Prediction of Transformation Products of Monensin by Electrochemistry Compared to Microsomal Assay and Hydrolysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrochemical Investigation
2.2. Microsomal Tests
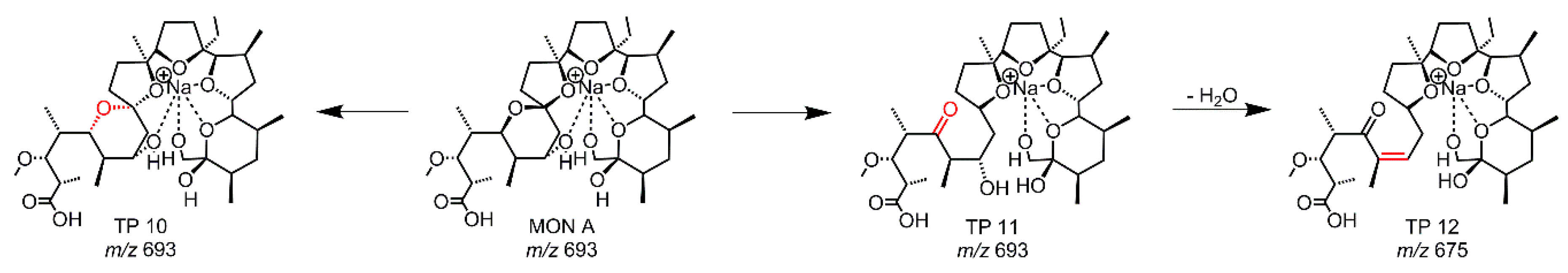
2.3. Hydrolysis of MON
3. Materials and Methods
3.1. Chemicals
3.2. Microsomal Sources and Incubation
3.3. EC/ESI-HRMS
3.4. Hydrolysis
3.5. LC/HRMS
3.6. EC/ESI-MS—LC/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bletsou, A.A.; Jeon, J.; Hollender, J.; Archontaki, E.; Thomaidis, N.S. Targeted and non-targeted liquid chromatography-mass spectrometric workflows for identification of transformation products of emerging pollutants in the aquatic environment. TrAC Trends Anal. Chem. 2015, 66, 32–44. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Fogg, L.A.; Blackwell, P.A.; Blackwell, P.; Kay, P.; Pemberton, E.J.; Croxford, A. Veterinary Medicines in the Environment. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2004; pp. 1–91. [Google Scholar]
- Farré, M.l.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Kantiani, L.; Llorca, M.; Sanchís, J.; Farré, M.; Barceló, D. Emerging food contaminants: A review. Analy. Bioanaly. Chem. 2010, 398, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Bartikova, H.; Podlipna, R.; Skalova, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. Chemical Transformations in the Environment. In Environmental Organic Chemistry for Engineers; Butterworth-Heinemann: Oxford, UK, 2017; pp. 305–353. [Google Scholar]
- Fatta-Kassinos, D.; Vasquez, M.I.; Kummerer, K. Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes-degradation, elucidation of byproducts and assessment of their biological potency. Chemosphere 2011, 85, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Pico, Y.; Barcelo, D. Transformation products of emerging contaminants in the environment and high-resolution mass spectrometry: A new horizon. Anal. Bioanal. Chem. 2015, 407, 6257–6273. [Google Scholar] [CrossRef] [PubMed]
- Agüera, A.; Martínez Bueno, M.J.; Fernández-Alba, A.R. New trends in the analytical determination of emerging contaminants and their transformation products in environmental waters. Environ. Sci. Pollut. Res. 2013, 20, 3496–3515. [Google Scholar] [CrossRef]
- Kotthoff, L.; Keller, J.; Lörchner, D.; Mekonnen, T.F.; Koch, M. Transformation products of organic contaminants and residues—overview of current simulation methods. Molecules 2019, 24, 753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Luo, G.; Ding, X.; Lu, C. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm. Sin. B 2012, 2, 549–561. [Google Scholar] [CrossRef]
- Brandon, E.F.A.; Raap, C.D.; Meijerman, I.; Beijnen, J.H.; Schellens, J.H.M. An update on in vitro test methods in human hepatic drug biotransformation research: Pros and cons. Toxicol. Appl. Pharmacol. 2003, 189, 233–246. [Google Scholar] [CrossRef]
- Jahn, S.; Karst, U. Electrochemistry coupled to (liquid chromatography/) mass spectrometry--current state and future perspectives. J. Chromatogr. A 2012, 1259, 16–49. [Google Scholar] [CrossRef] [PubMed]
- Portychova, L.; Schug, K.A. Instrumentation and applications of electrochemistry coupled to mass spectrometry for studying xenobiotic metabolism: A review. Anal. Chim. Acta 2017, 993, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bruins, A.P. An overview of electrochemistry combined with mass spectrometry. TrAC Trends Anal. Chem. 2015, 70, 14–19. [Google Scholar] [CrossRef]
- OECD. Test No. 111: Hydrolysis as a Function of pH. Available online: https://www.oecd-ilibrary.org/environment/test-no-111-hydrolysis-as-a-function-of-ph_9789264069701-en (accessed on 11 October 2018).
- Rocha, B.A.; Assis, M.D.; Peti, A.P.; Moraes, L.A.; Moreira, F.L.; Lopes, N.P.; Pospisil, S.; Gates, P.J.; de Oliveira, A.R. In vitro metabolism of monensin A: Microbial and human liver microsomes models. Xenobiotica 2014, 44, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Nebbia, C.; Ceppa, L.; Dacasto, M.; Nachtmann, C.; Carletti, M. Oxidative monensin metabolism and cytochrome P450 3A content and functions in liver microsomes from horses, pigs, broiler chicks, cattle and rats. J. Vet. Pharmacol. Therap. 2001, 24, 399–403. [Google Scholar] [CrossRef]
- Davison, K.L. Monensin absorption and metabolism in calves and chickens. J. Agri. Food Chem. 1984, 32, 1273–1277. [Google Scholar] [CrossRef]
- Donoho, A.; Manthey, J.; Occolowitz, J.; Zornes, L. Metabolism of monensin in the steer and rat. J. Agric. Food Chem. 1978, 26, 1090–1095. [Google Scholar] [CrossRef]
- Kiehl, D.E.; Julian, R.K.; Kennington, A.S. Electrospray ionization mass spectrometry with in-source collision-induced dissociation of monensin factors and related metabolites. Rapid Commun. Mass Spectrom. 1998, 12, 903–910. [Google Scholar] [CrossRef]
- Munaretto, J.S.; Yonkos, L.; Aga, D.S. Transformation of ionophore antimicrobials in poultry litter during pilot-scale composting. Environ. Pollut. 2016, 212, 392–400. [Google Scholar] [CrossRef]
- Sun, P.; Cabrera, M.L.; Huang, C.H.; Pavlostathis, S.G. Biodegradation of veterinary ionophore antibiotics in broiler litter and soil microcosms. Environ. Sci. Technol. 2014, 48, 2724–2731. [Google Scholar] [CrossRef]
- Arikan, O.A.; Mulbry, W.; Rice, C.; Lansing, S. The fate and effect of monensin during anaerobic digestion of dairy manure under mesophilic conditions. PLoS ONE 2018, 13, e0192080. [Google Scholar] [CrossRef] [PubMed]
- Sassman, S.A.; Lee, L.S. Sorption and degradation in soils of veterinary ionophore antibiotics: Monensin and lasalocid. Environ. Toxicol. Chem. 2007, 26, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yao, H.; Minakata, D.; Crittenden, J.C.; Pavlostathis, S.G.; Huang, C.H. Acid-catalyzed transformation of ionophore veterinary antibiotics: Reaction mechanism and product implications. Environ. Sci. Technol. 2013, 47, 6781–6789. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Pavlostathis, S.G.; Huang, C.H. Photodegradation of veterinary ionophore antibiotics under UV and solar irradiation. Environ. Sci. Technol. 2014, 48, 13188–13196. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Junior, J.N.; Rocha, B.A.; Assis, M.D.; Peti, A.P.F.; Moraes, L.A.B.; Iamamoto, Y.; Gates, P.J.; de Oliveira, A.R.M.; Lopes, N.P. Biomimetic oxidation studies of monensin A catalyzed by metalloporphyrins: Identification of hydroxyl derivative product by electrospray tandem mass spectrometry. Rev. Bras. Farmacogn. 2013, 23, 621–629. [Google Scholar] [CrossRef]
- Rocha, B.A.; de Oliveira, A.R.; Pazin, M.; Dorta, D.J.; Rodrigues, A.P.; Berretta, A.A.; Peti, A.P.; de Moraes, L.A.; Lopes, N.P.; Pospisil, S.; et al. Jacobsen catalyst as a cytochrome P450 biomimetic model for the metabolism of monensin A. Biomed. Res. Int. 2014, 2014, 152102. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, C.H.; Pavlostathis, S.G. Inhibition and biotransformation potential of veterinary ionophore antibiotics under different redox conditions. Environ. Sci. Technol. 2014, 48, 13146–13154. [Google Scholar] [CrossRef]
- Lopes, N.P.; Stark, C.B.; Hong, H.; Gates, P.J.; Staunton, J. Fragmentation studies on monensin A and B by accurate-mass electrospray tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2002, 16, 414–420. [Google Scholar] [CrossRef]
- Lopes, N.P.; Stark, C.B.W.; Gates, P.J.; Staunton, J. Fragmentation studies on monensin A by sequential electrospray mass spectrometry. Analyst 2002, 127, 503–506. [Google Scholar] [CrossRef]
- Simon, H.; Hoffmann, G.; Hubner, F.; Humpf, H.U.; Karst, U. Electrochemical simulation of metabolic reactions of the secondary fungal metabolites alternariol and alternariol methyl ether. Anal. Bioanal. Chem. 2016, 408, 2471–2483. [Google Scholar] [CrossRef]
- Keller, J.; Haase, H.; Koch, M. Electrochemical simulation of biotransformation reactions of citrinin and dihydroergocristine compared to UV irradiation and Fenton-like reaction. Anal. Bioanal. Chem. 2017, 409, 4037–4045. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
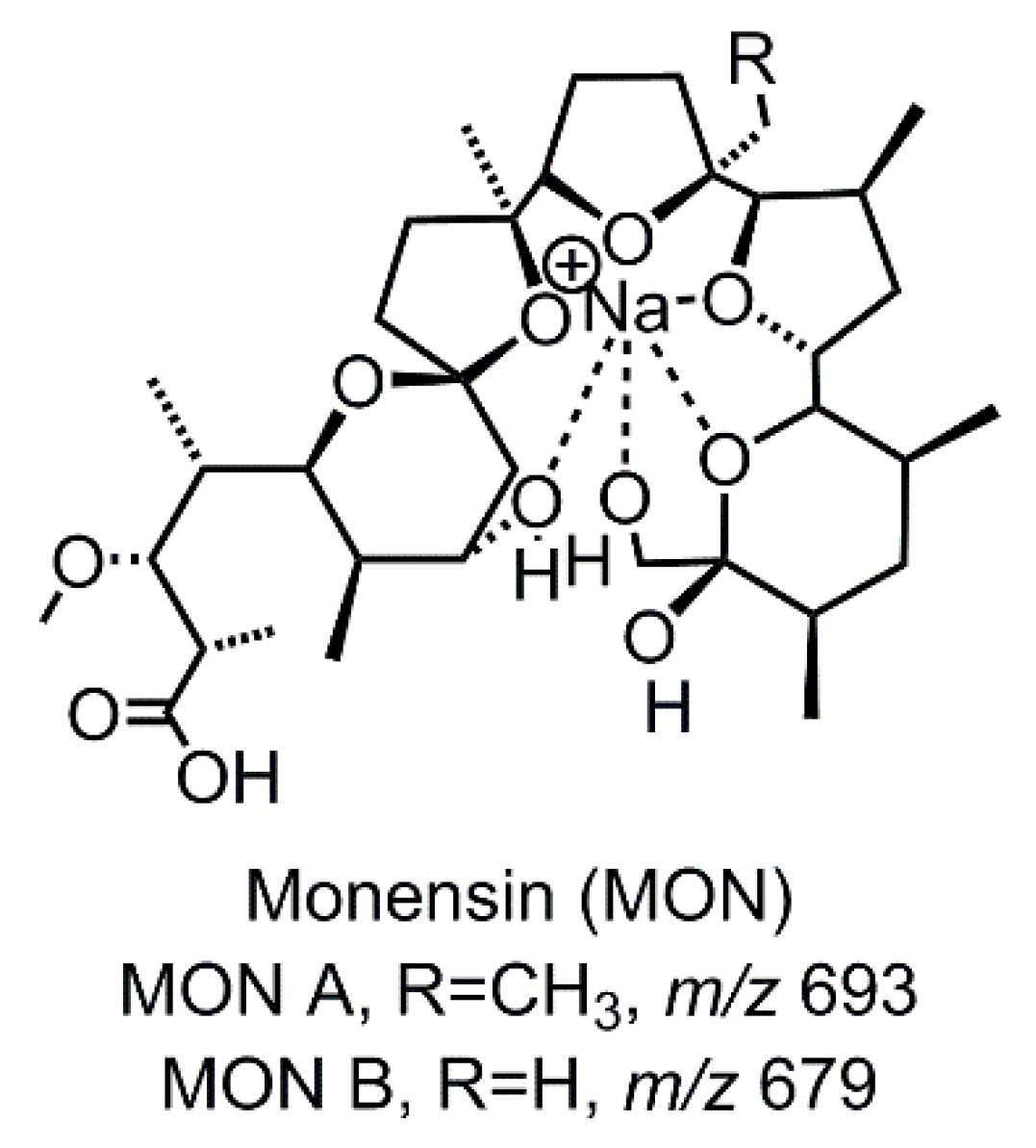
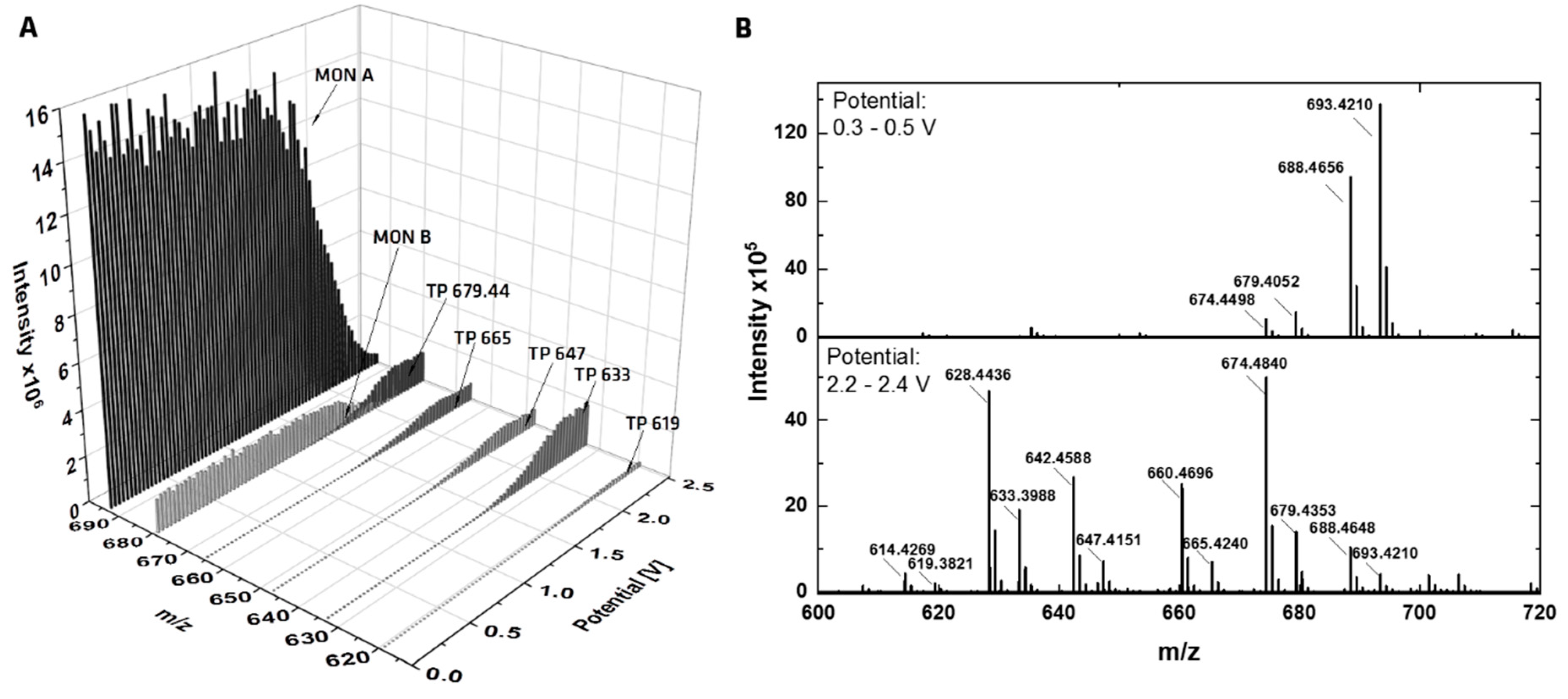

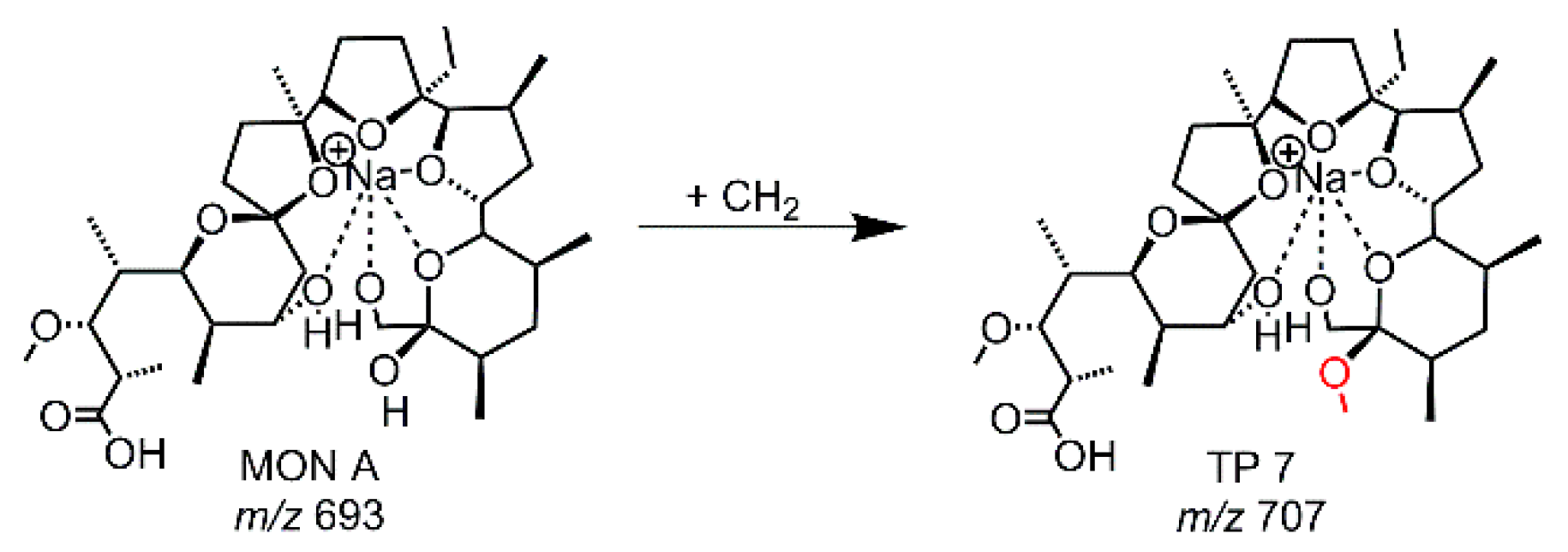
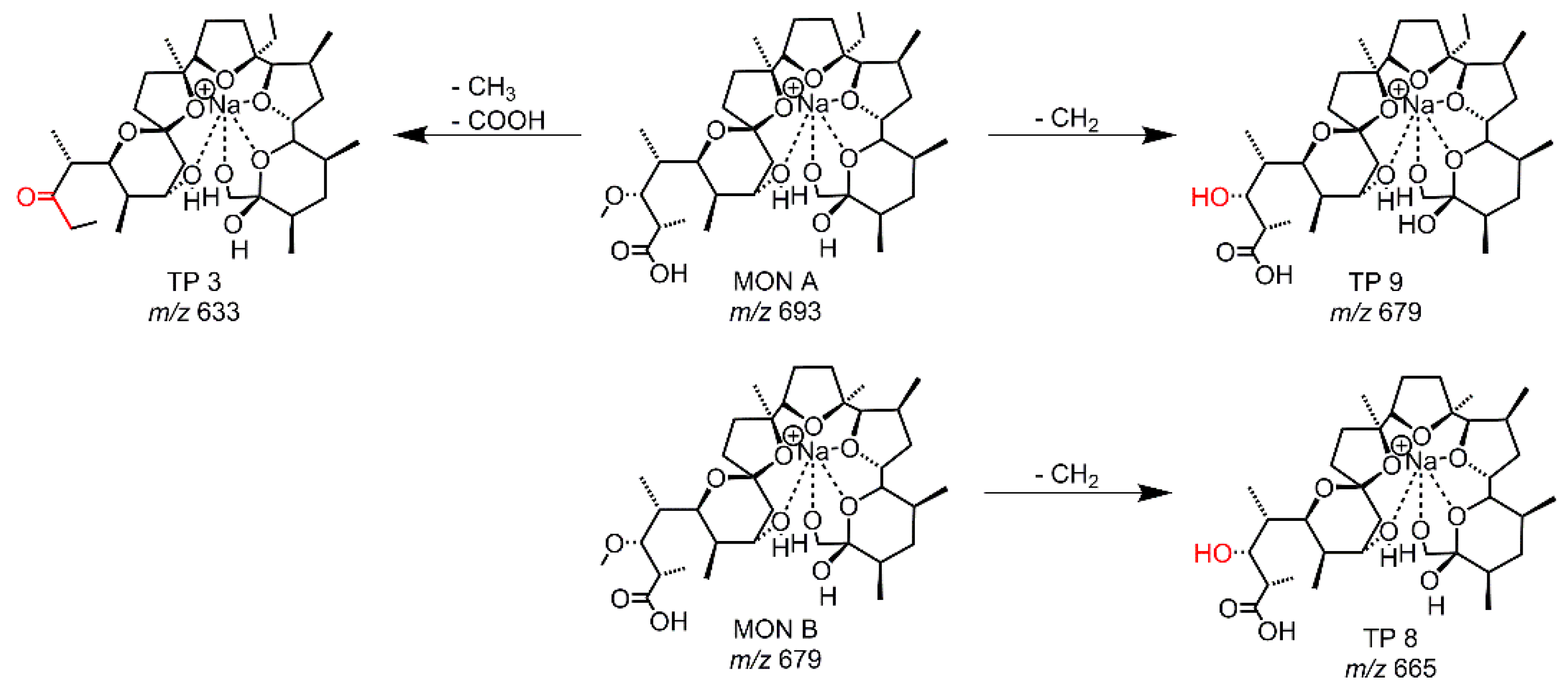
| TP Simulation Method | Compound | Retention Time [s] | calc. m/z | Molecular Formula | Suggested Transformation | TP Intensity |
|---|---|---|---|---|---|---|
| Std | MON A | 162 | 693.4184 | C36H62O11Na | - | s |
| Std | MON B | 120 | 679.4028 | C35H60O11Na | - | w |
| EC-GC | TP 1 | 92 | 619.3817 | C33H56O9Na | -CO2 -2x CH2 | w |
| EC-GC | TP 2 | 102 | 665.4235 | C35H62O10Na | -CO2 -2H +H2O | w |
| EC-GC, RLM | TP 3 | 109 | 633.3973 | C34H58O9Na | -CO2 -CH2 | s |
| EC-GC | TP 4 | 132 | 647.4130 | C35H60O9Na | -CO2 -2H | ms |
| EC-GC | TP 5 | 133 | 679.4392 | C36H64O10Na | -CO2 -2H +OCH3 +H | s |
| EC-GC | TP 6 | 144 | 679.4392 | C36H64O10Na | -CO2 -2H +OCH3 +H | ms |
| EC-MD | TP 7 | 107 | 707.4341 | C37H64O11Na | +CH2 | vw |
| RLM | TP 8 | 79 | 665.3877 | C34H58O11Na | -2 CH2 | vw |
| RLM | TP 9 | 95 | 679.4028 | C35H60O11Na | -CH2 | w |
| Hyd | TP 10 | 75 | 693.4184 | C36H62O11Na | diastereomer | s |
| Hyd | TP 11 | 99 | 693.4184 | C36H62O11Na | ring cleavage | ms |
| Hyd | TP 12 | 86 | 675.4079 | C36H60O10Na | -H2O | s |
| Day | ||||||||
|---|---|---|---|---|---|---|---|---|
| pH 3 | 1 | 2 | 3 | 4 | 5 | 8 | 15 | 30 |
| MON | x | x | x | x | x | x | x | x |
| TP10 | x | x | x | x | x | x | x | x |
| TP11 | x | x | x | x | x | x | x | x |
| TP12 | x | x | x | x | x | x | x | |
| Day | ||||||||
| pH 4 | 1 | 2 | 3 | 4 | 5 | 8 | 15 | 30 |
| MON | x | x | x | x | x | x | x | x |
| TP10 | x | x | x | x | x | x | x | |
| TP11 | x | x | x | x | x | x | ||
| TP12 | x | x | x | x | x | |||
| Day | ||||||||
| pH 5 | 1 | 2 | 3 | 4 | 5 | 8 | 15 | 30 |
| MON | x | x | x | x | x | x | x | x |
| TP10 | x | x | x | x | x | |||
| TP11 | x | x | ||||||
| TP12 | ||||||||
| Experiments Parameters | Mass Range Parameters | ||
|---|---|---|---|
| gas temperature | 350 °C | collision energy | 10 V |
| ion source gas 1 (nitrogen) | 20 L/min | declustering potential | 80 V |
| ion source gas 2 (nitrogen | 15 L/min | mass range | 200–1000 Da |
| curtain gas (nitrogen) | 25 L/min | ||
| ion spray voltage floating | +5500 V | ||
| V Potassium Biphthalate Buffer–0.1 M [mL] | V Hydrochloric Acid–0.1 M [mL] | V Sodium Hydroxide–0.1 M [mL] | pH Measured | |
|---|---|---|---|---|
| pH 3 | 5 | 2.032 | - | 3.06 |
| pH 4 | 5 | - | 0.04 | 4.02 |
| pH 5 | 5 | - | 2.385 | 5.04 |
| Experiments Parameters | Mass Range Parameters | ||
|---|---|---|---|
| gas temperature | 400 °C | MS 1 | |
| ion source gas 1 (nitrogen) | 50 L/min | collision energy | 10 V |
| ion source gas 2 (nitrogen | 55 L/min | declustering potential | 80 V |
| curtain gas (nitrogen) | 45 L/min | mass range | 100–800 Da |
| ion spray voltage floating | +5500 V | MS2 | |
| collision energy | 85 V | ||
| collision energy spread | 20 V | ||
| declustering potential | 80 V | ||
| mass range | 50–800 Da | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotthoff, L.; Lisec, J.; Schwerdtle, T.; Koch, M. Prediction of Transformation Products of Monensin by Electrochemistry Compared to Microsomal Assay and Hydrolysis. Molecules 2019, 24, 2732. https://doi.org/10.3390/molecules24152732
Kotthoff L, Lisec J, Schwerdtle T, Koch M. Prediction of Transformation Products of Monensin by Electrochemistry Compared to Microsomal Assay and Hydrolysis. Molecules. 2019; 24(15):2732. https://doi.org/10.3390/molecules24152732
Chicago/Turabian StyleKotthoff, Lisa, Jan Lisec, Tanja Schwerdtle, and Matthias Koch. 2019. "Prediction of Transformation Products of Monensin by Electrochemistry Compared to Microsomal Assay and Hydrolysis" Molecules 24, no. 15: 2732. https://doi.org/10.3390/molecules24152732
APA StyleKotthoff, L., Lisec, J., Schwerdtle, T., & Koch, M. (2019). Prediction of Transformation Products of Monensin by Electrochemistry Compared to Microsomal Assay and Hydrolysis. Molecules, 24(15), 2732. https://doi.org/10.3390/molecules24152732







