On the Effect of Aqueous Ammonia Soaking Pre-Treatment on Continuous Anaerobic Digestion of Digested Swine Manure Fibers
Abstract
1. Introduction
2. Results and Discussion
2.1. Influent Characterization
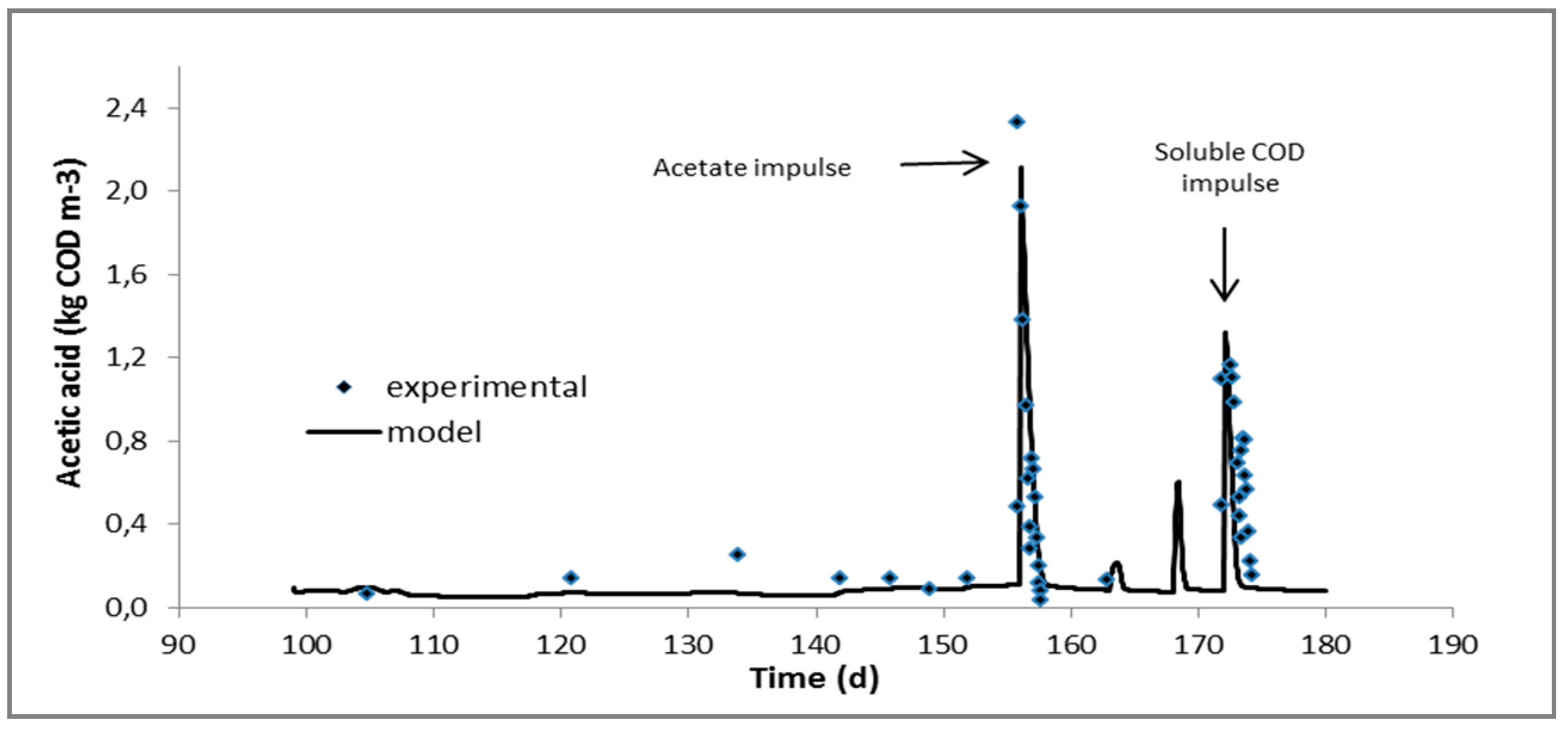
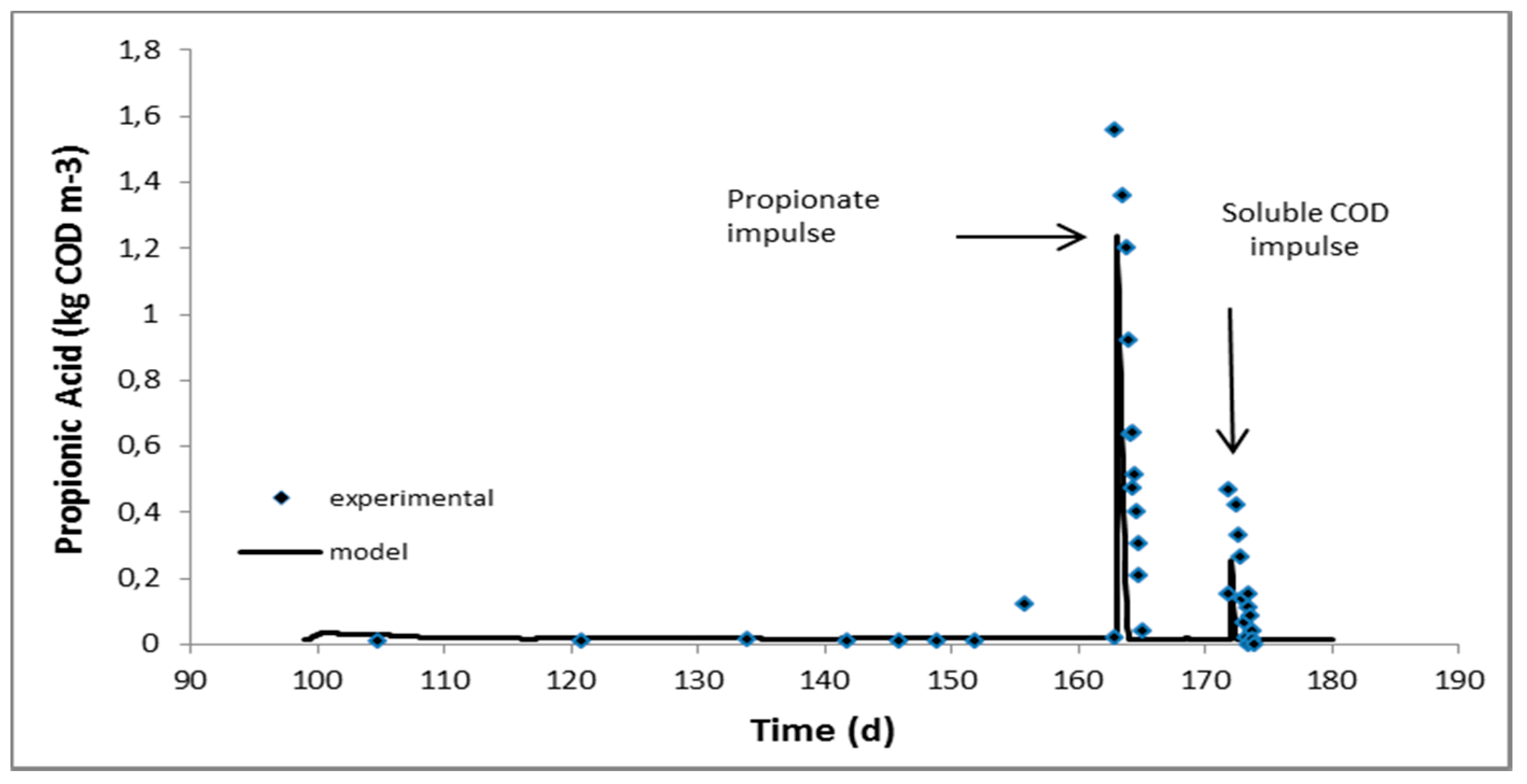
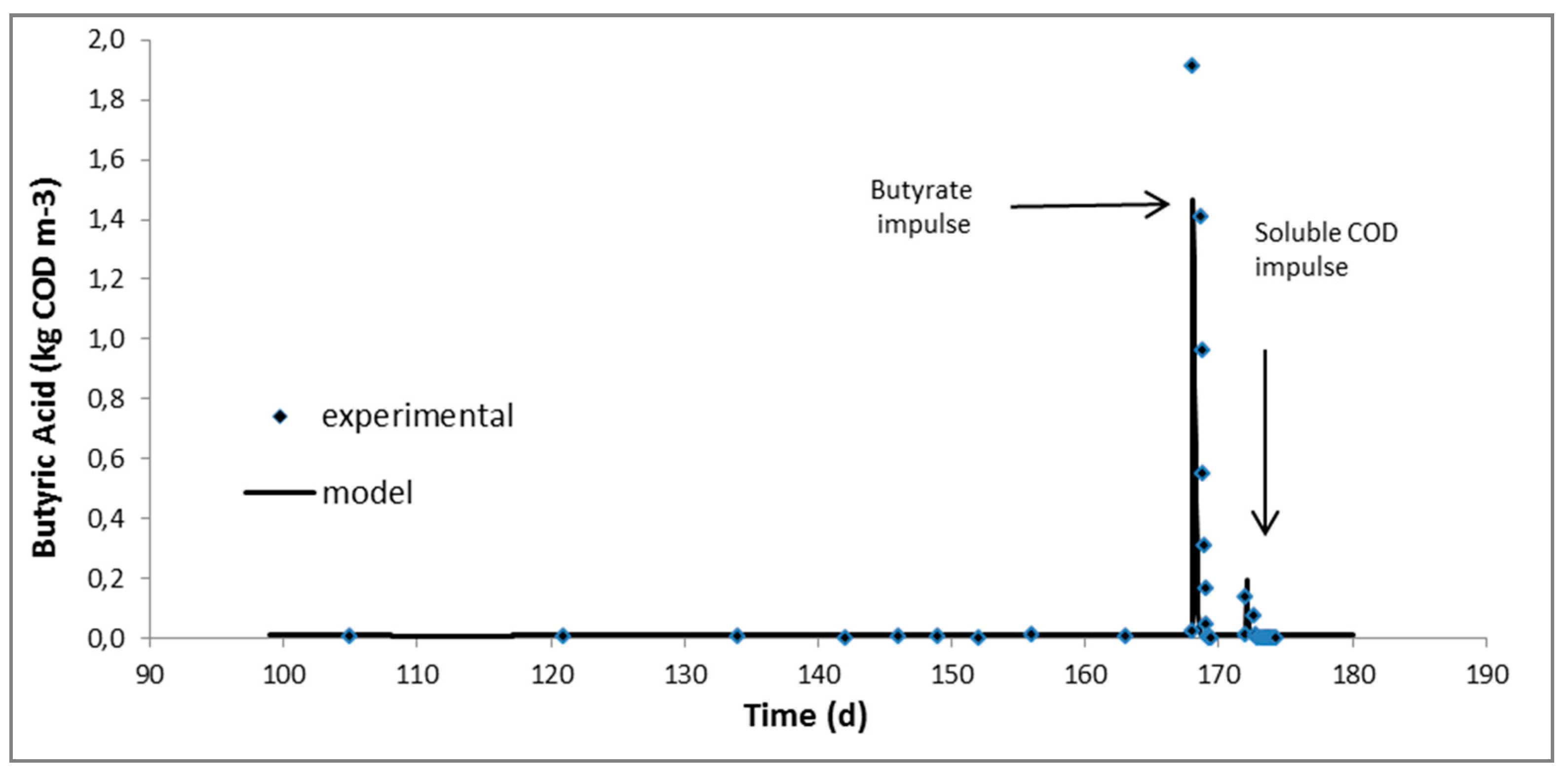
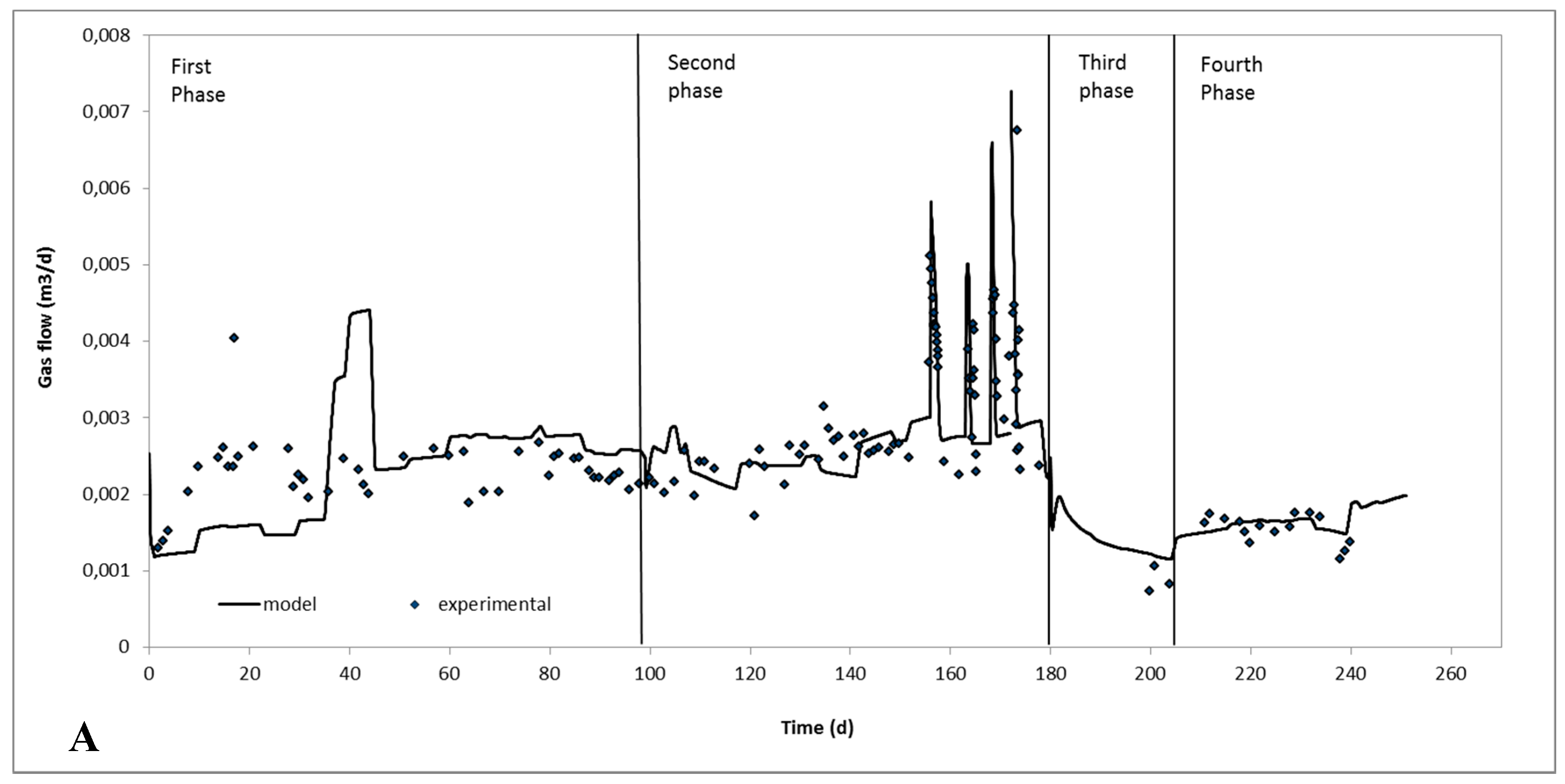
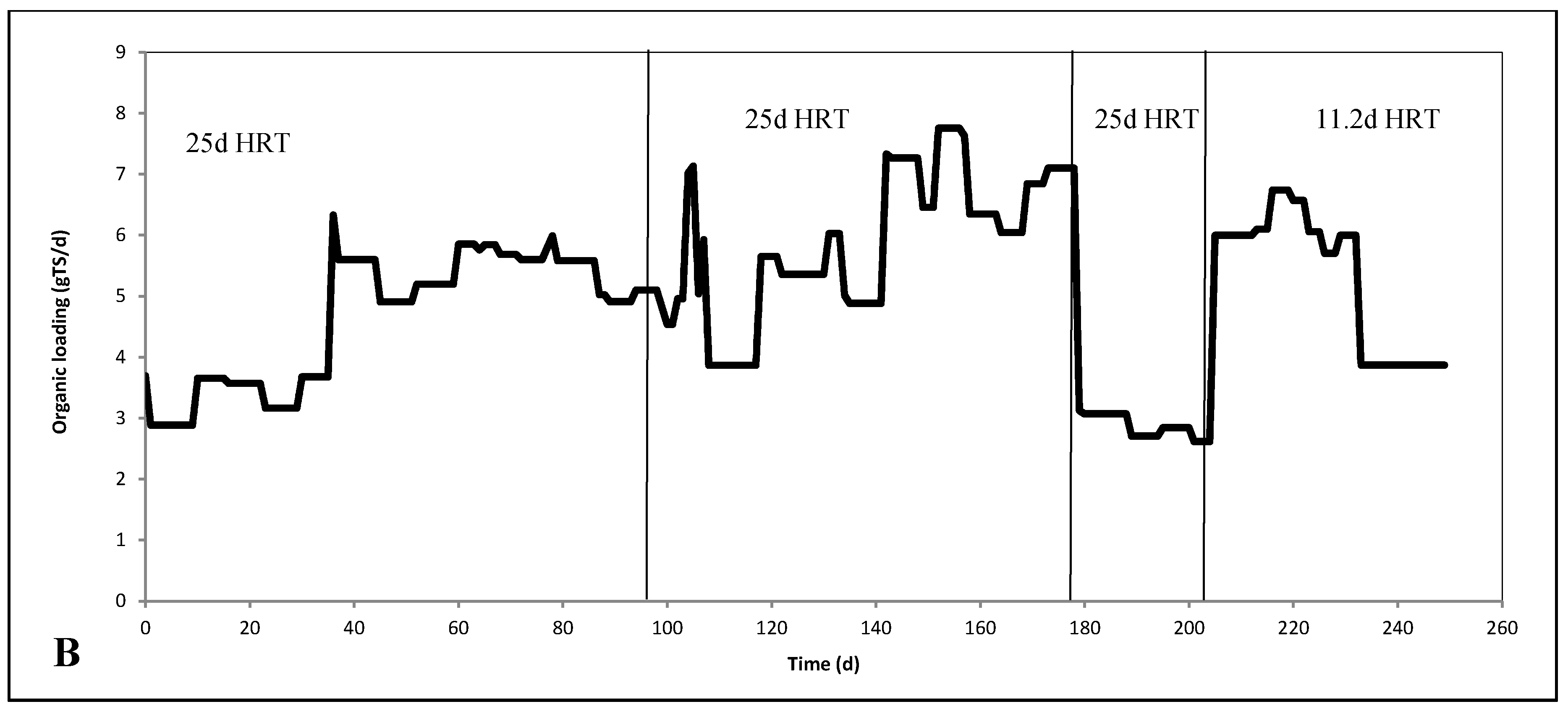
2.2. Continuous Experiments and Modeling Results
3. Materials and Methods
3.1. Feedstock
3.2. Analytical Methods
3.3. Experimental Set-Up
3.3.1. Ammonia Pre-Treatment
3.3.2. Continuous Experiments
- First Phase: manure A as influent at an HRT of 25 d.
- Second Phase: mixture of manure A and AAS-treated digested fibers (at a ratio of 0.52:0.48 on Total Solids basis) as influent at an HRT of 25 d.
- Third Phase: mixture of manure B and AAS-treated digested fibers (at a ratio of 0.52:0.48 on Total Solids basis) as influent at an HRT of 25 d.
- Fourth Phase: mixture of manure B and AAS-treated digested fibers (at a ratio of 0.52:0.48 on Total Solids basis) as influent at an HRT of 11.2 d.
3.4. Modeling
3.4.1. Correction of the HRT in the Kinetic Model
3.4.2. Organic and Inorganic Inputs in the Kinetic Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Birkmose, T.; Foged, H.L.; Hinge, J. State of Biogas Plants in European Agriculture; Danish Agricultural Advisory Service: Brussels, Belgium, 2007. [Google Scholar]
- Angelidaki, I.; Ellegaard, L. Codigestion of manure and organic wastes in centralized biogas plants. Status and Future Trends. Appl. Biochem. Biotechnol. 2003, 109, 95–105. [Google Scholar]
- Maibritt Hjorth, K.V.; Christensen, M.L.; Christensen, S.G.S. Solid-liquid Separation of Animal Slurry in Theory and Practice. In Sustainable Agriculture Volume 2; Springer: Dordrecht, The Netherlands, 2017; pp. 553–568. [Google Scholar]
- Jurado, E.; Gavala, H.N.; Rohold, L.; Skiadas, I. Cost-effective production of biogas from manure—Retrogas project. In Proceedings of the WasteEng10, Beijing, China, 17–19 May 2010; p. 224. [Google Scholar]
- Hartmann, H.; Angelidaki, I.; Ahring, B.K. Increase of anaerobic degradation of particulate organic matter in full-scale biogas plants by mechanical maceration. Water Sci. Technol. 2000, 41, 145–153. [Google Scholar]
- Bruni, E.; Jensen, A.P.; Angelidaki, I. Comparative study of mechanical, hydrothermal, chemical and enzymatic treatments of digested biofibers to improve biogas production. Bioresour. Technol. 2010, 101, 8713–8717. [Google Scholar]
- Kim, T.H.; Gupta, R.; Lee, Y. Pretreatment of Biomass by Aqueous Ammonia for bioethanol production. In Biofuels: Methods and Protocols; Springer: Dordrecht, The Netherlands, 2009; pp. 79–91. [Google Scholar]
- Jurado, E.; Skiadas, I.V.; Gavala, H.N. Enhanced methane productivity from manure fibers by aqueous ammonia soaking pretreatment. Appl. Energy 2013, 109, 104–111. [Google Scholar]
- Mirtsou-Xanthopoulou, C.; Jurado, E.; Skiadas, I.V.; Gavala, H.N. Effect of aqueous ammonia soaking on the methane yield and composition of digested manure fibers applying different ammonia concentrations and treatment durations. Energies 2014, 7, 4157–4168. [Google Scholar]
- Lymperatou, A.; Gavala, H.N.; Skiadas, I.V. Optimization of Aqueous Ammonia Soaking of manure fibers by Response Surface Methodology for unlocking the methane potential of swine manure. Bioresour. Technol. 2017, 244, 509–516. [Google Scholar]
- Gavala, H.; Angelidaki, I.; Ahring, B. Kinetics and modeling of anaerobic digestion process. In Biomethanation I; Springer: Dordrecht, The Netherlands, 2003; pp. 57–93. [Google Scholar]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No. 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar]
- Batstone, D.J.; Keller, J.; Steyer, J.P. A review of ADM1 extensions, applications, and analysis: 2002–2005. Water Sci. Technol. 2006, 54, 1–10. [Google Scholar]
- Astals, S.; Ariso, M.; Gali, A.; Mata-Alvarez, J. Co-digestion of pig manure and glycerine: Experimental and modelling study. J. Environ. Manage. 2011, 92, 1091–1096. [Google Scholar]
- Bulkowska, K.; Bialobrzewski, I.; Klimiuk, E.; Pokoj, T. Kinetic parameters of volatile fatty acids uptake in the ADM1 as key factors for modeling co-digestion of silages with pig manure, thin stillage and glycerine phase. Renew. Energy 2018, 126, 163–176. [Google Scholar]
- Gali, A.; Benabdallah, T.; Astals, S.; Mata-Alvarez, J. Modified version of ADM1 model for agro-waste application. Bioresour. Technol. 2009, 100, 2783–2790. [Google Scholar]
- Jablonski, S.J.; Biernacki, P.; Steinigeweg, S.; Lukaszewicz, M. Continuous mesophilic anaerobic digestion of manure and rape oilcake—Experimental and modelling study. Waste Manag. 2015, 35, 105–110. [Google Scholar]
- Mata-Alvarez, J.; Dosta, J.; Mace, S.; Astals, S. Codigestion of solid wastes: A review of its uses and perspectives including modeling. Crit. Rev. Biotechnol. 2011, 31, 99–111. [Google Scholar]
- Nordlander, E.; Thorin, E.; Yan, J. Investigating the possibility of applying an ADM1 based model to a full-scale co-digestion plant. Biochem. Eng. J. 2017, 120, 73–83. [Google Scholar]
- Jurado, E.; Antonopoulou, G.; Lyberatos, G.; Gavala, H.N.; Skiadas, I.V. Continuous anaerobic digestion of swine manure: ADM1-based modelling and effect of addition of swine manure fibers pretreated with aqueous ammonia soaking. Appl. Energy 2016, 172, 190–198. [Google Scholar]
- American Water Works Association; Water Environment Federation; American Public Health Association. Standard Methods for Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Haagensen, F.; Skiadas, I.V.; Gavala, H.N.; Ahring, B.K. Pre-treatment and ethanol fermentation potential of olive pulp at different dry matter concentrations. Biomass Bioenerg. 2009, 33, 1643–1651. [Google Scholar]
- Sluite, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. National Renewable Energy Laboratory (NREL) Home Page. Available online: https://www.nrel.gov/ (accessed on 4 July 2019).
- Kalfas, H.; Skiadas, I.V.; Gavala, H.N.; Stamatelatou, K.; Lyberatos, G. Application of ADM1 for the simulation of anaerobic digestion of olive pulp under mesophilic and thermophilic conditions. Water Sci. Technol. 2006, 54, 149–156. [Google Scholar]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Lyberatos, G. Modeling of fermentative hydrogen production from sweet sorghum extract based on modified ADM1. Int. J. Hydrogen Energy 2012, 37. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Characteristics, Particulate Matter | Manure A a (Used in Phases 1 and 2) | AAS-Pretreated Digested Fibers (Used in Phases 2, 3 and 4) | Manure B a (Used in Phases 3 and 4) |
|---|---|---|---|
| COD, kg/100 kgTS | 79.11 | 75.34 | 124.19 |
| Carbohydrates, kg COD/100 kg TS | 6.10 | 32.75 | 9.57 |
| Proteins, kg COD/100 kg TS | 28.00 | 21.41 | 43.95 |
| Lipids, kg COD/100 kg TS | 32.65 | 1.10 | 51.25 |
| Inerts, kg COD/100 kg TS | 12.37 | 20.08 | 19.42 |
| Characteristics, soluble matter | |||
| COD, kg/100 kgTS | 91.83 | 23.7 | 55.90 |
| Sugars, kg COD/100 kg TS | 0 | 0 | 0.0 |
| Aminoacids, kg COD/100 kg TS | 18.92 | 1.02 | 11.52 |
| Long chain fatty acids, kg COD/100 kg TS | 21.25 | 19.49 | 12.94 |
| Valeric acid, kg COD/100 kg TS | 0 | 0.66 | 0.0 |
| Butyric acid, kg COD/100 kg TS | 3.98 | 0.00 | 2.42 |
| Propionic acid, kg COD/100 kg TS | 8.46 | 0.34 | 5.15 |
| Acetic acid, kg COD/100 kg TS | 34.75 | 2.07 | 21.15 |
| Inerts, kg COD/100 kg TS | 4.46 | 0 | 2.71 |
| Inorganic carbon, kmole/100 kg TS | 0.53 | 0.05 | 0.32 |
| Inorganic phosphorus, kmole/100 kg TS | 9.59 × 10−3 | 6.52 × 10−3 | 0.0058 |
| Inorganic nitrogen (NH3-N), kmole/100 kg TS | 0.73 | 4.28 × 10−2 | 0.44 |
| Operating Conditions | Manure A (1st Phase) | Mixture of Manure A and AAS-Treated Digested Fibers (2nd Phase) | ||
|---|---|---|---|---|
| HRT, d | 24–25 | 24–25 | ||
| Flow rate, mL/d | 114–134 | 117–125 | ||
| Organic loading rate (OLR), g COD/l/d | 2.83–3.53 | 2.30–3.65 | ||
| Soluble OLR, g soluble COD/l/d | 1.52–1.90 | 1.00–1.58 | ||
| Steady state characteristics | Manure A (1st phase) | Mixture of manure and AAS-treated digested fibers (2nd phase ) | ||
| Experimental | Model | Experimental | Model | |
| Methane, % | 67.6 ± 2.1 | 64.2 ± 8.3 | 66.6 ± 1.8 | 67.0 ± 7.6 |
| Biogas production, m3/d | 2.32 × 10−3 ± 0.2 × 10−3 | 2.65 × 10−3 ± 0.1 × 10−3 | 2.70 × 10−3 ± 0.3 × 10−3 | 2.9 × 10−3 ± 0.2 × 10−3 |
| pH | 8.2 | 8.1 | 8.1 | 8.0 |
| Acetic acid, kgCOD/m3 | 0.142 ± 0.025 | 0.087 ± 0.013 | 0.161 ± 0.084 | 0.081 ± 0.018 |
| Propionic acid, kgCOD/m3 | 0.023 ± 0.005 | 0.018 ± 0.002 | 0.012 ± 0.003 | 0.019 ± 0.002 |
| Butyric acid, kgCOD/m3 | 0.026 ± 0.002 | 0.011 ± 0.001 | 0.007 ± 0.0001 | 0.012 ± 0.001 |
| Kinetic Parameter | Units | Value |
|---|---|---|
| Carbohydrates hydrolysis constant, khydr_ch | d−1 | << |
| Proteins hydrolysis constant, khydr_pr | d−1 | 3.0 × 10−3 |
| Lipids hydrolysis constant, khydr_li | d−1 | 2.8 × 10−4 |
| Maximum uptake rate of long chain fatty acids, km_fa | kg COD fa/kg COD x_fa/d | 0.93 |
| Maximum uptake rate of butyric acid, km_c4 | kg COD c4/kg COD x_c4/d | 13.1 |
| Maximum uptake rate of propionic acid, km_pro | kg COD pro/kg COD x_pro/d | 6.56 |
| Maximum uptake rate of acetic acid, km_ac | kg COD ac/kg COD x_ac/d | 45.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirtsou-Xanthopoulou, C.; Skiadas, I.V.; Gavala, H.N. On the Effect of Aqueous Ammonia Soaking Pre-Treatment on Continuous Anaerobic Digestion of Digested Swine Manure Fibers. Molecules 2019, 24, 2469. https://doi.org/10.3390/molecules24132469
Mirtsou-Xanthopoulou C, Skiadas IV, Gavala HN. On the Effect of Aqueous Ammonia Soaking Pre-Treatment on Continuous Anaerobic Digestion of Digested Swine Manure Fibers. Molecules. 2019; 24(13):2469. https://doi.org/10.3390/molecules24132469
Chicago/Turabian StyleMirtsou-Xanthopoulou, Chrysoula, Ioannis V. Skiadas, and Hariklia N. Gavala. 2019. "On the Effect of Aqueous Ammonia Soaking Pre-Treatment on Continuous Anaerobic Digestion of Digested Swine Manure Fibers" Molecules 24, no. 13: 2469. https://doi.org/10.3390/molecules24132469
APA StyleMirtsou-Xanthopoulou, C., Skiadas, I. V., & Gavala, H. N. (2019). On the Effect of Aqueous Ammonia Soaking Pre-Treatment on Continuous Anaerobic Digestion of Digested Swine Manure Fibers. Molecules, 24(13), 2469. https://doi.org/10.3390/molecules24132469






