Ursolic Acid and Its Derivatives as Bioactive Agents
Abstract
1. Introduction
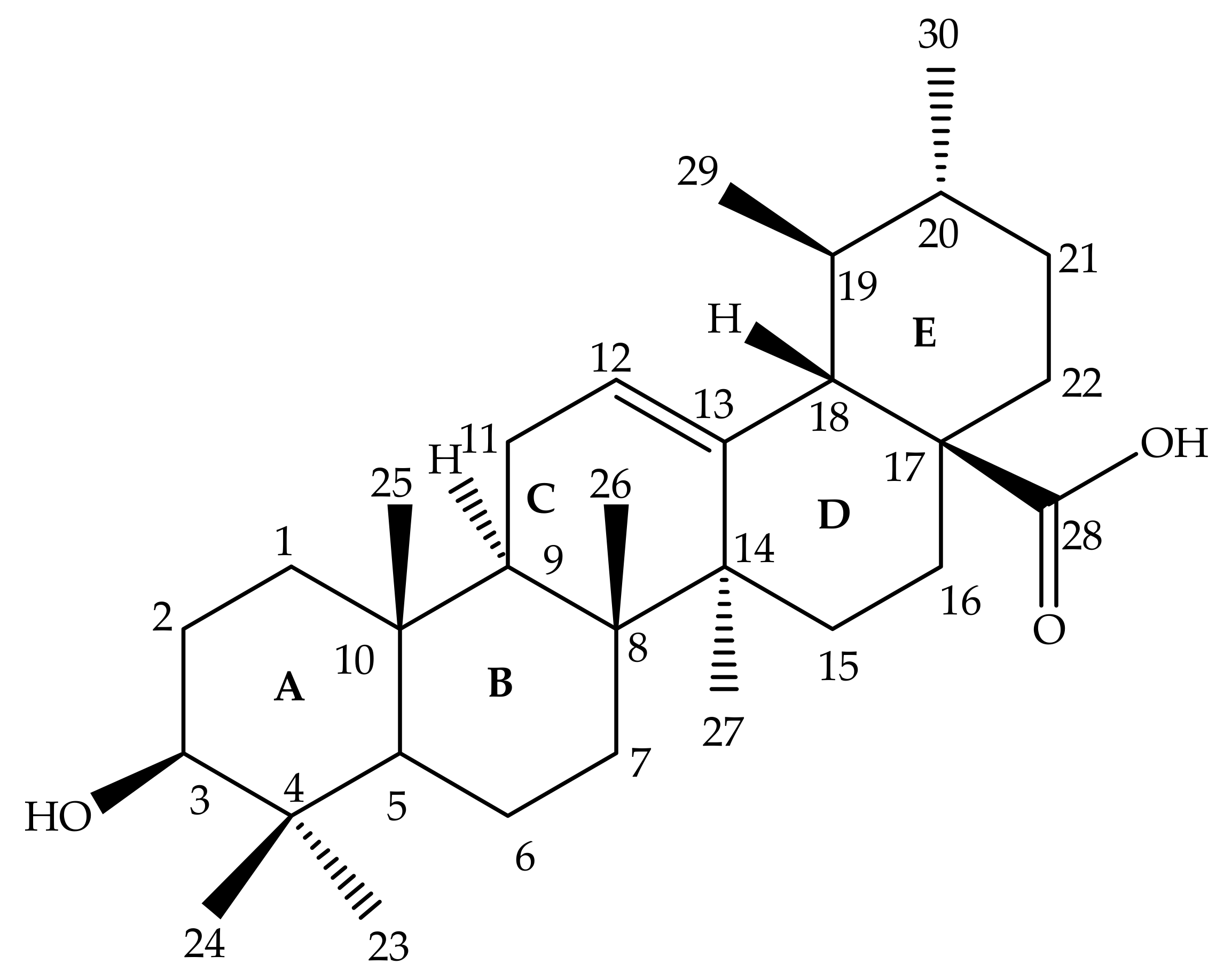
2. Chemistry of UA
3. Sources of UA and Its Biological Potency
4. Biological Effects and Clinical Trials of UA and Some Derivatives
4.1. Anti-Inflammatory
4.2. Anticancer Activity
4.3. Antibacterial
4.4. Anti-Diabetes
4.5. Neuroprotective Activity
4.6. Herbicidal Activity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Global Health Observation (GHO). Is WHO’s Portal Providing Access to Data and Analyses for Monitoring the Global Health Situation. Available online: https://www.who.int/gho/ncd/en/ (accessed on 28 March 2018).
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid.-Based Complementary Altern. Med. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as Potentially Cytotoxic Compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Thoppil, R.J.; Bishayee, A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228–249. [Google Scholar] [CrossRef]
- Sandjo, L.P.; Kuete, V. Triterpenes and Steroids from the Medicinal Plants of Africa; Elsevier: Amsterdam, The Netherlands, 2013; pp. 135–202. [Google Scholar]
- Babalola, I.T.; Shode, F.O. Ubiquitous ursolic acid: A potential pentacyclic triterpene natural product. J. Pharmacogn. Phytochem. 2013, 2, 214–222. [Google Scholar]
- Wang, C.-M.; Chen, H.-T.; Wu, Z.-Y.; Jhan, Y.-L.; Shyu, C.-L.; Chou, C.-H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules 2016, 21, 139. [Google Scholar] [CrossRef]
- Phillipson, J. Phytochemistry and medicinal plants. Phytochemistry 2001, 56, 237–243. [Google Scholar] [CrossRef]
- Mazumder, K.; Tanaka, K.; Fukase, K. Cytotoxic Activity of Ursolic Acid Derivatives Obtained by Isolation and Oxidative Derivatization. Molecules 2013, 18, 8929–8944. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liao, Y.; Fang, C.; Tsunoda, M.; Zhang, Y.; Song, Y.; Deng, S. Simultaneous analysis of ursolic acid and oleanolic acid in guava leaves using QuEChERS-based extraction followed by high-performance liquid chromatography. J. Anal. Methods Chem. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Meng, Y.; Song, Y.; Yan, Z.; Xia, Y. Synthesis and in vitro Cytotoxicity of Novel Ursolic Acid Derivatives. Molecules 2010, 15, 4033–4040. [Google Scholar] [CrossRef]
- Kwon, T.H.; Lee, B.M.; Chung, S.H.; Kim, D.H.; Lee, Y.S. Synthesis and NO production inhibitory activities of ursolic acid and oleanolic acid derivatives. Bull. Korean Chem. Soc. 2009, 30, 119–123. [Google Scholar] [CrossRef]
- Basir, D.; Julinar, J.; Agustriana, E.; Untari, B. Oxidation and Acetylation of Ursolic and Oleanolic Acids Isolated from Fragraea fragrans fruits; Antiproliferation of P388 Leukemia Cells. Indones. J. Chem. 2014, 14, 269–276. [Google Scholar] [CrossRef]
- Wang, Y.; He, Z.; Deng, S. Ursolic acid reduces the metalloprotease/anti-metalloprotease imbalance in cerebral ischemia and reperfusion injury. Drug Des. Devel. Ther. 2016, 10, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Ursolic acid and other pentacyclic triterpenoids: Anticancer activities and occurrence in berries. In Berries and Cancer Prevention; Seeram, N., Stoner, G., Eds.; Springer: New York, NY, USA, 2011; pp. 41–49. [Google Scholar]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.M.; Aimar, M.L.; Demmel, G.I.; Criado, S.G.; Ruiz, G.M.; Cantero, J.J.; Rossi, L.I.; Velasco, M.I. Determination of volatile organic compounds of Tagetes argentina Cabrera (Asteraceae) using HS-SPME analysis. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 463–469. [Google Scholar]
- Stiti, N.; Hartmann, M.-A. Nonsterol Triterpenoids as Major Constituents of Olea europaea. J. Lipids 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rali, S.; Oyedeji, O.O.; Aremu, O.O.; Oyedeji, A.O.; Nkeh-Chungag, B.N. Semisynthesis of derivatives of oleanolic acid from Syzygium aromaticum and their antinociceptive and anti-inflammatory properties. Mediators Inflamm. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Garcıa-Granados, A.; López, P.E.; Melguizo, E.; Parra, A.; Simeo, Y. Partial synthesis of C-ring derivatives from oleanolic and maslinic acids. Formation of several triene systems by chemical and photochemical isomerization processes. Tetrahedron 2004, 60, 1491–1503. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Vanga, N.R.; Jeengar, M.K.; Naidu, V.G.M. Synthesis of novel ring-A fused hybrids of oleanolic acid with capabilities to arrest cell cycle and induce apoptosis in breast cancer cells. Eur. J. Med. Chem. 2014, 74, 398–404. [Google Scholar] [CrossRef]
- Yap, W.H.; Lim, Y.M. Mechanistic Perspectives of Maslinic Acid in Targeting Inflammation. Biochem. Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xu, H.; Ma, X.; Zhan, R.; Chen, W. Triterpenoid Saponin Biosynthetic Pathway Profiling and Candidate Gene Mining of the Ilex asprella Root Using RNA-Seq. Int. J. Mol. Sci. 2014, 15, 5970–5987. [Google Scholar] [CrossRef] [PubMed]
- Fadipe, V.O.; Mongalo, N.I.; Opoku, A.R.; Dikhoba, P.M.; Makhafola, T.J. Isolation of anti-mycobacterial compounds from Curtisia dentata (Burm. f.) CA Sm (Curtisiaceae). BMC Complement. Altern. Med. 2017, 17, 306. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2010, 285, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Kaur, S.; Kweon, M.-H.; Adhami, V.M.; Afaq, F.; Mukhtar, H. Lupeol, a fruit and vegetable based triterpene, induces apoptotic death of human pancreatic adenocarcinoma cells via inhibition of Ras signaling pathway. Carcinogenesis 2005, 26, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.B.; Sarachine, M.J. Biological activities of lupeol. Int. J. Biomed. Pharm. Sci. 2009, 3, 46–66. [Google Scholar]
- Saratha, V.; Pillai, S.I.; Subramanian, S. Isolation and characterization of lupeol, a triterpenoid from Calotropis gigantea latex. Int. J. Pharm. Sci. Rev. Res. 2011, 10, 54–57. [Google Scholar]
- Wal, A.; Srivastava, R.S.; Wal, P.; Rai, A.; Sharma, S. Lupeol as a magical drug. Pharm. Biol. Eval. 2015, 2, 142–151. [Google Scholar]
- Manjula, K.; Rajendran, K.; Eevera, T.; Kumaran, S. Quantitative Estimation of Lupeol and Stigmasterol in Costus Igneus by High-Performance Thin-Layer Chromatography. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 197–212. [Google Scholar] [CrossRef]
- Laghari, A.H.; Memon, S.; Nelofar, A.; Khan, K.M. Alhagi maurorum: A convenient source of lupeol. Ind. Crop. Prod. 2011, 34, 1141–1145. [Google Scholar] [CrossRef]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef]
- Innocente, A.M.; Silva, G.N.S.; Cruz, L.N.; Moraes, M.S.; Nakabashi, M.; Sonnet, P.; Gosmann, G.; Garcia, C.R.S.; Gnoatto, S.C.B. Synthesis and Antiplasmodial Activity of Betulinic Acid and Ursolic Acid Analogues. Molecules 2012, 17, 12003–12014. [Google Scholar] [CrossRef]
- Tadesse, G.; Reneela, P.; Dekebo, A. Isolation and characterization of natural products from Helinus mystachnus (Rhamnaceae). J. Chem. Pharm. Res. 2012, 4, 1756–1762. [Google Scholar]
- Dewir, Y.H.; Singh, N.; Mngomezulu, S.; Omar, A.M.K. Micropropagation and detection of important triterpenes in in vitro and field grown plants of Syzygium cordatum. J. Med. Plants Res. 2011, 5, 3078–3083. [Google Scholar]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2015, 32, 273–327. [Google Scholar] [CrossRef]
- Rambabu, P.; Ramana, K.V.; Ganapaty, S. Dammarane and ceanothane triterpenes from Zizyphus xylopyra. Int. J. Chem. Sci. 2010, 8, 1231–1239. [Google Scholar]
- Ruan, J.; Zheng, C.; Qu, L.; Liu, Y.; Han, L.; Yu, H.; Zhang, Y.; Wang, T. Plant Resources, 13C-NMR Spectral Characteristic and Pharmacological Activities of Dammarane-Type Triterpenoids. Molecules 2016, 21, 1047. [Google Scholar] [CrossRef]
- Ganapaty, S.; Thomas, P.S.; Ramana, K.V.; Karagianis, G.; Waterman, P.G. Dammarane and Ceanothane Triterpenes from Zizyphus glabrata. Zeitschrift für Naturforschung B 2006, 61, 87–92. [Google Scholar] [CrossRef][Green Version]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2013, 30, 1028–1065. [Google Scholar] [CrossRef]
- Melo, M.N.; Ingólfsson, H.I.; Marrink, S.J. Parameters for Martini sterols and hopanoids based on a virtual-site description. J. Chem. Phys. 2015, 143, 243152. [Google Scholar] [CrossRef]
- Belin, B.J.; Busset, N.; Giraud, E.; Molinaro, A.; Silipo, A.; Newman, D.K. Hopanoid lipids: From membranes to plant–bacteria interactions. Nat. Rev. Genet. 2018, 16, 304–315. [Google Scholar] [CrossRef]
- Simonin, P.; Tindall, B.; Rohmer, M. Structure elucidation and biosynthesis of 31-methylhopanoids from Acetobacter europaeus. Studies on a new series of bacterial triterpenoids. JBIC J. Boil. Inorg. Chem. 1994, 225, 765–771. [Google Scholar] [CrossRef]
- Saenz, J.P.; Grosser, D.; Bradley, A.S.; Lagny, T.J.; Lavrynenko, O.; Broda, M.; Simons, K. Hopanoids as functional analogues of cholesterol in bacterial membranes. Proc. Natl. Acad. Sci. USA 2015, 112, 11971–11976. [Google Scholar] [CrossRef]
- Kamboj, A.; Saluja, A.K. Isolation of stigmasterol and β-sitosterol from petroleum ether extract of aerial parts of Ageratum conyzoides (Asteraceae). Int. J. Pharm. Pharm. Sci. 2011, 3, 94–96. [Google Scholar]
- Nirmal, S.A.; Pal, S.C.; Mandal, S.C.; Patil, A.N. Analgesic and anti-inflammatory activity of β-sitosterol isolated from Nyctanthes arbortristis leaves. Inflammopharmacology 2012, 20, 219–224. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Isolation of Stigmasterol and β-Sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 2012, 1, 239–242. [Google Scholar] [CrossRef]
- Dighe, S.B.; Kuchekar, B.S.; Wankhede, S.B. Analgesic and anti-inflammatory activity of β-sitosterol isolated from leaves of Oxalis corniculata. Int. J. Pharmacol. Res. 2016, 6, 109–113. [Google Scholar]
- Pironi, A.M.; de Araújo, P.R.; Fernandes, M.A.; Salgado, H.R.N.; Chorilli, M. Characteristics, biological properties and analytical methods of ursolic acid: A review. Crit. Rev. Anal. Chem. 2018, 48, 86–93. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef]
- López-Hortas, L.; Pérez-Larrán, P.; González-Muñoz, M.J.; Falqué, E.; Domínguez, H. Recent developments on the extraction and application of ursolic acid. A review. Food Res. Int. 2018, 103, 130–149. [Google Scholar] [CrossRef]
- Navin, R.; Kim, S.M. Therapeutic interventions using ursolic acid for cancer treatment. Med. Chem. 2016, 6, 339–344. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef]
- Zacchigna, M.; Cateni, F.; Drioli, S.; Procida, G.; Altieri, T. PEG–Ursolic Acid Conjugate: Synthesis and In Vitro Release Studies. Sci. Pharm. 2014, 82, 411–421. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Ali, I.; Khan, I.A.; Ali, Z.; Al-Sadi, A.M.; Ahmed, I. Ursolic acid derivatives for pharmaceutical use: A patent review (2012–2016). Expert Opin. Ther. Pat. 2017, 27, 1–38. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef]
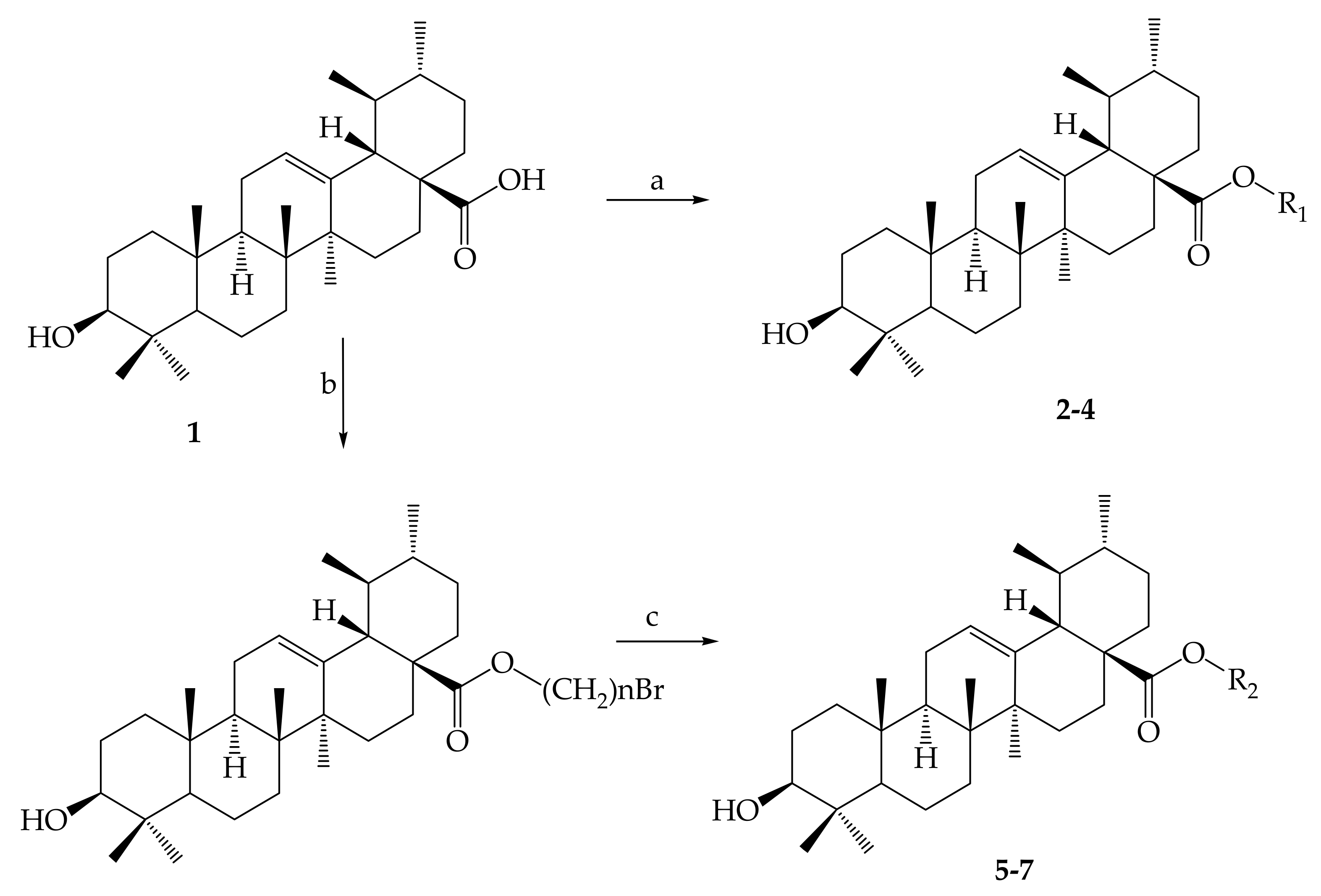
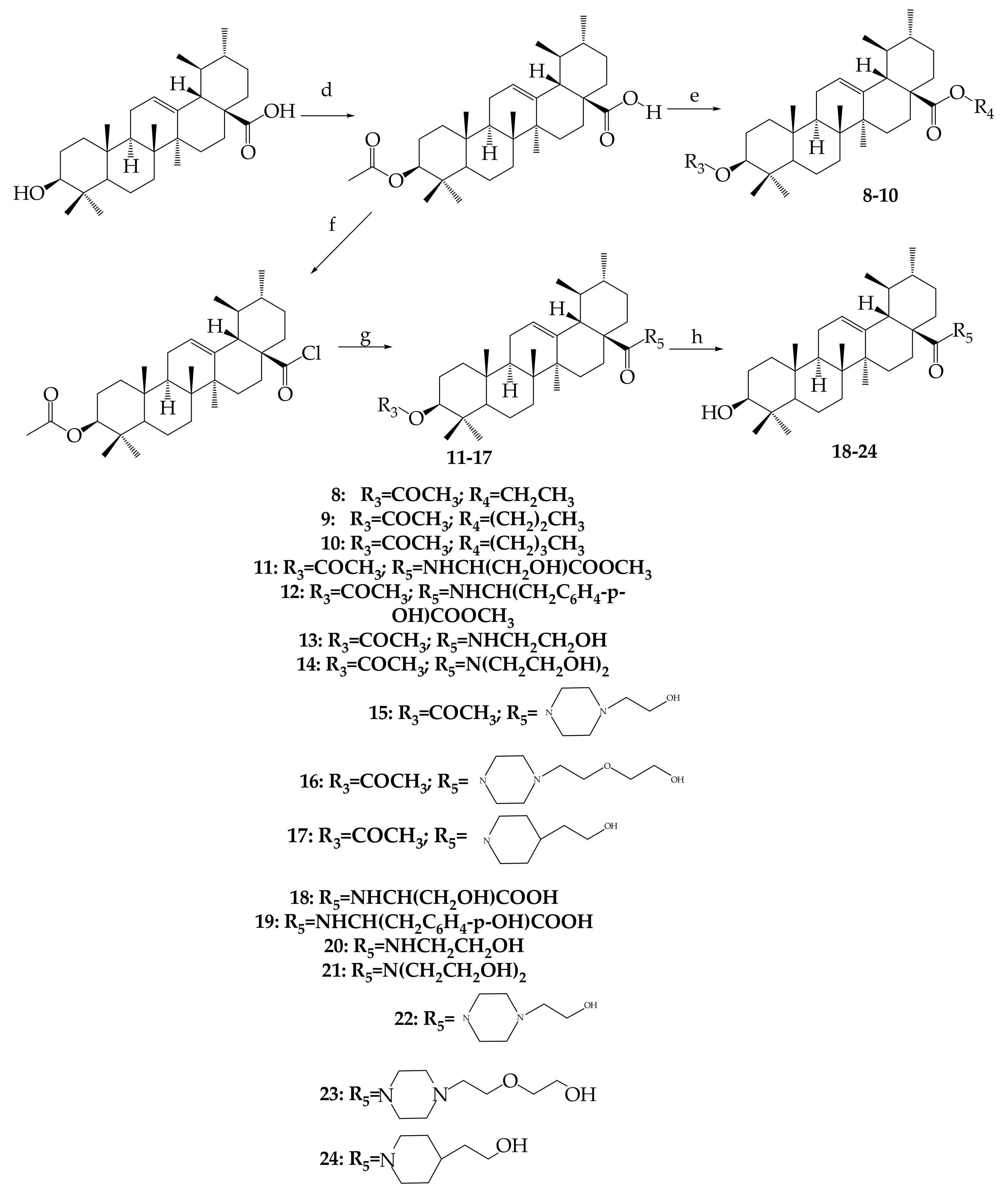
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in Medicinal Chemistry of Ursolic Acid Derivatives as Anticancer Agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef]
- Shao, J.W.; Dai, Y.C.; Xue, J.P.; Wang, J.C.; Lin, F.P.; Guo, Y.H. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef]
- Mendes, V.I.S.; Bartholomeusz, G.A.; Ayres, M.; Gandhi, V.; Salvador, J.A.R. Synthesis and cytotoxic activity of novel A-ring cleaved ursolic acid derivatives in human non-small cell lung cancer cells. Eur. J. Med. Chem. 2016, 123, 317–331. [Google Scholar] [CrossRef]
- Wu, P.P.; Zhang, B.J.; Cui, X.P.; Yang, Y.; Jiang, Z.Y.; Zhou, Z.H.; Zhong, Y.Y.; Mai, Y.Y.; Ouyang, Z.; Chen, H.-S.; et al. Synthesis and biological evaluation of novel ursolic acid analogues as potential α-glucosidase inhibitors. Sci. Rep. 2017, 7, 45578. [Google Scholar] [CrossRef]
- Batra, A.; Sastry, V.G. Extraction of ursolic acid from Ocimum sanctum and synthesis of its novel derivatives: Effects on extracellular homocysteine, dihydrofolate reductase activity and proliferation of HepG2 human hepatoma cells. Pteridines 2013, 24, 191–199. [Google Scholar] [CrossRef]
- Nascimento, P.G.D.; Lemos, T.L.; Bizerra, A.M.; Arriaga, A.M.; Ferreira, D.A.; Santiago, G.M.; Filho, R.B.; Costa, J.G.M. Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Zhao, Y.W.; Kuai, Z.Y.; Liu, L.W.; Li, W. Synthesis and antitumor activity evaluation of novel oleanolic acid derivatives. J. Asian Nat. Prod. Res. 2017, 19, 1000–1010. [Google Scholar] [CrossRef]
- Sahni, R.; Parcha, V.; Dobhal, Y.; Maithani, A. Isolation, characterization of ursolic acid and its synthetic modification as new neuro-protective agent for prevention of cognition defects and oxidative damage. Pharm. Biol. Eval. 2016, 3, 126–134. [Google Scholar]
- Wu, P.; Huang, T.; Li, D.; Hu, Q.; Cheng, A.; Jiang, Z.; Jiao, L.; Zhao, S.; Zhang, K. Synthesis and Evaluation of Novel Triterpene Analogues of Ursolic Acid as Potential Antidiabetic Agent. PLoS ONE 2015, 10, e0138767. [Google Scholar] [CrossRef]
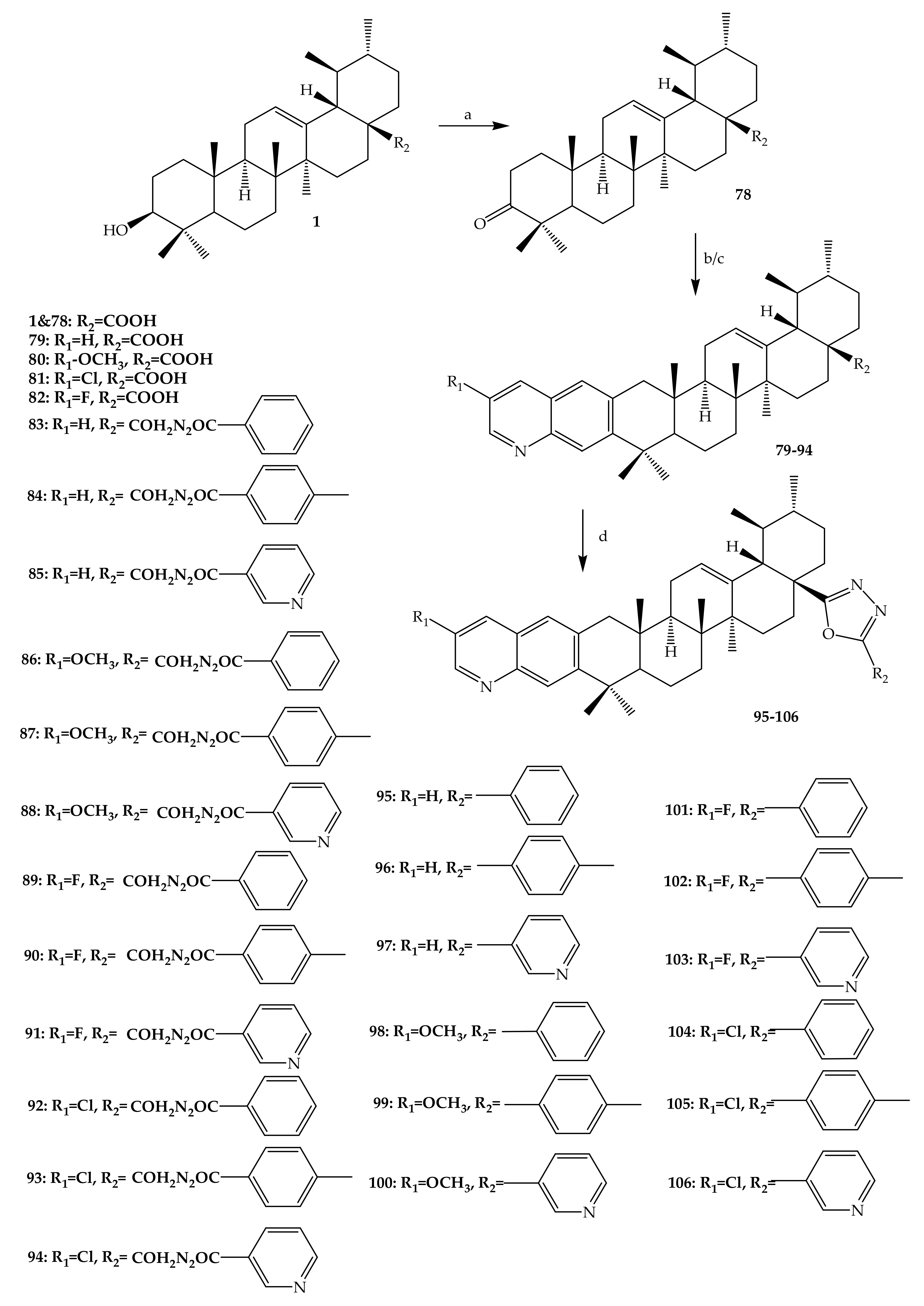
- Gu, W.; Jin, X.Y.; Li, D.D.; Wang, S.F.; Tao, X.B.; Chen, H. Bioorganic and Medicinal Chemistry Letters Design, synthesis and in vitro anticancer activity of novel quinoline and oxadiazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2017, 27, 4128–4132. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Smirnova, I.E.; Giniyatullina, G.V.; Medvedeva, N.I.; Yamansarov, E.Y.; Kazakov, D.V.; Kazakova, O.B.; Linh, P.T.; Viet, D.Q.; Huong, D.T. Inhibition of Alpha-Glucosidase by Synthetic Derivatives of Lupane, Oleanane, Ursane and Dammarane Triterpenoids. Nat. Prod. Commun. 2016, 11, 33–35. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Gul’nara, V.G.; Yamansarov, E.Y.; Tolstikov, G.A. Bioorganic and medicinal chemistry letters betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorg. Med. Chem. Lett. 2010, 20, 4088–4090. [Google Scholar] [CrossRef]
- Yu, S.-G.; Zhang, C.-J.; Xu, X.-E.; Sun, J.-H.; Zhang, L.; Yu, P.-F. Ursolic acid derivative ameliorates streptozotocin-induced diabestic bone deleterious effects in mice. Int. J. Clin. Exp. Pathol. 2015, 8, 3681–3690. [Google Scholar]
- Tian, T.; Liu, X.; Jingyang, E.L. Synthesis of novel oleanolic acid and ursolic acid in C-28 position derivatives as potential anticancer agents. Arch. Pharm. Res. 2017, 40, 458–468. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, H.; Ren, Y.; Wang, Z.; Zeng, P.; Li, X.; Wang, J.; Zheng, X. Anti-hepatocellular carcinoma activity and mechanism of chemopreventive compounds: Ursolic acid derivatives. Pharm. Boil. 2016, 54, 3189–3196. [Google Scholar] [CrossRef]
- Tuyen, P.; Xuan, T.; Tu Anh, T.; Mai Van, T.; Ahmad, A.; Elzaawely, A.; Khanh, T. Weed suppressing potential and isolation of potent plant growth inhibitors from Castanea crenata Sieb. et Zucc. Molecules 2018, 23, 345. [Google Scholar] [CrossRef]
- Gupta, A.; Maheta, P.; Chauhan, R.; Pandey, S.; Yadav, J.S.; Shah, S. Simultaneous Quantification of Bioactive Triterpene acids (Ursolic acid and Oleanolic acid) in Different Extracts of Eucalyptus globulus (L) by HPTLC Method. Pharmacogn. J. 2018, 10, 179–185. [Google Scholar] [CrossRef]
- Lawal, H.O.; Etatuvie, S.O.; Fawehinmi, A.B. Ethnomedicinal and pharmacological properties of Morinda lucida. J. Nat. Prod. 2012, 5, 93–99. [Google Scholar]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti-and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Rout, K.K.; Singh, R.K.; Barik, D.P.; Mishra, S.K. Thin-Layer Chromatographic separation and validated HPTLC Method for Quantification of Ursolic Acid in Various Ocimum Species. J. Food Drug Anal. 2012, 20, 865–871. [Google Scholar]
- Yamaguchi, H.; Noshita, T.; Kidachi, Y.; Umetsu, H.; Hayashi, M.; Komiyama, K.; Funayama, S.; Ryoyama, K. Isolation of Ursolic Acid from Apple Peels and Its Specific Efficacy as a Potent Antitumor Agent. J. Heal. Sci. 2008, 54, 654–660. [Google Scholar] [CrossRef]
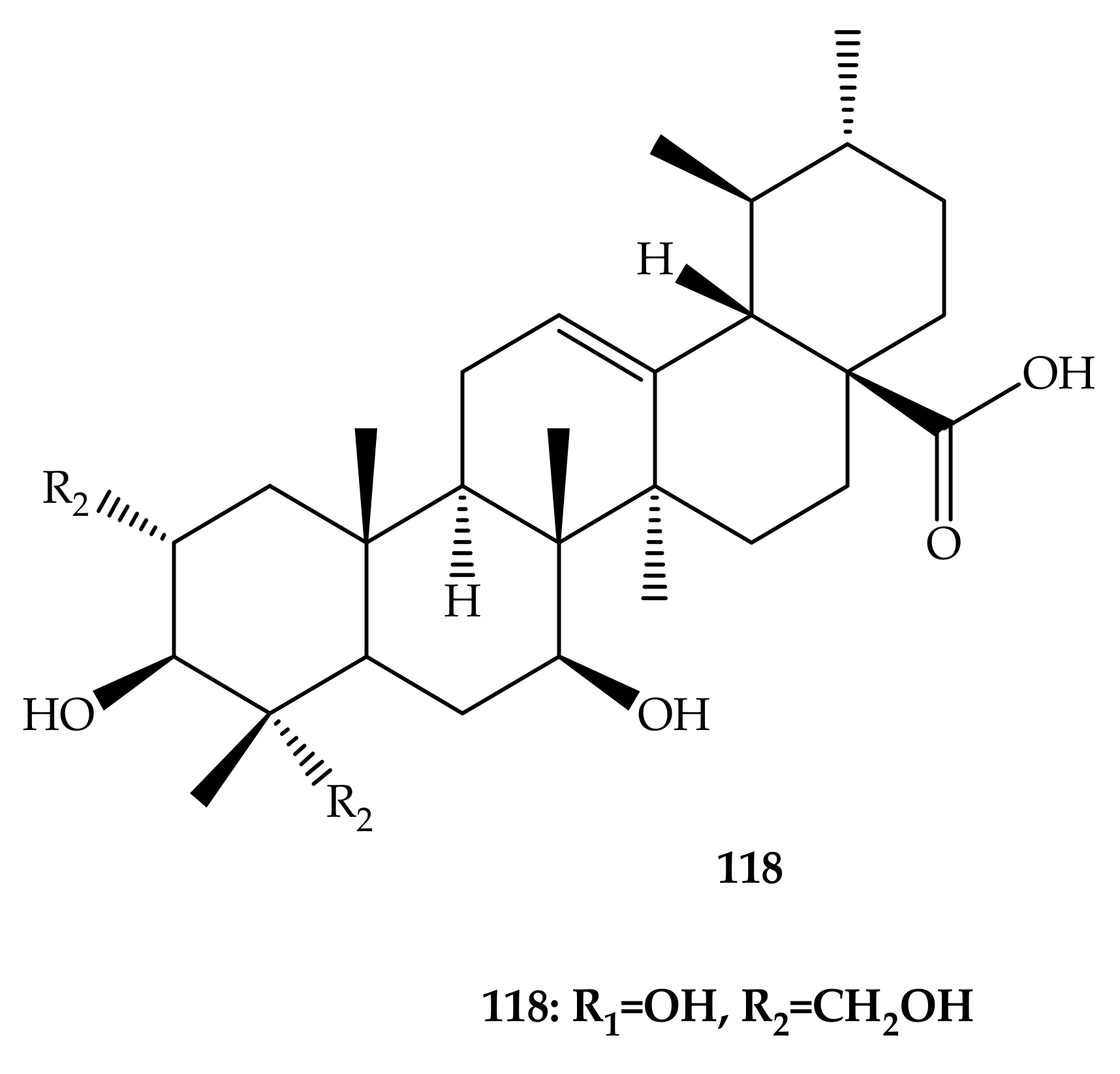
- Leal, A.S.; Wang, R.; Salvador, J.A.R.; Jing, Y. Synthesis of novel ursolic acid heterocyclic derivatives with improved abilities of antiproliferation and induction of p53, p21waf1 and NOXA in pancreatic cancer cells. Bioorg. Med. Chem. 2012, 20, 5774–5786. [Google Scholar] [CrossRef]
- Mngomezulu., S.T.; Oyedeji, A.O.; Shode, F.O.; Oyedeji, O.O.; Opoku, A.R.S. The cytotoxicity of Mimusops caffra-derived ursolic acid and its three triterpenoid semi-synthesized derivatives on HEK293 and HepG2 cells. In Chemistry for Clean and Healthy Planet; Ramasami, P., Gupta Bhowon, M., Jhaumeer Laulloo, S., Li Kam Wah, H., Eds.; Springer: New York, NY, USA, 2019; accepted. [Google Scholar]
- Ma, J.-Q.; Ding, J.; Zhang, L.; Liu, C.-M. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway. Environ. Toxicol. Pharmacol. 2014, 37, 975–983. [Google Scholar] [CrossRef]
- Yang, G.; Yang, T.; Zhang, W.; Lu, M.; Ma, X.; Xiang, G. In vitro and in vivo antitumor effects of folate-targeted ursolic acid stealth liposome. J. Agric. Food Chem. 2014, 62, 2207–2215. [Google Scholar] [CrossRef]
- Silva, M.G.V.; Vieira, I.; Mendes, F.N.P.; Albuquerque, I.L.; Dos Santos, R.N.; Silva, F.O.; Morais, S.M. Variation of Ursolic Acid Content in Eight Ocimum Species from Northeastern Brazil. Molecules 2008, 13, 2482–2487. [Google Scholar] [CrossRef]
- Jiménez-Arellanes, A.; Luna-Herrera, J.; Cornejo-Garrido, J.; López-García, S.; Castro-Mussot, M.E.; Meckes-Fischer, M.; Mata-Espinosa, D.; Marquina, B.; Torres, J.; Hernández-Pando, R. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement. Altern. Med. 2013, 13, 258. [Google Scholar] [CrossRef]
- Kataev, V.E.; Khaybullin, R.N.; Garifullin, B.F.; Sharipova, R.R. New Targets for Growth Inhibition of Mycobacterium tuberculosis: Why Do Natural Terpenoids Exhibit Antitubercular Activity? Russ. J. Bioorganic Chem. 2018, 44, 438–452. [Google Scholar] [CrossRef]
- Vetal, M.D.; Lade, V.G.; Rathod, V.K. Extraction of ursolic acid from Ocimum sanctum leaves: Kinetics and modeling. Food Bioprod. Process. 2012, 90, 793–798. [Google Scholar] [CrossRef]
- Bulus, T.; Atawodi, S.E.; Mamman, M. Acute toxicity effect of the aqueous extract of Terminalia avicennioides on white albino rats. Sci. World J. 2011, 6, 1–4. [Google Scholar]
- Pereira, S.R.; Fonseca, D.R.; Matias, R.; Correa, B.O.; Pedrinho, D.R. Phytochemistry and Allelophatic Potential of Torelliodora Eucalyptus Leaves on Germination and Initial Growth of Mutambo. Planta Daninha 2018, 36, 1–13. [Google Scholar] [CrossRef]
- Abu-Gharbieh, E.; Shehab, N.G.; Almasri, I.M.; Bustanji, Y. Antihyperuricemic and xanthine oxidase inhibitory activities of Tribulus arabicus and its isolated compound, ursolic acid: In vitro and in vivo investigation and docking simulations. PLoS ONE 2018, 13, e0202572. [Google Scholar] [CrossRef]
- Somova, L.; Nadar, A.; Rammanan, P.; Shode, F.; Shode, F. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef]
- Rodríguez-López, V.; Figueroa-Suárez, M.F.M.Z.; González Christen, J.; Cardoso-Taketa, A.T. Anti-inflammatory and antihistaminic activity of triterpenoids isolated from Bursera cuneata (Schldl.) Engl. J. Ethnopharmacol. 2019, 238, 111786. [Google Scholar]
- Costa, J.F.O.; Barbosa-Filho, J.M.; de Azevedo Maia, G.L.; Guimarães, E.T.; Meira, C.S.; Ribeiro-dos-Santos, R.; de Carvalho, L.C.P.; Soares, M.B.P. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474. [Google Scholar] [CrossRef]
- Zerin, T.; Lee, M.; Jang, W.S.; Nam, K.W.; Song, H.Y. Anti-inflammatory potential of ursolic acid in Mycobacterium tuberculosis-sensitized and Concanavalin A-stimulated cells. Mol. Med. Rep 2016, 13, 2736–2744. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Mulabagal, V.; Liu, Y.; Nair, M.G. Ursolic acid analogues: Non-phenolic functional food components in Jamaican raspberry fruits. Food Chem. 2009, 116, 633–637. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Chi, K.Q.; Wang, K.S.; Wu, J.; Liu, L.P.; Piao, H.R. Design, synthesis, evaluation, and molecular docking of ursolic acid derivatives containing a nitrogen heterocycle as anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2018, 28, 1797–1803. [Google Scholar] [CrossRef]
- Benetou, V.; Lagiou, A.; Lagiou, P. Chemoprevention of cancer: Current evidence and future prospects. F1000Research 2015, 4. [Google Scholar] [CrossRef]
- Rashid, S.; Dar, B.A.; Majeed, R.; Hamid, A.; Bhat, B.A. Synthesis and biological evaluation of ursolic acid-triazolyl derivatives as potential anti-cancer agents. Eur. J. Med. Chem. 2013, 66, 238–245. [Google Scholar] [CrossRef]
- Sultana, N. Triterpenes and Triterpenoids Clinically Useful with Multiple Targets in Cancer, Malaria and More Treatment: Focus on Potential Therapeutic Value. Int. J. Biochem. Res. Rev. 2017, 16, 1–35. [Google Scholar] [CrossRef]
- Ren, Y.; Kinghorn, A.D. Natural Product Triterpenoids and Their Semi-Synthetic Derivatives with Potential Anticancer Activity. Planta Medica 2019. [Google Scholar] [CrossRef]
- Yin, R.; Li, T.; Tian, J.X.; Xi, P.; Liu, R.H. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit. Rev. Food Sci. Nutr. 2018, 58, 568–574. [Google Scholar] [CrossRef]
- Sultana, N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J. Enzym. Inhib. Med. Chem. 2011, 26, 616–642. [Google Scholar] [CrossRef]
- Zhao, W.W.; Zan, K.; Wu, J.Y.; Gao, W.; Yang, J.; Ba, Y.Y.; Wu, X.; Chen, X.Q. Antibacterial triterpenoids from the leaves of Ilex hainanensis Merr. Nat. Prod. Res. 2018, 33, 2435–2439. [Google Scholar] [CrossRef]
- Park, S.-N.; Ahn, S.-J.; Kook, J.-K. Oleanolic acid and ursolic acid inhibit peptidoglycan biosynthesis in Streptococcus mutans UA159. Braz. J. Microbiol. 2015, 46, 613–617. [Google Scholar]
- Wang, L.-J.; Jiang, B.; Wu, N.; Shi, D.-Y. Natural and semisynthetic protein tyrosine phosphatase 1B (PTP1B) inhibitors as anti-diabetic agents. RSC Adv. 2015, 5, 48822–48834. [Google Scholar] [CrossRef]
- Wu, P.-P.; Zhang, K.; Lu, Y.-J.; He, P.; Zhao, S.-Q. In vitro and in vivo evaluation of the antidiabetic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2014, 80, 502–508. [Google Scholar] [CrossRef]
- Lo, S.-H.; Li, Y.; Niu, C.-S.; Cheng, K.C. Ursolic acid activates the TGR5 receptor to enhance GLP-1 secretion in type 1-like diabetic rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2017, 390, 1097–1104. [Google Scholar] [CrossRef]
- Zhang, T.; Su, J.; Wang, K.; Zhu, T.; Li, X. Ursolic acid reduces oxidative stress to alleviate early brain injury following experimental subarachnoid hemorrhage. Neurosci. Lett. 2014, 579, 12–17. [Google Scholar] [CrossRef]
- Zhuang, Z.; Zhou, M.-L.; You, W.-C.; Zhu, L.; Ma, C.-Y.; Sun, X.-J.; Shi, J.-X. Hydrogen-rich saline alleviates early brain injury via reducing oxidative stress and brain edema following experimental subarachnoid hemorrhage in rabbits. BMC Neurosci. 2012, 13, 47. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Cui, L.; Wang, L.; Liu, H.; Ji, H.; Du, Y. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res. 2013, 1497, 32–39. [Google Scholar] [CrossRef]
- DeMaagd, G.; Philip, A. Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharm. Ther. 2015, 40, 504–532. [Google Scholar]
- Huang, Y.; Nikolic, D.; Pendland, S.; Doyle, B.J.; Locklear, T.D.; Mahady, G.B. NIH Public Access. Pharm. Biol. 2010, 47, 18–25. [Google Scholar] [CrossRef]
- Rai, S.N.; Yadav, S.K.; Singh, D.; Singh, S.P. Ursolic acid attenuates oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in MPTP-induced Parkinsonian mouse model. J. Chem. Neuroanat. 2016, 71, 41–49. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 1–35. [Google Scholar] [CrossRef]
- Saidi, I.; El Ayeb-Zakhama, A.; Harzallah-Skhiri, F.; Ben Jannet, H. Phytotoxicity of pentacyclic triterpene acids from Citharexylum spinosum L. to radish, lettuce and canary grass. Allelopath. J. 2018, 45, 243–254. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.R.; Molinillo, J.M.G. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Stampar, F. European elderberry (Sambucus nigra L.) and American Elderberry (Sambucus canadensis L.): Botanical, chemical and health properties of flowers, berries and their products. Berries Prop. Consum. Nutr. 2012, 2012, 127–144. [Google Scholar]
- Basas-Jaumandreu, J.; de las Heras, F.X.C. Allelochemicals and esters from leaves and inflorescences of Sambucus nigra L. Phytochem. Lett. 2019, 30, 107–115. [Google Scholar] [CrossRef]
- Abu-Irmaileh, B.E.; Abu-Zarga, M.H. Selective growth inhibitory compounds isolated from shoots of Salvia syriaca L. Crop Res. 2015, 49, 86–90. [Google Scholar]
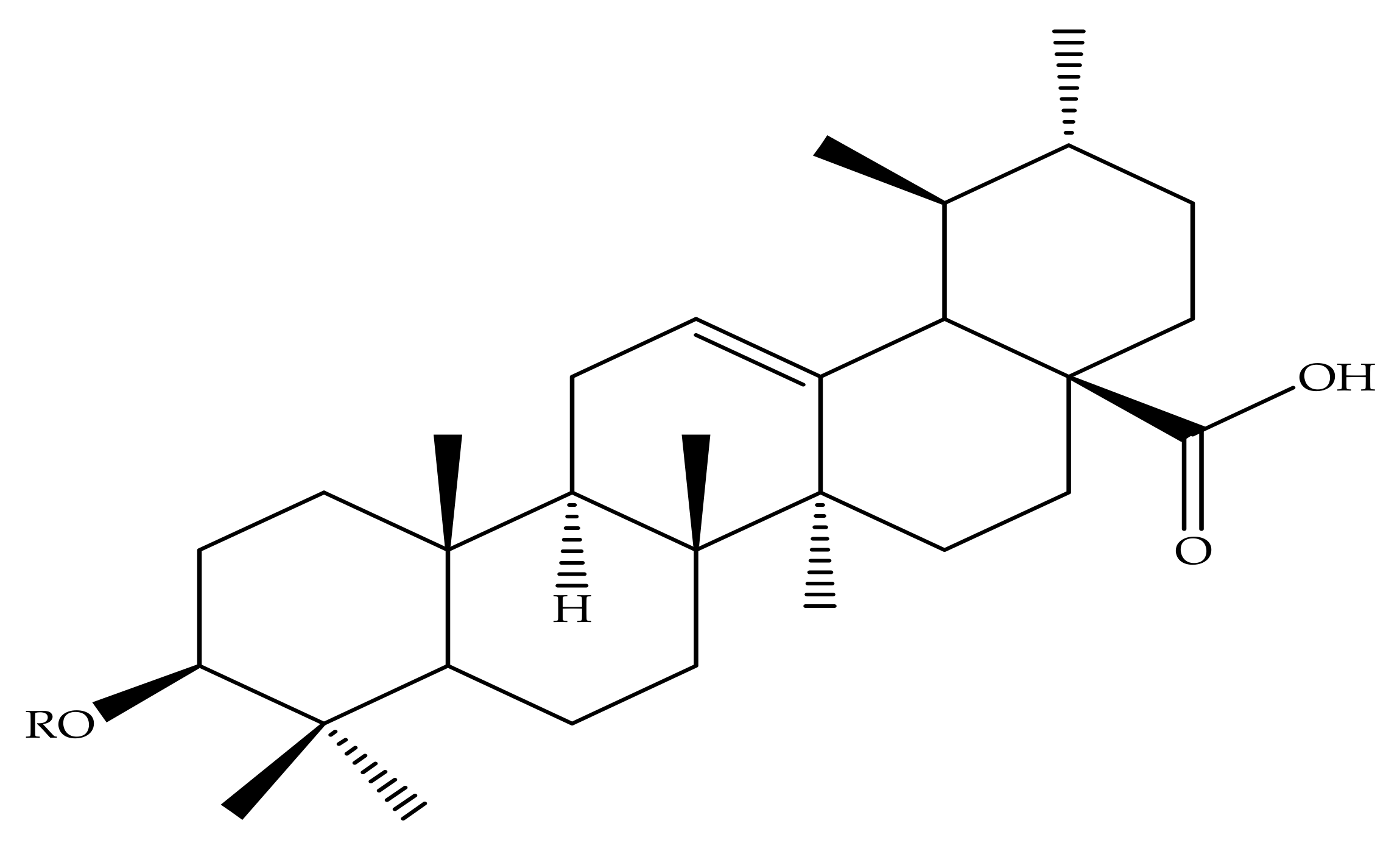
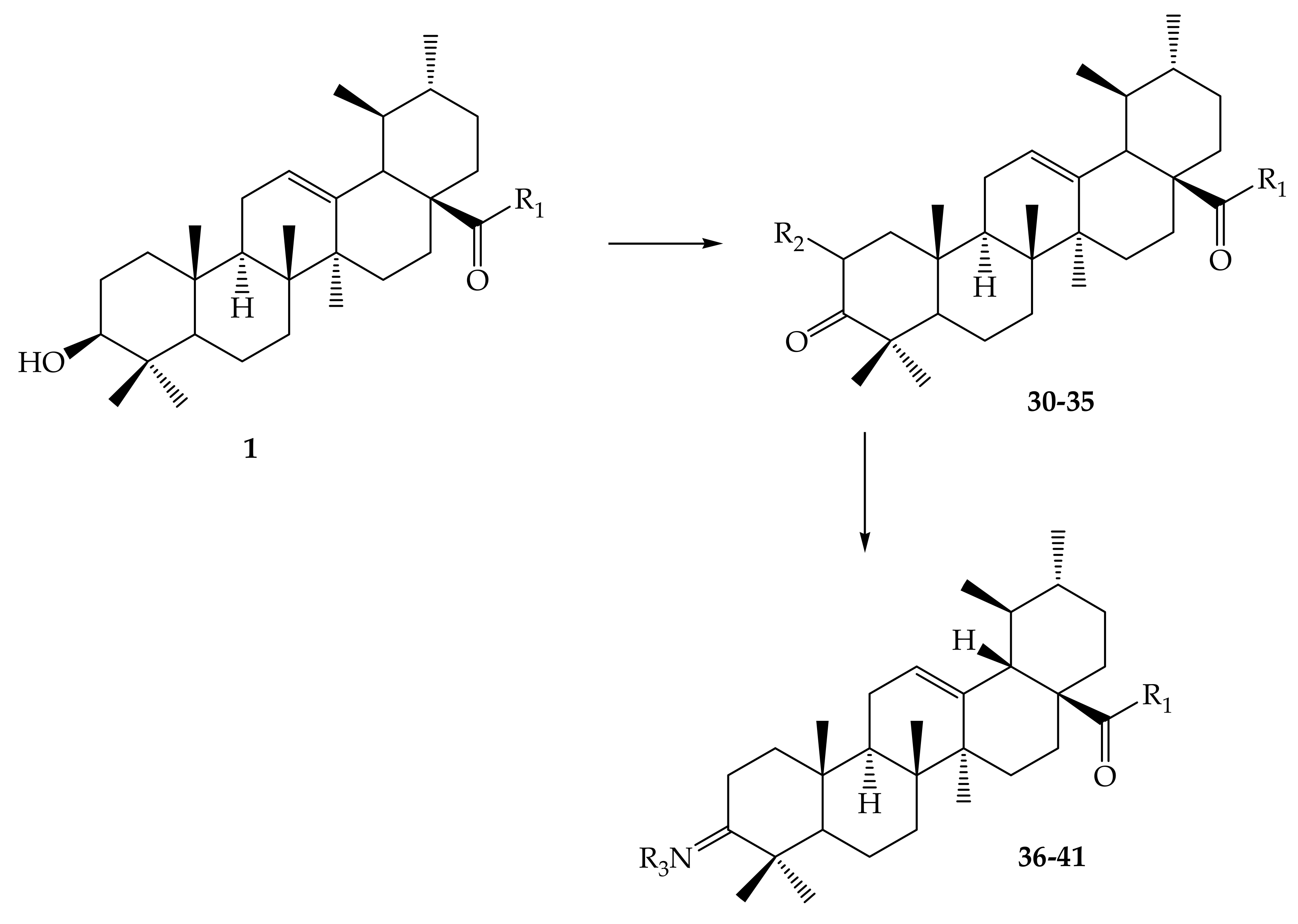
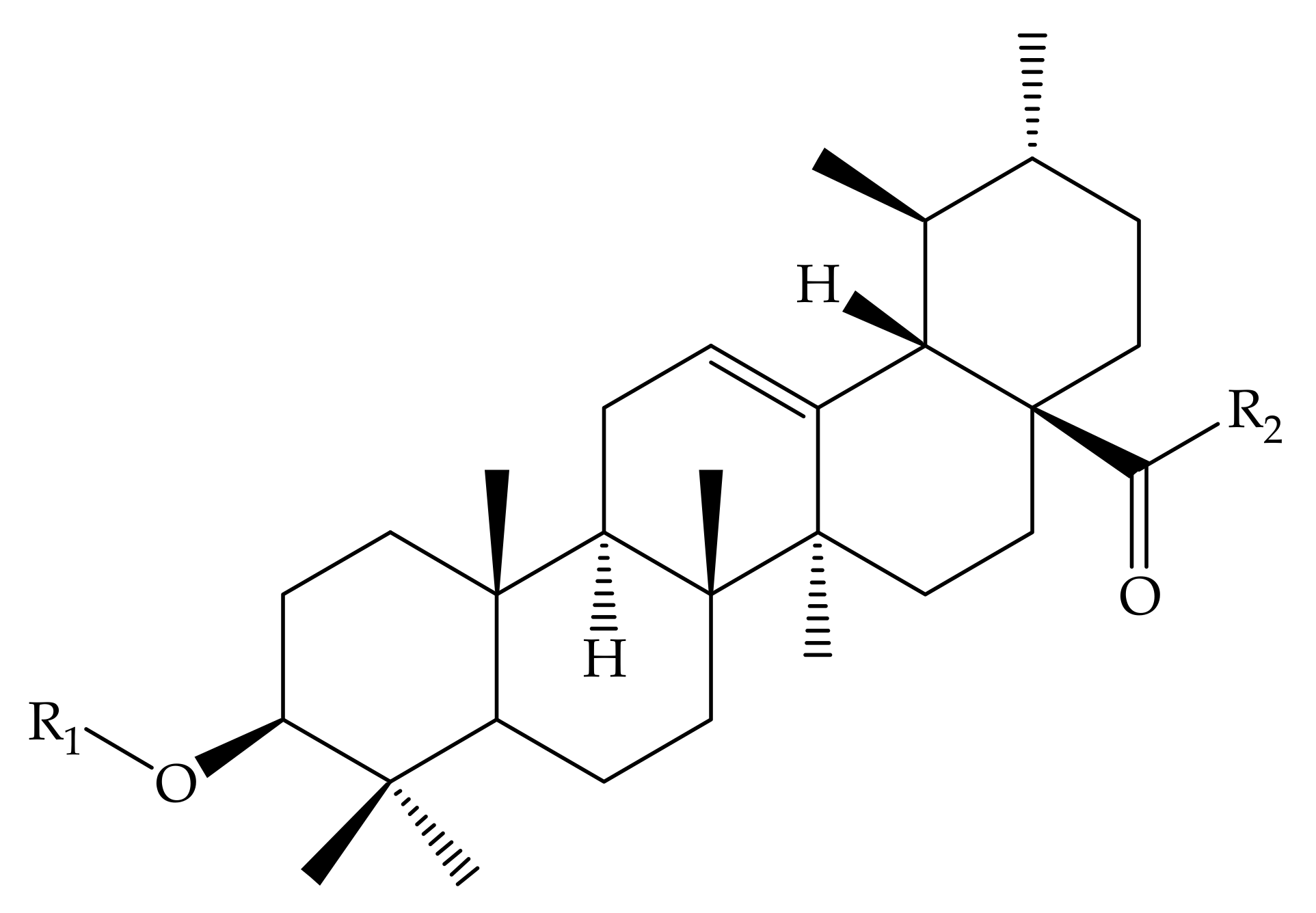


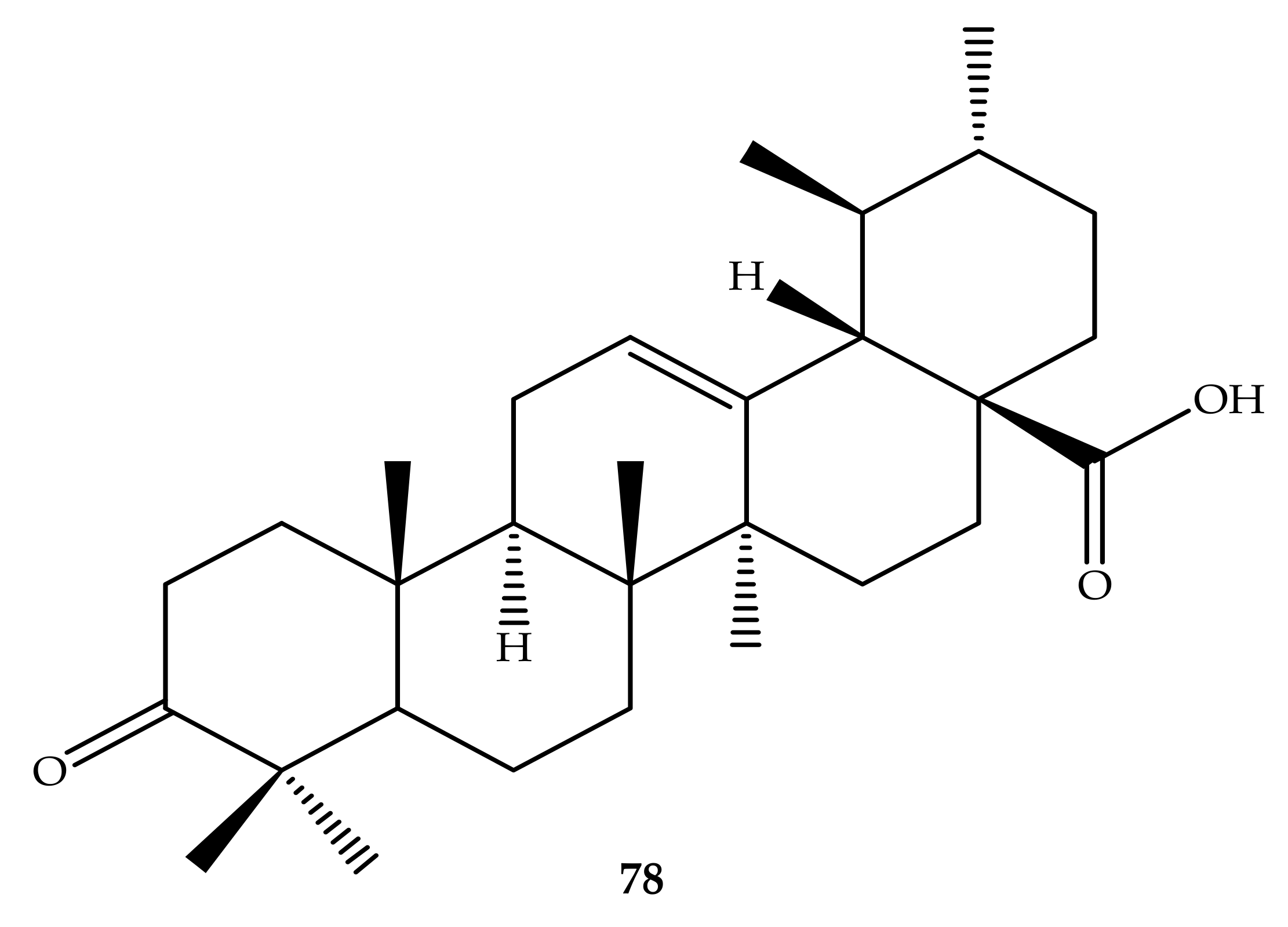

| Pentacyclic Triterpenes | Compound | Chemical Formula | Molecular Mass (g/mol) | Reference(s) |
|---|---|---|---|---|
| ursane | ||||
| ursolic acid | C30H48O3 | 456.71 | [7,10,11,12,13,14,15] | |
| uvaol | C30H50O2 | 442.72 | [16,17,18] | |
| α-amyrin | C30H50O | 426.70 | [17,18,19,20] | |
| oleanane | oleanolic acid | C30H48O3 | 456.71 | [13,14,15,20,21,22,23] |
| maslinic acid | C30H48O4 | 472.70 | [20,22,24] | |
| β-amyrin | C30H50O | 442.70 | [18,20,25] | |
| erythrodiol | C30H50O2 | 442.72 | [17,18,20] | |
| lupane | lupeol | C30H50O | 426.70 | [15,20,26,27,28,29,30,31,32,33,34] |
| betulin | C30H50O2 | 442.72 | [15,17] | |
| betulinic acid | C30H48O3 | 456.71 | [15,17,26,35,36,37] | |
| dammarane | dammarane | C30H54 | 414.75 | [38,39,40] |
| pseudojujubogenin-3-o-β-d-glucopyranoside | C36H58O10 | 650.85 | [41] | |
| hopane | C30H52 | 412.75 | [38,42] | |
| diploptene | C30H50 | 410.73 | [43,44] | |
| bacteriohopanetetrol | C35H62O4 | 546.89 | [43,45] | |
| sterols | cholesterol | C27H46O | 386.65 | [46] |
| ergosterol | C28H44O | 396.65 | [43] | |
| β-sitosterol | C29H50O | 414.71 | [47,48,49,50] |
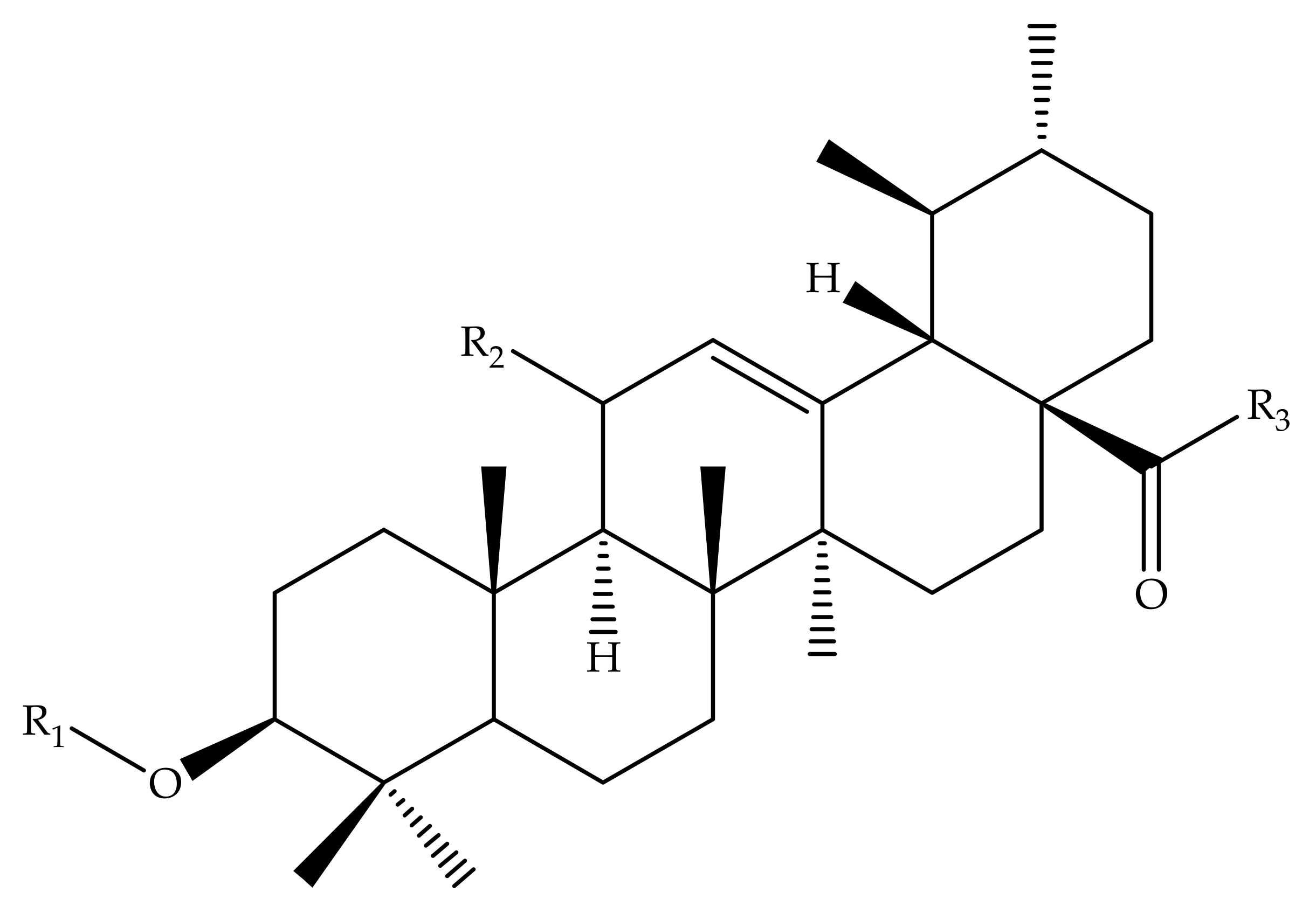
| Compound Number | R1 | R2 | R3 |
|---|---|---|---|
| 1 | OH | - | - |
| 30 | OCH3 | CHO | - |
| 31 | OC2H5 | CHO | - |
| 32 | OCH(CH3)2 | CHO | - |
| 33 | 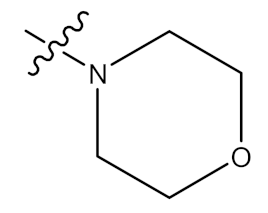 | CHO | - |
| 34 | 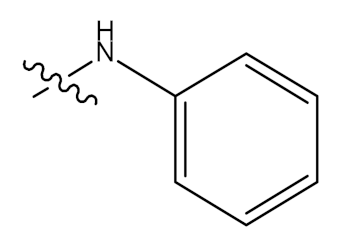 | CHO | - |
| 35 | OCH2CH2CH3 | CHO | - |
| 36 | OH | - | OCOCH3 |
| 37 | Cl | - | OCOCH3 |
| 38 |  | - | OCOCH3 |
| 39 | 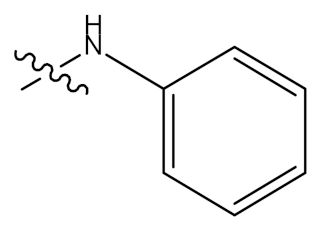 | - | OCOCH3 |
| 40 |  | - | OCOCH3 |
| 41 | 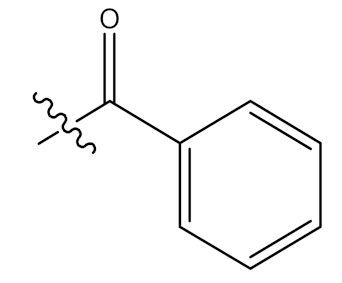 | - | OCOCH3 |
| Plants Species (Family) | Plant Part (Solvent Crude Fraction) | UA Content (mg or g) | Type of Study | Biological Effects | Reference(s) |
|---|---|---|---|---|---|
| Fragrae fragrans (Gentianaceae) | fruits (methanol) | 91 g | in vitro | antiproliferation | [14] |
| Saurauja roxburghii (Actinidiaceae) | leaves (methanol) | nr | in vitro | cytotoxicityagainst glioma cells | [10] |
| Ocimum sanctum (Lamiaceae) | whole plant (methanol, acetone, acetonitrile and ethyl acetate) | 11.21 mg | in vitro | anticancer and antiproliferation | [63,87,88] |
| Eucalyptus (Myrtaceae) | leaves (acetone) | nr | in vivo | neuro-protective agent | [66,75,89] |
| Malus pumila (Rosaceae) | fruits | nr | in vitro | antitumor | [79] |
| Tribulus arabicus (Zygophyllaceae) | aerial parts (ethanol) | 1 g | in vitro and in vivo | antihyperuricemic activity | [90] |
| Panax ginseng (Araliaceae) | roots | nr | in vivo | antihypertensive, antihyperlipidemic and antioxidant effects | [91] |
| Bursera cuneata (Burseraceae) | aerial parts (dichloromethane) | 33.3 mg | in vitro and in vivo | anti-inflammatory and antihistaminic activity | [92] |
| Sambucus australis (Adoxaceae) | aerial parts (ethanol) | 180 mg | in vitro | antibacterial and antioxidant | [64] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. https://doi.org/10.3390/molecules24152751
Mlala S, Oyedeji AO, Gondwe M, Oyedeji OO. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules. 2019; 24(15):2751. https://doi.org/10.3390/molecules24152751
Chicago/Turabian StyleMlala, Sithenkosi, Adebola Omowunmi Oyedeji, Mavuto Gondwe, and Opeoluwa Oyehan Oyedeji. 2019. "Ursolic Acid and Its Derivatives as Bioactive Agents" Molecules 24, no. 15: 2751. https://doi.org/10.3390/molecules24152751
APA StyleMlala, S., Oyedeji, A. O., Gondwe, M., & Oyedeji, O. O. (2019). Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules, 24(15), 2751. https://doi.org/10.3390/molecules24152751





