Analysis of the Overlapped Electrochemical Signals of Hydrochlorothiazide and Pyridoxine on the Ethylenediamine-Modified Glassy Carbon Electrode by Use of Chemometrics Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Voltammetry at a Modified Electrode
2.2. Selection of Buffer Solution
2.3. Cyclic Voltammetry to Study the Adsorptivity of the Electrode Reaction
2.4. Linearity Ranges and Limits of Detection for Drugs
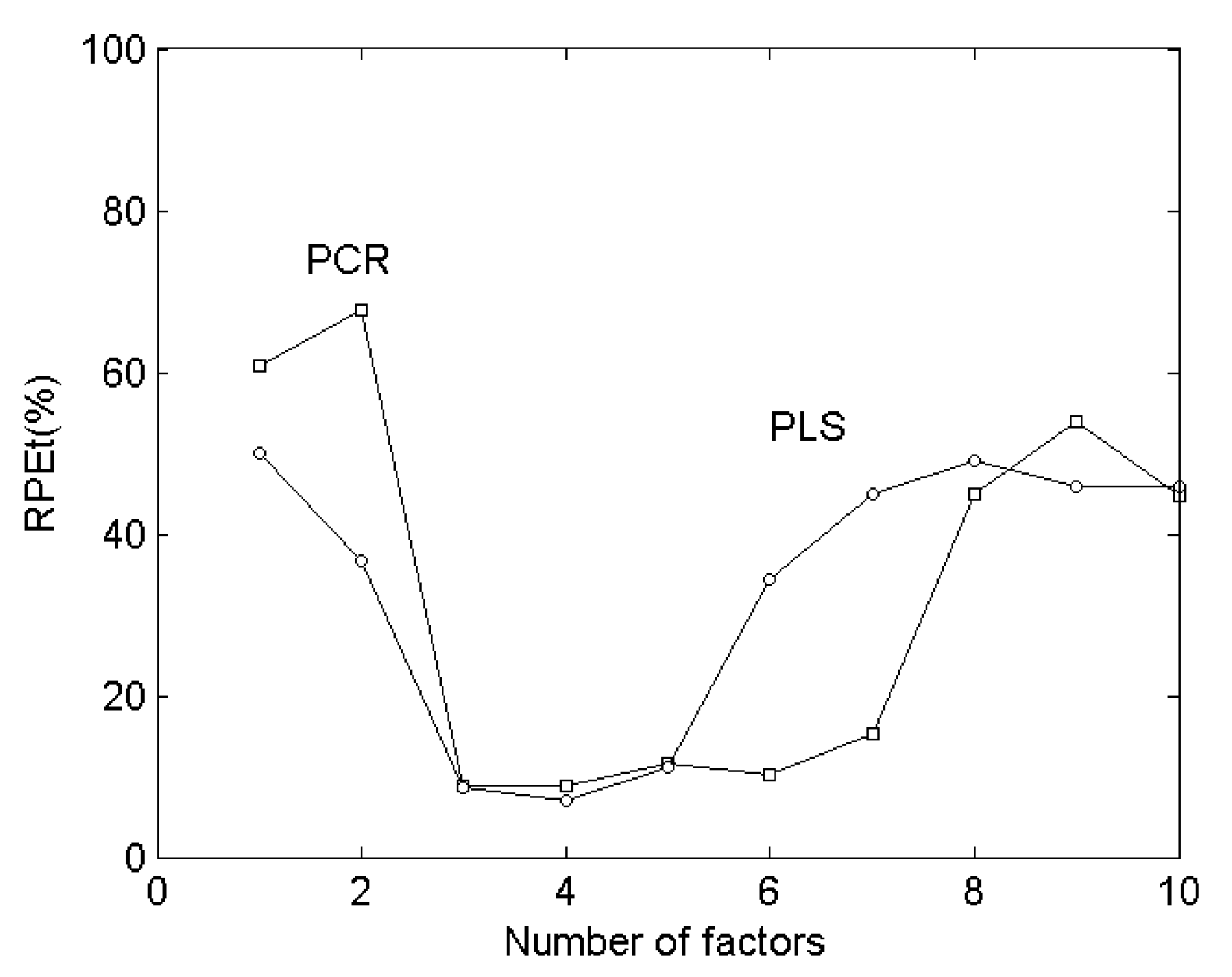
2.5. Prediction of Synthetic Mixtures of Drug Compounds
3. Detection of the Drugs in Real Samples
4. Materials and Methods
4.1. Reagents and Apparatus
4.2. Procedure
4.2.1. Modification of Glassy Carbon Electrode
4.2.2. Procedure
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global burden of hypertension: Analysis of population-based studies from 89 countries. J. Hypertens. 2015, 33, E2. [Google Scholar] [CrossRef]
- Mendis, S.; Lindholm, L.H.; Mancia, G.; Whitworth, J.; Alderman, M.; Lim, S.; Heagerty, T. World health organization (who) and international society of hypertension (ish) risk prediction charts: Assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J. Hypertens. 2007, 25, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Tientcheu, D.; Ayers, C.; Das, S.R.; McGuire, D.K.; de Lemos, J.A.; Khera, A.; Kaplan, N.; Victor, R.; Vongpatanasin, W. Target Organ Complications and Cardiovascular Events Associated With Masked Hypertension and White-Coat Hypertension: Analysis From the Dallas Heart Study. J. Am. Soc. Hypertens. 2015, 66, 2159–2169. [Google Scholar] [CrossRef][Green Version]
- Farkas, Z.C.; Chugh, P.; Frager, S.; Haq, K.F.; Khan, M.A.; Solanki, S.; Esses, E.; Veillette, G.; Bodin, R. Periampullary Variceal Bleeding: An Atypical Complication of Portal Hypertension. Case Rep. Gastrointest. Med. 2018, 2018, 4643695. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Choi, G.S.; Lim, S.M.; Kim, Y.J.; Song, T.J. Interarm Systolic and Diastolic Blood Pressure Difference Is Diversely Associated With Cerebral Atherosclerosis in Noncardioembolic Stroke Patients. Am. J. Hypertens. 2017, 31, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Webster, C.; Gallacher, J. Neighbourhood walkability and incidence of hypertension: Findings from the study of 429,334 UK Biobank participants. Int. J. Hyg. Envir. Heal. 2018, 221, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Neutel, J. State of Hypertension Control. In Combination Therapy in Hypertension; Springer Healthcare Ltd.: Orange, CA, USA, 2011; pp. 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Wang, L.; Zhang, M.; Huang, Z.; Deng, Q.; Zhou, M.; Chen, Z.; Wang, L. Burden of hypertension in China: A nationally representative survey of 174,621 adults. Int. J. Cardiol. 2017, 227, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Berzigotti, A.; Garcia-Pagan, J.C.; Abraldes, J.G. The management of portal hypertension: Rational basis, available treatments and future options☆. J. Hepatol. 2008, 48, S68–S92. [Google Scholar] [CrossRef]
- Tavassoli, Z.; Taghdir, M.; Ranjbar, B. Renin inhibition by soyasaponin I: A potent native anti-hypertensive compound. J. Biomol. Struct. Dyn. 2018, 36, 166–176. [Google Scholar] [CrossRef]
- Oparil, S.; Schmieder, R.E. New approaches in the treatment of hypertension. Circ. Res. 2015, 116, 1074–1095. [Google Scholar] [CrossRef]
- Wenyi, F.; Yiqun, W.U.; Yang, C.; Yang, C.; Xueying, Q.; Xun, T.; Dafang, C.; Siyan, Z.; Yonghua, H. The efficacy and safety of compound anti-hypertensive tablets (NO.O) in patients with elderly isolated systolic hypertension. Mod. Prev. Med. 2012, 39, 1008–1010. [Google Scholar]
- Ma, L.; Han, R.; Li, L.; Li, Z.; Sun, F.; Diao, L.; Tang, Z. Trends in the prevalence of antihypertensive drug treatment in the Beijing Longitudinal Study of Aging. Arch. Gerontol. Geriat. 2018, 74, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Huang, J. Clinical Application and Research Progress of the Compound Antihypertensive. Drug Eval. 2011, 08, 8–13. [Google Scholar] [CrossRef]
- Li, B.; Yang, Z.B.; Lei, S.S.; Su, J.; Jin, Z.W.; Chen, S.H.; Lv, G.Y. Combined Antihypertensive Effect of Paeoniflorin Enriched Extract and Metoprolol in Spontaneously Hypertensive Rats. Pharmacogn. Mag. 2018, 14, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Gao, J.L.; Jin, G.Q. Effects and Expense of Blood Pressure Control on Hypertensive Patients Using Different Treatment Methods: A Comparative Study. Chin. Gen. Pract. 2016, 19, 96–99. [Google Scholar] [CrossRef]
- Lu, X.M. Simultaneous Determination of Content and Uniformity of Hydrochlorothiazide and Promethazine Hydro- chloride in Compound Kendir Leaves Tablets I by HPLC. Chin. Pharm. 2014, 17, 1891–1893. [Google Scholar]
- Long, Z.; Gamache, P.; Guo, Z.; Pan, Y.; Zhang, Y.; Liu, X.; Jin, Y.; Liu, L.; Liang, L.; Li, R. A highly sensitive high performance liquid chromatography-electrochemical detection method for the determination of five phenolic compounds from salvia miltiorrhiza. Se pu Chin. J. Chromatogr. 2017, 35, 897–905. [Google Scholar] [CrossRef]
- Sanford, A.A.; Isenberg, S.L.; Carter, M.D.; Mojica, M.A.; Mathews, T.P.; Harden, L.A.; Takeoka, G.R.; Thomas, J.D.; Pirkle, J.L.; Johnson, R.C. Quantitative HPLC-MS/MS analysis of toxins in soapberry seeds: Methylenecyclopropylglycine and hypoglycin A. Food Chem. 2018, 264, S0308814618307349. [Google Scholar] [CrossRef]
- Wilson, I.D.; Plumb, R.; Granger, J.; Major, H.; Williams, R.; Lenz, E.M. Hplc-ms-based methods for the study of metabonomics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 817, 67–76. [Google Scholar] [CrossRef]
- Kotrebai, M.; Birringer, M.; Tyson, J.F.; Block, E.; Uden, P.C. Selenium speciation in enriched and natural samples by hplc-icp-ms and hplc-esi-ms with perfluorinated carboxylic acid ion-pairing agents. Analyst 2000, 125, 71–78. [Google Scholar] [CrossRef]
- Her, N.; Amy, G.; Mcknight, D.; Sohn, J.; Yoon, Y. Characterization of dom as a function of mw by fluorescence eem and hplc-sec using uva, doc, and fluorescence detection. Water Res. 2003, 37, 4295–4303. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, L.; Lin, J. Post-column detection of benzenediols and 1,2,4-benzenetriol based on acidic potassium permanganate chemiluminescence. Talanta 2006, 68, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Gil-Alegre, M.E.; Barone, M.L.; Torres-Suárez, A.L. Extraction and determination by liquid chromatography and spectrophotometry of naloxone in microparticles for drug-addiction treatment. J. Sep. Sci. 2005, 28, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ji, M.; Fang, X.; Liu, Y.; Yu, Z.; Cao, Y.; Sun, A.; Zhao, L.; Zhang, Y. Capillary electrophoresis—Mass spectrometry metabolomics analysis revealed enrichment of hypotaurine in rat glioma tissues. Anal. Biochem. 2017, 537. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, R.; King, R.C.; Olah, T.V.; Merkle, K. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun. Mass Spectrom. 1999, 13, 1175–1185. [Google Scholar] [CrossRef]
- Erdem, A.; Karadeniz, H.; Caliskan, A. Dendrimer modified graphite sensors for detection of anticancer drug Daunorubicin by voltammetry and electrochemical impedance spectroscopy. Analyst 2011, 136, 1041–1045. [Google Scholar] [CrossRef]
- Ferris, M.J.; Calipari, E.S.; Yorgason, J.L.; Jones, S.R. Examining the Complex Regulation and Drug-InducedPlasticity of Dopamine Release and Uptake Using Voltammetry in BrainSlices. ACS Chem. Neurosci. 2013, 4, 693–703. [Google Scholar] [CrossRef]
- Norouzi, P.; Haji-Hashemi, H.; Larijani, B.; Aghazadeh, M.; Pourbasheer, E.; Ganjali, M.R. Application of New Advanced Electrochemical Methods Combine with Nano-Based Materials Sensor in Drugs Analysis. Curr. Anal. Chem. 2016, 13, 70–80. [Google Scholar] [CrossRef]
- Dinc, E. Spectral analysis of benazepril hydrochloride and hydrochlorothiazide in pharmaceutical formulations by three chemometric techniques. Anal. Lett. 2002, 35, 1021–1039. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhang, Z.L.; Pang, D.W. Electrochemical oxidation of theophylline at multi-wall carbon nanotube modified glassy carbon electrodes. J. Electroanal. Chem. 2005, 581, 303–309. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Kokulnathan, T.; Chen, S.M.; Karthik, R.; Chiu, T.W. Ecofriendly preparation of graphene sheet decorated with ethylenediamine copper (II) complex composite fabricated electrode for selective detection of hydroquinone in water. Inorg. Chem. Front. 2017, 5, 490–500. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Chen, Q.; Wang, Y.; Fu, Y. Selective response of antigen-antibody reactions on chiral surfaces modified with 1,2-diphenylethylenediamine enantiomers. Surf. Interface Anal. 2012, 44, 170–174. [Google Scholar] [CrossRef]
- Yang, G.; Liu, B.; Dong, S. Covalent modification of glassy carbon electrode during electrochemical oxidation process of 4-aminobenzylphosphonic acid in aqueous solution. J. Electroanal. Chem. 2005, 585, 301–305. [Google Scholar] [CrossRef]
- Sumalekshmy, S.; Gopidas, K.R. Reaction of aromatic amines with Cu(ClO4)2 in acetonitrile as a facile route to amine radical cation generation. Chem. Phys. Lett. 2005, 413, 294–299. [Google Scholar] [CrossRef]
- Morris, S.A.; Wang, J.; Zheng, N. The Prowess of Photogenerated Amine Radical Cations in Cascade Reactions: From Carbocycles to Heterocycles. Acc. Chem. Res. 2016, 49, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, J.; Nguyen, T.H.; Zheng, N. The chemistry of amine radical cations produced by visible light photoredox catalysis. Beilstein J. Org. Chem. 2013, 9, 1977–2001. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Jia, N.; Chen, L.; Tan, L.; Yao, S. Electrochemical impedance spectroscopy study on polymerization of\r, l\r, -lysine on electrode surface and its application for immobilization and detection of suspension cells. Anal. Chem. 2014, 86, 6940–6947. [Google Scholar] [CrossRef] [PubMed]
- Tabaraki, R.; Khodabakhshi, M. Multidye Biosorption: Wavelet Neural Network Modeling and Taguchi L 16 Orthogonal Array Design. Clean Soil Air Water 2017, 45, 1500499. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, L.; Kokot, S. Simultaneous determination of nitrobenzene and nitro-substituted phenols by differential pulse voltammetry and chemometrics. Anal. Chim. Acta 2001, 431, 101–113. [Google Scholar] [CrossRef]
- Ghasemi, J.; Niazi, A. Simultaneous determination of cobalt and nickel. comparison of prediction ability of pcr and pls using original, first and second derivative spectra. Microchem. J. 2001, 68, 1–11. [Google Scholar] [CrossRef]
- Crouch, S.R.; Coello, J.; Maspoch, S.; Porcel, M. Evaluation of classical and three-way multivariate calibration procedures in kinetic-spectrophotometric analysis. Anal. Chim. Acta 2000, 424, 115–126. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Elghobashy, M.R.; Yehia, A.M.; Mostafa, A.A. Simultaneous determination of metformin hydrochloride and pioglitazone hydrochloride in binary mixture and in their ternary mixture with pioglitazone acid degradate using spectrophotometric and chemometric methods. Drug Test. Anal. 2010, 1, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Henseler, J.; Hubona, G.; Ray, P. Using PLS Path Modeling in New Technology Research: Updated Guidelines. Ind. Manage. Data Syst. 2016, 116, 2–20. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Zhang, R.Q.; Ge, J.; Chen, W.C.; Yang, W.Y.; Du, Y.P. Calibration transfer based on the weight matrix (CTWM) of PLS for near infrared (NIR) spectral analysis. Anal. Methods 2018, 10, 2169–2179. [Google Scholar] [CrossRef]
Sample Availability: Samples of the hydrochlorothiazide and pyridoxine are available from the authors. |


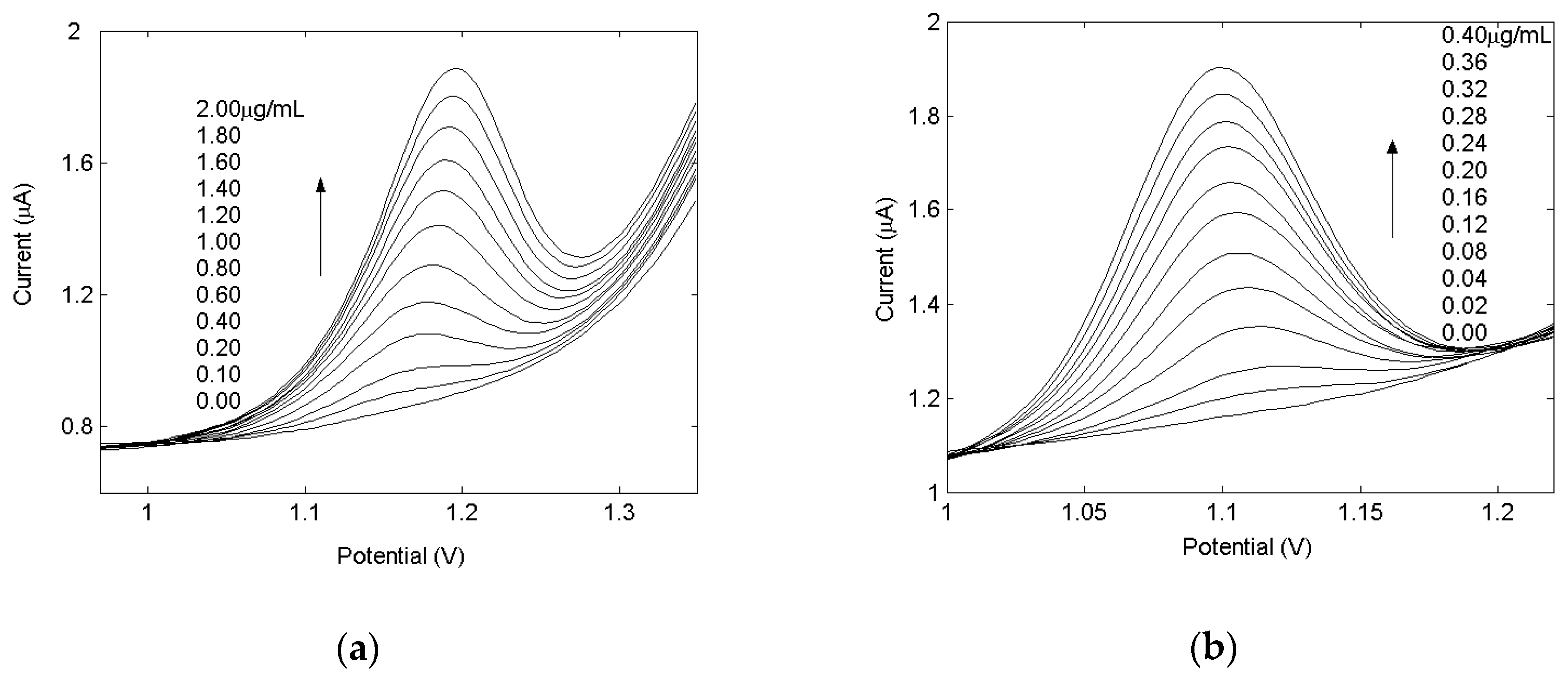
| Parameters | Pyridoxine | Hydrochlorothiazide |
|---|---|---|
| Sample number (n) | 11 | 11 |
| Linear range (μg/mL) | 0.02–0.40 | 0.10–2.0 |
| Intercept (nA) | 14.38 | 12.12 |
| Slope(nA∙mL/μg) | 0.60 | 0.20 |
| Correlation coefficient | 0.9995 | 0.9995 |
| Limit of detection (ng/mL) | 7.00 | 37.60 |
| Sample | Added | Found (CLS) | Found (PLS) 1 | Found (PCR)2 | ||||
|---|---|---|---|---|---|---|---|---|
| PX | HC | PX | HC | PX | HC | PX | HC | |
| 1 | 0.025 | 0.100 | 0.212 | 0.322 | 0.025 | 0.173 | 0.024 | 0.143 |
| 2 | 0.025 | 0.275 | 0.194 | 0.418 | 0.026 | 0.260 | 0.027 | 0.187 |
| 3 | 0.050 | 0.600 | 0.150 | 0.641 | 0.041 | 0.572 | 0.043 | 0.506 |
| 4 | 0.050 | 0.900 | 0.111 | 0.834 | 0.049 | 0.891 | 0.049 | 0.840 |
| 5 | 0.110 | 0.100 | 0.239 | 0.195 | 0.133 | 0.068 | 0.134 | 0.057 |
| 6 | 0.110 | 0.275 | 0.210 | 0.372 | 0.105 | 0.272 | 0.106 | 0.251 |
| 7 | 0.110 | 0.600 | 0.173 | 0.558 | 0.113 | 0.563 | 0.114 | 0.542 |
| 8 | 0.110 | 0.900 | 0.141 | 0.759 | 0.101 | 0.888 | 0.101 | 0.843 |
| 9 | 0.170 | 0.100 | 0.255 | 0.162 | 0.188 | 0.811 | 0.189 | 0.096 |
| 10 | 0.170 | 0.275 | 0.228 | 0.303 | 0.173 | 0.264 | 0.174 | 0.278 |
| 11 | 0.170 | 0.600 | 0.194 | 0.508 | 0.164 | 0.568 | 0.165 | 0.552 |
| 12 | 0.170 | 0.900 | 0.156 | 0.680 | 0.162 | 0.873 | 0.162 | 0.858 |
| RPES (%)3 | 51.8 | 23.2 | 8.18 | 5.47 | 8.41 | 9.33 | ||
| Recovery (%)4 | 277.4 | 134.4 | 100.1 | 99.19 | 100.5 | 91.58 | ||
| RPET (%)3 | 46.8 | 7.04 | 8.86 | |||||
| Sample | Target values | Found by this method | Recovery(%) | |||
|---|---|---|---|---|---|---|
| PX | HC | PX | HC | PX | HC | |
| Tablet 11 | 0.5 | 1.6 | 0.47 ± 0.03 | 1.48 ± 0.02 | 94.0 | 92.5 |
| Tablet 22 | 0.5 | 1.6 | 0.44 ± 0.04 | 1.69 ± 0.03 | 88.0 | 105.6 |
| Tablet 33 | 1.0 | 3.1 | 1.01 ± 0.02 | 3.29 ± 0.01 | 101.0 | 106.1 |
| Tablet 44 | 1.0 | 3.1 | 1.04 ± 0.02 | 3.25 ± 0.03 | 104.0 | 104.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, Y.; Chen, S.; You, Y.; Qiu, P.; Ni, Y. Analysis of the Overlapped Electrochemical Signals of Hydrochlorothiazide and Pyridoxine on the Ethylenediamine-Modified Glassy Carbon Electrode by Use of Chemometrics Methods. Molecules 2019, 24, 2536. https://doi.org/10.3390/molecules24142536
Zhang Y, Zhou Y, Chen S, You Y, Qiu P, Ni Y. Analysis of the Overlapped Electrochemical Signals of Hydrochlorothiazide and Pyridoxine on the Ethylenediamine-Modified Glassy Carbon Electrode by Use of Chemometrics Methods. Molecules. 2019; 24(14):2536. https://doi.org/10.3390/molecules24142536
Chicago/Turabian StyleZhang, Yue, Yan Zhou, Shujun Chen, Yashi You, Ping Qiu, and Yongnian Ni. 2019. "Analysis of the Overlapped Electrochemical Signals of Hydrochlorothiazide and Pyridoxine on the Ethylenediamine-Modified Glassy Carbon Electrode by Use of Chemometrics Methods" Molecules 24, no. 14: 2536. https://doi.org/10.3390/molecules24142536
APA StyleZhang, Y., Zhou, Y., Chen, S., You, Y., Qiu, P., & Ni, Y. (2019). Analysis of the Overlapped Electrochemical Signals of Hydrochlorothiazide and Pyridoxine on the Ethylenediamine-Modified Glassy Carbon Electrode by Use of Chemometrics Methods. Molecules, 24(14), 2536. https://doi.org/10.3390/molecules24142536






