Synthesis and Regeneration of A MXene-Based Pollutant Adsorbent by Mechanochemical Methods
Abstract
:1. Introduction
2. Results
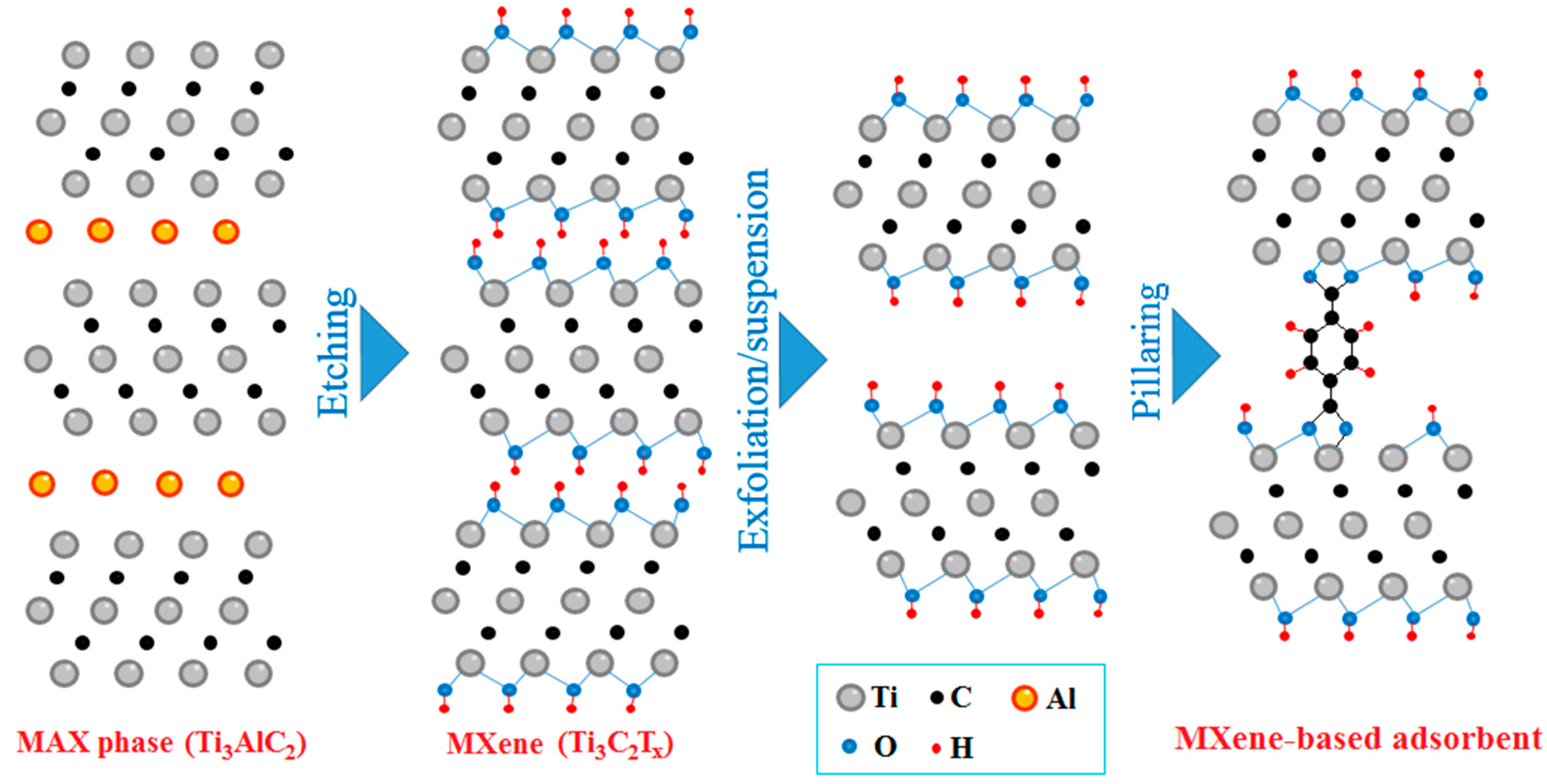
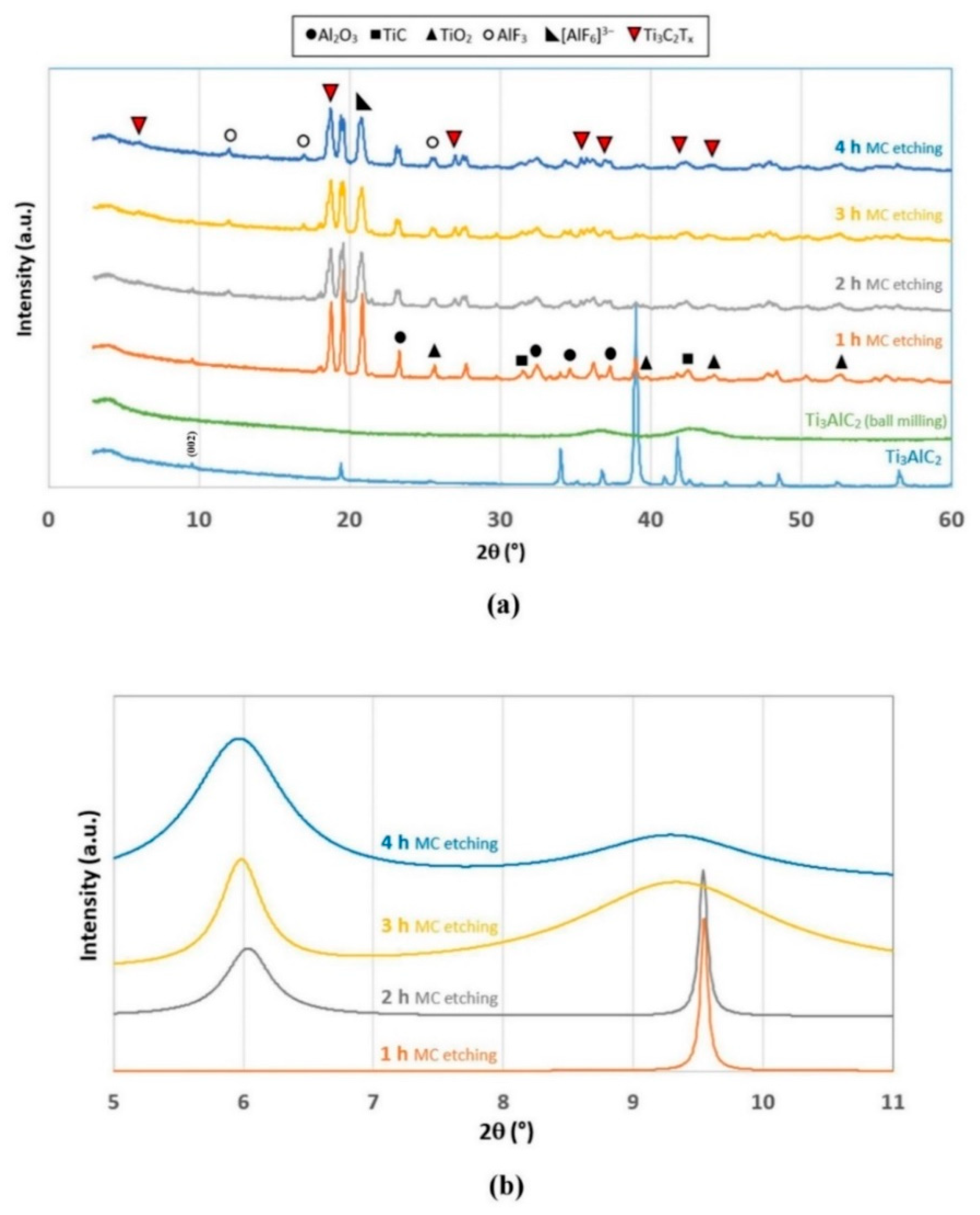
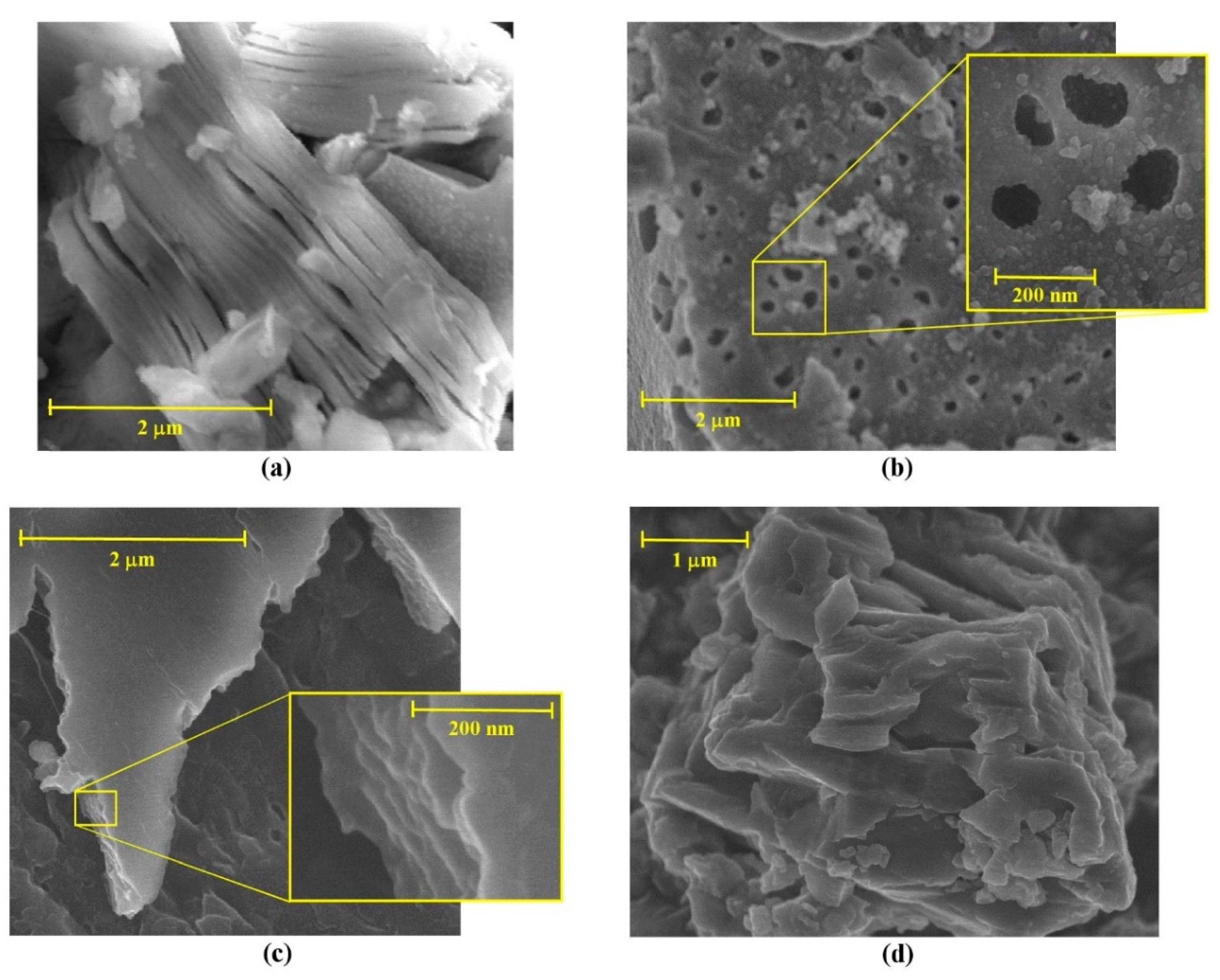
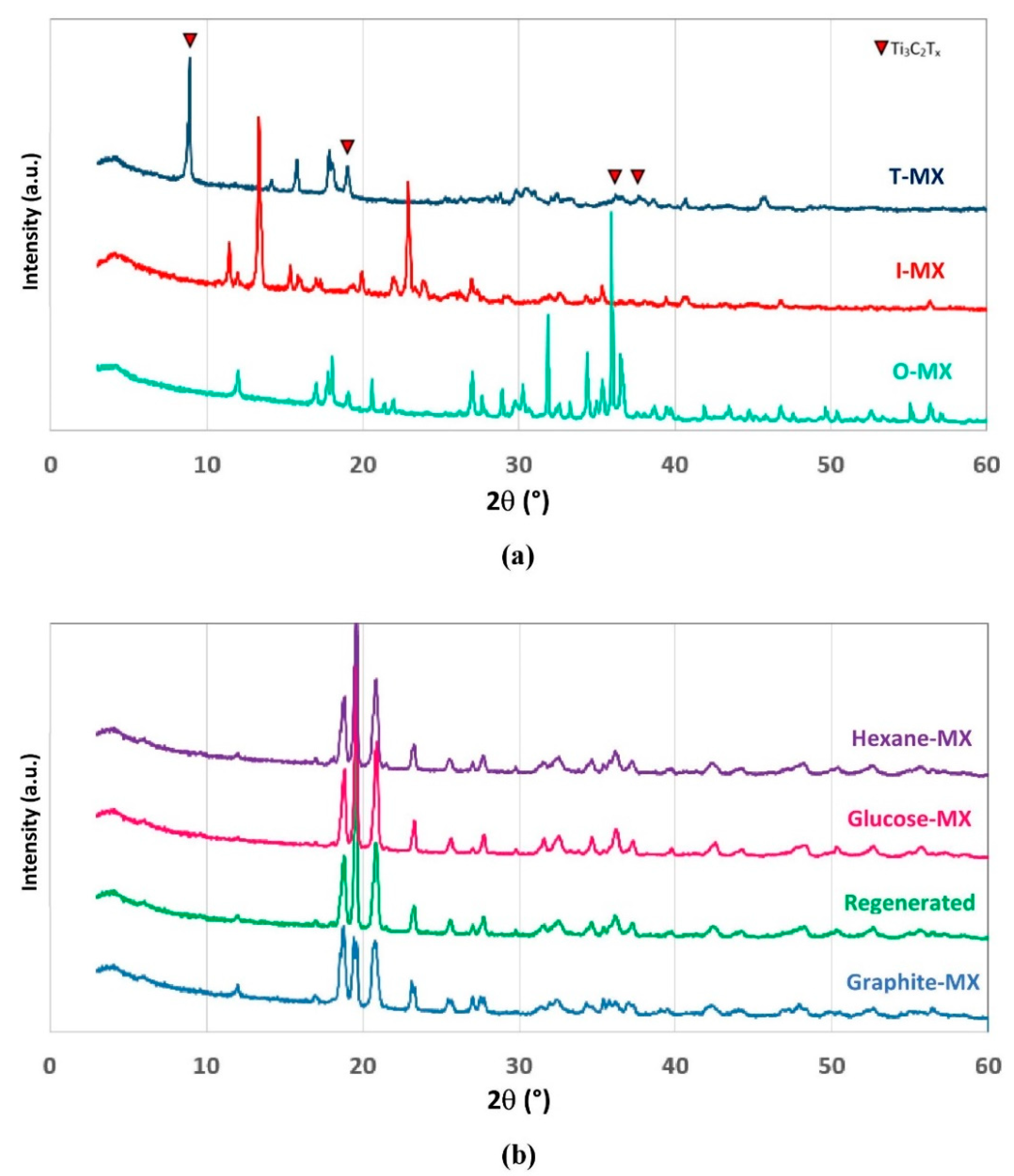
2.1. Mechanochemical Etching
2.2. Adsorbent Synthesis and Performance
2.3. Mechanochemical Regeneration of Spent Adsorbent
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mechanochemical Synthesis of Ti3AlC2 MAX Phase
4.3. Mechanochemical Etching of Ti3AlC2 MAX Phase for Ti3C2Tx MXene Synthesis
4.4. Adsorbent Preparation
4.5. Adsorption of Methylene Blue onto Terephthalate-MXene
4.6. Characterization of the Materials
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liang, L.; Li, C.; Hou, T.; Zhong, Z.; Chen, D.; Li, S.; Hu, Z.; Yang, H.; Ye, X. Preparation of poly (allylthiourea-co-acrylic acid) derived carbon materials and their applications in wastewater treatment. Molecules 2019, 24, 957. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Abdullah, A.Z.; Gholami, Z.; Salamatinia, B. Enhancing reactive blue 4 adsorption through chemical modification of chitosan with hexadecylamine and 3-aminopropyl triethoxysilane. J. Water Process. Eng. 2017, 15, 49–54. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Abdullah, A.Z.; Salamatinia, B.; Gholami, Z. Oil palm biomass as an adsorbent for heavy metals. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2014; Volume 232, pp. 61–88. ISBN 978-3-319-06746-9. [Google Scholar]
- Vakili, M.; Deng, S.; Shen, L.; Shan, D.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents for eliminating dyes from aqueous solutions. Sep. Purif. Rev. 2019, 48, 1–13. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Baláž, M.; Ficeriová, J.; Briančin, J. Influence of milling on the adsorption ability of eggshell waste. Chemosphere 2016, 146, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef]
- Lei, Z.; Cagnetta, G.; Li, X.; Qu, J.; Li, Z.; Zhang, Q.; Huang, J. Enhanced adsorption of potassium nitrate with potassium cation on H3PO4 modified kaolinite and nitrate anion into Mg-Al layered double hydroxide. Appl. Clay Sci. 2018, 154, 10–16. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, D.; Wei, F.; Chen, N.; Liang, Z.; Luo, Y. Removal of Congo red dye from aqueous solution with nickel-based metal-organic framework/graphene oxide composites prepared by ultrasonic wave-assisted ball milling. Ultrason. Sonochem. 2017, 39, 845–852. [Google Scholar] [CrossRef]
- Welham, N.J.; Setoudeh, N. Highly adsorbent carbon formed by ball milling. Carbon 2005, 43, 892–894. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Russ. Chem. Rev. 2006, 75, 177–189. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, G.; Huang, J.; Lu, M.; Wang, B.; Wang, Y.; Deng, S.; Yu, G. Defect engineered oxides for enhanced mechanochemical destruction of halogenated organic pollutants. Chemosphere 2017, 184, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Cagnetta, G.; Huang, J.; Yu, G. Mechanochemical enhancement of the natural attenuation capacity of soils using two organophosphate biocides as models. J. Hazard. Mater. 2018, 360, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-74854-0. [Google Scholar]
- Wieczorek-Ciurowa, K.; Gamrat, K. Mechanochemical syntheses as an example of green processes. J. Therm. Anal. Calorim. 2007, 88, 213–217. [Google Scholar] [CrossRef]
- Cagnetta, G.; Liu, H.; Zhang, K.; Huang, J.; Wang, B.; Deng, S.; Wang, Y.; Yu, G. Mechanochemical conversion of brominated POPs into useful oxybromides: A greener approach. Sci. Rep. 2016, 6, 28394. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, G.; Robertson, J.; Huang, J.; Zhang, K.; Yu, G. Mechanochemical destruction of halogenated organic pollutants: A critical review. J. Hazard. Mater. 2016, 313, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, G.; Huang, J.; Yu, G. A mini-review on mechanochemical treatment of contaminated soil: From laboratory to large-scale. Crit. Rev. Environ. Sci. Technol. 2018, 48, 723–771. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. MXenes: A new family of two-dimensional materials. ChemInform 2014, 26, 992–1005. [Google Scholar]
- Eklund, P.; Rosen, J.; Persson, P.O.Å. Layered ternary Mn+1AXn phases and their 2D derivative MXene: An overview from a thin-film perspective. J. Phys. D Appl. Phys. 2017, 50, 113001. [Google Scholar] [CrossRef]
- Zhu, J.F.; Qi, G.Q.; Wang, F.; Yang, H.B. Synthesis of Ti3AlC2/Al2O3 nanopowders by mechano-chemical reaction. Adv. Powder Technol. 2010, 21, 578–581. [Google Scholar] [CrossRef]
- Srivastava, P.; Mishra, A.; Mizuseki, H.; Lee, K.-R.; Singh, A.K. Mechanistic insight into the chemical exfoliation and functionalization of Ti3C2 MXene. Acs Appl. Mater. Interf. 2016, 8, 24256–24264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pan, L.; Tang, H.; Du, F.; Guo, Y.; Qiu, T.; Yang, J. Synthesis of two-dimensional Ti3C2TxMXene using HCl + LiF etchant: Enhanced exfoliation and delamination. J. Alloy. Compd. 2017, 695, 818–826. [Google Scholar] [CrossRef]
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes”. Dalton Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef] [PubMed]
- Rajavel, K.; Ke, T.; Yang, K.; Lin, D. Condition optimization for exfoliation of two dimensional titanium carbide (Ti3C2Tx). Nanotechnology 2018, 29, 095605. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Nie, P.; Wang, J.; Dou, H.; Zhang, X. Few-layer MXenes delaminated via high-energy mechanical milling for enhanced sodium-ion batteries performance. Acs Appl. Mater. Interf. 2017, 9, 39610–39617. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.-C.; Zhang, X.; Zhou, Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, N.; Zhou, Z. Adsorptive environmental applications of MXene nanomaterials: A review. Rsc Adv. 2018, 8, 19895–19905. [Google Scholar] [CrossRef]
- Xu, M.; Lei, S.; Qi, J.; Dou, Q.; Liu, L.; Lu, Y.; Huang, Q.; Shi, S.; Yan, X. Opening magnesium storage capability of two-dimensional MXene by intercalation of cationic surfactant. Acs Nano 2018, 12, 3733–3740. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yuan, H.; Jin, C.; Zhang, L.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.; et al. Pillared structure design of MXene with ultralarge interlayer spacing for high-performance lithium-ion capacitors. Acs Nano 2017, 11, 2459–2469. [Google Scholar] [CrossRef]
- Wei, Z.; Peigen, Z.; Wubian, T.; Xia, Q.; Yamei, Z.; ZhengMing, S. Alkali treated Ti3C2Tx MXenes and their dye adsorption performance. Mater. Chem. Phys. 2018, 206, 270–276. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, G.; Liu, K.; Deng, S.; Wang, B.; Huang, J.; Wang, Y. Integrated adsorption and visible-light photodegradation of aqueous clofibric acid and carbamazepine by a Fe-based metal-organic framework. Chem. Eng. J. 2017, 330, 157–165. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Ibrahim, M.H.; Abdullah, A.Z. Elimination of reactive blue 4 from aqueous solutions using 3-aminopropyl triethoxysilane modified chitosan beads. Carbohydr. Polym. 2015, 132, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Deng, S.; Li, T.; Wang, W.; Wang, W.; Yu, G. Novel crosslinked chitosan for enhanced adsorption of hexavalent chromium in acidic solution. Chem. Eng. J. 2018, 347, 782–790. [Google Scholar] [CrossRef]
- Cagnetta, G.; Huang, J.; Lomovskiy, I.O.; Yu, G. Tailoring the properties of a zero-valent iron-based composite by mechanochemistry for nitrophenols degradation in wastewaters. Environ. Technol. 2017, 38, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, G.; Huang, J.; Wang, B.; Deng, S.; Yu, G. A comprehensive kinetic model for mechanochemical destruction of persistent organic pollutants. Chem. Eng. J. 2016, 291, 30–38. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Abdullah, A.Z.; Salamatinia, B.; Gholami, Z. Chitosan hydrogel beads impregnated with hexadecylamine for improved reactive blue 4 adsorption. Carbohydr. Polym. 2016, 137, 139–146. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds MC etched MXene, T-MX, I-MX andO-MX are available from the authors |
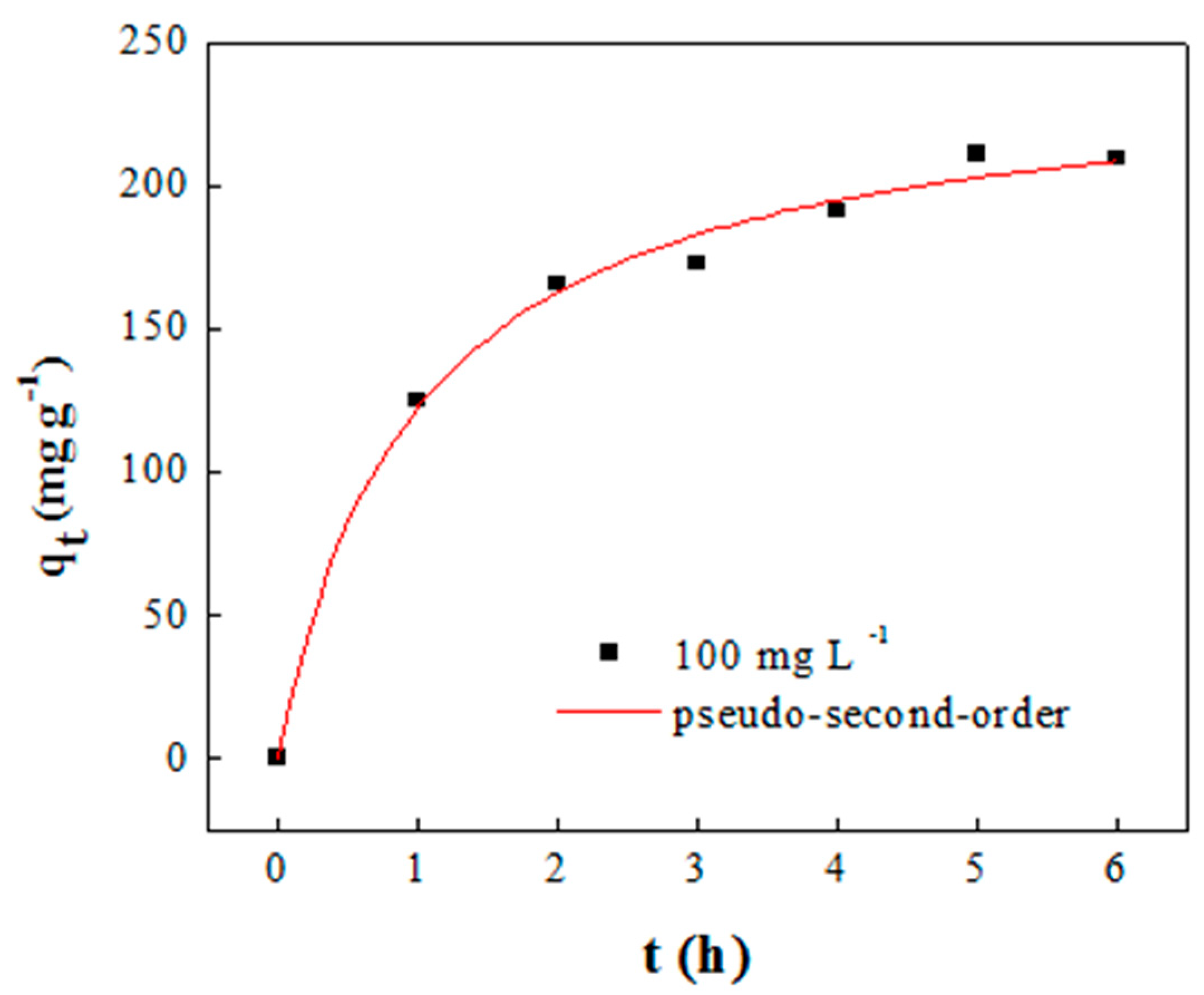
| Adsorption Capacity (mg g−1) | Pseudo-First-Order * | Pseudo-Second-Order ** | ||||||
|---|---|---|---|---|---|---|---|---|
| 209.5 | qe | K1 | R2 | χ2 | qe | v0 | R2 | χ2 |
| 242.89 | 0.837 | 0.982 | 95.35 | 205.60 | 246.77 | 0.993 | 38.58 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakili, M.; Cagnetta, G.; Huang, J.; Yu, G.; Yuan, J. Synthesis and Regeneration of A MXene-Based Pollutant Adsorbent by Mechanochemical Methods. Molecules 2019, 24, 2478. https://doi.org/10.3390/molecules24132478
Vakili M, Cagnetta G, Huang J, Yu G, Yuan J. Synthesis and Regeneration of A MXene-Based Pollutant Adsorbent by Mechanochemical Methods. Molecules. 2019; 24(13):2478. https://doi.org/10.3390/molecules24132478
Chicago/Turabian StyleVakili, Mohammadtaghi, Giovanni Cagnetta, Jun Huang, Gang Yu, and Jing Yuan. 2019. "Synthesis and Regeneration of A MXene-Based Pollutant Adsorbent by Mechanochemical Methods" Molecules 24, no. 13: 2478. https://doi.org/10.3390/molecules24132478
APA StyleVakili, M., Cagnetta, G., Huang, J., Yu, G., & Yuan, J. (2019). Synthesis and Regeneration of A MXene-Based Pollutant Adsorbent by Mechanochemical Methods. Molecules, 24(13), 2478. https://doi.org/10.3390/molecules24132478