Iridium(III) Complexes Targeting Apoptotic Cell Death in Cancer Cells
Abstract
1. Introduction
2. Mechanism of Apoptosis-Targeted Cell Death in Cancer Cells
2.1. B-Cell Lymphoma 2 (Bcl-2) Family Proteins
2.2. Caspase Family Proteins
2.3. Protein Kinase Pathways
2.4. Extrinsic Apoptosis Pathway
2.5. Intrinsic Apoptosis Pathway
3. Dilemma of Conventional Apoptotic Cell-Targeted Anticancer Agents
4. Characteristics of Transition Metal-Based Compounds
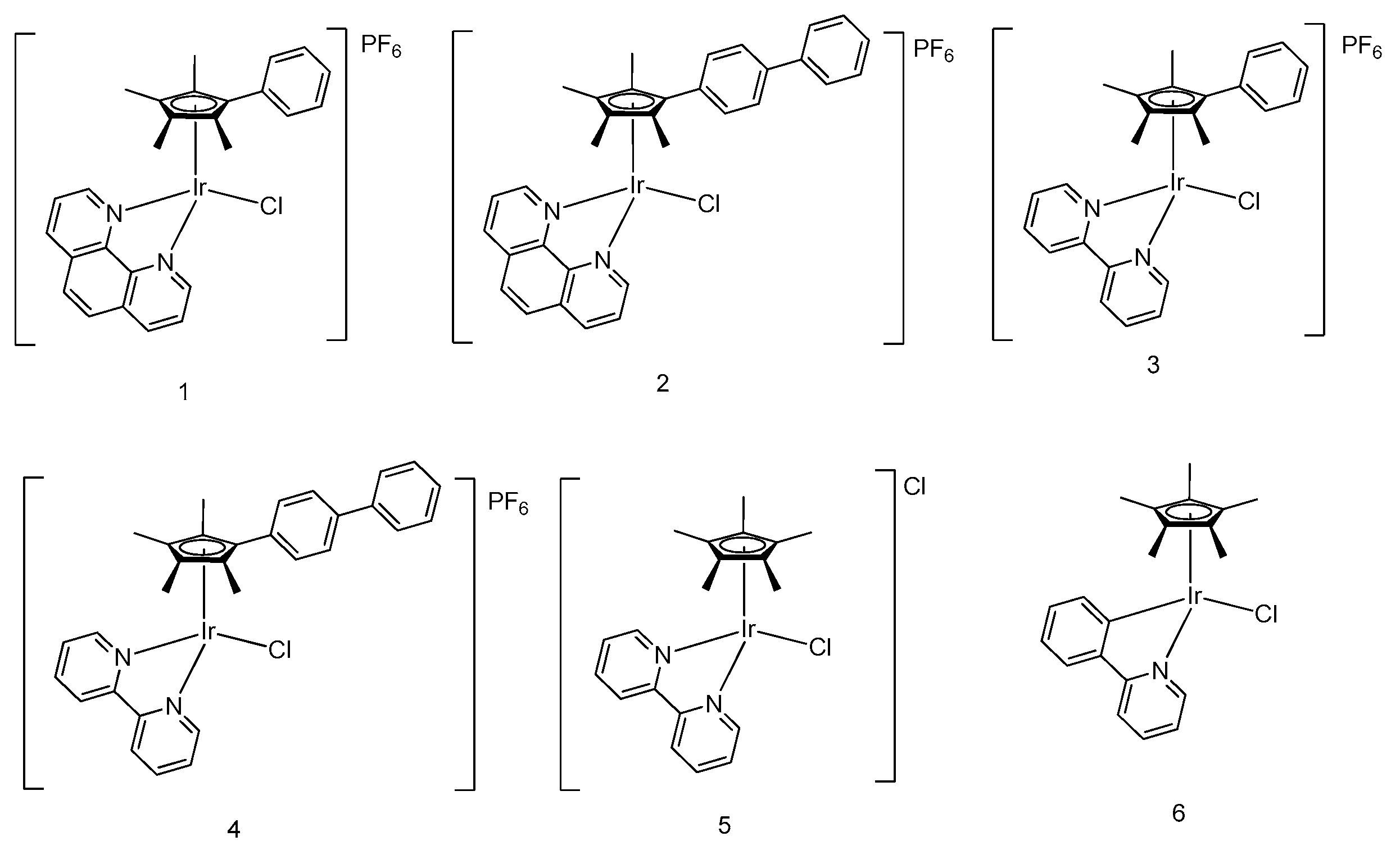
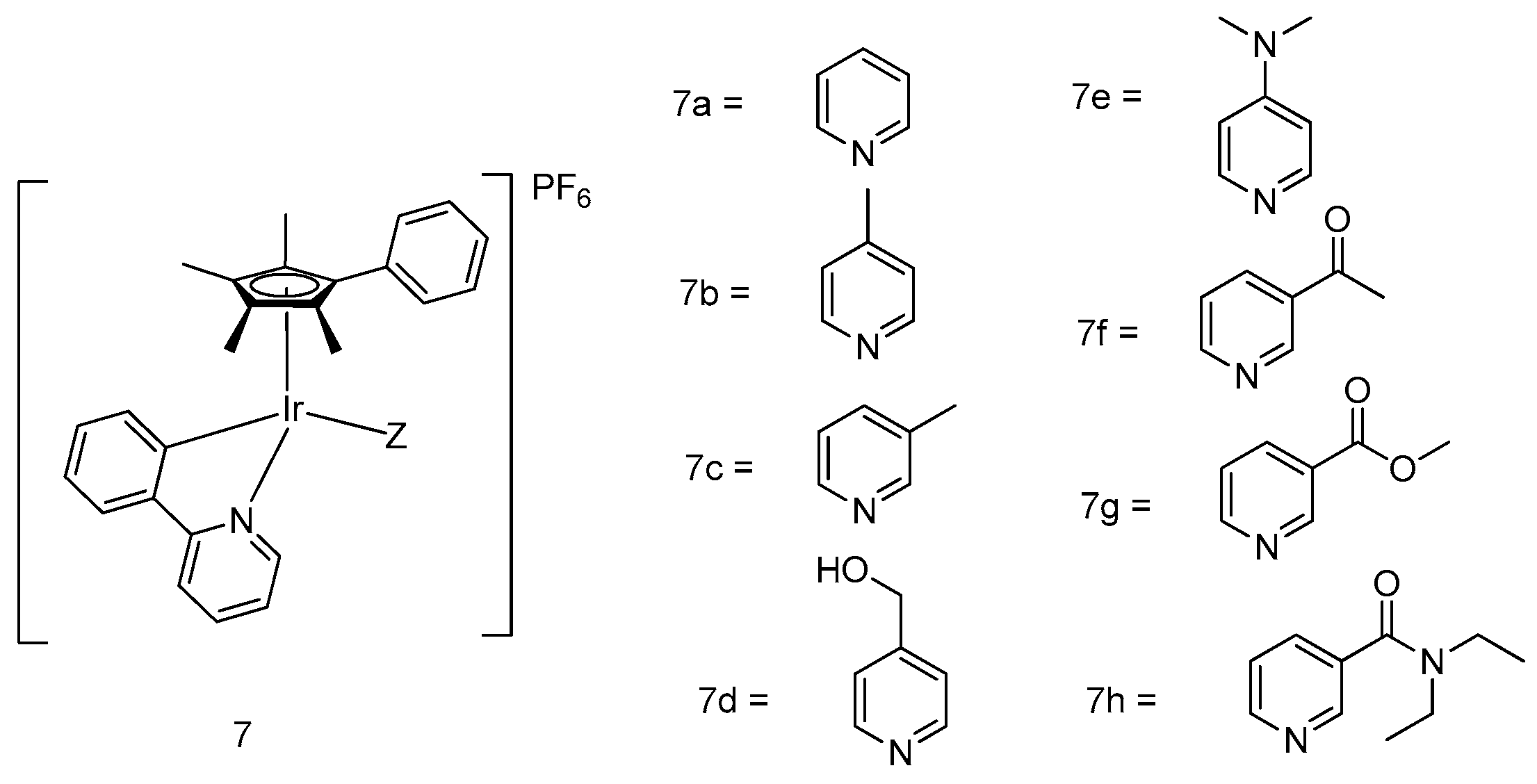
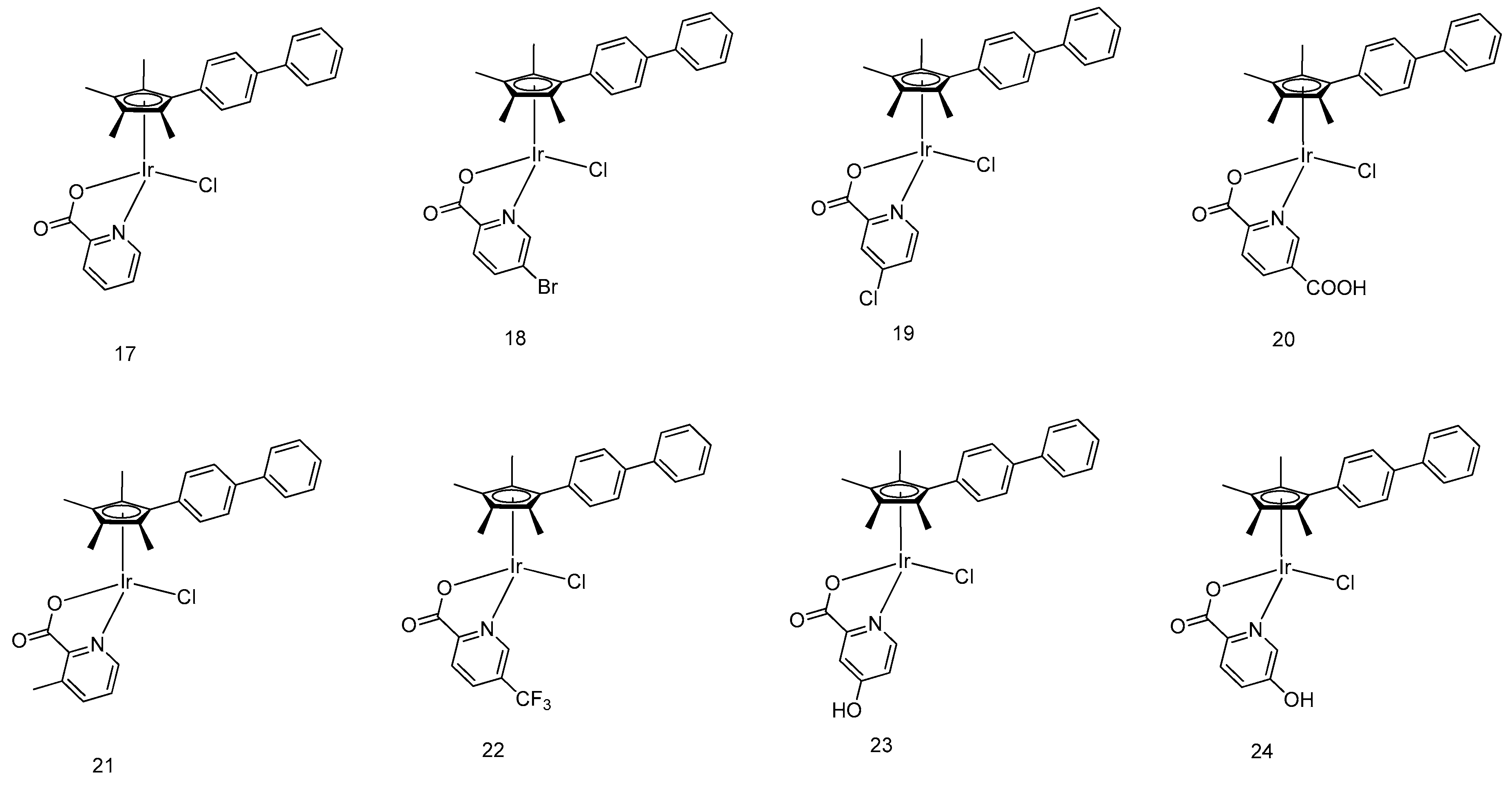
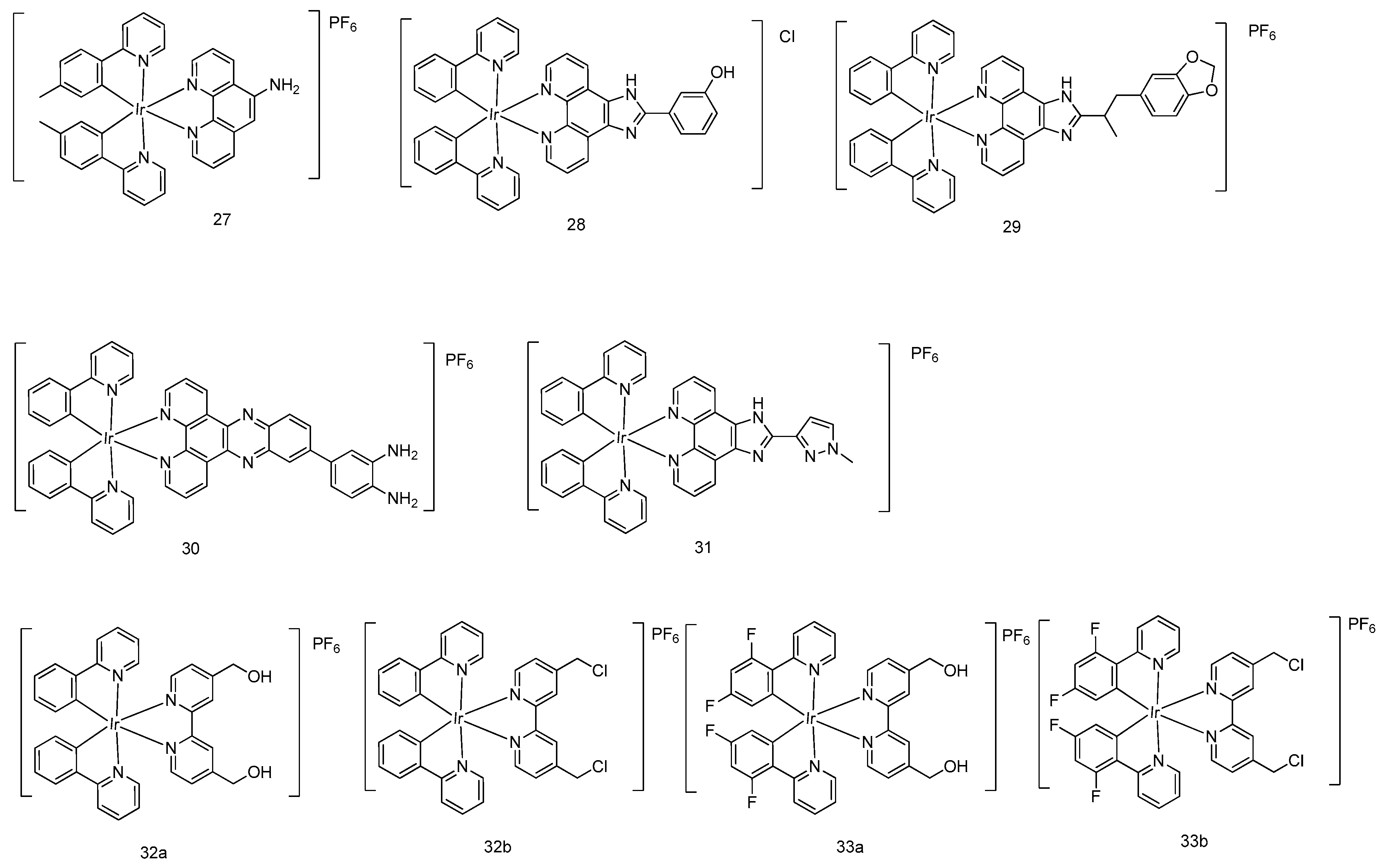
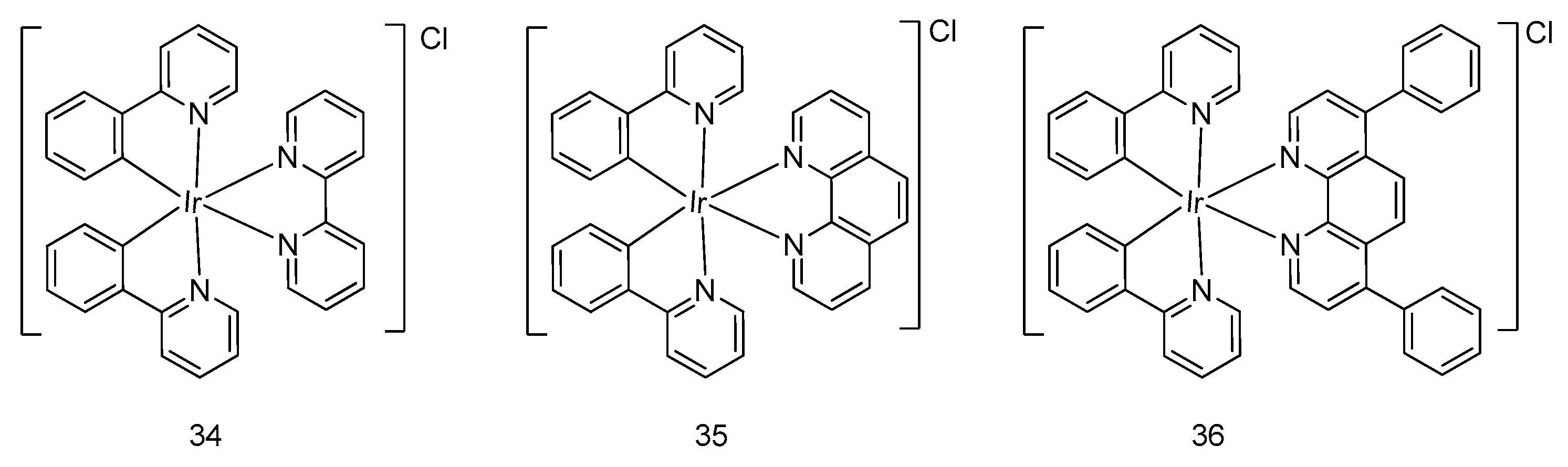
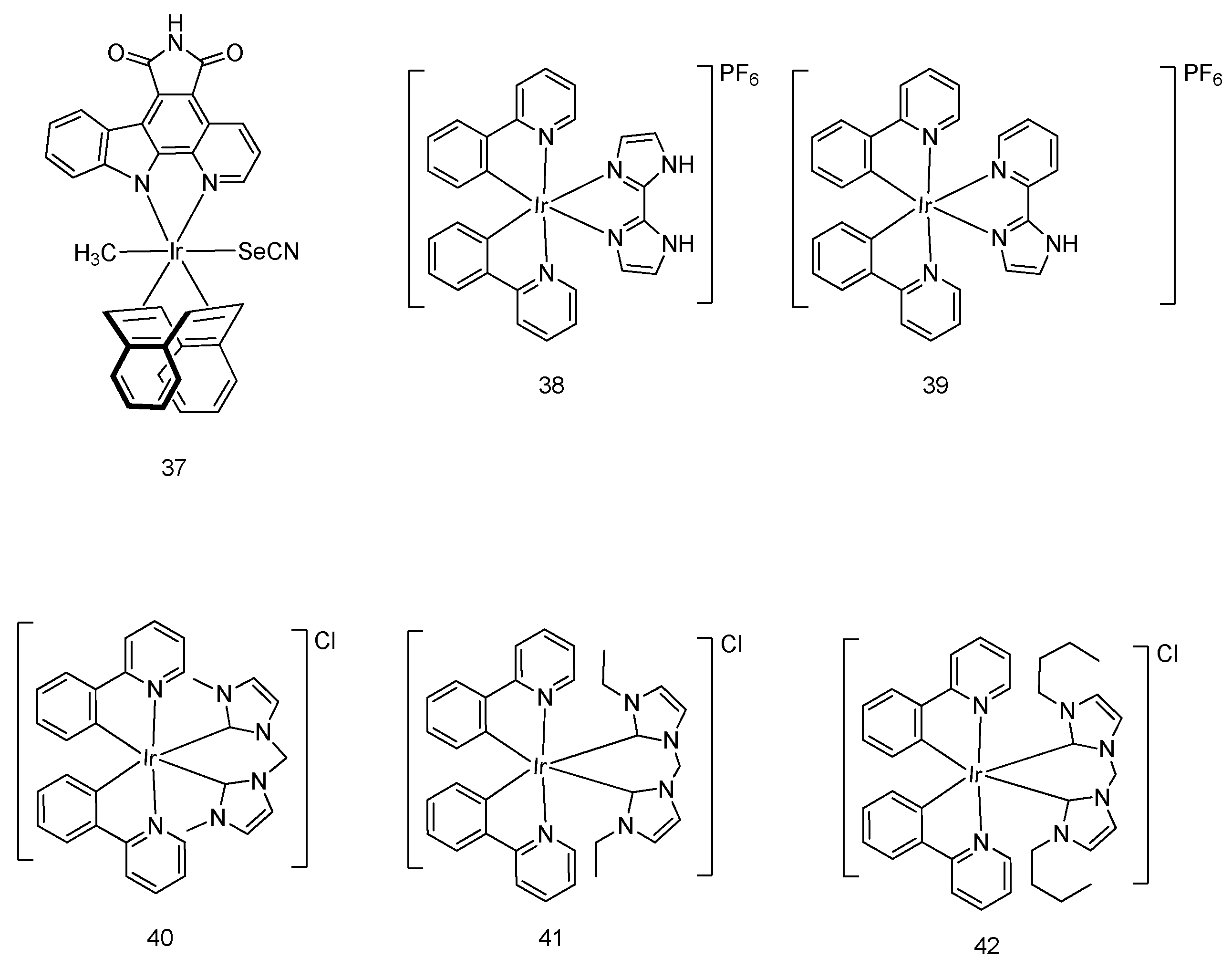
5. Iridium(III) Complexes with Anticancer Activity
5.1. Development of Organometallic Half-Sandwich Iridium(III) Compounds as Antitumor Agents
5.2. Development of Cyclometalated Octahedral Iridium(III) Compounds as Antitumor Agents
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lopez, J.; Tait, S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957. [Google Scholar] [CrossRef] [PubMed]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.-R.; Cao, J.-J.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Valproic Acid-Functionalized Cyclometalated Iridium(III) Complexes as Mitochondria-Targeting Anticancer Agents. Chem. Eur. J. 2017, 23, 15166–15176. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.J.; Lord, R.M.; Wilson, R.L.; Phillips, R.M.; Sridharan, V.; McGowan, P.C. Synthesis of iridium and ruthenium complexes with (N,N), (N,O) and (O,O) coordinating bidentate ligands as potential anti-cancer agents. Dalton Trans. 2012, 41, 13800–13802. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.K.-W. Luminescent Rhenium(I) and Iridium(III) Polypyridine Complexes as Biological Probes, Imaging Reagents, and Photocytotoxic Agents. Acc. Chem. Res 2015, 48, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lebrun, V.; Kitanosono, T.; Mallin, H.; Köhler, V.; Häussinger, D.; Hilvert, D.; Kobayashi, S.; Ward, T.R. Upregulation of an Artificial Zymogen by Proteolysis. Angew. Chem. Int. Ed. 2016, 55, 11587–11590. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, C.; Yuan, B.; Chen, Y.; Liu, X.; Ji, L.; Chao, H. Enhanced cancer therapy by the marriage of metabolic alteration and mitochondrial-targeted photodynamic therapy using cyclometalated Ir(iii) complexes. Chem. Commun. 2017, 53, 9878–9881. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.-Y.; Yip, A.M.-H.; Liu, H.-W.; Lo, K.K.-W. Installing an additional emission quenching pathway in the design of iridium(III)-based phosphorogenic biomaterials for bioorthogonal labelling and imaging. Biomaterials 2016, 103, 305–313. [Google Scholar] [CrossRef]
- Petrini, A.; Pettinari, R.; Marchetti, F.; Pettinari, C.; Therrien, B.; Galindo, A. n.; Scopelliti, R.; Riedel, T.; Dyson, P.J. Cytotoxic Half-Sandwich Rh(III) and Ir(III) β-Diketonates. Inorg. Chem. 2017, 56, 13600–13612. [Google Scholar] [CrossRef]
- Wenger, O.S. Proton-coupled electron transfer with photoexcited ruthenium(II), rhenium(I), and iridium(III) complexes. Coord. Chem. Rev. 2015, 282, 150–158. [Google Scholar] [CrossRef]
- Susnow, N.; Zeng, L.; Margineantu, D.; Hockenbery, D.M. Bcl-2 family proteins as regulators of oxidative stress. Semin Cancer Biol. 2009, 19, 42–49. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Asweto, C.O.; Wu, J.; Alzain, M.A.; Hu, H.; Andrea, S.; Feng, L.; Yang, X.; Duan, J.; Sun, Z. Cellular pathways involved in silica nanoparticles induced apoptosis: A systematic review of in vitro studies. Environ. Toxicol. Pharmacol. 2017, 56, 191–197. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, D.-W.; Yang, L.-Y.; Fu, L.; Zhu, X.-J.; Wong, W.-K.; Jiang, F.-L.; Liu, Y. A novel bifunctional mitochondria-targeted anticancer agent with high selectivity for cancer cells. Sci. Rep. 2015, 5, 13543. [Google Scholar] [CrossRef] [PubMed]
- Bouchier-Hayes, L.; Lartigue, L.; Newmeyer, D.D. Mitochondria: Pharmacological manipulation of cell death. J. Clin. Investig. 2005, 115, 2640–2647. [Google Scholar] [CrossRef] [PubMed]
- Cross, T.G.; Scheel-Toellner, D.; Henriquez, N.V.; Deacon, E.; Salmon, M.; Lord, J.M. Serine/Threonine Protein Kinases and Apoptosis. Exp. Cell Res. 2000, 256, 34–41. [Google Scholar] [CrossRef]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015, 460, 72–81. [Google Scholar] [CrossRef]
- van Rijt, S.H.; Peacock, A.F.A.; Johnstone, R.D.L.; Parsons, S.; Sadler, P.J. Organometallic Osmium(II) Arene Anticancer Complexes Containing Picolinate Derivatives. Inorg. Chem. 2009, 48, 1753–1762. [Google Scholar] [CrossRef]
- Kostrhunova, H.; Florian, J.; Novakova, O.; Peacock, A.F.A.; Sadler, P.J.; Brabec, V. DNA Interactions of Monofunctional Organometallic Osmium(II) Antitumor Complexes in Cell-Free Media. J. Med. Chem. 2008, 51, 3635–3643. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M. Cyclometalation Using d-Block Transition Metals: Fundamental Aspects and Recent Trends. Chem. Res. 2010, 110, 576–623. [Google Scholar] [CrossRef] [PubMed]
- Meggers, E. Exploring biologically relevant chemical space with metal complexes. Curr. Opin. Chem. Biol. 2007, 11, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Cutillas, N.; Yellol, G.S.; de Haro, C.; Vicente, C.; Rodríguez, V.; Ruiz, J. Anticancer cyclometalated complexes of platinum group metals and gold. Coord. Chem. Rev. 2013, 257, 2784–2797. [Google Scholar] [CrossRef]
- Almodares, Z.; Lucas, S.J.; Crossley, B.D.; Basri, A.M.; Pask, C.M.; Hebden, A.J.; Phillips, R.M.; McGowan, P.C. Rhodium, Iridium, and Ruthenium Half-Sandwich Picolinamide Complexes as Anticancer Agents. Inorg. Chem. 2014, 53, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiao, L.; Ji, L.; Chao, H. Phosphorescent iridium(III) complexes as multicolor probes for specific mitochondrial imaging and tracking. Biomaterials 2014, 35, 2–13. [Google Scholar] [CrossRef]
- Payne, R.; Govender, P.; Therrien, B.; Clavel, C.M.; Dyson, P.J.; Smith, G.S. Neutral and cationic multinuclear half-sandwich rhodium and iridium complexes coordinated to poly(propyleneimine) dendritic scaffolds: Synthesis and cytotoxicity. J. Organomet. Chem. 2013, 729, 20–27. [Google Scholar] [CrossRef]
- Ye, R.-R.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Coumarin-appended phosphorescent cyclometalated iridium(iii) complexes as mitochondria-targeted theranostic anticancer agents. Dalton Trans. 2016, 45, 13042–13051. [Google Scholar] [CrossRef]
- Aird, R.E.; Cummings, J.; Ritchie, A.A.; Muir, M.; Morris, R.E.; Chen, H.; Sadler, P.J.; Jodrell, D.I. In vitro and in vivo activity and cross resistance profiles of novel ruthenium (II) organometallic arene complexes in human ovarian cancer. Br. J. Cancer 2002, 86, 1652–1657. [Google Scholar] [CrossRef]
- Loughrey, B.T.; Healy, P.C.; Parsons, P.G.; Williams, M.L. Selective Cytotoxic Ru(II) Arene Cp* Complex Salts [R-PhRuCp*]+X− for X = BF4−, PF6−, and BPh4−. Inorg. Chem. 2008, 47, 8589–8591. [Google Scholar] [CrossRef]
- Mendoza-Ferri, M.G.; Hartinger, C.G.; Mendoza, M.A.; Groessl, M.; Egger, A.E.; Eichinger, R.E.; Mangrum, J.B.; Farrell, N.P.; Maruszak, M.; Bednarski, P.J.; et al. Transferring the Concept of Multinuclearity to Ruthenium Complexes for Improvement of Anticancer Activity. J. Med. Chem. 2009, 52, 916–925. [Google Scholar] [CrossRef]
- Liu, Z.; Habtemariam, A.; Pizarro, A.M.; Fletcher, S.A.; Kisova, A.; Vrana, O.; Salassa, L.; Bruijnincx, P.C.A.; Clarkson, G.J.; Brabec, V.; et al. Organometallic Half-Sandwich Iridium Anticancer Complexes. J. Med. Chem. 2011, 54, 3011–3026. [Google Scholar] [CrossRef]
- Liu, Z.; Habtemariam, A.; Pizarro, A.M.; Clarkson, G.J.; Sadler, P.J. Organometallic Iridium(III) Cyclopentadienyl Anticancer Complexes Containing C,N-Chelating Ligands. Organometallics 2011, 30, 4702–4710. [Google Scholar] [CrossRef]
- Liu, Z.; Romero-Canelón, I.; Habtemariam, A.; Clarkson, G.J.; Sadler, P.J. Potent Half-Sandwich Iridium(III) Anticancer Complexes Containing C∧N-Chelated and Pyridine Ligands. Organometallics 2014, 33, 5324–5333. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, L.; Tian, Z.; Tian, M.; Zhang, S.; Xu, Z.; Gong, P.; Zheng, X.; Zhao, J.; Liu, Z. Significant effects of counteranions on the anticancer activity of iridium(iii) complexes. Chem. Commun. 2018, 54, 4421–4424. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Tian, Z.; Tian, M.; Tian, L.; Zhao, W.; Liu, Z. Half-sandwich iridium N-heterocyclic carbene anticancer complexes. Dalton Trans. 2017, 46, 6870–6883. [Google Scholar] [CrossRef]
- Li, J.; Tian, M.; Tian, Z.; Zhang, S.; Yan, C.; Shao, C.; Liu, Z. Half-Sandwich Iridium(III) and Ruthenium(II) Complexes Containing P^P-Chelating Ligands: A New Class of Potent Anticancer Agents with Unusual Redox Features. Inorg. Chem. 2018, 57, 1705–1716. [Google Scholar] [CrossRef]
- Romero-Canelón, I.; Mos, M.; Sadler, P.J. Enhancement of Selectivity of an Organometallic Anticancer Agent by Redox Modulation. J. Med. Chem. 2015, 58, 7874–7880. [Google Scholar] [CrossRef]
- Kong, D.; Guo, L.; Tian, M.; Zhang, S.; Tian, Z.; Yang, H.; Tian, Y.; Liu, Z. Lysosome-targeted potent half-sandwich iridium(III) α-diimine antitumor complexes. Appl. Organomet. Chem. 2019, 33, e4633. [Google Scholar] [CrossRef]
- Hao, H.; Liu, X.; Ge, X.; Zhao, Y.; Tian, X.; Ren, T.; Wang, Y.; Zhao, C.; Liu, Z. Half-sandwich iridium(III) complexes with α-picolinic acid frameworks and antitumor applications. J. Inorg. Biochem. 2019, 192, 52–61. [Google Scholar] [CrossRef]
- Venkatesh, V.; Berrocal-Martin, R.; Wedge, C.J.; Romero-Canelón, I.; Sanchez-Cano, C.; Song, J.-I.; Coverdale, J.P.C.; Zhang, P.; Clarkson, G.J.; Habtemariam, A.; et al. Mitochondria-targeted spin-labelled luminescent iridium anticancer complexes. Chem. Sci. 2017, 8, 8271–8278. [Google Scholar] [CrossRef]
- Lu, L.; Liu, L.-J.; Chao, W.-c.; Zhong, H.-J.; Wang, M.; Chen, X.-P.; Lu, J.-J.; Li, R.-N.; Ma, D.-L.; Leung, C.-H. Identification of an iridium(III) complex with anti-bacterial and anti-cancer activity. Sci. Rep. 2015, 5, 14544. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, Z.; Lin, K.; Wang, J. A phosphorescent cyclometalated iridium(III) complex as mitochondria-targeted theranostic anticancer agent. Inorg. Chem. Commun. 2018, 94, 75–79. [Google Scholar] [CrossRef]
- Yi, Q.-Y.; Wan, D.; Tang, B.; Wang, Y.-J.; Zhang, W.-Y.; Du, F.; He, M.; Liu, Y.-J. Synthesis, characterization and anticancer activity in vitro and in vivo evaluation of an iridium (III) polypyridyl complex. Eur. J. Med. Chem. 2018, 145, 338–349. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yi, Q.-Y.; Zhang, W.-Y.; Du, F.; He, M.; Liu, Y.-J. The induction of apoptosis in BEL-7402 cells by an iridium(III) complex through lysosome–mitochondria pathway. Polyhedron 2018, 156, 320–331. [Google Scholar] [CrossRef]
- Liang, Z.-H.; Wan, D.; Yi, Q.-Y.; Zhang, W.-Y.; Liu, Y.-J. A cyclometalated iridium(III) complex induces apoptosis and autophagy through inhibition of the PI3K/AKT/mTOR pathway. Transit. Met. Chem. 2018, 43, 243–257. [Google Scholar] [CrossRef]
- Cao, J.-J.; Tan, C.-P.; Chen, M.-H.; Wu, N.; Yao, D.-Y.; Liu, X.-G.; Ji, L.-N.; Mao, Z.-W. Targeting cancer cell metabolism with mitochondria-immobilized phosphorescent cyclometalated iridium(iii) complexes. Chem. Sci. 2017, 8, 631–640. [Google Scholar] [CrossRef]
- Cao, R.; Jia, J.; Ma, X.; Zhou, M.; Fei, H. Membrane Localized Iridium(III) Complex Induces Endoplasmic Reticulum Stress and Mitochondria-Mediated Apoptosis in Human Cancer Cells. J. Med. Chem. 2013, 56, 3636–3644. [Google Scholar] [CrossRef]
- Kastl, A.; Wilbuer, A.; Merkel, A.L.; Feng, L.; Di Fazio, P.; Ocker, M.; Meggers, E. Dual anticancer activity in a single compound: Visible-light-induced apoptosis by an antiangiogenic iridium complex. Chem. Commun. 2012, 48, 1863–1865. [Google Scholar] [CrossRef]
- Tammela, T.; Zarkada, G.; Wallgard, E.; Murtomäki, A.; Suchting, S.; Wirzenius, M.; Waltari, M.; Hellström, M.; Schomber, T.; Peltonen, R.; et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008, 454, 656. [Google Scholar] [CrossRef]
- Chen, M.-H.; Zheng, Y.; Cai, X.-J.; Zhang, H.; Wang, F.-X.; Tan, C.-P.; Chen, W.-H.; Ji, L.-N.; Mao, Z.-W. Inhibition of autophagic flux by cyclometalated iridium(iii) complexes through anion transportation. Chem. Sci. 2019, 10, 3315–3323. [Google Scholar] [CrossRef]
- Li, Y.; Tan, C.-P.; Zhang, W.; He, L.; Ji, L.-N.; Mao, Z.-W. Phosphorescent iridium(III)-bis-N-heterocyclic carbene complexes as mitochondria-targeted theranostic and photodynamic anticancer agents. Biomaterials 2015, 39, 95–104. [Google Scholar] [CrossRef]




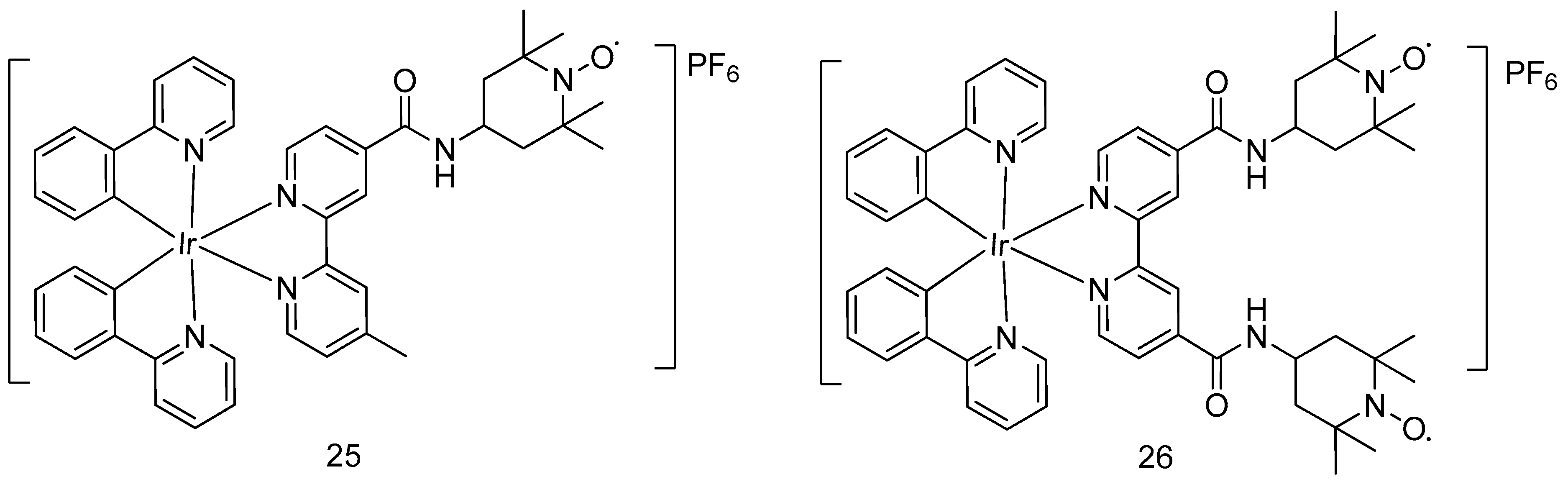
| Cell Lines | IC50/μM | ||
|---|---|---|---|
| 25 | 26 | Cisplatin | |
| A2780 | 14.5 ± 0.5 | 3.0 ± 0.2 | 1.2 ± 0.2 |
| A2780Cis | 8.6 ± 0.1 | 2.57 ± 0.08 | 13.4 ± 0.3 |
| A549 | 13.8 ± 0.5 | 7.5 ± 0.6 | 3.2 ± 0.1 |
| PC3 | 16.21 ± 0.08 | 0.53 ± 0.02 | 4.1 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.-L.; Wu, C.; Wu, K.-J.; Leung, C.-H. Iridium(III) Complexes Targeting Apoptotic Cell Death in Cancer Cells. Molecules 2019, 24, 2739. https://doi.org/10.3390/molecules24152739
Ma D-L, Wu C, Wu K-J, Leung C-H. Iridium(III) Complexes Targeting Apoptotic Cell Death in Cancer Cells. Molecules. 2019; 24(15):2739. https://doi.org/10.3390/molecules24152739
Chicago/Turabian StyleMa, Dik-Lung, Chun Wu, Ke-Jia Wu, and Chung-Hang Leung. 2019. "Iridium(III) Complexes Targeting Apoptotic Cell Death in Cancer Cells" Molecules 24, no. 15: 2739. https://doi.org/10.3390/molecules24152739
APA StyleMa, D.-L., Wu, C., Wu, K.-J., & Leung, C.-H. (2019). Iridium(III) Complexes Targeting Apoptotic Cell Death in Cancer Cells. Molecules, 24(15), 2739. https://doi.org/10.3390/molecules24152739







