Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress
Abstract
1. Introduction
2. Biosynthetic Pathway of Polyphenols in Plants
3. Physiological Roles of Phenolics in Plants
4. Abiotic Stresses and Their Toxic Effects on Plants
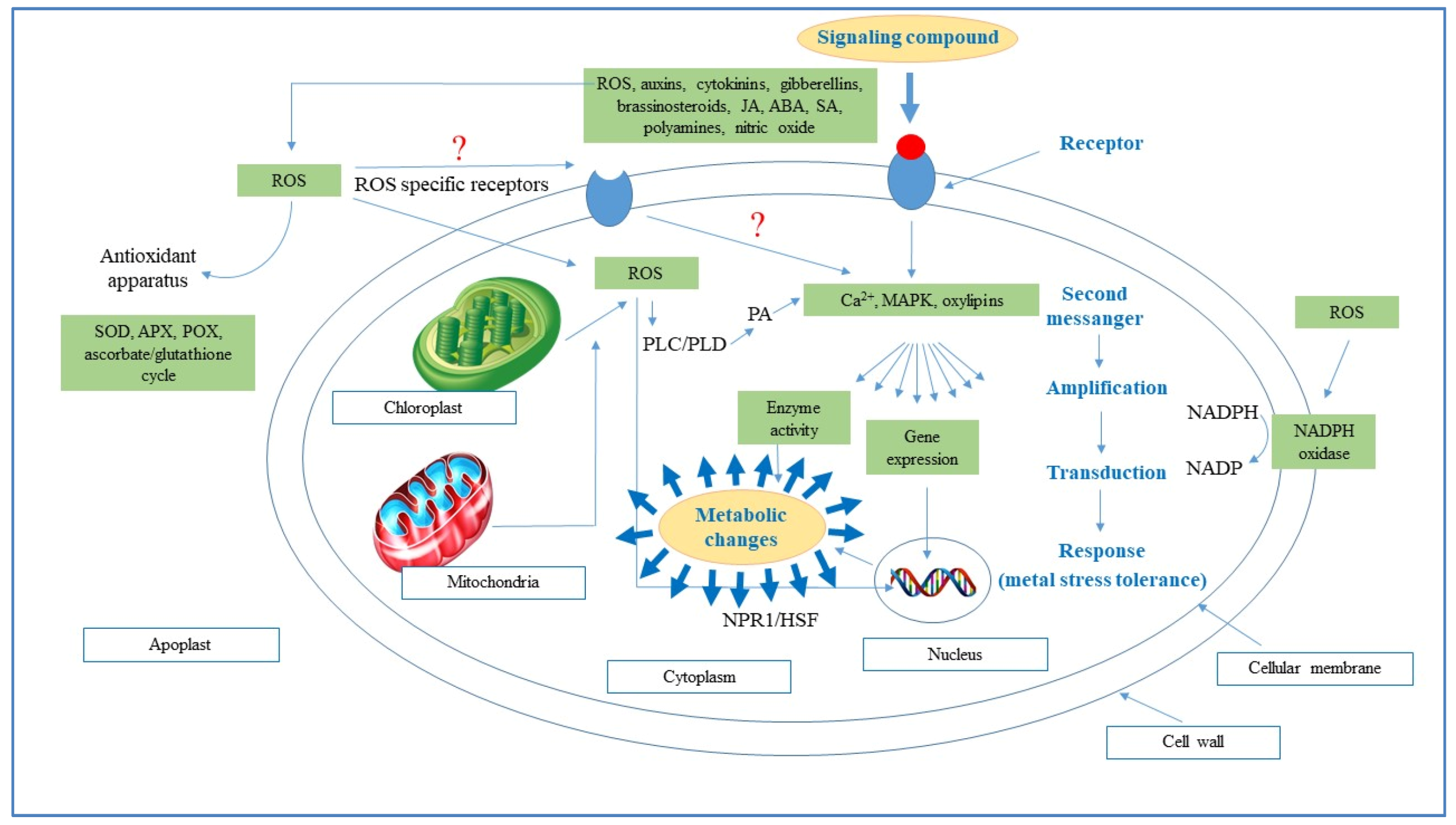
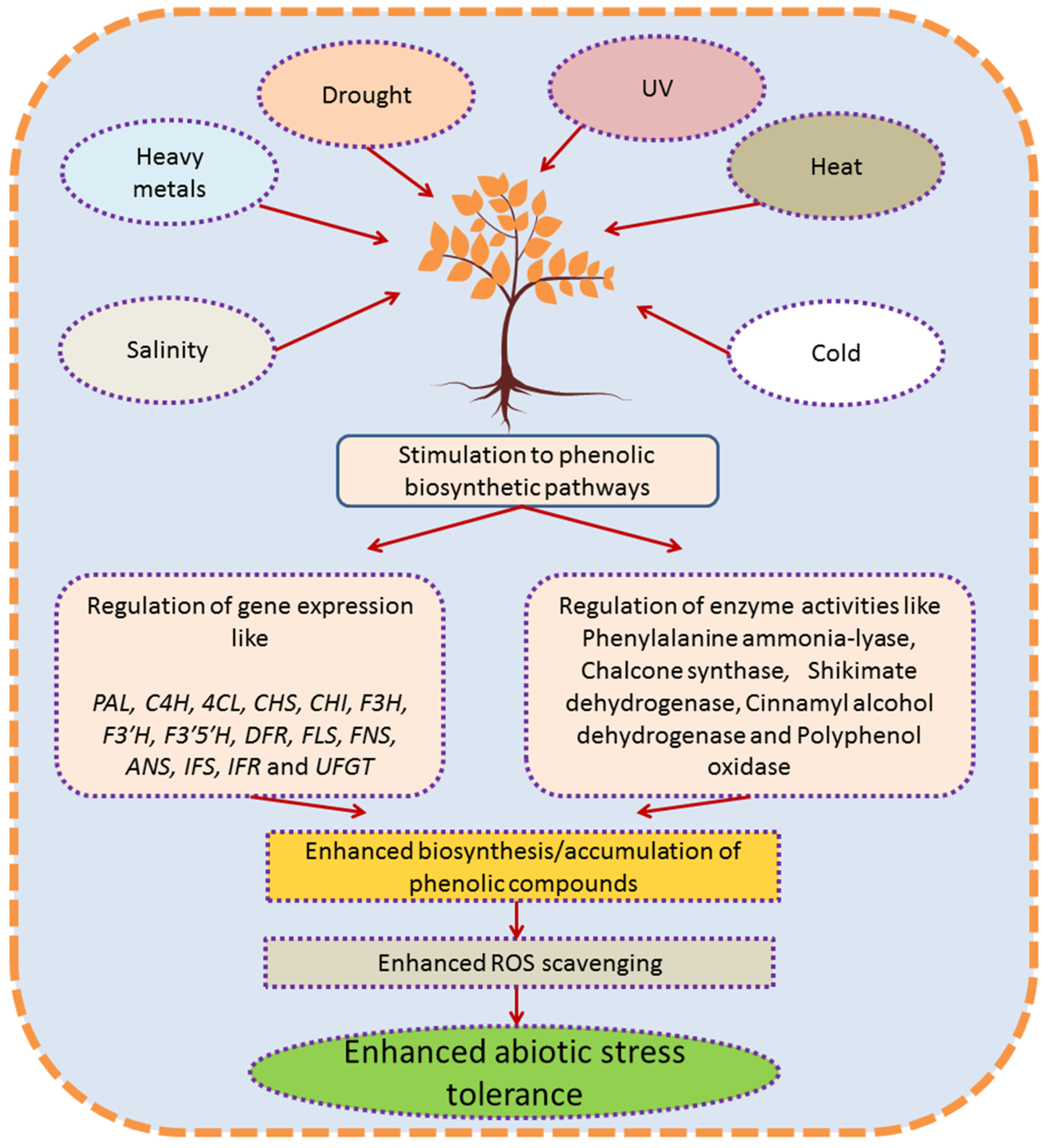
5. Response and Role of Endogenous Phenolics in Plants against Abiotic Stress
5.1. Heavy Metal
5.2. Drought
5.3. Salinity
5.4. UV Light
5.5. Other Abiotic Factors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dresselhaus, T.; Hückelhoven, R. Biotic and Abiotic Stress Responses in Crop Plants. Agronomy 2018, 8, 267. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Plant Sci. 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kreps, J.A.; Wu, Y.; Chang, H.-S.; Zhu, T.; Wang, X.; Harper, J.F. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002, 130, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. Genetic engineering for modern agriculture: Challenges and perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.-B.; Guo, Q.-J.; Chu, L.-Y.; Zhao, X.-N.; Su, Z.-L.; Hu, Y.-C.; Cheng, J.-F. Understanding molecular mechanism of higher plant plasticity under abiotic stress. Colloids Surf. B Biointerfaces 2007, 54, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, S.S.; Gill, R. Plant Adaptation to Environmental Change: Significance of Amino Acids and Their Derivatives; CABI: Wallingford, UK, 2014. [Google Scholar]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Tanveer, M.; Ashraf, U.; Hussain, S.; Shahzad, B.; Khan, I.; Wang, L. Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ. Sci. Pollut. Res. 2016, 23, 17132–17141. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Ur Rehman, S.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment-A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Kumar, R.; Shahzad, B.; Thukral, A.K.; Bhardwaj, R. Brassinosteroid-mediated pesticide detoxification in plants: A mini-review. Cogent Food Agric. 2018, 4, 1436212. [Google Scholar] [CrossRef]
- Fahad, S.; Rehman, A.; Shahzad, B.; Tanveer, M.; Saud, S.; Kamran, M.; Ihtisham, M.; Khan, S.U.; Turan, V.; Ur Rahman, M.H. Rice Responses and Tolerance to Metal/Metalloid Toxicity. In Advances in Rice Research for Abiotic Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–312. [Google Scholar]
- Soares, C.; Carvalho, M.E.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Guo, H.; Feng, X.; Hong, C.; Chen, H.; Zeng, F.; Zheng, B.; Jiang, D. Malate secretion from the root system is an important reason for higher resistance of Miscanthus sacchariflorus to cadmium. Physiol. Planta 2017, 159, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, H.; Hong, C.; Jiang, D.; Zheng, B. Exogenous malic acid alleviates cadmium toxicity in Miscanthus sacchariflorus through enhancing photosynthetic capacity and restraining ROS accumulation. Ecotoxicol. Environ. Saf. 2017, 141, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, L.; Guo, H.; Wang, Y.; Yuan, H.; Yan, D.; Zheng, B. Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Braz. J. Bot. 2017, 40, 841–851. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A. Salicylic acid and jasmonates: Approaches in abiotic stress tolerance. J. Plant Biochem. Physiol. 2013, 1, 4. [Google Scholar] [CrossRef]
- Rao, K.M.; Raghavendra, A.; Reddy, K.J. Physiology and Molecular Biology of Stress Tolerance in Plants; Springer Science & Business Media: New York, NY, USA, 2006. [Google Scholar]
- Kaur, G.; Kumar, S.; Nayyar, H.; Upadhyaya, H. Cold stress injury during the pod-filling phase in chickpea (Cicer arietinum L.): Effects on quantitative and qualitative components of seeds. J. Agron. Crop Sci. 2008, 194, 457–464. [Google Scholar]
- Mantri, N.; Patade, V.; Penna, S.; Ford, R.; Pang, E. Abiotic stress responses in plants: Present and future. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 1–19. [Google Scholar]
- Shahzad, B.; Cheema, S.; Farooq, M.; Cheema, Z.; Rehman, A.; Abbas, T. Growth Stimulating Influence of Foliage Applied Brassica Water Extracts on Morphological and Yield Attributes of Bread Wheat under Different Fertilizer Regimes. Planta Daninha 2018, 36, e018178331. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 26. [Google Scholar] [CrossRef]
- Pereira, A. Plant abiotic stress challenges from the changing environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar] [CrossRef]
- Raskin, I. Role of salicylic acid in plants. Ann. Rev. Plant Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Yalpani, N.; Enyedi, A.J.; León, J.; Raskin, I. Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 1994, 193, 372–376. [Google Scholar] [CrossRef]
- Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. In Fruit and Vegetable Phytochemicals Chemistry, Nutritional Value, and Stability; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar]
- Boudet, A.M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kohli, S.K.; Bali, S.; Sharma, M.; Kumar, R.; Bhardwaj, R.; Thukral, A.K. Differential distribution of polyphenols in plants using multivariate techniques. Biotech. Res. Innov. 2019, 3, 1–21. [Google Scholar] [CrossRef]
- Tanase, C.; Bujor, O.-C.; Popa, V.I. Phenolic Natural Compounds and Their Influence on Physiological Processes in Plants. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–58. [Google Scholar] [CrossRef]
- Bujor, O.-C.; Talmaciu, I.A.; Volf, I.; Popa, V.I. Biorefining to recover aromatic compounds with biological properties. TAPPI J. 2015, 14, 187–193. [Google Scholar]
- Andersen, C.P. Source–sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cardinali, A.; Ruta, C.; Fortunato, I.M.; Lattanzio, V.M.T.; Linsalata, V.; Cicco, N. Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ. Exp. Bot. 2009, 65, 54–62. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef] [PubMed]
- Oszmanski, J. Polyphenols as antioxidants in food. Przem. Spo. 1995, 3, 94–96. [Google Scholar]
- Halvorson, J.J.; Gonzalez, J.M.; Hagerman, A.E.; Smith, J.L. Sorption of tannin and related phenolic compounds and effects on soluble-N in soil. Soil Biol. Biochem. 2009, 41, 2002–2010. [Google Scholar] [CrossRef]
- Seneviratne, G.; Jayasinghearachchi, H.S. Mycelial colonization by bradyrhizobia and azorhizobia. J. Biosci. 2003, 28, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Farooq, M.; Naveed, M.; Nawaz, A.; Shahzad, B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur. J. Agron. 2018, 94, 98–107. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Naveed, M.; Ozturk, L.; Nawaz, A. Pseudomonas aided zinc application improves the productivity and biofortification of bread wheat. Crop Pasture Sci. 2018, 69, 659–672. [Google Scholar] [CrossRef]
- Zhang, J.; Subramanian, S.; Stacey, G.; Yu, O. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J. 2009, 57, 171–183. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J. Exp. Bot. 2001, 52, 419–426. [Google Scholar] [CrossRef]
- Van Der Meer, I.M.; Stam, M.E.; Van Tunen, A.J.; Mol, J.N.; Stuitje, A.R. Antisense inhibition of flavonoid biosynthesis in petunia anthers results in male sterility. Plant Cell 1992, 4, 253–262. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Baleroni, C.R.S.; Ferrarese, M.L.L.; Souza, N.E.; Ferrarese-Filho, O. Lipid Accumulation during Canola Seed Germination in Response to Cinnamic Acid Derivatives. Biol. Planta 2000, 43, 313–316. [Google Scholar] [CrossRef]
- Shankar, S.; Girish, R.; Karthik, N.; Rajendran, R.; Mahendran, V. Allelopathic effects of phenolics and terpenoids extracted from Gimelina arborea on germination of Black gram (Vigna mungo) and Green gram (Vigna radiata). Allelopath. J. 2009, 23, 323–332. [Google Scholar]
- Chen, Z.; Yu, L.; Wang, X.; Gu, Z.; Beta, T. Changes of phenolic profiles and antioxidant activity in canaryseed (Phalaris canariensis L.) during germination. Food Chem. 2016, 194, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Balas, A.; Popa, V. Bioactive compounds extracted from Picea abies bark. In Proceedings of the 10th European Workshop on Lignocellulosics and Pulp, Stockholm, Sweden, 25–28 August 2008; pp. 345–356. [Google Scholar]
- Tobe, K.; Zhang, L.; Qiu, G.Y.; Shimizu, H.; Omasa, K. Characteristics of seed germination in five non-halophytic Chinese desert shrub species. J. Arid. Environ. 2001, 47, 191–201. [Google Scholar] [CrossRef]
- Tanase, C.; Boz, I.; Stingu, A.; Volf, I.; Popa, V.I. Physiological and biochemical responses induced by spruce bark aqueous extract and deuterium depleted water with synergistic action in sunflower (Helianthus annuus L.) plants. Ind. Crops Prod. 2014, 60, 160–167. [Google Scholar] [CrossRef]
- Tanase, C.; Bara, C.I.; Popa, V.I. Cytogenetical effect of some polyphenol compounds separated from industrial by-products on. Cell. Chem. Technol. 2015, 49, 799–805. [Google Scholar]
- Moreland, D.E.; Novitzky, W.P. Effects of Phenolic Acids, Coumarins, and Flavonoids on Isolated Chloroplasts and Mitochondria. In Allelochemicals: Role in Agriculture and Forestry; American Chemical Society: Washington, DC, USA, 1987; pp. 247–261. [Google Scholar] [CrossRef]
- Amarowicz, R.; Weidner, S. Biological activity of grapevine phenolic compounds. In Grapevine Molecular Physiology & Biotechnology; Springer: New York, NY, USA, 2009; pp. 389–405. [Google Scholar]
- Wani, S.H.; Sah, S. Biotechnology and abiotic stress tolerance in rice. J. Rice Res. 2014, 2, e105. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Farooq, M.; Asif, M.; Ozturk, L. Supra-optimal growth temperature exacerbates adverse effects of low Zn supply in wheat. J. Plant Nutr. Soil Sci. 2019. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; Palma, J.M.; Rodriguez-Ruiz, M. Plant peroxisomes: A nitro-oxidative cocktail. Redox Biol. 2017, 11, 535–542. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, B.K.; Askerlund, P.; Bykova, N.V.; Egsgaard, H.; Moller, I.M. Identification of oxidised proteins in the matrix of rice leaf mitochondria by immunoprecipitation and two-dimensional liquid chromatography-tandem mass spectrometry. Phytochemistry 2004, 65, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Buchert, F.; Forreiter, C. Singlet oxygen inhibits ATPase and proton translocation activity of the thylakoid ATP synthase CF1CFo. FEBS Lett. 2010, 584, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.N.; Simonian, R.A.; Skulachev, V.P.; Starkov, A.A. Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria. FEBS Lett. 1997, 415, 87–90. [Google Scholar] [CrossRef]
- Foyer, C.H.; Bloom, A.J.; Queval, G.; Noctor, G. Photorespiratory metabolism: Genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol. 2009, 60, 455–484. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Thukral, A.K.; Bhardwaj, R. Epibrassinolide-imidacloprid interaction enhances non-enzymatic antioxidants in Brassica juncea L. Ind. J. Plant Physiol. 2016, 21, 70–75. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, S.; Kumar, V.; Kanwar, M.K.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R.; Alam, P.; Ahmad, P. Pre-sowing Seed Treatment with 24-Epibrassinolide Ameliorates Pesticide Stress in Brassica juncea L. through the Modulation of Stress Markers. Front. Plant Sci. 2016, 7, 1569. [Google Scholar] [CrossRef] [PubMed]
- Ancillotti, C.; Bogani, P.; Biricolti, S.; Calistri, E.; Checchini, L.; Ciofi, L.; Gonnelli, C.; Del Bubba, M. Changes in polyphenol and sugar concentrations in wild type and genetically modified Nicotiana langsdorffii Weinmann in response to water and heat stress. Plant Physiol. Biochem. 2015, 97, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, B.; Huang, B. Differential Heat-Induced Changes in Phenolic Acids Associated with Genotypic Variations in Heat Tolerance for Hard Fescue. Crop Sci. 2019, 59, 667–674. [Google Scholar] [CrossRef]
- Smirnov, O.E.; Kosyan, A.M.; Kosyk, O.I.; Taran, N.Y. Response of phenolic metabolism induced by aluminium toxicity in Fagopyrum esculentum moench. plants. Ukr. Biochem. J. 2015, 87, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D. Potential of salt and drought stress to increase pharmaceutical significant secondary compounds in plants. Landbauforschung Volkenrode 2008, 58, 139. [Google Scholar]
- Schroeter, H.; Boyd, C.; Spencer, J.P.; Williams, R.J.; Cadenas, E.; Rice-Evans, C. MAPK signaling in neurodegeneration: Influences of flavonoids and of nitric oxide. Neurobiol. Aging 2002, 23, 861–880. [Google Scholar] [CrossRef]
- Sharma, A.; Yuan, H.; Kumar, V.; Ramakrishnan, M.; Kohli, S.K.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Zheng, B. Castasterone attenuates insecticide induced phytotoxicity in mustard. Ecotoxicol. Environ. Saf. 2019, 179, 50–61. [Google Scholar] [CrossRef]
- Perin, E.C.; Da Silva Messias, R.; Borowski, J.M.; Crizel, R.L.; Schott, I.B.; Carvalho, I.R.; Rombaldi, C.V.; Galli, V. ABA-dependent salt and drought stress improve strawberry fruit quality. Food Chem. 2019, 271, 516–526. [Google Scholar] [CrossRef]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of exogenous Ca2+ on phenolic accumulation and physiological changes in germinated wheat (Triticum aestivum L.) under UV-B radiation. Food Chem. 2019, 288, 368–376. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Jia, H.; Sun, X.; Shangguan, L.; Mu, Q.; Wang, B.; Fang, J. Comparative transcriptome analysis of grapevine in response to copper stress. Sci. Rep. 2015, 5, 17749. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Q.; Liu, G.; Xu, N.; Yang, Y.; Zeng, W.; Chen, A.; Wang, S. Integrated analysis of transcriptomic and metabolomic data reveals critical metabolic pathways involved in polyphenol biosynthesis in Nicotiana tabacum under chilling stress. Funct. Plant Biol. 2018, 46, 30–43. [Google Scholar] [CrossRef]
- Pandey, N.; Sharma, C.P. Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci. 2002, 163, 753–758. [Google Scholar] [CrossRef]
- Villiers, F.; Ducruix, C.; Hugouvieux, V.; Jarno, N.; Ezan, E.; Garin, J.; Junot, C.; Bourguignon, J. Investigating the plant response to cadmium exposure by proteomic and metabolomic approaches. Proteomics 2011, 11, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef]
- Kaur, R.; Yadav, P.; Sharma, A.; Kumar Thukral, A.; Kumar, V.; Kaur Kohli, S.; Bhardwaj, R. Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd(II) toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florencio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free. Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. S. Afr. J. Bot. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- Trejo-Tapia, G.; Jimenez-Aparicio, A.; Rodriguez-Monroy, M.; De Jesus-Sanchez, A.; Gutierrez-Lopez, G. Influence of cobalt and other microelements on the production of betalains and the growth of suspension cultures of Beta vulgaris. Plant Cell Tiss. Org. Cult. 2001, 67, 19–23. [Google Scholar] [CrossRef]
- Zafari, S.; Sharifi, M.; Ahmadian Chashmi, N.; Mur, L.A. Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds and amino acids. Plant Physiol. Biochem. 2016, 99, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kısa, D.; Elmastaş, M.; Öztürk, L.; Kayır, Ö. Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl. Biol. Chem. 2016, 59, 813–820. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia Obovata under Cd and Zn stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Keilig, K.; Ludwig-Mueller, J. Effect of flavonoids on heavy metal tolerance in Arabidopsis thaliana seedlings. Bot. Stud. 2009, 50, 311–318. [Google Scholar]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Štork, F.; Bačkor, M. Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 2009, 320, 231. [Google Scholar] [CrossRef]
- Mishra, B.; Sangwan, N.S. Amelioration of cadmium stress in Withania somnifera by ROS management: Active participation of primary and secondary metabolism. Plant Growth Regul. 2019, 87, 403–412. [Google Scholar] [CrossRef]
- Mishra, B.; Sangwan, R.S.; Mishra, S.; Jadaun, J.S.; Sabir, F.; Sangwan, N.S. Effect of cadmium stress on inductive enzymatic and nonenzymatic responses of ROS and sugar metabolism in multiple shoot cultures of Ashwagandha (Withania somnifera Dunal). Protoplasma 2014, 251, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Poonam, R.K.; Bhardwaj, R.; Sirhindi, G. Castasterone regulated polyphenolic metabolism and photosynthetic system in Brassica juncea plants under copper stress. J. Pharmacogn. Phytochem. 2015, 4, 282–289. [Google Scholar]
- Kaur, P.; Bali, S.; Sharma, A.; Vig, A.P.; Bhardwaj, R. Effect of earthworms on growth, photosynthetic efficiency and metal uptake in Brassica juncea L. plants grown in cadmium-polluted soils. Environ. Sci. Pollut. Res. 2017, 24, 13452–13465. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Bali, S.; Sharma, A.; Vig, A.P.; Bhardwaj, R. Role of earthworms in phytoremediation of cadmium (Cd) by modulating the antioxidative potential of Brassica juncea L. Appl. Soil Ecol. 2018, 124, 306–316. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Kumar, V.; Kaur, P.; Bhardwaj, R. Synergistic effect of 24-epibrassinolide and salicylic acid on photosynthetic efficiency and gene expression in Brassica juncea L. under Pb stress. Turk. J. Biol. 2017, 41, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.; Kaufman, P.B.; Warber, S.; Bolling, S.; Chang, S.C. Antioxidant capacity of polyphenolic extracts from leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) subjected to drought and cold stress. J. Agric. Food Chem. 2003, 51, 3973–3976. [Google Scholar] [CrossRef]
- Ballizany, W.L.; Hofmann, R.W.; Jahufer, M.Z.Z.; Barrett, B.A. Multivariate associations of flavonoid and biomass accumulation in white clover (Trifolium repens) under drought. Funct. Plant Biol. 2012, 39, 167–177. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Differential responses of phenolic compounds of Brassica napus under drought stress. Iran. J. Plant Physiol. 2018, 8, 2417–2425. [Google Scholar]
- Li, M.; Li, Y.; Zhang, W.; Li, S.; Gao, Y.; Ai, X.; Zhang, D.; Liu, B.; Li, Q. Metabolomics analysis reveals that elevated atmospheric CO2 alleviates drought stress in cucumber seedling leaves. Anal. Biochem. 2018, 559, 71–85. [Google Scholar] [CrossRef]
- Nichols, S.N.; Hofmann, R.W.; Williams, W.M. Physiological drought resistance and accumulation of leaf phenolics in white clover interspecific hybrids. Environ. Exp. Bot. 2015, 119, 40–47. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Moreno, D.A.; Ferreres, F.; Rubio-Wilhelmi Mdel, M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Alegre, L.; Van Breusegem, F.; Munne-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, S.; Tabatabaei, B.E.; Saeidi, G.; Goli, S.A. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Garcia-Calderon, M.; Pons-Ferrer, T.; Mrazova, A.; Pal’ove-Balang, P.; Vilkova, M.; Perez-Delgado, C.M.; Vega, J.M.; Eliasova, A.; Repcak, M.; Marquez, A.J.; et al. Modulation of phenolic metabolism under stress conditions in a Lotus japonicus mutant lacking plastidic glutamine synthetase. Front. Plant Sci. 2015, 6, 760. [Google Scholar] [CrossRef]
- Silva, F.L.B.; Vieira, L.G.E.; Ribas, A.F.; Moro, A.L.; Neris, D.M.; Pacheco, A.C. Proline accumulation induces the production of total phenolics in transgenic tobacco plants under water deficit without increasing the G6PDH activity. Theor. Exp. Plant Physiol. 2018, 30, 251–260. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Khalil, N.; Fekry, M.; Bishr, M.; El-Zalabani, S.; Salama, O. Foliar spraying of salicylic acid induced accumulation of phenolics, increased radical scavenging activity and modified the composition of the essential oil of water stressed Thymus vulgaris L. Plant Physiol. Biochem. 2018, 123, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Zhawar, V.K. Phenolic parameters under exogenous ABA, water stress, salt stress in two wheat cultivars varying in drought tolerance. Ind. J. Plant Physiol. 2015, 20, 151–156. [Google Scholar] [CrossRef]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, K.; Taïbi, F.; Ait Abderrahim, L.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; Abreu, C.E.B.D.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of Flavonols over Hydroxycinnamic Acids Favors Oxidative Damage Protection under Abiotic Stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NtMYB4 and NtCHS1 Are Critical Factors in the Regulation of Flavonoid Biosynthesis and Are Involved in Salinity Responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef]
- Rossi, L.; Borghi, M.; Francini, A.; Lin, X.; Xie, D.Y.; Sebastiani, L. Salt stress induces differential regulation of the phenylpropanoid pathway in Olea europaea cultivars Frantoio (salt-tolerant) and Leccino (salt-sensitive). J. Plant Physiol. 2016, 204, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Golkar, P.; Taghizadeh, M. In vitro evaluation of phenolic and osmolite compounds, ionic content, and antioxidant activity in safflower (Carthamus tinctorius L.) under salinity stress. Plant Cell Tiss. Org. Cult. 2018, 134, 357–368. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Chen, D.; Li, Z.; Peng, R.; Yao, Q. A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell Tiss. Org. Cult. 2016, 125, 387–398. [Google Scholar] [CrossRef]
- Yan, J.; Wang, B.; Jiang, Y.; Cheng, L.; Wu, T. GmFNSII-controlled soybean flavone metabolism responds to abiotic stresses and regulates plant salt tolerance. Plant Cell Physiol. 2014, 55, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Ben-Abdallah, S.; Zorrig, W.; Amyot, L.; Renaud, J.; Hannoufa, A.; Lachâal, M.; Karray-Bouraoui, N. Potential production of polyphenols, carotenoids and glycoalkaloids in Solanum villosum Mill. under salt stress. Biologia 2019, 74, 309–324. [Google Scholar] [CrossRef]
- Scagel, C.F.; Lee, J.; Mitchell, J.N. Salinity from NaCl changes the nutrient and polyphenolic composition of basil leaves. Ind. Crops Prod. 2019, 127, 119–128. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under salinity stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef]
- Dkhil, B.B.; Denden, M. Effect of salt stress on growth, anthocyanins, membrane permeability and chlorophyll fluorescence of Okra (Abelmoschus esculentus L.) seedlings. Am. J. Plant Physiol. 2012, 7, 174–183. [Google Scholar] [CrossRef]
- Eryılmaz, F. The Relationships between Salt Stress and Anthocyanin Content in Higher Plants. Biotechnol. Biotechnol. Equip. 2006, 20, 47–52. [Google Scholar] [CrossRef]
- Aloisi, I.; Parrotta, L.; Ruiz, K.B.; Landi, C.; Bini, L.; Cai, G.; Biondi, S.; Del Duca, S. New Insight into Quinoa Seed Quality under Salinity: Changes in Proteomic and Amino Acid Profiles, Phenolic Content, and Antioxidant Activity of Protein Extracts. Front. Plant Sci. 2016, 7, 656. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Borgognone, D.; Rouphael, Y.; Cardarelli, M.; Bernardi, J.; Colla, G. Mild Potassium Chloride Stress Alters the Mineral Composition, Hormone Network, and Phenolic Profile in Artichoke Leaves. Front. Plant Sci. 2016, 7, 948. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, P.; Gu, Z.; Tao, Y.; Shen, C.; Zhou, Y.; Han, Y.; Yang, R. Ca2+ involved in GABA signal transduction for phenolics accumulation in germinated hulless barley under NaCl stress. Food Chem. X 2019, 2, 100023. [Google Scholar] [CrossRef]
- Çoban, Ö.; Göktürk Baydar, N. Brassinosteroid effects on some physical and biochemical properties and secondary metabolite accumulation in peppermint (Mentha piperita L.) under salt stress. Ind. Crops Prod. 2016, 86, 251–258. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Niazi, A.; Moghadam, A. Phenylalanine ammonia lyase isolation and functional analysis of phenylpropanoid pathway under salinity stress in ‘Salvia’ species. Aust. J. Crop Sci. 2015, 9, 656–665. [Google Scholar]
- Daayf, F.; Lattanzio, V. Recent Advances in Polyphenol Research; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Kolb, C.A.; Kaser, M.A.; Kopecky, J.; Zotz, G.; Riederer, M.; Pfundel, E.E. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant. Physiol. 2001, 127, 863–875. [Google Scholar] [CrossRef]
- Ghasemi, S.; Kumleh, H.H.; Kordrostami, M. Changes in the expression of some genes involved in the biosynthesis of secondary metabolites in Cuminum cyminum L. under UV stress. Protoplasma 2019, 256, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Siddiqui, S.; Upadhyay, N.; Soni, J. Effects of ultraviolet irradiation, pulsed electric field, hot water and ethanol vapours treatment on functional properties of mung bean sprouts. J. Food Sci. Technol. 2014, 51, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Veronneau, P.Y.; Rolland, D.; Roussel, D. Preharvest Ultraviolet C Irradiation Increased the Level of Polyphenol Accumulation and Flavonoid Pathway Gene Expression in Strawberry Fruit. J. Agric. Food Chem. 2017, 65, 9970–9979. [Google Scholar] [CrossRef] [PubMed]
- Demkura, P.V.; Abdala, G.; Baldwin, I.T.; Ballare, C.L. Jasmonate-dependent and -independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defense. Plant Physiol. 2010, 152, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Berli, F.J.; Fanzone, M.; Piccoli, P.; Bottini, R. Solar UV-B and ABA are involved in phenol metabolism of Vitis vinifera L. increasing biosynthesis of berry skin polyphenols. J. Agric. Food Chem. 2011, 59, 4874–4884. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Llorens, L.; Koufogianni, A.; Diaz, L.; Font, J.; Gonzalez, J.A.; Verdaguer, D. Interactive effects of UV radiation and reduced precipitation on the seasonal leaf phenolic content/composition and the antioxidant activity of naturally growing Arbutus unedo plants. J. Photochem. Photobiol. B 2015, 153, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Rodriguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef]
- Liu, M.; Cao, B.; Zhou, S.; Liu, Y. Responses of the flavonoid pathway to UV-B radiation stress and the correlation with the lipid antioxidant characteristics in the desert plant Caryopteris mongolica. Acta Ecol. Sin. 2012, 32, 150–155. [Google Scholar] [CrossRef]
- Nascimento, L.; Leal-Costa, M.V.; Menezes, E.A.; Lopes, V.R.; Muzitano, M.F.; Costa, S.S.; Tavares, E.S. Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. J. Photochem. Photobiol. B 2015, 148, 73–81. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Bruckova, K.; Brestic, M.; Hemmerich, I.; Rauh, C.; Simko, I. Shift in accumulation of flavonoids and phenolic acids in lettuce attributable to changes in ultraviolet radiation and temperature. Sci. Hortic. 2018, 239, 193–204. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, J.E.; Oh, M.M. Growth and phenolic compounds of Lactuca sativa L. grown in a closed-type plant production system with UV-A, -B, or -C lamp. J. Sci. Food Agric. 2014, 94, 197–204. [Google Scholar] [CrossRef]
- Huyskens-Keil, S.; Eichholz, I.; Kroh, L.; Rohn, S. UV-B induced changes of phenol composition and antioxidant activity in black currant fruit (Ribes nigrum L.). J. App. Bot. Food Qual. 2012, 81, 140–144. [Google Scholar]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; De Oliveira, J.F.; Melo, P.; Santos, C. Tomato plants use non-enzymatic antioxidant pathways to cope with moderate UV-A/B irradiation: A contribution to the use of UV-A/B in horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Weng, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of UV-B radiation on phenolic accumulation, antioxidant activity and physiological changes in wheat (Triticum aestivum L.) seedlings. Food Biosci. 2019, 30, 100409. [Google Scholar] [CrossRef]
- Alonso, R.; Berli, F.J.; Fontana, A.; Piccoli, P.; Bottini, R. Malbec grape (Vitis vinifera L.) responses to the environment: Berry phenolics as influenced by solar UV-B, water deficit and sprayed abscisic acid. Plant Physiol. Biochem. 2016, 109, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Swieca, M. Elicitation with abiotic stresses improves pro-health constituents, antioxidant potential and nutritional quality of lentil sprouts. Saudi J. Biol. Sci. 2015, 22, 409–416. [Google Scholar] [CrossRef]
- Wang, L.; Shan, T.; Xie, B.; Ling, C.; Shao, S.; Jin, P.; Zheng, Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2019, 272, 530–538. [Google Scholar] [CrossRef]
- Mahdavi, V.; Farimani, M.M.; Fathi, F.; Ghassempour, A. A targeted metabolomics approach toward understanding metabolic variations in rice under pesticide stress. Anal. Biochem. 2015, 478, 65–72. [Google Scholar] [CrossRef]
- Korosi, L.; Bouderias, S.; Csepregi, K.; Bognar, B.; Teszlak, P.; Scarpellini, A.; Castelli, A.; Hideg, E.; Jakab, G. Nanostructured TiO2-induced photocatalytic stress enhances the antioxidant capacity and phenolic content in the leaves of Vitis vinifera on a genotype-dependent manner. J. Photochem. Photobiol. B 2019, 190, 137–145. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Singh, R.; Thukral, A.K.; Bhardwaj, R. Effect of seed pre-soaking with 24-epibrassinolide on growth and photosynthetic parameters of Brassica juncea L. in imidacloprid soil. Ecotoxicol. Environ. Saf. 2016, 133, 195–201. [Google Scholar] [CrossRef]
- Zlotek, U.; Szymanowska, U.; Baraniak, B.; Karas, M. Antioxidant activity of polyphenols of adzuki bean (Vigna angularis) germinated in abiotic stress conditions. Acta Sci. Pol. Technol. Aliment. 2015, 14, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Commisso, M.; Toffali, K.; Strazzer, P.; Stocchero, M.; Ceoldo, S.; Baldan, B.; Levi, M.; Guzzo, F. Impact of phenylpropanoid compounds on heat stress tolerance in carrot cell cultures. Front. Plant Sci. 2016, 7, 1439. [Google Scholar] [CrossRef] [PubMed]
- Cingoz, G.S.; Gurel, E. Effects of salicylic acid on thermotolerance and cardenolide accumulation under high temperature stress in Digitalis trojana Ivanina. Plant Physiol. Biochem. 2016, 105, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.; Yaish, M.W. Antifreeze proteins in overwintering plants: A tale of two activities. Trends Plant. Sci. 2004, 9, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Post. Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Yuan, H.; Kanwar, M.K.; Bhardwaj, R.; Thukral, A.K.; Zheng, B. Jasmonic Acid Seed Treatment Stimulates Insecticide Detoxification in Brassica juncea L. Front. Plant Sci. 2018, 9, 1609. [Google Scholar] [CrossRef]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Mandoulakani, B.A. Pharmaceutical important phenolic compounds overproduction and gene expression analysis in Dracocephalum kotschyi hairy roots elicited by SiO2 nanoparticles. Ind. Crops Prod. 2019, 133, 435–446. [Google Scholar] [CrossRef]
- Girilal, M.; Fayaz, A.M.; Elumalai, L.K.; Sathiyaseelan, A.; Gandhiappan, J.; Kalaichelvan, P.T. Comparative Stress Physiology Analysis of Biologically and Chemically Synthesized Silver Nanoparticles on Solanum lycopersicum L. Colloid Interface Sci. Commun. 2018, 24, 1–6. [Google Scholar] [CrossRef]
- Raigond, P.; Raigond, B.; Kaundal, B.; Singh, B.; Joshi, A.; Dutt, S. Effect of zinc nanoparticles on antioxidative system of potato plants. J. Environ. Biol. 2017, 38, 435–439. [Google Scholar] [CrossRef]
- Singh, O.S.; Pant, N.C.; Laishram, M.L.; Tewari, R.D.; Joshi, K.; Pandey, C. Effect of CuO nanoparticles on polyphenols content and antioxidant activity in Ashwagandha (Withania somnifera L. Dunal). J. Pharmacogn. Phytochem. 2018, 7, 3433–3439. [Google Scholar]
| Plant Species | Heavy Metal | Response of Endogenous Phenolics and Related Parameters | Reference |
|---|---|---|---|
| Brassica juncea | Cu | Increase in contents of total phenols, anthocyanins and other phenolic compounds like catechin, caffeic acid, coumaric acid, kaempferol. | [103] |
| Cr | Increase in total contents of phenols, flavonoids and anthocyanins, accompanied by enhanced expressions of PAL and CHS. | [72] | |
| Cr | Increase in anthocyanins accompanied by up-regulation of CHS gene. | [93] | |
| Cd | Increase in the contents of total flavonoids and anthocyanins. | [90] | |
| Cd | Increase in total contents of flavonoids and anthocyanins, accompanied by enhanced expressions of PAL and CHS. | [104] | |
| Cd | Increase in total contents of phenols, polyphenols, flavonoids and anthocyanins. | [105] | |
| Pb | Increase in total contents of phenols, flavonoids and anthocyanins, accompanied by enhanced expressions of PAL and CHS. | [106] | |
| Pb | Increase in total contents of phenols, polyphenols, flavonoids and anthocyanins. | [89] | |
| Fagopyrum esculentum | Al | Increase in total phenolic, flavonoid and anthocyanin contents. Increase in the activity of PAL enzyme. | [77] |
| Kandelia obovata | Cd and Zn | Enhanced levels of total phenolics accompanied by increased activities of phenol metabolic enzymes like shikimate dehydrogenase, cinnamyl alcohol dehydrogenase and polyphenol oxidase. | [97] |
| Prosopis farcta | Pb | Increase in total contents of phenols accompanied by enhanced activity of PAL enzyme. Contents of other phenolic compounds were also increased including ferulic acid, cinnamic acid, caffeic acid, daidzein, vitexin, resveratrol, myricetin, quercetin, kaempferol, naringinine, luteolin and diosmin. | [95] |
| Vitis vinifera | Cu | Enhanced transcript levels of various genes encoding enzymes involved in biosynthesis of phenolics (PAL, C4H, CHS, F3H, DFR) and down-regulation of UFGT and ANR. | [85] |
| Withania somnifera | Cd | Increase in total contents of flavonoids and phenolics | [101] |
| Zea mays | Cu, Pb, Cd | Increase in the contents of total phenols and some polyphenols like chlorogenic and vanillic acid. | [96] |
| Plant Species | Response of Endogenous Phenolics and Related Parameters | Reference |
|---|---|---|
| Achillea spp. | Increase in the contents of chlorogenic acid, caffeic acid, rutin, luteolin-7-O-glycoside, 1,3-dicaffeoylquinic acid, luteolin, apigenin and kaempferol under 21 days exposure of drought. Enhanced transcript levels of PAL, CHS, CHI, F3H, F3′H, F3′5′H and FLS. | [82] |
| Increase in contents of total phenols and flavonoids. | [115] | |
| Brassica napus | Increase in contents of total phenols, flavonoid and flavonol. Increase in PAL enzyme activity accompanied by enhanced expression of PAL. | [110] |
| Chrysanthemum morifolium | Increase in contents of total phenolics, anthocyanins, chlorogenic acid, luteolin, rutin, ferulic acid, apigenin and quercetin. Enhanced expression of PAL, CHI, and F3H, particularly in cultivar Taraneh. | [116] |
| Cucumis sativus | Up-regulation of phenolic metabolites including vanillic acid and 4-hydroxycinnamic acid. | [111] |
| Fragaria ananassa | Enhanced transcript levels of PAL, C4H, 4CL, DFR, ANS, FLS and UFGT. | [81] |
| Lactuca sativa | Increase in the contents of phenolic compounds such as caftaric acid and rutin. | [117] |
| Larrea spp. | Increase in the contents of polyphenols including flavonoids, proanthocyanidins and flavonols. | [118] |
| Lotus japonicus | Increase in the contents of kaempferol and quercetine. Up-regulation of the expression of PAL, C4H, 4CL, CHS, CHI, DFR, IFS and IFR | [119] |
| Nicotiana tabacum | Increase in PAL enzyme activity and lignin content. | [120] |
| Ocimum spp. | Increase in content of total phenols | [121] |
| Thymus vulgaris | Increase in the contents of total flavonoids and polyphenols. | [122] |
| Triticum aestivum | Increase in content of total phenols | [123] |
| Increase in the total contents of phenolics, flavonoids and anthocyanins. Enhanced expression of genes like CHS, CHI, F3H, FNS, FLS, DFR and ANS. | [84] | |
| Vitis vinifera | Increase in the contents of polyphenols including 4-coumaric acid, caffeic acid, ferulic acid, cis-resveratrol-3-O-glucoside, trans-resveratrol-3-O-glucoside, catechin, epicatechin, caftaric acid, epicatechin gallate, kaempferol-3-O-glucoside, cyanidin-3-O-glucoside, quercetin-3-O-glucoside and quercetin-3-O glucuronide. | [124] |
| Increase in anthocyanin content accompanied by up-regulation of associated biosynthetic genes like UFGT, CHS and F3H. | [125] |
| Plant Species | Response of Endogenous Phenolics and Related Parameters | Reference |
|---|---|---|
| Amaranthus tricolor | Increase in contents of total phenolics, hydroxybenzoic acids (gallic acid, vanilic acid, syringic acid, p-hydroxybenzoic acid, ellagic acid), hydroxycinnamic acids (caffeic acid, chlorogenic acid, p-coumaric acid, m-coumaric acid, ferulic acid, sinapic acid, trans-cinnamic acid) and flavonoids (iso-quercetin, hyperoside, rutin) | [140] |
| Asparagus aethiopicus | Increase in the levels of phenolics like robinin, rutin, apigein, chlorogenic acid and caffeic acid. | [134] |
| Carthamus tinctorius | Increase in contents of total phenols and flavonoids. | [136] |
| Chenopodium quinoa | Increase in total polyphenol and flavonoid contents. | [143] |
| Cynara cardunculus | Increase in contents of phenolic compounds like luteolin-O-glucoside, apigenin 6-c-glucoside 8-c-arabinoside, gallocatechin, leucocyanidin and quercitrin. Decrease in contents of compounds like apigenin, chrysin, genistein, daidzein and ferulic acid | [144] |
| Fragaria ananassa | Enhanced transcript levels of PAL, C4H, F3H, DFR and FLS. | [81] |
| Hordeum vulgare | Increase of total phenolic contents. | [145] |
| Mentha piperita | Increase of total phenolic contents. | [146] |
| Ocimum basilicum | Increase in the contents of various phenolic compounds like caffeic acis, caftaric acid, cinnamyl malic acid, feruloyl tartaric acid, quercetin-rutinoside and rosmarinic acid. | [139] |
| Olea europaea | Increase in contents of total phenolics, kaempf erol and quercetin. Regulation of transcript levels of PAL, C4H, 4CL, CHS and CHI. | [133] |
| Salvia mirzayanii | Increase of total phenolic contents. | [132] |
| Salvia mirzayanii and Salvia acrosiphon | Increase in total phenolic content and PAL activity accompanied by enhanced expression of PAL. | [147] |
| Solanum lycopersicon | Increase in total caffeoylquinic acid content | [129] |
| Solanum villosum | Increase in total phenolic, caffeic acid, and quercetin 3-β-D-glucoside contents. Up-regulation of the expression of PAL and FLS | [138] |
| Thymus spp. | Increase in the contents of various phenolic compounds like caffeic acid, gallic acid, trans-2-hydroxycinnamic acid, cinnamic acid, rosmarinic acid, rutin, syringic acid, vanillic acid, apigenin, quercitrin, naringenin and luteolin. | [131] |
| Triticum aestivum | Increase in contents of total phenols | [123] |
| Plant Species | Response of Endogenous Phenolics and Related Parameters | Reference |
|---|---|---|
| Arbutus unedo | Increase in contents of phenolic compounds like theogallin, avicularin and juglanin. | [158] |
| Brassica oleracea | Increase in contents of gallic acid and sinapic acid. | [159] |
| Caryopteris mongolica | Increase in contents of flavonoids and anthocyanidins, accompanied by PAL and CHI activity. | [160] |
| Cuminum cyminum | Increase in contents of total phenolics and anthocyanins, accompanied by enhanced gene expression of DAHP and PAL. | [153] |
| Fragaria x ananassa | Increase in contents of kaempferol, ellagic acid and, glucoside derivative of cyaniding, pelargonidin and quercetin. Up-regulation of key genes involved in flavonoid pathway including CHS, CHI, FHT, DFR, FLS and FGT. | [155] |
| Kalanchoe pinnata | Increase in contents of total flavonoids and quercitrin. | [161] |
| Lactuca sativa | Increase in contents of total phenolics, flavonoids and anthocyanins. Contents of phenolic acids were also increased including rosmarinic acid, vanillic acid, p-anisic acid, methoxycinnamic acid and chlorogenic acid. | [162] |
| Increase in total anthocyanin and phenolic contents. This is accompanied by enhanced activity of PAL enzyme and up-regulation of PAL expression. | [163] | |
| Ribes nigrum | Increase in contents of flavonols, anthocyanins, hydroxycinnamic and hydroxybenzoic acids. | [164] |
| Solanum lycopersicum | Increase in total phenolic content | [165] |
| Triticum aestivum | After 3 days of UV exposure, increase in contents of total phenolics, ferulic acid, p-coumaric acid and vanillic acid, whereas no change in the contents of p-hydroxybenzoic acid, syringic acid and sinapic acid. Alterations in the transcript levels of PAL, C4H, 4CL, and COMT | [83] |
| Triticum aestivum | Increase in contents of free, bound and total phenolics accompanied by enhanced PAL activity. | [166] |
| Vigna radiata | Increase in total flavonoid and phenol content, accompanied by enhanced activities of PAL and CHI enzymes. | [154] |
| Vitis vinifera | Increase in contents of astilbin, quercetin and kaempferol. | [167] |
| Increase in contents of phenolic compounds like cyaniding, petunidin, peonidin, malvidin, quercetin, myricetin, kaempferol, procyanidin, gallic acid, protocatechuic acid and vanillic acid. | [157] |
| Plant Species | Abiotic Factor | Response of Endogenous Phenolics and Related Parameters | Reference |
|---|---|---|---|
| Brassica juncea | Insecticide | Increase in total phenol and polyphenol contents. | [73] |
| Insecticide | Increase in total phenol, polyphenol and anthocyanin contents accompanied by enhanced expression of PAL and CHS. | [74] | |
| Insecticide | Increase in total phenol and anthocyanin contents. | [178] | |
| Insecticide | Increase in total phenol and anthocyanin contents accompanied by enhanced expression of PAL and CHS. | [80] | |
| Dracocephalum kotschyi | Silicon dioxide NP | Increase in total phenol, total flavonoid, rosmarinic acid and xantomicrol contents, accompanied by up-regulation of the gene expression of PAL and RAS. | [179] |
| Festuca trachyphylla | Heat | Increase in the contents of phenolic compounds like 4-hydroxybenzoic acid, benzoic acid, caffeic acid, coumaric acid, cinnamic acid, gallic acid, homovanillic acid, ferulic acid, salicylic acid and vanillic acid. | [76] |
| Lens culinaris | Heat | Enhanced levels of total phenolics and flavonoids. Increase in the contents of gallic acid, salicylic acid, chlorogenic acid, ferulic acid and naringenin, | [168] |
| Nicotiana tabacum | Chilling | Alteration in the contents of various metabolites of phenylalanine metabolic pathway. Enhanced expression of PAL, HCT and CAD. | [86] |
| Nicotiana langsdorffii | Heat | Increase in the contents of total polyphenols and individual contents of p-coumaric acid, chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid and ferulic acid. | [75] |
| Oryza sativa | Insecticide | Increase in the contents of phenylalanine, p-hydroxybenzoic acid and ferulic acid | [170] |
| Prunus persica | Chilling | Increase in the activities of enzymes like PAL, C4H, 4CL and CHI. Increase in the contents of phenolic compounds like protocatechuic acid, catechin, cholorogenic acid, neocholorogenic acid, quercetin-3- rutinoside, quercetin-3-glucoside, kaempferol-3- rutinoside | [169] |
| Solanum lycopersicon | Heat | Increase in total flavonol content | [129] |
| Silver NP | Increase in total phenolic content. | [180] | |
| Solanum tuberosum | Zinc NP | Increase in contents of total phenolics and anthocyanins. | [181] |
| Vigna angularis | Heat | Increase in the contents of anthocyanins and flavonoids. | [173] |
| Vitis vinifera | Titanium NP | Increase in contents of total phenolics, caftaric acid, quercetin derivatives and kaempferol derivatives. | [171] |
| Withania somnifera | Copper NP | Increase in contents of total phenolics and flavonoids. | [182] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. https://doi.org/10.3390/molecules24132452
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules. 2019; 24(13):2452. https://doi.org/10.3390/molecules24132452
Chicago/Turabian StyleSharma, Anket, Babar Shahzad, Abdul Rehman, Renu Bhardwaj, Marco Landi, and Bingsong Zheng. 2019. "Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress" Molecules 24, no. 13: 2452. https://doi.org/10.3390/molecules24132452
APA StyleSharma, A., Shahzad, B., Rehman, A., Bhardwaj, R., Landi, M., & Zheng, B. (2019). Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules, 24(13), 2452. https://doi.org/10.3390/molecules24132452