Downscaling of Industrial Turbo-Distillation to Laboratory Turbo-Clevenger for Extraction of Essential Oils. Application of Concepts of Green Analytical Chemistry
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Kinetics
2.2. Composition of Essential Oils
2.3. Green Process Assessment
3. Materials and Methods
3.1. Plant Material
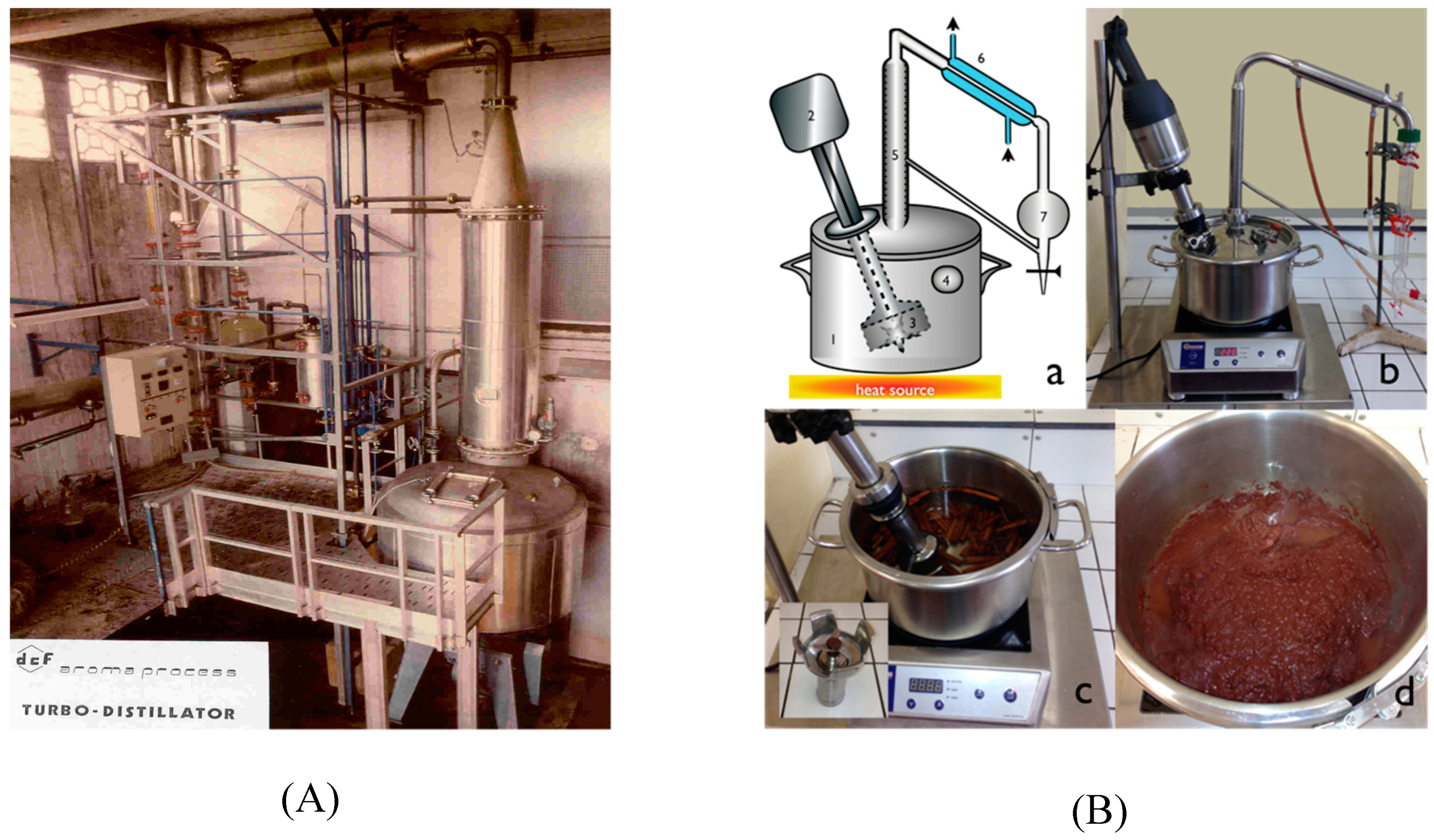
3.2. Turbo-Clevenger (TC) Apparatus and Procedure
3.3. Hydrodistillation (HD) Apparatus and Procedure
3.4. Gas Chromatography Analysis and Compound Identification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tyskiewicz, K.; Gieysztor, R.; Konkol, M.; Szalas, J.; Roj, E. Essential oils from Humulus Lupulus scCO2 Extract by Hydrodistillation and Microwave Assisted Hydrodistillation. Molecules 2018, 23, 2866. [Google Scholar] [CrossRef] [PubMed]
- Banozic, M.; Banjari, I.; Jakovljevic, M.; Subaric, D.; Tomas, S.; Babic, J.; Jokic, S. Optimization of Ultrasound-Assisted Extraction of Some Bioactive Compounds from Tobacco Waste. Molecules 2019, 24, 1611. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.P. Device for High Speed Production of Aromatic Essential Oils from Perfume-Generating Plants or Parts Thereof. U.S. Patent 4,406,745, 27 September 1983. [Google Scholar]
- Périno-Issartier, S.; Ginies, C.; Cravotto, G.; Chemat, F. A comparison of essential oils obtained from lavandin via different extraction processes: Ultrasound, microwave, turbohydrodistillation, steam and hydrodistillation. J. Chromatogr. A 2013, 1305, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Périno, S.; Pierson, J.T.; Ruiz, K.; Cravotto, G.; Chemat, F. Laboratory to pilot scale: Microwave extraction for polyphenols lettuce. Food Chem. 2016, 204, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Filly, A.; Fernandez, X.; Minuti, M.; Visinoni, F.; Cravotto, G.; Chemat, F. Solvent free microwave extraction of essential oil from aromatic herbs: From laboratory to pilot and industrial sclae. Food Chem. 2014, 150, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Ramos, L.; Ramos, J.J.; Brinkman, U.A. Miniaturization in sample treatment for environmental analysis. Anal. Bioanal. Chem. 2005, 381, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Périno-Issartier, S.; Petitcolas, E. “In situ” extraction of essential oils by use of Dean-Stark glassware and a Vigreux column inside a microwave oven: A procedure for teaching green analytical chemistry. Anal. Bioanal. Chem. 2012, 404, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Al Bittar, S.; Périno-Issartier, S.; Dangles, O.; Chemat, F. An innovative grape juice enriched in polyphenols by microwave-assisted extraction. Food Chem. 2013, 141, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Périno-Issartier, S.; Huma, Z.; Abert-Vian, M.; Chemat, F. Solvent Free Microwave-Assisted Extraction of Antioxidants from Sea Buckthorn (Hippophae rhamnoides) Food By-Products. Food Bioprocess Technol. 2011, 4, 1020–1028. [Google Scholar] [CrossRef]
- Abert-Vian, M.; Fernandez, X.; Visinoni, F.; Chemat, F. Microwave Hydrodiffusion and gravity, a new technique for extraction of essential oil. J. Chromatogr. A 2008, 1190, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. An original solvent free microwave extraction of essential oils from spices. Flavour Fragr. J. 2004, 19, 134–138. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
 hydrodistillation
hydrodistillation  ).
).
 hydrodistillation
hydrodistillation  ).
).
| N°. | Compounds a | RI b | Brazilian Pepper | Cinnamon | Chinese Star Anise | |||
|---|---|---|---|---|---|---|---|---|
| HP5MS | HD (%) | TC (%) | HD (%) | TC (%) | HD (%) | TC (%) | ||
| Monoterpenes | 86.25 ± 0.05 | 87.06 ± 0.05 | 0.36 ± 0.01 | 0.99 ± 0.02 | 2.2 ± 0.04 | 4 ± 0.02 | ||
| 1 | α-pinene | 928 | 7.83 ± 0.11 | 8.67 ± 0.12 | 0.12 ± 0.01 | 0.41 ± 0.02 | 0.10 ± 0.08 | 0.30 ± 0.05 |
| 2 | Sabinene | 966 | 1.33 ± 0.01 | 1.39 ± 0.01 | - | - | tr. | 0.10 ± 0.01 |
| 3 | β-pinene | 970 | 0.57 ± 0.01 | 0.73 ± 0.01 | 0.11 ± 0.01 | 0.32 ± 0.01 | tr. | 0.10 ± 0.01 |
| 4 | β-myrcene | 987 | 4.24 ± 0.02 | 4.50 ± 0.02 | tr. | tr. | 0.10 ± 0.01 | 0.20 ± 0.01 |
| 5 | α-phellandrene | 1005 | 54.0 ± 0.24 | 54.25 ± 0.19 | - | - | 0.10 ± 0.03 | 0.10 ± 0.01 |
| 6 | p-cymene | 1020 | 1.65 ± 0.01 | 1.59 ± 0.01 | tr. | tr. | 0.10 ± 0.06 | tr. |
| 7 | Limonene + β-phellandrene | 1024 | 15.48 ± 0.04 | 14.87 ± 0.02 | 0.13 ± 0.02 | 0.26 ± 0.02 | 1.80 ± 0.01 | 3.10 ± 0.02 |
| 8 | α-terpinolene | 1080 | 1.15 ± 0.01 | 1.06 ± 0.01 | - | - | tr. | 0.10 ± 0.01 |
| Oxygenated monoterpenes | 0.68 ± 0.01 | 0.28 ± 0.01 | 6.02 ± 0.02 | 8.11 ± 0.03 | 1.1 ± 0.01 | 0.8 ± 0.01 | ||
| 9 | Eucalyptol | 1026 | - | - | 0.85 ± 0.01 | 1.19 ± 0.03 | 0.20 ± 0.01 | 0.10 ± 0.01 |
| 10 | Linalool | 1099 | 0.47±0.01 | 0.18±0.01 | 0.25 ± 0.01 | 0.3 ± 0.01 | 0.70 ± 0.02 | 0.60 ± 0.03 |
| 11 | 4-terpineol | 1173 | 0.21±0.01 | 0.10±0.01 | 0.40 ± 0.01 | 0.37 ± 0.01 | 0.20 ± 0.01 | 0.10 ± 0.01 |
| 12 | α-terpineol | 1190 | tr. | tr. | 0.58 ± 0.01 | 0.52 ± 0.01 | - | - |
| 13 | Bornyl acetate | 1276 | tr. | tr. | 0.37 ± 0.04 | 0.79 ± 0.06 | - | - |
| 14 | Cinnamyl acetate | 1445 | - | - | 3.57 ± 0.02 | 4.94 ± 0.03 | - | - |
| Sesquiterpenes | 5.6 ± 0.03 | 6.13 ± 0.02 | - | - | - | 0.5 ± 0.02 | ||
| 15 | trans β-caryophyllene | 1405 | 1.50 ± 0.02 | 1.69 ± 0.01 | - | - | - | - |
| 16 | δ-elemene | 1326 | 0.15 ± 0.01 | 0.15 ± 0.01 | - | - | - | - |
| 17 | (E)-α-bergamoten | 1424 | 0.13 ± 0.01 | 0.11 ± 0.01 | - | - | tr. | 0.50 ± 0.02 |
| 18 | Germacrene D | 1467 | 2.27 ± 0.07 | 2.30 ± 0.06 | - | - | - | - |
| 19 | δ-cadinene | 1507 | 0.68 ± 0.02 | 1.08 ± 0.03 | tr. | tr. | - | - |
| 20 | γ-elemene | 1541 | 0.87 ± 0.06 | 0.80 ± 0.01 | - | - | - | - |
| Other oxygenated compounds | 2.81 ± 0.06 | 2.52±0.03 | 93.15 ± 0.06 | 89.37 ± 0.02 | 94.9 ± 0.07 | 89.7 ± 0.08 | ||
| 21 | Benzaldehyde | 960 | - | - | 0.11 ± 0.01 | 0.34 ± 0.02 | - | - |
| 22 | 3-phenylpropanal | 1160 | - | - | 0.48 ± 0.03 | 0.36 ± 0.02 | - | - |
| 23 | Estragole | 1194 | - | - | - | - | 5.30 ± 0.03 | 4.10 ± 0.06 |
| 24 | Cis-cinnamaldehyde | 1214 | - | - | 0.5 ± 0.02 | 0.5 ± 0.02 | - | - |
| 25 | Anisaldehyde | 1247 | - | - | - | - | 2.60 ± 0.04 | 0.20 ± 0.01 |
| 26 | trans-cinnamaldehyde | 1266 | - | - | 91.44 ± 0.25 | 87.56 ± 0.20 | - | - |
| 27 | Isoestragole | 1288 | - | - | - | - | 87.00 ± 0.15 | 85.40 ± 0.18 |
| 28 | 2H-1-benzopyranone | 1432 | - | - | 0.62 ± 0.01 | 0.61 ± 0.01 | - | - |
| 29 | Elemol | 1540 | 0.66 ± 0.05 | 0.52 ± 0.07 | - | - | - | - |
| 30 | Spathulenol | 1564 | 0.56 ± 0.02 | 0.57 ± 0.02 | - | - | - | - |
| 31 | γ-eudesmol | 1620 | 0.34 ± 0.11 | 0.27 ± 0.01 | - | - | - | - |
| 32 | β-eudesmol | 1644 | 1.25 ± 0.07 | 1.16 ± 0.04 | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Périno, S.; Chemat-Djenni, Z.; Petitcolas, E.; Giniès, C.; Chemat, F. Downscaling of Industrial Turbo-Distillation to Laboratory Turbo-Clevenger for Extraction of Essential Oils. Application of Concepts of Green Analytical Chemistry. Molecules 2019, 24, 2734. https://doi.org/10.3390/molecules24152734
Périno S, Chemat-Djenni Z, Petitcolas E, Giniès C, Chemat F. Downscaling of Industrial Turbo-Distillation to Laboratory Turbo-Clevenger for Extraction of Essential Oils. Application of Concepts of Green Analytical Chemistry. Molecules. 2019; 24(15):2734. https://doi.org/10.3390/molecules24152734
Chicago/Turabian StylePérino, Sandrine, Zoubida Chemat-Djenni, Emmanuel Petitcolas, Christian Giniès, and Farid Chemat. 2019. "Downscaling of Industrial Turbo-Distillation to Laboratory Turbo-Clevenger for Extraction of Essential Oils. Application of Concepts of Green Analytical Chemistry" Molecules 24, no. 15: 2734. https://doi.org/10.3390/molecules24152734
APA StylePérino, S., Chemat-Djenni, Z., Petitcolas, E., Giniès, C., & Chemat, F. (2019). Downscaling of Industrial Turbo-Distillation to Laboratory Turbo-Clevenger for Extraction of Essential Oils. Application of Concepts of Green Analytical Chemistry. Molecules, 24(15), 2734. https://doi.org/10.3390/molecules24152734






