Technology and Process Design for Phenols Recovery from Industrial Chicory (Chicorium intybus) Leftovers
Abstract
1. Introduction
2. Results and Discussion
2.1. Water Content Determination
2.2. Extraction Conditions
2.3. Extraction Yield and Phenolic Content
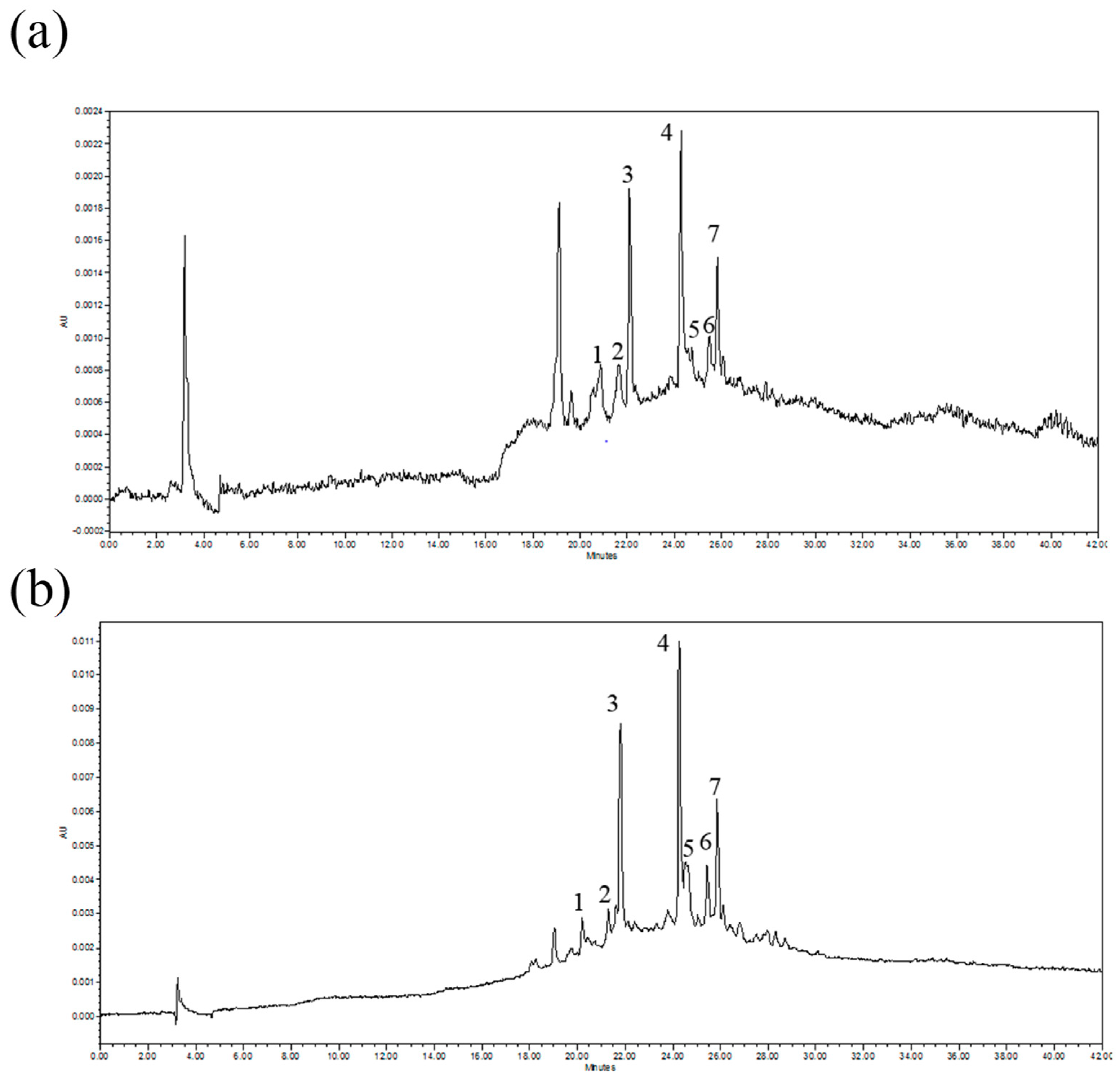
2.4. HPLC Analysis
2.5. Process Design for Scaling Up from Laboratory-Sized Research to Industrial Production
3. Materials and Methods
3.1. Materials
3.2. Water Content Determination
3.3. Experimental Methods and Reactors
3.3.1. Maceration at 40 °C
3.3.2. UAE
3.3.3. Maceration at 75 °C
3.3.4. Combined MW/US Procedure
3.3.5. MAE Under Pressure
3.3.6. Exhaustive Protocol
3.4. Total Phenolic Assay
3.5. Extract Purification
3.6. HPLC Analyses
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, N.; Tixier, A.S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuel. Bioprod. Bior. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Zhang, L.F.; Liu, Z.L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.S.; Le Bourvellec, C.; Renard, C.; Chemat, F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Binello, A.; Cravotto, G.; Boffa, L.; Stevanato, L.; Bellumori, M.; Innocenti, M.; Mulinacci, N. Efficient and selective green extraction of polyphenols from lemon balm. C. R. Chim. 2017, 20, 921–926. [Google Scholar] [CrossRef]
- Chemat, F.; Zill e, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. The combined use of microwaves and ultrasound: Improved tools in process chemistry and organic synthesis. Chem-Eur. J. 2007, 13, 1903–1909. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications-a review. Int. J. Food Sci. Tech. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Linseisen, J.; Rohrmann, S. Biomarkers of dietary intake of flavonoids and phenolic acids for studying diet-cancer relationship in humans. Eur. J. Nutr. 2008, 47, 60–68. [Google Scholar] [CrossRef]
- Jaberian, H.; Piri, K.; Nazari, J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem. 2013, 136, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Dalar, A.; Konczak, I. Cichorium intybus from Eastern Anatolia: Phenolic composition, antioxidant and enzyme inhibitory activities. Ind. Crop. Prod. 2014, 60, 79–85. [Google Scholar] [CrossRef]
- Innocenti, M.; Gallori, S.; Giaccherini, C.; Ieri, F.; Vincieri, F.F.; Mulinacci, N. Evaluation of the phenolic content in the aerial parts of different varieties of Cichorium intybus L. J. Agr. Food Chem. 2005, 53, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, P.N.; Low, H.K.; Manikandan, J.; Tan, B.K.H.; Tan, C.H. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2007, 111, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Spina, M.; Cuccioloni, M.; Sparapani, L.; Acciarri, S.; Eleuteri, A.M.; Fioretti, E.; Angeletti, M. Comparative evaluation of flavonoid content in assessing quality of wild and cultivated vegetables for human consumption. J. Sci.Food Agr. 2008, 88, 294–304. [Google Scholar] [CrossRef]
- Azay-Milhau, J.; Ferrare, K.; Leroy, J.; Aubaterre, J.; Tournier, M.; Lajoix, A.D.; Tousch, D. Antihyperglycemic effect of a natural chicoric acid extract of chicory (Cichorium intybus L.): A comparative in vitro study with the effects of caffeic and ferulic acids. J. Ethnopharmacol. 2013, 150, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Carazzone, C.; Mascherpa, D.; Gazzani, G.; Papetti, A. Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 2013, 138, 1062–1071. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.; Carvalho, A.M.; Sanchez-Mata, M.C.; Camara, M.; Fernandez-Ruiz, V.; Pardo-de-Santayana, M.; Tardio, J. Mediterranean non-cultivated vegetables as dietary sources of compounds with antioxidant and biological activity. Lwt-Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef]
- Montefusco, A.; Semitaio, G.; Marrese, P.P.; Iurlaro, A.; De Caroli, M.; Piro, G.; Dalessandro, G.; Lenucci, M.S. Antioxidants in Varieties of Chicory (Cichorium intybus L.) and Wild Poppy (Papaver rhoeas L.) of Southern Italy. J. Chem. 2015. [Google Scholar] [CrossRef]
- Papetti, A.; Daglia, M.; Grisoli, P.; Dacarro, C.; Gregotti, C.; Gazzani, G. Anti- and pro-oxidant activity of Cichorium genus vegetables and effect of thermal treatment in biological systems. Food Chem. 2006, 97, 157–165. [Google Scholar] [CrossRef]
- Ricca, E.; Curcio, S.; Calabro, V.; Iorio, G. Inulin Extraction from Chicory Roots: Transport Phenomena and Optimization. J. Biotechnol. 2010, 150, S324. [Google Scholar] [CrossRef]
- Zhu, Z.Z.; Bals, O.; Grimi, N.; Vorobiev, E. Pilot scale inulin extraction from chicory roots assisted by pulsed electric fields. Int. J. Food Sci. Tech. 2012, 47, 1361–1368. [Google Scholar] [CrossRef]
- Heimler, D.; Isolani, L.; Vignolini, P.; Romani, A. Polyphenol content and antiradical activity of Cichorium intybus L. from biodynamic and conventional farming. Food Chem. 2009, 114, 765–770. [Google Scholar] [CrossRef]
- Baiano, A.; Bevilacqua, L.; Terracone, C.; Conto, F.; Del Nobile, M.A. Single and interactive effects of process variables on microwave-assisted and conventional extractions of antioxidants from vegetable solid wastes. J. Food Eng. 2014, 120, 135–145. [Google Scholar] [CrossRef]
- Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: Extraction and energy consumption optimization. Ultrason. Sonochem. 2016, 32, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Vauchel, P.; Colli, C.; Pradal, D.; Philippot, M.; Decossin, S.; Dhulster, P.; Dimitrov, K. Comparative LCA of ultrasound-assisted extraction of polyphenols from chicory grounds under different operational conditions. J. Clean. Prod. 2018, 196, 1116–1123. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A Comparative Analysis of the ‘Green’ Techniques Applied for Polyphenols Extraction from Bioresources. Chem. Biodivers. 2015, 12, 1635–1651. [Google Scholar] [CrossRef]
- Rosello-Soto, E.; Galanakis, C.M.; Brncic, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzer, K.; Tiwari, B.K.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Tech. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Liu, C.Z. Microwave-assisted extraction of solanesol from tobacco leaves. J. Chromatogr. A 2006, 1129, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.J.; Niu, G.G.; Liu, H.Z. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crop. Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. F. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Zeb, A.; Haq, A.; Murkovic, M. Effects of microwave cooking on carotenoids, phenolic compounds and antioxidant activity of Cichorium intybus L. (chicory) leaves. Eur. Food Res. Techn. 2019, 245, 365–374. [Google Scholar] [CrossRef]
- Bahri, M.; Hance, P.; Grec, S.; Quillet, M.C.; Trotin, F.; Hilbert, J.L.; Hendriks, T. A “Novel” Protocol for the Analysis of Hydroxycinnamic Acids in Leaf Tissue of Chicory (Cichorium intybus L., Asteraceae). Sci. World J. 2012. [Google Scholar] [CrossRef][Green Version]
- Ferioli, F.; D’Antuono, L.F. An update procedure for an effective and simultaneous extraction of sesquiterpene lactones and phenolics from chicory. Food Chem. 2012, 135, 243–250. [Google Scholar] [CrossRef]
- Mascherpa, D.; Carazzone, C.; Marrubini, G.; Gazzani, G.; Papetti, A. Identification of Phenolic Constituents in Cichorium endivia Var. crispum and Var. latifolium Salads by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ioniziation Tandem Mass Spectrometry. J. Agr. Food Chem. 2012, 60, 12142–12150. [Google Scholar] [CrossRef]
- Sinkovic, L.; Demsar, L.; Znidarcic, D.; Vidrih, R.; Hribar, J.; Treutter, D. Phenolic profiles in leaves of chicory cultivars (Cichorium intybus L.) as influenced by organic and mineral fertilizers. Food Chem. 2015, 166, 507–513. [Google Scholar] [CrossRef]
- Cefola, M.; Carbone, V.; Minasi, P.; Pace, B. Phenolic profiles and postharvest quality changes of fresh-cut radicchio (Cichorium intybus L.): Nutrient value in fresh vs. stored leaves. J. Food Compos. Anal. 2016, 51, 76–84. [Google Scholar] [CrossRef]
- Tardugno, R.; Pozzebon, M.; Beggio, M.; Del Turco, P.; Pojana, G. Polyphenolic profile of Cichorium intybus L. endemic varieties from the Veneto region of Italy. Food Chem. 2018, 266, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research Inc. Available online: www.grandviewresearch.com (accessed on 6 August 2018).
- Tabasso, S.; Mariatti, F.; Grillo, G.; Boffa, L.; Tibaldi, P.; Cravotto, G. Sustainable microwave-assisted aerobic oxidation of biomass into bio-aromatics and organic acids. Ind. Eng. Chem. Res. 2019, 58, 8578–8584. [Google Scholar] [CrossRef]
- Cravotto, G.; Mariatti, F.; Gunjevic, V.; Secondo, M.; Villa, M.; Parolin, J.; Cavaglià, G. Pilot Scale Cavitational Reactors and Other Enabling Technologies to Design the Industrial Recovery of Polyphenols from Agro-Food By-Products, a Technical and Economical Overview. Foods 2018, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Extraction Method | Sample a–l | Temp. (°C) | Time (min) | Yield (% w/w) | TPC (GAE mg/g DE) | TPC (GAE mg/g DM) | TPC (GAE g/kg FM) |
|---|---|---|---|---|---|---|---|
| Maceration | M40-H2O a | 40 | 15 | 33.9 ± 0.53 | 30.4 ± 1.13 ef | 10.3 ± 0.12 | 0.72 |
| M40-EtOH 60% b | 44.6 ± 0.46 c | 34.6 ± 0.80 cdf | 15.4 ± 0.37 c | 1.08 | |||
| UAE | US-H2O c | 40 | 15 | 42.8 ± 1.15 be | 35.2 ± 0.61 bdf | 15.0 ± 0.26 b | 1.05 |
| US-EtOH 60% d | 50.5 ± 1.36 | 37.0 ± 0.28 bc | 18.7 ± 0.15 e | 1.31 | |||
| Maceration | M75-H2O e | 75 | 15 | 40.5 ± 0.48 c | 29.3 ± 0.36 a | 11.9 ± 0.08 d | 0.83 |
| M75-EtOH 60% f | 57.1 ± 0.49 | 32.5 ± 1.16 abc | 18.5 ± 0.37 | 1.30 | |||
| MW/US | MW/US-H2O g | 75 | 15 | 66.9 ± 0.85 j | 41.7 ± 0.37 | 27.9 ± 0.24 | 1.95 |
| MW/US-EtOH 60% h | 87.0 ± 1.48 | 49.7 ± 0.44 | 43.3 ± 0.29 jl | 3.03 | |||
| MW/US-EtOH 60%after SPE i | x | x | 5.0% * | 168.8 ± 1.06 | x | x | |
| MAE | MW-sbc-H2O j | 150 | 15 | 65.4 ± 0.79 g | 67.5 ± 1.17 | 44.2 ± 0.65 hl | 3.09 |
| MW-sbc-H2O after SPE k | x | x | 11.6% * | 258.6 ± 1.16 | x | x | |
| Exhaustivemethod | EM-EtOH 75% l | 85 | 15 | 95.9 ± 1.19 | 46.1 ± 0.79 | 44.2 ± 0.18 hj | 3.09 |
| Peak | Identif. | Compound | RT (min) | λ max (nm) | λ for Eq. (nm) | Equation Curve (mg/mL) | Lin. Range (mg/mL) | R2 | LOD (mg/mL) | LOQ (mg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ST | Chlorogenic acid | 20.0 | 215, 240, 326 | 340 | y = 2,078,561.4x − 652.1 | 0.003–0.200 | 0.9998 | 0.001 | 0.003 |
| 2 | ST | p-Hydroxybenzoic acid | 21.0 | 256 | 280 | y = 1,001,749.2x + 1456.8 | 0.004–1.27 | 1.0000 | 0.001 | 0.004 |
| 3 | ST | Caffeic acid | 21.8 | 217, 240, 298, 324 | 340 | y = 4,699,291.5x − 3720.2 | 0.002–0.200 | 0.9999 | 0.001 | 0.002 |
| 4 | ST | Luteolin-3-glucoside | 23.9 | 203, 254, 348 | 340 | y = 3,376,292.6x − 5788.6 | 0.004–0.200 | 0.9999 | 0.002 | 0.004 |
| 5 | ST | p-Coumaric acid | 24.7 | 217, 235, 323 | 280 | y = 4,304,744.2x − 1271.3 | 0.0015–0.160 | 0.9999 | 0.0007 | 0.0015 |
| 6 | R * | Chicoric acid | 25.4 | 241, 305, 327 | - | - | - | - | - | - |
| 7 | ST | Apigenin-3-glucoside | 25.8 | 266, 308, 337.6 | 340 | y = = 1,870,507.7x − 434.2 | 0.003–0.200 | 0.9999 | 0.001 | 0.003 |
| Sample | Chlorogenic Acid | p-Hydroxy-benzoic Acid | Caffeic Acid | Luteolin-3-glucoside | p-Coumaric Acid | Apigenin-3-glucoside |
|---|---|---|---|---|---|---|
| M40-H2O | * <LOQ | * <LOQ | 2.83 | 1.19 | 0.69 | 1.06 |
| M40-EtOH 60% | * <LOQ | 2.55 | 1.86 | 1.39 | ** N.D. | 0.90 |
| US-H2O | * <LOQ | 2.63 | 1.96 | 1.33 | ** N.D. | 0.73 |
| US-EtOH 60% | * <LOQ | 2.72 | 1.74 | 1.06 | ** N.D. | 0.72 |
| M75-H2O | 3.52 | 1.99 | 2.45 | 1.68 | 0.64 | 1.49 |
| M75-EtOH 60% | 1.92 | 2.67 | 1.94 | 1.63 | ** N.D. | 1.16 |
| MW/US-H2O | 0.82 | 2.84 | 1.76 | 1.32 | ** N.D. | 2.76 |
| MW/US-EtOH 60% | 1.10 | 3.14 | 2.86 | 2.42 | 1.30 | 1.79 |
| MW/US-EtOH 60% after SPE | 9.75 | 6.44 | 6.17 | 5.56 | 4.02 | 6.27 |
| MW-sbc-H2O | 3.45 | 4.27 | 3.16 | 3.57 | 1.97 | 5.09 |
| MW-sbc-H2O after SPE | 12.2 | 9.35 | 8.75 | 7.06 | 4.36 | 15.8 |
| EM-EtOH 75% | 1.70 | 2.35 | 2.59 | 1.80 | 0.57 | 4.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cova, C.M.; Boffa, L.; Pistocchi, M.; Giorgini, S.; Luque, R.; Cravotto, G. Technology and Process Design for Phenols Recovery from Industrial Chicory (Chicorium intybus) Leftovers. Molecules 2019, 24, 2681. https://doi.org/10.3390/molecules24152681
Cova CM, Boffa L, Pistocchi M, Giorgini S, Luque R, Cravotto G. Technology and Process Design for Phenols Recovery from Industrial Chicory (Chicorium intybus) Leftovers. Molecules. 2019; 24(15):2681. https://doi.org/10.3390/molecules24152681
Chicago/Turabian StyleCova, Camilla Maria, Luisa Boffa, Marco Pistocchi, Silver Giorgini, Rafael Luque, and Giancarlo Cravotto. 2019. "Technology and Process Design for Phenols Recovery from Industrial Chicory (Chicorium intybus) Leftovers" Molecules 24, no. 15: 2681. https://doi.org/10.3390/molecules24152681
APA StyleCova, C. M., Boffa, L., Pistocchi, M., Giorgini, S., Luque, R., & Cravotto, G. (2019). Technology and Process Design for Phenols Recovery from Industrial Chicory (Chicorium intybus) Leftovers. Molecules, 24(15), 2681. https://doi.org/10.3390/molecules24152681