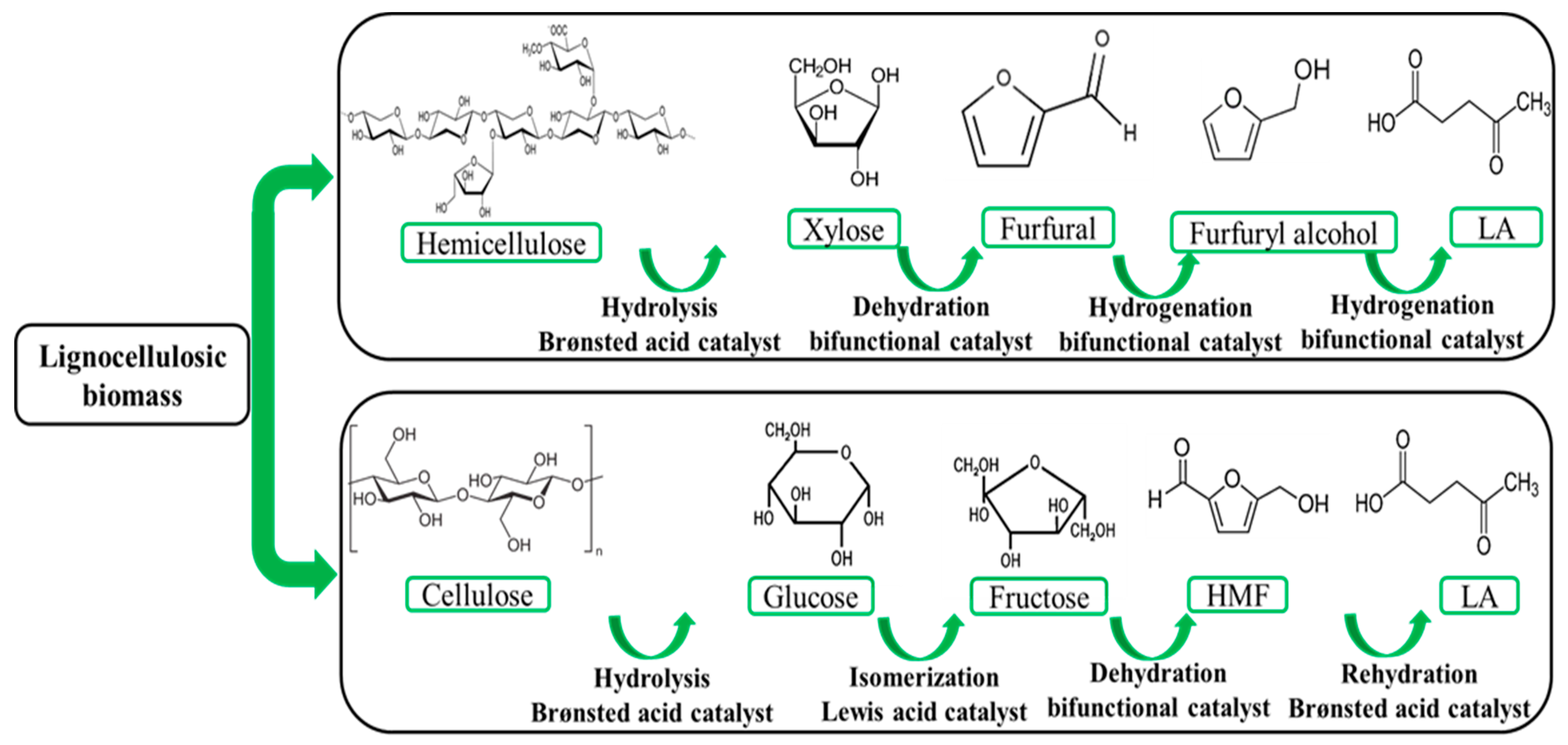
3. LA Production from Second Generation of Biomass: Food Waste and Lignocellulosic Biomass
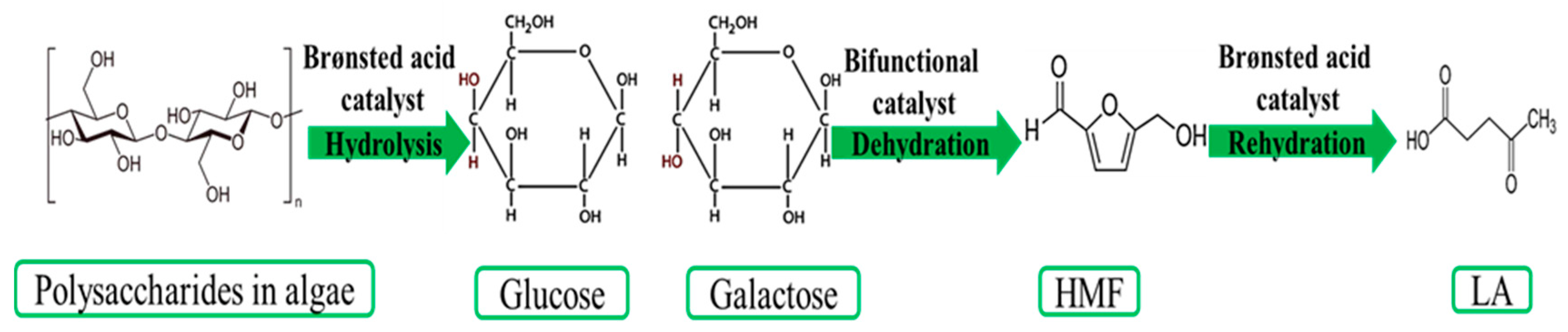
4. LA Production from Third Generation of Biomass: Algal Biomass
5. Conclusions
- (1)
- Pretreatment of biomass seems to be a compulsory step for improving the yield of LA and reaction rate [32]. Some of the studies performed pretreatment before starting real hydrolysis reaction. There are different methods for biomass pretreatment, which depend on the type of the raw starting compounds. Dilute acid pretreatment was demonstrated as the most common method, especially for second generation biomass. Pretreatment causes an increase in cellulose percentage in the feedstock, and most of the glucose could remain in the pretreated feedstock. Therefore, pretreatment could act as a desirable economic method, which leads to higher efficient LA production. According to the type of biomass, it could be more utile if other types of pretreatment, such as mechanical communication, steam explosion, CO2 explosion, pyrolysis, organosolv, and biological pretreatment processes are taken into the account in order to remove some destructive components, such as lignin and inorganic salts [107].
- (2)
- Water seems to be a preferable solvent for the LA production from biomass. Water or supercritical water in the reaction condition is safe and environmental friendly. In addition, it has lots of advantages, such as high thermal conductivity and low viscosity. On the other hand, in some cases, water cannot be an appropriate solvent, due to the insolubility of polymeric feedstock, especially when heterogeneous catalyst is used, limited mass transfer and instability of some water-sensitive catalysts, such as metal chloride [108]. Therefore, using the most suitable ionic and organic solvents could be critical. In recent years, study on ionic liquids has attracted great attention, owing their wide performance as solvents as well as catalysts. However, the harmful effect of this class of solvent, such as toxicity, explosivity, biodegradability, and their high cost limits them from plentiful use [109].
- (3)
- According to what was reported in the recent year literatures, homogeneous catalysts, especially HCl and H2SO4, were widely used for the conversion of all three generation biomasses. Homogeneous catalysts may be recovered from the reaction solution by using the distillation method, but the challenge of reactor corrosion makes the process outrageously expensive. Replacing homogeneous acid catalysts with green and efficient heterogeneous catalysts can be useful for hydrolysis process in the future. In addition, in recent years, using heterogeneous catalyst in LA production especially from second generation biomass, has gradually increased. Normally, solid catalysts are tunable in the aspect of acidity and reaction condition and they could be easily recovered [110]. Furthermore, heterogeneous catalysts do not have the problem of reactor corrosion and they could be a promising catalyst for industrial use [111]. However, using heterogeneous catalysts still have some limitations, such as limited mass transfer and the deposit of some solid byproducts, such as humins and big organic components on the surface of the catalyst and deactivation over a long period of time.
- (1)
- Development of the green heterogeneous catalysts focusing on the important factors, such as surface area, pore size and structure, accessibility of acid sites, recovery and recyclability, and lifetime is the trend for future biomass direct hydrolysis.
- (2)
- Using some novel non-terrestrial resources, such as macroalgae, can demonstrate an important achievement. Therefore, further study is still compulsory for developing an environmental-friendly process with new high recyclable catalysts, which increase the LA yield and progressively target towards raw and cheap biomass feedstock, and finally the possibility to scale up the process going beyond the economic and technological barriers.
- (3)
- Separation and purification of LA from reaction solution, especially while using organic solvent is still a challenge for having a cost-effective application. One potential way to solve this problem is producing a higher LA concentration in the product stream, which can decrease the amount of waste-solvent and reduce the consumption of energy. Moreover, solid acid catalysts are preferred in the separation process. However, work on this research area is still needed.
- (4)
- Formation of by-products, such as thermal-table humins, is still a bottleneck for the industrial scale production of LA. This problem is more relevant while using lignocellulosic biomass (owing to the presence of lignin) as the feedstock. Since humins are prone to blocking and deactivating the catalyst active sites, especially for heterogeneous catalysts, it can limit the scale-up on larger scale. Performing reaction at low temperature, high acid concentration, and using low biomass concentration could be some possible ways of preventing the formation of humins. However, more studies are still needed in order to completely inhibit the formation of humins. In addition, conversion of humins to some new carbon components is suggested for future work.
Author Contributions
Funding
Conflicts of Interest
References
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Valie Added Chemicals from Biomass: Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Department of Energy: Washington, DC, USA, 2004; pp. 1–76. [Google Scholar]
- Boisen, A.; Christensen, T.B.; Fu, W.; Gorbanev, Y.Y.; Hansen, T.S.; Jensen, J.S.; Klitgaard, S.K.; Pedersen, S.; Riisager, A.; Ståhlberg, T.; et al. Process integration for the conversion of glucose to 2, 5-furandicarboxylic acid. Chem. Eng. Res. Des. 2009, 87, 1318–1327. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.J.; Raghavan, V. Review: Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Zhang, X.; Wilson, K.; Lee, A.F. Heterogeneously Catalyzed Hydrothermal Processing of C5–C6 Sugars. Chem. Rev. 2016, 116, 12328–12368. [Google Scholar] [CrossRef] [PubMed]
- Vennestrøm, P.N.R.; Osmundsen, C.M.; Christensen, C.H.; Taarning, E. Beyond petrochemicals: The renewable chemicals industry. Angew. Chem. Int. Ed. 2011, 50, 10502–10509. [Google Scholar] [CrossRef] [PubMed]
- Pileidis, F.D.; Titirici, M.M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef] [PubMed]
- Morone, A.; Apte, M.; Pandey, R.A. Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renew. Sustain. Energy Rev. 2015, 51, 548–565. [Google Scholar] [CrossRef]
- Dayma, G.; Halter, F.; Foucher, F.; Togbé, C.; Mounaim-Rousselle, C.; Dagaut, P. Experimental and detailed kinetic modeling study of ethyl pentanoate (Ethyl Valerate) oxidation in a jet stirred reactor and laminar burning velocities in a spherical combustion chamber. Energy Fuels 2012, 26, 4735–4748. [Google Scholar] [CrossRef]
- Petrus, L.; Louis, J.; Ayoub, P.M.; Clarke, L.; Price, R.; Gosselink, H.; Lange, J.-P. Valeric Biofuels: A Platform of Cellulosic Transportation Fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar]
- Luo, W.; Deka, U.; Beale, A.M.; Van Eck, E.R.H.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Ruthenium-catalyzed hydrogenation of levulinic acid: Influence of the support and solvent on catalyst selectivity and stability. J. Catal. 2013, 301, 175–186. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Marelli, M.; Psaro, R.; Ravasio, N.; Zaccheria, F. New generation biofuels: γ-Valerolactone into valeric esters in one pot. RSC Adv. 2013, 3, 1302–1306. [Google Scholar] [CrossRef]
- Bozell, J.J. Connecting Biomass and Petroleum Processing with a Chemical Bridge an Atomic View of Quantum. Science 2010, 329, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.Q.; Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2014, 1110, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone-a sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Pan, T.; Deng, J.; Xu, Q.; Xu, Y.; Guo, Q.X.; Fu, Y. Catalytic conversion of biomass-derived levulinic acid to valerate esters as oxygenated fuels using supported ruthenium catalysts. Green Chem. 2013, 15, 2967–2974. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Martin Alonso, D.; Gürbüz, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Yoshida, R.; Sun, D.; Yamada, Y.; Sato, S.; Hutchings, G.J. Vapor-phase hydrogenation of levulinic acid to γ-valerolactone over Cu-Ni bimetallic catalysts. Catal. Commun. 2017, 97, 79–82. [Google Scholar] [CrossRef]
- Mawhood, R.; Gazis, E.; De Jong, S.; Hoefnagels, R.; Slade, R. Production pathways for renewable jet fuel: A review of commercialization. Biofuels Bioprod. Bioref. 2016, 10, 462–484. [Google Scholar] [CrossRef]
- Roy Goswami, S.; Dumont, M.J.; Raghavan, V. Starch to value added biochemicals. Starch Staerke 2016, 68, 274–286. [Google Scholar] [CrossRef]
- Yan, K.; Jarvis, C.; Gu, J.; Yan, Y. Production and catalytic transformation of levulinic acid: A platform for speciality chemicals and fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Martínez, J.J.; Silva, L.; Rojas, H.A.; Romanelli, G.P.; Santos, L.A.; Ramalho, T.C.; Brijaldo, M.H.; Passos, F.B. Reductive amination of levulinic acid to different pyrrolidones on Ir/SiO2-SO3H: Elucidation of reaction mechanism. Catal. Today 2017, 296, 118–126. [Google Scholar] [CrossRef]
- He, D.; Horváth, I.T. Application of silica-supported Shvo’s catalysts for transfer hydrogenation of levulinic acid with formic acid. J. Organomet. Chem. 2017, 847, 263–269. [Google Scholar] [CrossRef]
- Wu, G.; Guan, N.; Di, L.; Cao, J.; Song, S.; Yao, S.; Li, L. Heterostructured Ni/NiO composite as a robust catalyst for the hydrogenation of levulinic acid to γ-valerolactone. Appl. Catal. B Environ. 2017, 217, 115–124. [Google Scholar]
- Kang, S.; Yu, J. Effect of Methanol on Formation of Levulinates from Cellulosic Biomass. Ind. Eng. Chem. Res. 2015, 54, 11552–11559. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Gupta, K.; Tyagi, D.; Rai, R.K.; Mobin, S.M.; Singh, S.K. Ruthenium and Formic Acid Based Tandem Catalytic Transformation of Bioderived Furans to Levulinic Acid and Diketones in Water. ChemCatChem 2015, 7, 4050–4058. [Google Scholar] [CrossRef]
- Yan, Z.; Lin, L.; Liu, S.; March, R.V.; Re, V.; Recei, M.; June, V. Synthesis of γ-Valerolactone by Hydrogenation of Biomass-derived Levulinic Acid over Ru/C Catalyst. Energy Fuels 2009, 48, 3853–3858. [Google Scholar] [CrossRef]
- Windom, B.C.; Lovestead, T.M.; Mascal, M.; Nikitin, E.B.; Bruno, T.J. Advanced distillation curve analysis on ethyl levulinate as a diesel fuel oxygenate and a hybrid biodiesel fuel. Energy Fuels 2011, 25, 1878–1890. [Google Scholar] [CrossRef]
- Girisuta, B. Levulinic Acid from Lignocellulosic Biomass; University of Groningen: Groningen, Holland, 2007; ISBN 1226-086X. [Google Scholar]
- Chen, S.S.; Maneerung, T.; Tsang, D.C.W.; Ok, Y.S.; Wang, C.H. Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem. Eng. J. 2017, 328, 246–273. [Google Scholar] [CrossRef]
- Hayes, D.J.; Fitzpatrick, S.; Hayes, M.H.B.; Ross, J.R.H. The Biofine Process–Production of Levulinic Acid, Furfural, and Formic Acid from Lignocellulosic Feedstocks, in Biorefineries-Industrial Processes and Products: Status Quo and Future Directions. Biorefineries Ind. Process. Prod. 2006, 1, 139–164. [Google Scholar]
- Galia, A.; Schiavo, B.; Antonetti, C.; Galletti, A.M.R.; Interrante, L.; Lessi, M.; Scialdone, O.; Valenti, M.G. Autohydrolysis pretreatment of Arundo donax: A comparison between microwave-assisted batch and fast heating rate flow-through reaction systems. Biotechnol. Biofuels 2015, 8, 1–18. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Rackemann, B.D.; Doherty, W. A Review on the Production of Levulinic Acid and Furanics from Sugars. Int. Sugar J. 2013, 115, 28–34. [Google Scholar]
- Jadhav, H.; Pedersen, C.M.; Sølling, T.; Bols, M. 3-deoxy-glucosone is an intermediate in the formation of furfurals from D-glucose. ChemSusChem 2011, 4, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pidko, E.A.; Hensen, E.J.M. Molecular aspects of glucose dehydration by chromium chlorides in ionic liquids. Chem. A Eur. J. 2011, 17, 5281–5288. [Google Scholar] [CrossRef] [PubMed]
- Roy Goswami, S.; Dumont, M.J.; Raghavan, V. Microwave Assisted Synthesis of 5-Hydroxymethylfurfural from Starch in AlCl3·6H2O/DMSO/ [BMIM] Cl System. Ind. Eng. Chem. Res. 2016, 55, 4473–4481. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.J. Levulinic Acid Production from Starch Using Microwave and Oil Bath Heating: A Kinetic Modeling Approach. Ind. Eng. Chem. Res. 2016, 55, 8941–8949. [Google Scholar] [CrossRef]
- Sweygers, N.; Alewaters, N.; Dewil, R.; Appels, L. Microwave effects in the dilute acid hydrolysis of cellulose to 5-hydroxymethylfurfural. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Fang, Q.; Hanna, M.A. Experimental studies for levulinic acid production from whole kernel grain sorghum. Bioresour. Technol. 2002, 81, 187–192. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhou, N.; Liu, R.; Peng, Z.; Xu, Y. Concentrated Levulinic Acid Production from Sugar Cane Molasses. Energy Fuels 2018, 32, 3526–3531. [Google Scholar] [CrossRef]
- Kang, S.; Yu, J. Maintenance of a Highly Active Solid Acid Catalyst in Sugar Beet Molasses for Levulinic Acid Production. Sugar Tech 2018, 20, 182–193. [Google Scholar] [CrossRef]
- He, Y.; Hoff, T.C.; Emdadi, L.; Wu, Y.; Bouraima, J.; Liu, D. Catalytic consequences of micropore topology, mesoporosity, and acidity on the hydrolysis of sucrose over zeolite catalysts. Catal. Sci. Technol. 2014, 4, 3064–3073. [Google Scholar] [CrossRef]
- Zhou, L.; Shi, M.; Cai, Q.; Wu, L.; Hu, X.; Yang, X.; Chen, C.; Xu, J. Hydrolysis of hemicellulose catalyzed by hierarchical H-USY zeolites—The role of acidity and pore structure. Microporous Mesoporous Mater. 2013, 169, 54–59. [Google Scholar] [CrossRef]
- Pang, Q.; Wang, L.; Yang, H.; Jia, L.; Pan, X.; Qiu, C. Cellulose-derived carbon bearing-Cl and-SO3H groups as a highly selective catalyst for the hydrolysis of cellulose to glucose. RSC Adv. 2014, 40, 41212–41218. [Google Scholar] [CrossRef]
- Murphy, R.; Woods, J.; Black, M.; McManus, M. Global developments in the competition for land from biofuels. Food Policy 2011, 36, S52–S61. [Google Scholar] [CrossRef]
- Van Eijck, J.; Romijn, H. Prospects for Jatropha biofuels in Tanzania: An analysis with Strategic Niche Management. Energy Policy 2008, 36, 311–325. [Google Scholar] [CrossRef]
- Jefferies, D.; Muñoz, I.; Hodges, J.; King, V.J.; Aldaya, M.; Ercin, A.E.; Milà, I.; Canals, L.; Hoekstra, A.Y. Water footprint and life cycle assessment as approaches to assess potential impacts of products on water consumption. Key learning points from pilot studies on tea and margarine. J. Clean. Prod. 2012, 33, 155–166. [Google Scholar] [CrossRef]
- Van der Horst, D.; Vermeylen, S. Spatial scale and social impacts of biofuel production. Biomass Bioenergy 2011, 35, 2435–2443. [Google Scholar] [CrossRef]
- Bohre, A.; Saha, B.; Abu-Omar, M.M. Catalytic Upgrading of 5-Hydroxymethylfurfural to Drop-in Biofuels by Solid Base and Bifunctional Metal-Acid Catalysts. ChemSusChem 2015, 8, 4022–4029. [Google Scholar] [CrossRef]
- Morales, M.; Quintero, J.; Conejeros, R.; Aroca, G. Life cycle assessment of lignocellulosic bioethanol: Environmental impacts and energy balance. Renew. Sustain. Energy Rev. 2015, 42, 1349–1361. [Google Scholar] [CrossRef]
- Lange, J.P.; Van Der Heide, E.; Van Buijtenen, J.; Price, R. Furfural—A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Kang, S.; Yu, J. An intensified reaction technology for high levulinic acid concentration from lignocellulosic biomass. Biomass Bioenergy 2016, 95, 214–220. [Google Scholar] [CrossRef]
- Cozier, M. Business highlights: Collaboration: Bigger and beta. Biofuels Bioprod. Biorefining 2014, 8, 743. [Google Scholar]
- Zheng, Y.; Pan, Z.; Zhang, R.; Wang, D. Enzymatic saccharification of dilute acid pretreated saline crops for fermentable sugar production. Appl. Energy 2009, 86, 2459–2465. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Hou, W.; Shen, H. Direct conversion of lignocellulose to levulinic acid catalyzed by ionic liquid. Carbohydr. Polym. 2018, 181, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, B.; Jin, S. Production of levulinic acid from steam exploded rice straw via solid superacid, S2O82-/ZrO2-SiO2-Sm2O3. Bioresour. Technol. 2011, 102, 3568–3570. [Google Scholar] [CrossRef]
- Ma, L.T.; Zhao, Z.M.; Yu, B.; Chen, H.Z. Enzymatic Pretreatment Coupled with the Addition of p-Hydroxyanisole Increased Levulinic Acid Production from Steam-Exploded Rice Straw Short Fiber. Appl. Biochem. Biotechnol. 2016, 180, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.; Pulidindi, I.N.; Mishra, R.K.; Gedanken, A. Development of Ga Salt of Molybdophosphoric Acid for Biomass Conversion to Levulinic Acid. Energy Fuels 2016, 30, 10583–10591. [Google Scholar] [CrossRef]
- Elumalai, S.; Agarwal, B.; Sangwan, R.S. Thermo-chemical pretreatment of rice straw for further processing for levulinic acid production. Bioresour. Technol. 2016, 218, 232–246. [Google Scholar] [CrossRef]
- Kumar, S.; Ahluwalia, V.; Kundu, P.; Sangwan, R.S.; Kansal, S.K.; Runge, T.M.; Elumalai, S. Improved levulinic acid production from agri-residue biomass in biphasic solvent system through synergistic catalytic effect of acid and products. Bioresour. Technol. 2018, 251, 143–150. [Google Scholar] [CrossRef]
- Yan, L.; Yang, N.; Pang, H.; Liao, B. Production of levulinic acid from bagasse and paddy straw by liquefaction in the presence of hydrochloride acid. Clean Soil Air Water 2008, 36, 158–163. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, H.; Guo, Y.; Zhuang, G.; Bai, Z.; Zhang, H.; Feng, C.; Dong, Y. Dilute-acid conversion of cotton straw to sugars and levulinic acid via 2-stage hydrolysis. Ind. Crops Prod. 2013, 46, 205–209. [Google Scholar] [CrossRef]
- Won, K.Y.; Um, B.H.; Kim, S.W.; Oh, K.K. Fractionation of barley straw with dilute sulfuric acid for improving hemicellulose recovery. Korean J. Chem. Eng. 2012, 29, 614–620. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Shah, F.U.; Muhammad, N. Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr. Polym. 2018, 181, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Rodriguez, M.E.; York, S.W.; Preston, J.F.; Ingram, L.O. Use of UV absorbance to monitor furans in dilute acid hydrolysates of biomass. Biotechnol. Prog. 2000, 16, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; González-Muñoz, M.J.; Vila, C.; Santos, V.; Parajó, J.C. Manufacture of levulinic acid from pine wood hemicelluloses: A kinetic assessment. Ind. Eng. Chem. Res. 2013, 52, 3951–3957. [Google Scholar] [CrossRef]
- Runge, T.; Zhang, C. Two-stage acid-catalyzed conversion of carbohydrates into levulinic acid. Ind. Eng. Chem. Res. 2012, 51, 3265–3270. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sharypov, V.I.; Grishechko, L.I.; Celzard, A. Integrated catalytic process for obtaining liquid fuels from renewable lignocellulosic biomass. Kinet. Catal. 2013, 54, 344–352. [Google Scholar] [CrossRef]
- Zhi, Z.; Li, N.; Qiao, Y.; Zheng, X.; Wang, H.; Lu, X. Kinetic study of levulinic acid production from corn stalk at relatively high temperature using FeCl3 as catalyst: A simplified model evaluated. Ind. Crops Prod. 2015, 76, 672–680. [Google Scholar] [CrossRef]
- Alipour, S.; Omidvarborna, H. Enzymatic and catalytic hybrid method for levulinic acid synthesis from biomass sugars. J. Clean. Prod. 2017, 143, 490–496. [Google Scholar] [CrossRef]
- Liang, C.; Hu, Y.; Wang, Y.; Wu, L.; Zhang, W. Production of levulinic acid from corn cob residue in a fed-batch acid hydrolysis process. Process Biochem. 2018, 73, 124–131. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, C.; Li, J.; Xu, S.; Hu, C. Synergistic Effect of Different Species in Stannic Chloride Solution on the Production of Levulinic Acid from Biomass. ACS Sustain. Chem. Eng. 2019, 7, 5176–5183. [Google Scholar] [CrossRef]
- Hartono, C.D.; Marlie, K.J.; Putro, J.N.; Soetardjo, F.E.; Ju, Y.H.; Sirodj, D.A.N.; Ismadji, S. Levulinic acid from corncob by subcritical water process. Int. J. Ind. Chem. 2016, 7, 401–409. [Google Scholar] [CrossRef]
- Girisuta, B.; Dussan, K.; Haverty, D.; Leahy, J.J.; Hayes, M.H.B. A kinetic study of acid catalysed hydrolysis of sugar cane bagasse to levulinic acid. Chem. Eng. J. 2013, 217, 61–70. [Google Scholar] [CrossRef]
- Lopes, E.S.; Dominices, K.M.C.; Lopes, M.S.; Tovar, L.P.; Filho, R.M. A green chemical production: Obtaining levulinic acid from pretreated sugarcane bagasse. Chem. Eng. Trans. 2017, 57, 145–150. [Google Scholar]
- Jeong, H.; Jang, S.K.; Hong, C.Y.; Kim, S.H.; Lee, S.Y.; Lee, S.M.; Choi, J.W.; Choi, I.G. Levulinic acid production by two-step acid-catalyzed treatment of Quercus mongolica using dilute sulfuric acid. Bioresour. Technol. 2017, 225, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Park, S.Y.; Ryu, G.H.; Choi, J.H.; Kim, J.H.; Choi, W.S.; Lee, S.M.; Choi, J.W.; Choi, I.G. Catalytic conversion of hemicellulosic sugars derived from biomass to levulinic acid. Catal. Commun. 2018, 117, 19–25. [Google Scholar] [CrossRef]
- Ya’aini, N.; Amin, N.A.S.; Asmadi, M. Optimization of levulinic acid from lignocellulosic biomass using a new hybrid catalyst. Bioresour. Technol. 2012, 116, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.A.S.; Amin, N.A.S. Optimization of Biomass Conversion to Levulinic Acid in Acidic Ionic Liquid and Upgrading of Levulinic Acid to Ethyl Levulinate. Bioenergy Res. 2017, 10, 50–63. [Google Scholar] [CrossRef]
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. One-pot conversion of oil palm empty fruit bunch and mesocarp fiber biomass to levulinic acid and upgrading to ethyl levulinate via indium trichloride-ionic liquids. J. Clean. Prod. 2017, 168, 1251–1261. [Google Scholar] [CrossRef]
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. Optimisation studies on the conversion of oil palm biomass to levulinic acid and ethyl levulinate via indium trichloride-ionic liquids: A response surface methodology approach. Ind. Crops Prod. 2019, 128, 221–234. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; Antonetti, C.; Ribechini, E.; Colombini, M.P.; Nassi o Di Nasso, N.; Bonari, E. From giant reed to levulinic acid and gamma-valerolactone: A high yield catalytic route to valeric biofuels. Appl. Energy 2013, 102, 157–162. [Google Scholar] [CrossRef]
- Antonetti, C.; Bonari, E.; Licursi, D.; Di Nasso, N.N.; Galletti, A.M.R.; Cravotto, G.; Chemat, F. Hydrothermal conversion of giant reed to furfural and levulinic acid: Optimization of the process under microwave irradiation and investigation of distinctive agronomic parameters. Molecules 2015, 20, 21232–21353. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lappalainen, K.; Markkola, A.; Rusanen, A.; Wäli, P.; Vogeler, N.; Ruotsalainen, A.L.; Kärkkäinen, J.; Lassi, U.; Niemelä, M. Microwave-assisted conversion of novel biomass materials into levulinic acid. Biomass Convers. Biorefinery 2018, 8, 965–970. [Google Scholar]
- Zhou, C.; Yu, X.; Ma, H.; He, R.; Vittayapadung, S. Optimization on the conversion of bamboo shoot shell to levulinic acid with environmentally benign acidic ionic liquid and response surface analysis. Chin. J. Chem. Eng. 2013, 21, 544–550. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Martinez-Jimenez, F.; Rezende, C.A.; Tompsett, G.; Timko, M.; Forster-Carneiro, T. Subcritical water hydrolysis of sugarcane bagasse: An approach on solid residues characterization. J. Supercrit. Fluids 2016, 108, 69–78. [Google Scholar] [CrossRef]
- Pagán-Torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-hydroxymethylfurfural from glucose using a combination of lewis and brønsted acid catalysts in water in a biphasic reactor with an alkylphenol solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Chidambaram, M.; Bell, A.T. A two-step approach for the catalytic conversion of glucose to 2, 5-dimethylfuran in ionic liquids. Green Chem. 2010, 12, 1253–1262. [Google Scholar] [CrossRef]
- Saha, B.; Abu-Omar, M.M. Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents. Green Chem. 2014, 16, 24–38. [Google Scholar] [CrossRef]
- Farrán, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernáiz, M.J.; Linhardt, R.J. Green Solvents in Carbohydrate Chemistry: From Raw Materials to Fine Chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D.J. Microwave Assisted Acid Hydrolysis of Brown Seaweed Ascophyllum nodosum for Bioethanol Production and Characterization of Alga Residue. ACS Sustain. Chem. Eng. 2015, 3, 1359–1365. [Google Scholar] [CrossRef]
- Guo, X.; Gu, D.; Wang, M.; Huang, Y.; Li, H.; Dong, Y.; Tian, J.; Wang, Y.; Yang, Y. Characterization of active compounds from: Gracilaria lemaneiformis inhibiting the protein tyrosine phosphatase 1B activity. Food Funct. 2017, 8, 3271–3275. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Murphy, J.D. Inhibition of thermochemical treatment on biological hydrogen and methane co-production from algae-derived glucose/glycine. Energy Convers. Manag. 2018, 158, 201–209. [Google Scholar] [CrossRef]
- Kwon, O.M.; Kim, D.H.; Kim, S.K.; Jeong, G.T. Production of sugars from macro-algae Gracilaria verrucosa using combined process of citric acid-catalyzed pretreatment and enzymatic hydrolysis. Algal Res. 2016, 13, 293–297. [Google Scholar] [CrossRef]
- Wyman, C.E. Ethanol from lignocellulosic biomass: Technology, economics, and opportunities. Bioresour. Technol. 1994, 50, 3–15. [Google Scholar] [CrossRef]
- Jambo, S.A.; Abdulla, R.; Mohd Azhar, S.H.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A review on third generation bioethanol feedstock. Renew. Sustain. Energy Rev. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Sweygers, N.; Somers, M.H.; Appels, L. Optimization of hydrothermal conversion of bamboo (Phyllostachys aureosulcata) to levulinic acid via response surface methodology. J. Environ. Manag. 2018, 219, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yu, I.K.M.; Cho, D.W.; Wang, D.; Tsang, D.C.W.; Zhang, S.; Ding, S.; Wang, L.; Ok, Y.S. Microwave-assisted low-temperature hydrothermal treatment of red seaweed (Gracilaria lemaneiformis) for production of levulinic acid and algae hydrochar. Bioresour. Technol. 2019, 273, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Nunraksa, N.; Rattanasansri, S.; Praiboon, J.; Chirapart, A. Proximate composition and the production of fermentable sugars, levulinic acid, and HMF from Gracilaria fisheri and Gracilaria tenuistipitata cultivated in earthen ponds. J. Appl. Phycol. 2019, 31, 683–690. [Google Scholar] [CrossRef]
- Jeong, G.T.; Ra, C.H.; Hong, Y.K.; Kim, J.K.; Kong, I.S.; Kim, S.K.; Park, D.H. Conversion of red-algae Gracilaria verrucosa to sugars, levulinic acid and 5-hydroxymethylfurfural. Bioprocess Biosyst. Eng. 2015, 38, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Park, M.R.; Kim, S.K.; Jeong, G.T. Optimization of the levulinic acid production from the red macroalga, Gracilaria verrucosa using methanesulfonic acid. Algal Res. 2018, 31, 116–121. [Google Scholar] [CrossRef]
- Jeong, G.T.; Park, D.H. Production of Sugars and Levulinic Acid from Marine Biomass Gelidium amansii. Appl. Biochem. Biotechnol. 2010, 161, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, S.W.; Kim, J.W.; Kim, T.H.; Kim, J.S. Optimization of levulinic acid production from Gelidium amansii. Renew. Energy 2013, 54, 173–179. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.B.; Kim, S.K.; Park, D.H.; Jeong, G.T. Optimization and Evaluation of Sugars and Chemicals Production from Green Macro-algae Enteromorpha intestinalis. Bioenergy Res. 2016, 9, 1155–1166. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Yang, J.; Qi, X. Catalytic production of levulinic acid and ethyl levulinate from uniconazole-induced duckweed (Lemna minor). Bioresour. Technol. 2018, 255, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Kuttiraja, M.; Binod, P.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Dilute acid pretreatment and enzymatic saccharification of sugarcane tops for bioethanol production. Bioresour. Technol. 2011, 102, 10915–10921. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.B.; Raines, R.T. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K.R. Assessing the greenness of some typical laboratory ionic liquid preparations. Green Chem. 2010, 12, 17–30. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Weingarten, R.; Conner, W.C.; Huber, G.W. Production of levulinic acid from cellulose by hydrothermal decomposition combined with aqueous phase dehydration with a solid acid catalyst. Energy Environ. Sci. 2012, 5, 7559–7574. [Google Scholar] [CrossRef]
| Biomass | Solvent | Homo Cat | Hetero Cat | Temperature (°C) | Time (min) | Other | Yield of LA | Ref |
|---|---|---|---|---|---|---|---|---|
| Normal corn starch | H2O | HCl | - | 165 | 15 | mw | 53–55% | [38] |
| High-amylose corn starch | H2O | HCl | - | 165 | 15 | mw | 53–55% | [38] |
| Waxy corn starch | H2O | HCl | - | 165 | 15 | mw | 53–55% | [38] |
| kernel sorghum grain | H2O | H2SO4 | - | 200 | 40 | 33% | [40] | |
| Sugar cane molasses | H2O + LA | H2SO4 | - | 180 | 180 | 30% | [41] | |
| Sugar beet molasses | - | - | Amberlyst-36TM | 140 | 180 | PRET | 78 mol% | [42] |
| Biomass | Solvent | Ionic Liquid | Homo Cat | Hetero Cat | T (°C) | Time (min) | Other | Yield of LA | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Rice straw | H2O | [C3SO3Hmim]HSO4 | - | - | 180 | 30 | 21% | [56] | |
| Rice straw | H2O | - | S2O82−/ZrO2–SiO2–Sm2O3 | 200 | 10 | 22% | [57] | ||
| Rice straw | H2O | - | S2O82−/ZrO2–SiO2–Sm2O3 | 200 | 15 | ENZ PRET | 25% | [58] | |
| Rice straw | H2O | - | GaHPMo | 175 | 360 | 46% | [59] | ||
| Rice straw | H2O + THF + DMSO | HCl | - | 180 | 120 | PRET H2SO4 | 21% | [60] | |
| Rice straw | H2O + DCM | HCl, FA, LA | - | 200 | 180 | 16.6% | [61] | ||
| Paddy Straw | H2O | HCl | - | 220 | 45 | 24% | [62] | ||
| Cotton straw | H2O | H2SO4 | - | 180 | 60 | 9.5% | [63] | ||
| Barley straw | H2O | H2SO4 | - | 158 | 15 | 0.03 g/L | [64] | ||
| Bamboo | H2O | [C4(Mim)2][(2HSO4)(H2SO4)4] | - | - | 100 | 60 | 47.5% | [65] | |
| Eucalyptus wood chips | H2O + MeOH | H2SO4 | - | 180 | 90 | 66 mol% | [25] | ||
| Eucalyptus wood | H2O | H2SO4 | - | 170 | 300 | PRET | 105 g/L | [53] | |
| Pinus pinaster wood | H2O | H2SO4 | - | 135 | 600 | 66% | [67] | ||
| Poplar wood chips | H2O | H2SO4 | - | 190 | 50 | 17.8% | [68] | ||
| Aspen, pine, fir, birch wood | H2O | H2SO4 | - | 220 | 120 | 24% | [69] | ||
| Corn stalk | H2O | FeCl3 | - | 230 | 10 | 48.7% | [70] | ||
| Corn Stover | H2O | [BMIMSO3H] HSO4 | - | - | 95 | 60 | 70% | [71] | |
| Corncob residues | H2O | H2SO4 | - | 180 | 50 | 7StageH | 107.9 g/L | [72] | |
| Corncob residues | H2O | SnCl4 | - | 180 | 60 | 64.6 mol% | [73] | ||
| Corncob | H2O | - | Acid modified zeolite | 200 | 60 | 52.48ppm | [74] | ||
| Sugarcane bagasse | H2O | H2SO4 | - | 150 | 360 | 63 mol% | [75] | ||
| Sugarcane bagasse | H2O | H2SO4 | - | 170 | 75 | PRET H2SO4 NaOH | 55.00 ± 0.36% | [76] | |
| Quercus mongolica | H2O | H2SO4 | - | 200 | 10 | PRET H2SO4 | 16.5% (g/100 g biomass) | [77] | |
| Quercus mongolica | H2O | - | modified zeolite Y | 190 | 180 | PRET H2SO4 | 4.6% | [78] | |
| Empty fruit bunch | H2O | - | hybrid of HY zeolite and CrCl3 | 145 | 146 | 53% | [79] | ||
| Kenaf | H2O | - | hybrid of HY zeolite and CrCl3 | 145.2 | 146.7 | 66% | [79] | ||
| Oil palm fronds | H2O | [SMIM][FeCl4] | - | - | 154.5 | 222 | 25% | [80] | |
| Oil palm empty fruit bunch | H2O | InCl3−[HMIM][HSO4] | - | - | 160 | 300 | 12% | [81] | |
| Mesocarp fiber | H2O | InCl3−[HMIM][HSO4] | - | - | 160 | 300 | 13% | [81] | |
| Oil palm empty fruit bunch | H2O | InCl3−[HMIM][HSO4] | - | - | 177 | 288 | 17.7% | [82] | |
| Mesocarp fiber | H2O | InCl3−[HMIM][HSO4] | - | - | 177 | 288 | 18.4% | [82] | |
| Giant reed | H2O | HCl | - | 180 | 60 | PRET | 23% | [83] | |
| Giant reed | H2O | HCl | - | 180 | 20 | mw | 21% | [84] | |
| Carbohydrate-rich potato peel waste | H2O | H2SO4 | CrCl3 orAlCl3 | 180 | 15 | mw | 49% | [85] | |
| Fungus Cortinarius armillatus | H2O | H2SO4 | CrCl3 orAlCl3 | 180 | 40 | mw | 62% | [85] | |
| Bamboo shoot shell | H2O | [C4mim] HSO4 | - | - | 145 | 104 | 71 mol% | [86] |
| Biomass | Solvent | Homogeneous Catalyst | T (°C) | Time (min) | Other | Yield of LA | Ref |
|---|---|---|---|---|---|---|---|
| Gracilaria lemaneiformis | H2O | H2SO4 | 180 | 20 | mw | 16.3% | [99] |
| Gracilaria fisheri | H2O | H2SO4 | 95 | 150 | 3.66 g L−1 | [100] | |
| Gracilaria. tenuistipitata | H2O | H2SO4 | 95 | 150 | 6.12 g L−1 | [100] | |
| Gracilaria verrucosa | H2O | H2SO4 | 180.9 | 50 | 19% | [101] | |
| Gracilaria verrucosa | H2O | MSA (methanesulfonic acid) | 180 | 20 | 22% | [102] | |
| Gelidium amansii | H2O | H2SO4 | 160 | 43.1 | 9.7 g/L | [103] | |
| Gelidium amansii | H2O | H2SO4 | 180 | 48 | PRET | 43% | [104] |
| Enteromorpha intestinalis | H2O | H2SO4 | 175 | 35 | 4% | [105] | |
| Lemna minor | H2O | HCl | 180 | 150 | 262 g/kg | [106] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signoretto, M.; Taghavi, S.; Ghedini, E.; Menegazzo, F. Catalytic Production of Levulinic Acid (LA) from Actual Biomass. Molecules 2019, 24, 2760. https://doi.org/10.3390/molecules24152760
Signoretto M, Taghavi S, Ghedini E, Menegazzo F. Catalytic Production of Levulinic Acid (LA) from Actual Biomass. Molecules. 2019; 24(15):2760. https://doi.org/10.3390/molecules24152760
Chicago/Turabian StyleSignoretto, Michela, Somayeh Taghavi, Elena Ghedini, and Federica Menegazzo. 2019. "Catalytic Production of Levulinic Acid (LA) from Actual Biomass" Molecules 24, no. 15: 2760. https://doi.org/10.3390/molecules24152760
APA StyleSignoretto, M., Taghavi, S., Ghedini, E., & Menegazzo, F. (2019). Catalytic Production of Levulinic Acid (LA) from Actual Biomass. Molecules, 24(15), 2760. https://doi.org/10.3390/molecules24152760