Diacetylcurcumin: Its Potential Antiarthritic Effect on a Freund’s Complete Adjuvant-Induced Murine Model
Abstract
:1. Introduction
2. Results
2.1. Acute Toxicity Studies
2.2. Anti-Inflammatory Activity
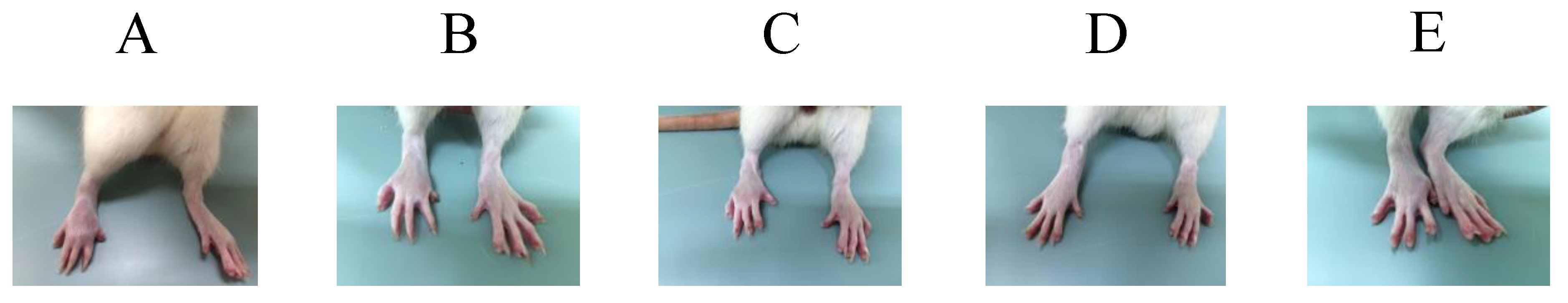
2.2.1. Behavior of Controls
2.2.2. Effect of the Oral Administration of the Diacetylcurcumin and Curcumin against FCA-Induced Arthritis
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Extraction and Purification of Curcumin
4.3. Synthesis of Diacetylcurcumin
4.4. HPLC and NMR Analysis
4.5. Pharmacological Effect
4.5.1. Animals
4.5.2. Acute Toxicity Studies
4.5.3. Anti-Inflammatory Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asteriou, E.; Gkoutzourelas, A.; Mavropoulos, A.; Katsiari, C.; Sakkas, L.I.; Bogdanos, D.P. Curcumin for the Management of Periodontitis and Early ACPA-Positive Rheumatoid Arthritis: Killing Two Birds with One Stone. Nutrients 2018, 10, 908. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- OMS 2019. Available online: https://www.who.int/chp/topics/rheumatic/en/ (accessed on 7 May 2019).
- Dudics, S.; Langan, D.; Meka, R.R.; Venkatesha, S.H.; Berman, B.M.; Che, C.T.; Moudgil, K.D. Natural products for the treatment of autoimmune arthritis: Their mechanisms of action, targeted delivery, and interplay with the host microbiome. Int. J. Mol. Sci. 2018, 19, 2508. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, S.D.; Sethi, R.; Srimal, R.C. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J. Med. Res. 1980, 71, 632–634. [Google Scholar]
- Dulbecco, P.; Savarino, V. Therapeutic potential of curcumin in digestive diseases. World J. Gastroenterol. 2013, 19, 9256–9270. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a mayor constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–143. [Google Scholar]
- Taylor, R.A.; Leonard, M.C. Curcumin for inflammatory bowel disease: A review of human studies. Altern. Med. Rev. 2011, 16, 152–156. [Google Scholar]
- Huang, G.; Xu, Z.; Huang, Y.; Duan, X.; Gong, W.; Zhang, Y.; Fan, J.; He, F. Curcumin protects against collagen-induced arthritis via suppression of BAFF production. J. Clin. Immunol. 2013, 33, 550–557. [Google Scholar] [CrossRef]
- Kamarudin, T.A.; Othman, F.; Mohd Ramli, E.S.; Md Isa, N.; Das, S. Protective effect of curcumin on experimentally induced arthritic rats: Detailed histopathological study of the joints and white blood cell count. EXCLI J. 2012, 11, 226–236. [Google Scholar] [PubMed]
- Yang, Y.; Wu, X.; Wei, Z.; Dou, Y.; Zhao, D.; Wang, T.; Bian, D.; Tong, B.; Xia, Y.; Xia, Y.; et al. Oral curcumin has anti-arthritic efficacy through somatostatin generation via cAMP/PKA and Ca2+/CaMKII signaling pathways in the small intestine. Pharmacol. Res. 2015, 95–96, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Banji, D.; Pinnapureddy, J.; Banji, O.J.F.; Saidulu, A.; Hayath, M.S. Synergistic activity of curcumin with methotrexate in ameliorating freund’s complete adjuvant induced arthritis with reduced hepatotoxicity in experimental animals. Eur. J. Pharmacol. 2011, 668, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Li, X. Curcumin, and active constituent of the ancient medicinal herb Curcuma longa L.: Some uses and the establishment and biological basis of medical efficacy. CNS Neurol. Disord. Drug Targets 2013, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Slifirski, P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim. Pol. 2012, 59, 201–212. [Google Scholar] [CrossRef]
- Srimal, R.C.; Dhawan, B.N. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef]
- Qian, J.; Chen, X.; Shu, S.; Zhang, W.; Fang, B.; Chen, X.; Zhao, Y.; Liu, Z.; Liang, G. Design and synthesis novel di-carbonyl analogs of curcumin (DACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI). Eur. J. Med. Chem. 2019, 167, 414–425. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Orlandi Sardi, J.C.; Polaquini, C.R.; Almeida Freires, I.; Câmara de Calvalho Galvâo, L.; Goldoni Lazarini, J.; Silva Torrezan, G.; Regasini, L.O.; Luiz Rosalen, P. Antibacterial activity of diacetylcurcumin against Staphylococcus aureus results in decreased biofilm and cellular adhesion. J. Med. Microbiol. 2017, 66, 816–824. [Google Scholar]
- Mishra, S.; Karmodiya, K.; Surolia, N.; Surolia, A. Synthesis and exploration of novel curcumin analogues as anti-malarial agents. Bioorg. Med. Chem. 2008, 16, 2894–2902. [Google Scholar] [CrossRef]
- Sahoo, B.K.; Ghosh, K.S.; Bera, R.; Dasgupta, S. Studies on the interaction of diacetylcurcumin with thymus-DNA. Chem. Phys. 2008, 351, 163–169. [Google Scholar] [CrossRef]
- Basile, V.; Ferrari, E.; Lazzari, S.; Belluti, S.; Pignedoli, F.; Imbriano, C. Curcumin derivative: Molecular basis of their anti-cancer activity. Biochem. Pharmacol. 2009, 78, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Basu, N.; Ghatak, N.; Gujral, P.K. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982, 12, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.N.; Badyal, D.K.; Bala, S.; Toloue, M. Evaluation of the in vivo anti-inflammatory and analgesic and in vitro anti-cancer activities of curcumin and its derivatives. Nat. Prod. Commun. 2013, 8, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Bagati, A.; Gupta, J. Comparison of anti-inflammatory activity of curcumin analogues. Indian Drugs 1995, 32, 502–505. [Google Scholar]
- Escobedo-Martínez, C.; Guzmán-Gutiérrez, S.L.; Hernández-Méndez, M.M.; Cassani, J.; Trujillo-Valdivia, A.; Orozco-Castellanos, L.M.; Enríquez, R.G. Heliopsis longipes: Anti-arthritic activity evaluated in a Freund’s adjuvant-induced models in rodents. Rev. Bras. Farmacogn. 2017, 27, 214–219. [Google Scholar] [CrossRef]
- Kumar, V.L.; Roy, S. Protective effect of latex of Calotropis procera in Freund’s Complete Adjuvant induced monoarthritis. Phytother. Res. 2009, 23, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Newbould, B.B. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br. J. Pharmacol. 1963, 21, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhou, Y.; Zhan, R.; Sun, Z.; Cheng, L.F. Antiarthritic activity of Qi-Wu rheumatism granule (a Chinese herbal compound) on complete Freund’s adjuvant-induced arthritis in rats. Evid. Based Complement. Alternat. Med. 2017, 2017, 1960517. [Google Scholar] [CrossRef]
- Barlett, R.R.; Schleyerbach, R. Immunopharmacological profile of a novel isoxazole derivative, HWA 486, with potential antirheumatic activity-I. disease modifying action on adjuvant arthritis of the rat. Int. J. Immunopharmacol. 1985, 7, 7–18. [Google Scholar] [CrossRef]
- Perper, R.J.; Álvarez, B.; Colombo, C.; Schroder, H. The use of a standardized adjuvant arthritis assay to differentiate between anti-inflammatory and immunosuppressive agents. Exp. Biol. Med. 1971, 137, 506–512. [Google Scholar] [CrossRef]
- Schleyerbach, R.; Wedde, H. Alterations in the gastro-intestinal functions during the development of adjuvant disease in rats. Agents Actions. 1984, 15, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Vajragupta, O.; Boochoong, P.; Watanabe, H.; Tohda, M.; Kummasud, N.; Sumanont, Y. Manganese complexes of curcumin and its derivatives: Evaluation for the radical scavenging ability and neuroprotective activity. Free Radic. Biol. Med. 2003, 35, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animal. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Lorke, D. A New Approach to Practical Acute Toxicity Testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| 1H a | Curcumin | Diacetylcurcumin | ||||||
|---|---|---|---|---|---|---|---|---|
| Chemical Shift | Multiplicity | Integral | Coupling Constant (Hz) | Chemical Shift | Multiplicity | Integral | Coupling Constant (Hz) | |
| CH3-2‴ | s | 6 | 2.33 | s | 6 | |||
| CH3-2″ | 3.93 | s | 6 | 3.88 | s | 6 | ||
| H-4 | 5.82 | s | 1 | 5.85 | s | 1 | ||
| H-2/H-6 | 6.48 | d | 2 | J = 15.72 | 6.56 | d | 2 | J = 15.86 |
| H-5′ | 6.91 | d | 2 | J = 8.01 | 7.06 | d | 2 | J = 8.11 |
| H-2’/H-6′ | 7.07 | m | 4 | |||||
| H-2′ | 7.12 | d | 2 | J = 1.81 | ||||
| H-6′ | 7.15 | dd | 2 | J = 8.15, 1.82 | ||||
| H-1/H-7 | 7.57 | d | 2 | J = 15.72 | 7.61 | d | 2 | J = 15.78 |
| O-Haryl | 8.04 | Wide signal | 2 | |||||
| O-H | 16.11 | Wide signal | 1 | 15.85 | Wide signal | 1 | ||
| Carbon a | Chemical Shift | |
|---|---|---|
| Curcumin | Diacetylcurcumin | |
| C-2‴ | 20.87 | |
| C-2″ | 55.42 | 56.11 |
| C-4 | 100.62 | 102.01 |
| C-2′ | 109.66 | 111.63 |
| C-5′ | 114.99 | 123.48 |
| C-2 and C-6 | 120.75 | 124.45 |
| C-6′ | 122.40 | 121.27 |
| C-1′ | 126.55 | 134.15 |
| C-1 and C-7 | 140.17 | 140.14 |
| C-4′ | 147.12 | 141.49 |
| C-3′ | 148.31 | 151.59 |
| C-1‴ | 169.00 | |
| C-3 and C-5 | 182.76 | 183.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobedo-Martínez, C.; Guzmán-Gutiérrez, S.L.; Carrillo-López, M.I.; Deveze-Álvarez, M.A.; Trujillo-Valdivia, A.; Meza-Morales, W.; Enríquez, R.G. Diacetylcurcumin: Its Potential Antiarthritic Effect on a Freund’s Complete Adjuvant-Induced Murine Model. Molecules 2019, 24, 2643. https://doi.org/10.3390/molecules24142643
Escobedo-Martínez C, Guzmán-Gutiérrez SL, Carrillo-López MI, Deveze-Álvarez MA, Trujillo-Valdivia A, Meza-Morales W, Enríquez RG. Diacetylcurcumin: Its Potential Antiarthritic Effect on a Freund’s Complete Adjuvant-Induced Murine Model. Molecules. 2019; 24(14):2643. https://doi.org/10.3390/molecules24142643
Chicago/Turabian StyleEscobedo-Martínez, Carolina, Silvia Laura Guzmán-Gutiérrez, María Isabel Carrillo-López, Martha Alicia Deveze-Álvarez, Alfonso Trujillo-Valdivia, William Meza-Morales, and Raúl G. Enríquez. 2019. "Diacetylcurcumin: Its Potential Antiarthritic Effect on a Freund’s Complete Adjuvant-Induced Murine Model" Molecules 24, no. 14: 2643. https://doi.org/10.3390/molecules24142643
APA StyleEscobedo-Martínez, C., Guzmán-Gutiérrez, S. L., Carrillo-López, M. I., Deveze-Álvarez, M. A., Trujillo-Valdivia, A., Meza-Morales, W., & Enríquez, R. G. (2019). Diacetylcurcumin: Its Potential Antiarthritic Effect on a Freund’s Complete Adjuvant-Induced Murine Model. Molecules, 24(14), 2643. https://doi.org/10.3390/molecules24142643