Stable Deuterium Labeling of Histidine-Rich Lysine-Based Dendrimers
Abstract
1. Introduction
2. Synthesis and Sample Preparation
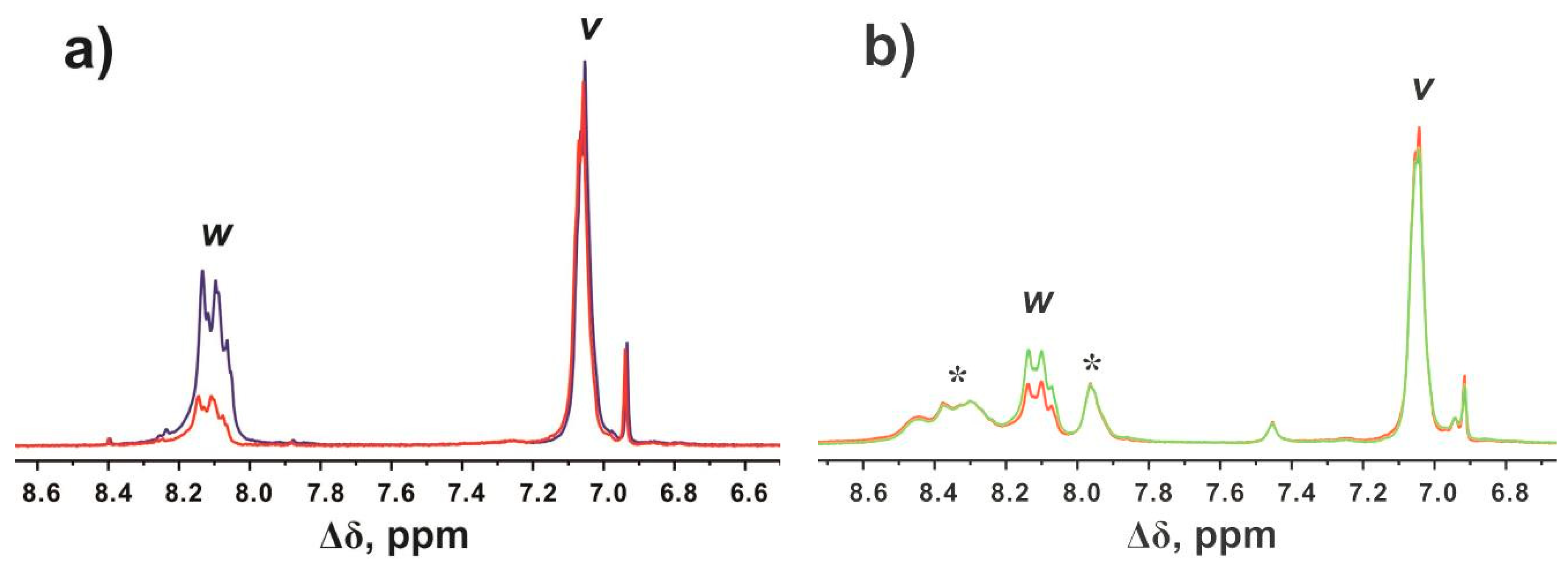
3. H and 13C Spectral Characterization
4. Deuteration (Hydrogen–Deuterium Exchange)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hsu, H.J.; Bugno, J.; Lee, S.R.; Hong, S. Dendrimer-based nanocarriers: A versatile platform for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1409. [Google Scholar] [CrossRef] [PubMed]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed]
- Lallana, E.; Sousa-Herves, A.; Fernandez-Trillo, F.; Riguera, R.; Fernandez-Megia, E. Click chemistry for drug delivery nanosystems. Pharm. Res. 2012, 29, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef]
- Felczak, A.; Wrońska, N.; Janaszewska, A.; Klajnert, B.; Bryszewska, M.; Appelhans, D.; Voit, B.; Różalska, S.; Lisowska, K. Antimicrobial activity of poly (propylene imine) dendrimers. New J. Chem. 2012, 36, 2215–2222. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Szulc, A.; Pulaski, L.; Appelhans, D.; Voit, B.; Klajnert-Maculewicz, B. Sugar-modified poly (propylene imine) dendrimers as drug delivery agents for cytarabine to overcome drug resistance. Int. J. Pharm. 2016, 513, 572–583. [Google Scholar] [CrossRef]
- Kuanga, T.; Fub, D.; Changb, L.; Yangb, Z.; Chenc, Z.; Jine, L.; Chenb, F.; Peng, X. Recent progress in dendrimer-based gene delivery systems. Curr. Org. Chem. 2016, 20, 1820–1826. [Google Scholar] [CrossRef]
- Milanizadeh, S.; Parhiz, H.; Abnous, K.; Tabatabai, S.M.; Ramezani, M.; Amel Farzad, S.; Hashemi, M. Gene delivery efficiency and cytotoxicity of heterocyclic amine-modified PAMAM and PPI dendrimers. Mater. Sci. Eng. C 2016, 61, 791–800. [Google Scholar]
- Gorzkiewicz, M.; Appelhans, D.; Boye, S.; Lederer, A.; Voit, B.; Klajnert-Maculewicz, B. Effect of the structure of therapeutic adenosine analogues on stability and surface electrostatic potential of their complexes with poly (propyleneimine) dendrimers. Macromol. Rapid Commun. 2019, 1900181. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.S.; Yu, G.S.; Bae, Y.M.; Kim, J.Y.; Choi, J.S.; Han, J. Amino acid-modified bioreducible poly(amidoamine) dendrimers: Synthesis, characterization and in vitro evaluation. Macromol. Res. 2012, 20, 1156–1162. [Google Scholar]
- Mutalik, S.; Nayak, U.Y.; Kalra, R.; Kumar, A.; Kulkarni, R.V.; Parekh, H.S. Sonophoresis-mediated permeation and retention of peptide dendrimers across human epidermis. Ski. Res. Technol. 2012, 18, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Neelov, I.M.; Janaszewska, A.; Klajnert, B.; Bryszewska, M.; Makova, N.Z.; Hicks, D.; Pearson, H.A.; Vlasov, G.P.; Ilyash, M.Y.; Vasilev, D.S.; et al. Molecular properties of lysine dendrimers and their Interactions with Aβ-peptides and neuronal cells. Curr. Med. Chem. 2013, 20, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Neelov, I.; Falkovich, S.; Markelov, D.; Paci, E.; Darinskii, A.; Tenhu, H. Molecular dynamics of lysine dendrimers. computer simulation and NMR. In Dendrimers in Biomedical Applications; The Royal Society of Chemistry: London, UK, 2013; pp. 99–114. ISBN 978-1-84973-611-4. [Google Scholar]
- Neelov, I.M.; Markelov, D.A.; Falkovich, S.G.; Ilyash, M.Y.; Okrugin, B.M.; Darinskii, A.A. Mathematical simulation of lysine dendrimers: temperature dependences. Polym. Sci. Ser. C 2013, 55, 154–161. [Google Scholar] [CrossRef]
- Markelov, D.A.; Falkovich, S.G.; Neelov, I.M.; Ilyash, M.Y.; Matveev, V.V.; Lähderanta, E.; Ingman, P.; Darinskii, A.A. Molecular dynamics simulation of spin–lattice NMR relaxation in poly-L-lysine dendrimers: Manifestation of the semiflexibility effect. Phys. Chem. Chem. Phys. 2015, 17, 3214–3226. [Google Scholar] [CrossRef] [PubMed]
- Markelov, D.A.; Dolgushev, M.; Lähderanta, E. Chapter One-NMR Relaxation in Dendrimers. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 91, pp. 1–66. ISBN 0066-4103. [Google Scholar]
- Yang, J.; Zhang, Q.; Chang, H.; Cheng, Y. Surface-engineered dendrimers in gene delivery. Chem. Rev. 2015, 115, 5274–5300. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.; Dehghan, G.; Abedi-Gaballu, F.; Kashanian, S.; Baradaran, B.; Dolatabadi, J.E.N.; Losic, D. Surface functionalized dendrimers as controlled-release delivery nanosystems for tumor targeting. Eur. J. Pharm. Sci. 2018, 122, 311–330. [Google Scholar] [CrossRef]
- LeMaster, D.M. Deuterium labelling in NMR structural analysis of larger proteins. Q. Rev. Biophys. 1990, 23, 133–174. [Google Scholar] [CrossRef]
- Sattler, M.; Fesik, S.W. Use of deuterium labeling in NMR: Overcoming a sizeable problem. Structure 1996, 4, 1245–1249. [Google Scholar] [CrossRef]
- Atzrodt, J.; Derdau, V.; Kerr, W.J.; Reid, M. Deuterium-and tritium-labelled compounds: applications in the life sciences. Angew. Chemie Int. Ed. 2018, 57, 1758–1784. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Z.; Su, C.; Zhao, X.; Gao, Q.; Ning, G.-H.; Zhu, H.; Tang, W.; Leng, K.; Fu, W.; et al. Controllable deuteration of halogenated compounds by photocatalytic D2O splitting. Nat. Commun. 2018, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Khandogin, J.; Brooks, C.L. Toward the accurate first-principles prediction of ionization equilibria in proteins. Biochemistry 2006, 45, 9363–9373. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Sugiyama, A.; Niidome, T.; Aoyagi, H. Characters of dendritic poly(L-lysine) analogues with the terminal lysines replaced with arginines and histidines as gene carriers in vitro. Biomaterials 2004, 25, 537–544. [Google Scholar] [CrossRef]
- Yu, G.S.; Bae, Y.M.; Choi, H.; Kong, B.; Choi, I.S.; Choi, J.S. Synthesis of PAMAM dendrimer derivatives with enhanced buffering capacity and remarkable gene transfection efficiency. Bioconjugate Chem. 2011, 22, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Wang, F.; Chen, Y.; Wang, H.; Cheng, Y.; Wang, Y. Synergistic effect of amino acids modified on dendrimer surface in gene delivery. Biomaterials 2014, 35, 9187–9198. [Google Scholar]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J.H.; Chapman, B.E.; Crompton, M.W.; Norton, R.S.; Teh, J.S. Hydrogen–deuterium exchange of the C-2 protons of histidine and histidine peptides and proteins. J. Chem. Soc. Perkin Trans. 2 1980, 693–700. [Google Scholar] [CrossRef]
- Cebo, M.; Kielmas, M.; Adamczyk, J.; Cebrat, M.; Szewczuk, Z.; Stefanowicz, P. Hydrogen-deuterium exchange in imidazole as a tool for studying histidine phosphorylation. Anal. Bioanal. Chem. 2014, 406, 8013–8020. [Google Scholar] [CrossRef]
- Matthews, H.R.; Matthews, K.S.; Opella, S.J. Selectively deuterated amino acid analogues synthesis, Incorporation into proteins and NMR properties. BBA-Gen. Subj. 1977, 497, 1–13. [Google Scholar] [CrossRef]
- Miyagi, M.; Nakazawa, T. Determination of pKa values of individual histidine residues in proteins using mass spectrometry. Anal. Chem. 2008, 80, 6481–6487. [Google Scholar] [CrossRef] [PubMed]
- Cobb, N.J.; Apostol, M.I.; Chen, S.; Smirnovas, V.; Surewicz, W.K. Conformational stability of mammalian prion protein amyloid fibrils is dictated by a packing polymorphism within the core region. J. Biol. Chem. 2014, 289, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Takesada, H.; Nakanishi, M.; Tsuboi, M.; Ajisaka, K. Hydrogen-deuterium exchange in the histidine residues of bovine α-lactalbumin. J. Biochem. 1976, 80, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Cesarotti, E.; Rimoldi, I.; Zerla, D.; Aldini, G. Histidine and deuterium labelled histidine by asymmetric catalytic reduction with gaseous H2 or D2; the role of strong non-coordinating acids. Tetrahedron Asymmetry 2008, 19, 273–278. [Google Scholar] [CrossRef]
- Hayashi, N.; Kuyama, H.; Nakajima, C.; Kawahara, K.; Miyagi, M.; Nishimura, O.; Matsuo, H.; Nakazawa, T. Imidazole C-2 hydrogen/deuterium exchange reaction at histidine for probing protein structure and function with matrix-assisted laser desorption ionization mass spectrometry. Biochemistry 2014, 53, 1818–1826. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyagi, M.; Wan, Q.; Ahmad, M.F.; Gokulrangan, G.; Tomechko, S.E.; Bennett, B.; Dealwis, C. Histidine hydrogen-deuterium exchange mass spectrometry for probing the microenvironment of histidine residues in dihydrofolate reductase. PLoS ONE 2011, 6, e17055. [Google Scholar] [CrossRef] [PubMed]
- Sheveleva, N.N.; Markelov, D.A.; Vovk, M.A.; Mikhailova, M.E.; Tarasenko, I.I.; Neelov, I.M.; Lähderanta, E. NMR studies of excluded volume interactions in peptide dendrimers. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Sheveleva, N.N.; Markelov, D.A.; Vovk, M.A.; Mikhailova, M.E.; Tarasenko, I.I.; Tolstoy, P.M.; Neelov, I.M.; Lahderanta, E. Lysine-based dendrimer with double arginine residues. RSC Adv. 2019, 9, 18018–18026. [Google Scholar] [CrossRef]
- Falkovich, S.; Markelov, D.; Neelov, I.; Darinskii, A. Are structural properties of dendrimers sensitive to the symmetry of branching? Computer simulation of lysine dendrimers. J. Chem. Phys. 2013, 139, 064903. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Lys-2His dendrimers are available from the authors. |
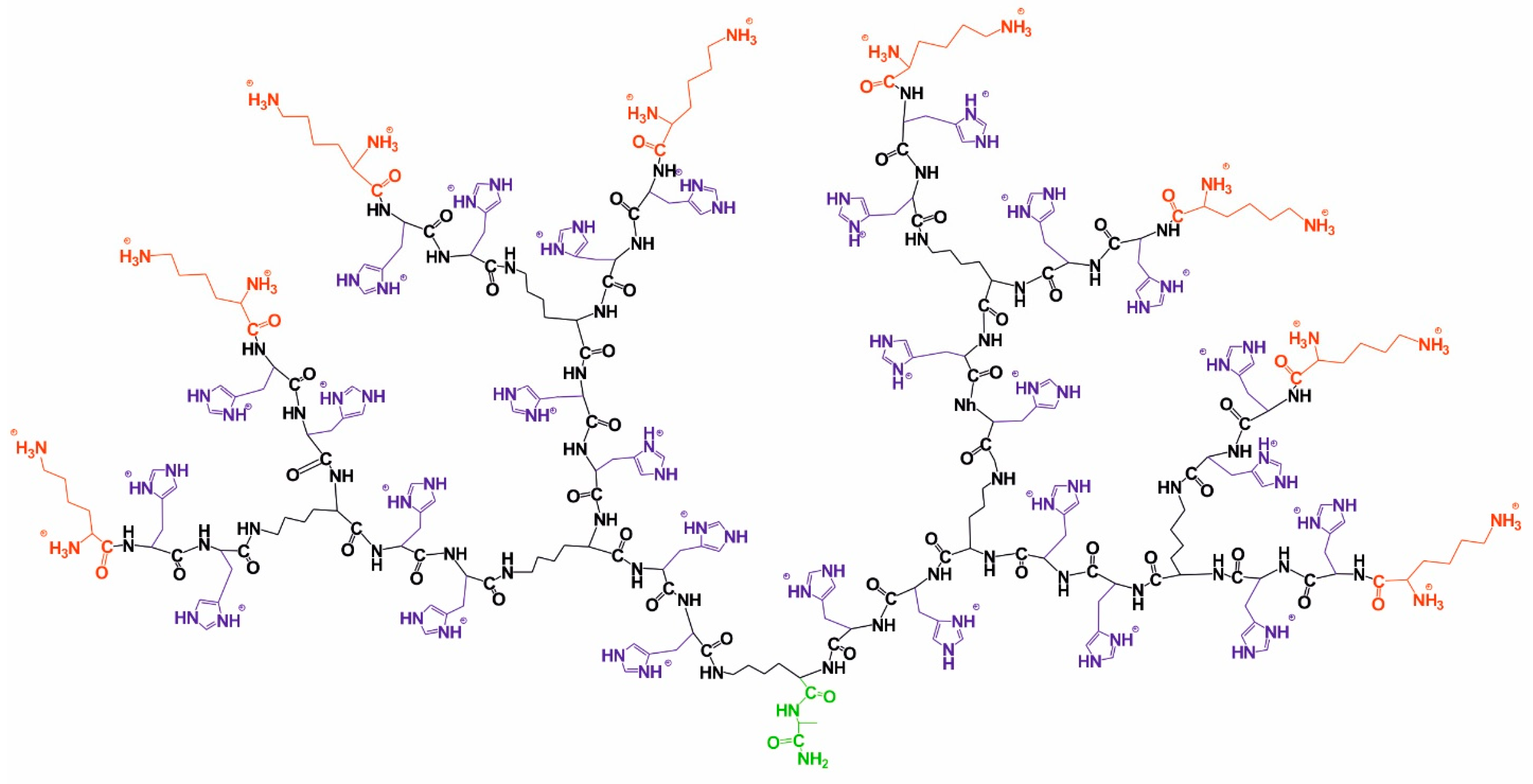
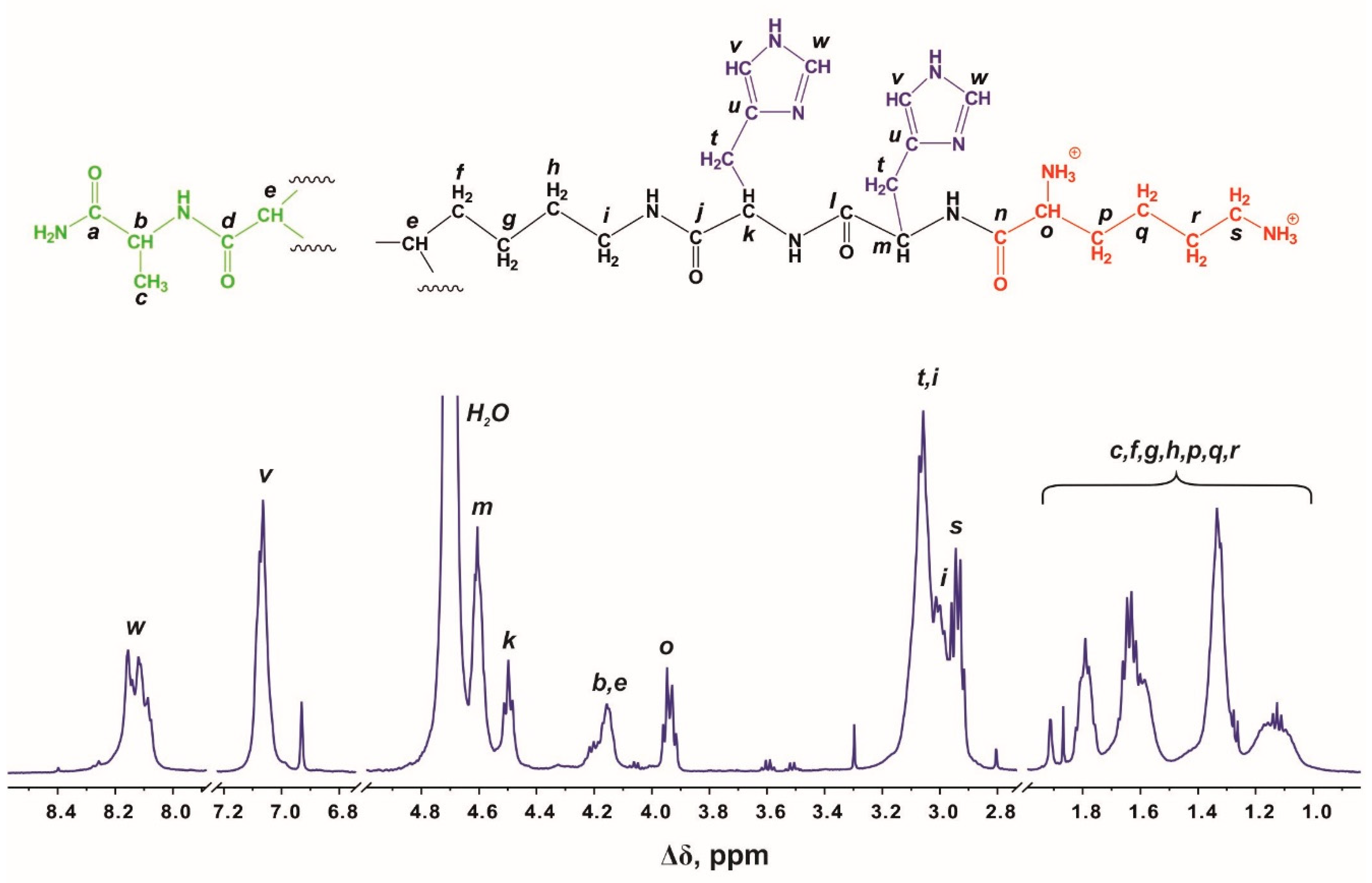
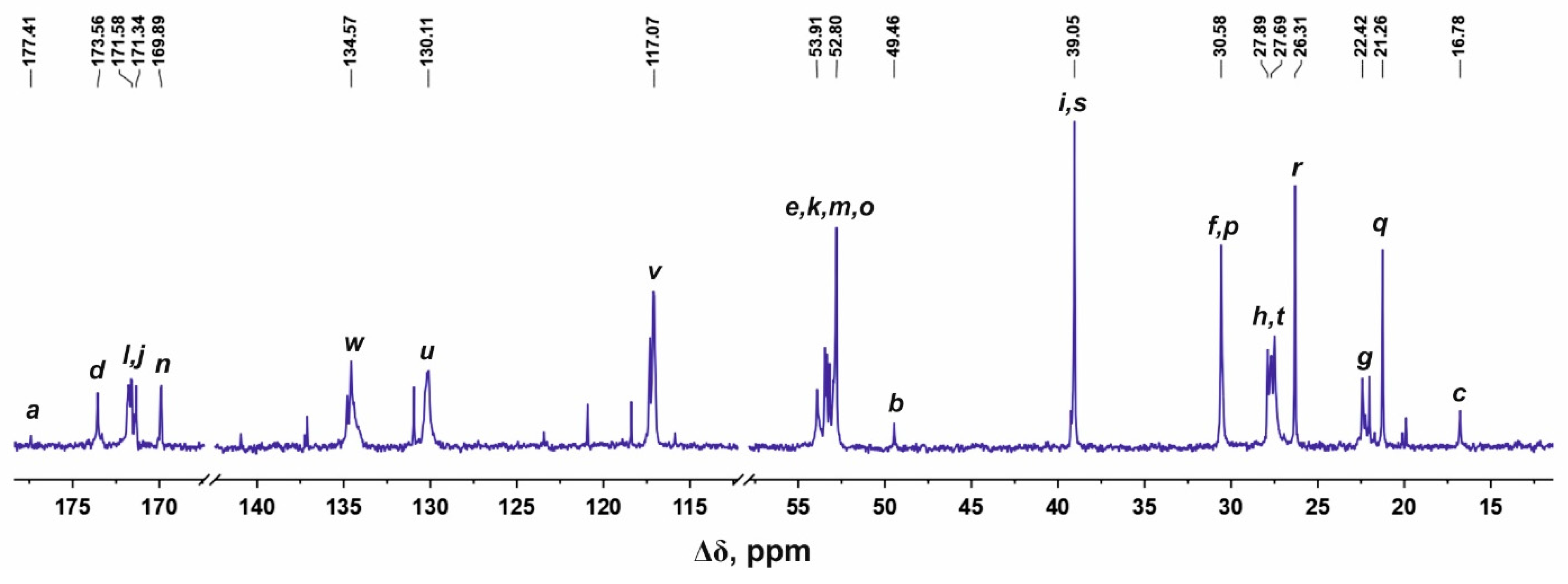
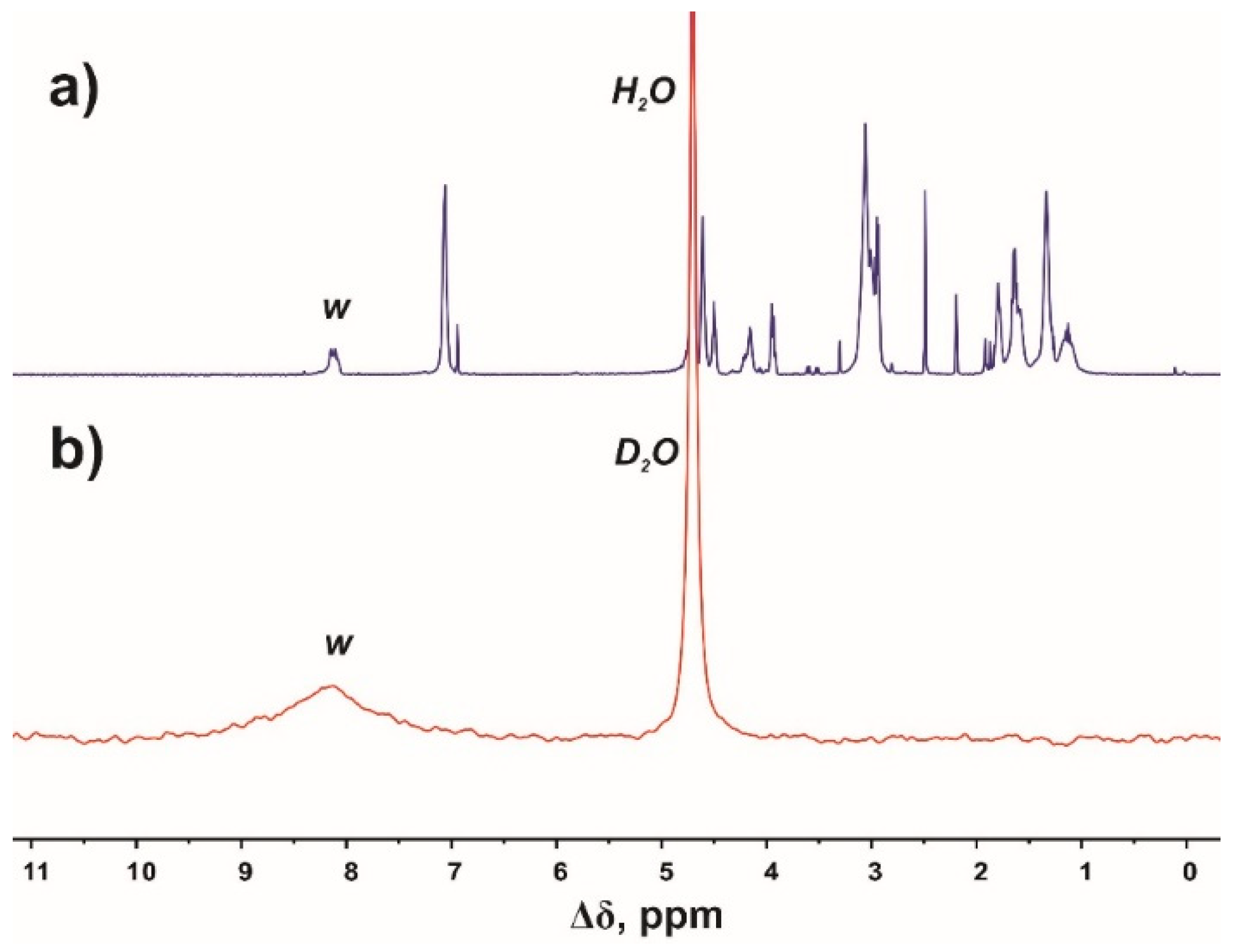

| Peak | Type of Group | Chemical Shift, ppm | Integral Value | Number of Protons in Groups |
|---|---|---|---|---|
| w | CH-(N) (in imidazole ring) | 8.12 | 26 | 28 |
| v | CH-(N) (in imidazole ring) | 7.06 | 26 | 28 |
| m,k,b,e,o | CH-(N) | 4.64–3.88 | 44 | 44 |
| i,t,s | CH2-(N) | 3.22–2.83 | 86 | 86 |
| c,f,g,h,p,q,r | CH2, CH3a | 1.93–1.00 | 102 | 90 + 3 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheveleva, N.N.; Markelov, D.A.; Vovk, M.A.; Tarasenko, I.I.; Mikhailova, M.E.; Ilyash, M.Y.; Neelov, I.M.; Lahderanta, E. Stable Deuterium Labeling of Histidine-Rich Lysine-Based Dendrimers. Molecules 2019, 24, 2481. https://doi.org/10.3390/molecules24132481
Sheveleva NN, Markelov DA, Vovk MA, Tarasenko II, Mikhailova ME, Ilyash MY, Neelov IM, Lahderanta E. Stable Deuterium Labeling of Histidine-Rich Lysine-Based Dendrimers. Molecules. 2019; 24(13):2481. https://doi.org/10.3390/molecules24132481
Chicago/Turabian StyleSheveleva, Nadezhda N., Denis A. Markelov, Mikhail A. Vovk, Irina I. Tarasenko, Mariya E. Mikhailova, Maxim Yu Ilyash, Igor M. Neelov, and Erkki Lahderanta. 2019. "Stable Deuterium Labeling of Histidine-Rich Lysine-Based Dendrimers" Molecules 24, no. 13: 2481. https://doi.org/10.3390/molecules24132481
APA StyleSheveleva, N. N., Markelov, D. A., Vovk, M. A., Tarasenko, I. I., Mikhailova, M. E., Ilyash, M. Y., Neelov, I. M., & Lahderanta, E. (2019). Stable Deuterium Labeling of Histidine-Rich Lysine-Based Dendrimers. Molecules, 24(13), 2481. https://doi.org/10.3390/molecules24132481






