2-Ketogluconate Kinase from Cupriavidus necator H16: Purification, Characterization, and Exploration of Its Substrate Specificity
Abstract
1. Introduction
2. Results and Discussion
2.1. Cloning, Overexpression, Purification, and Characterization of KGUK from C. necator
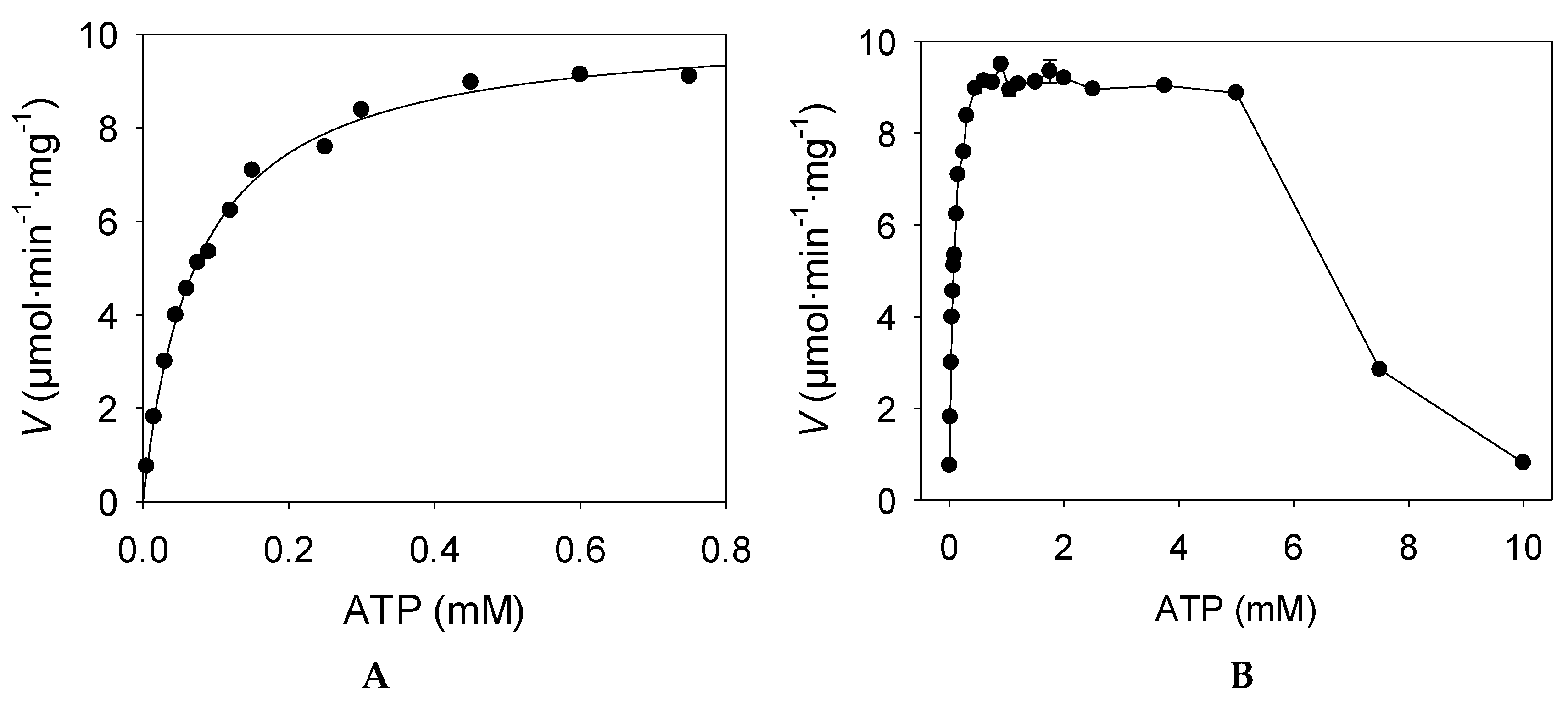
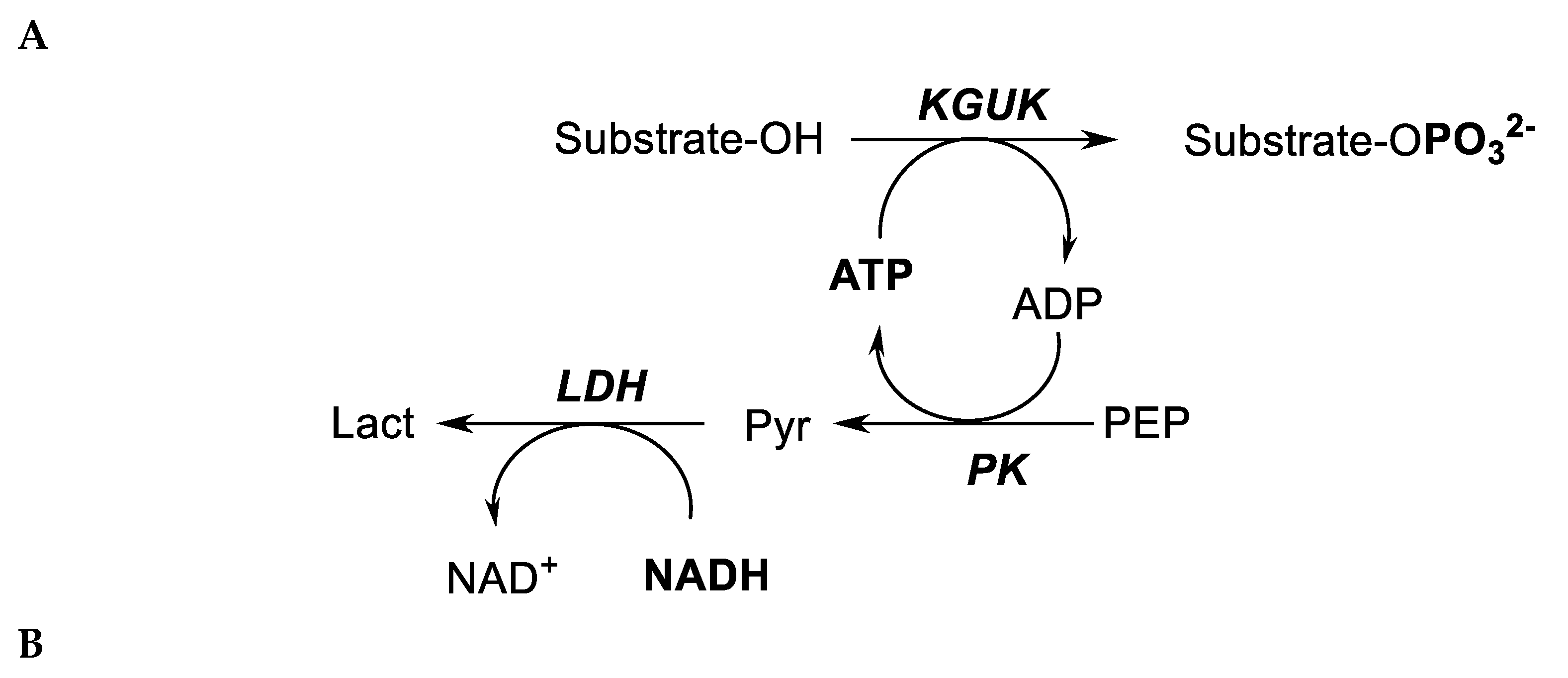
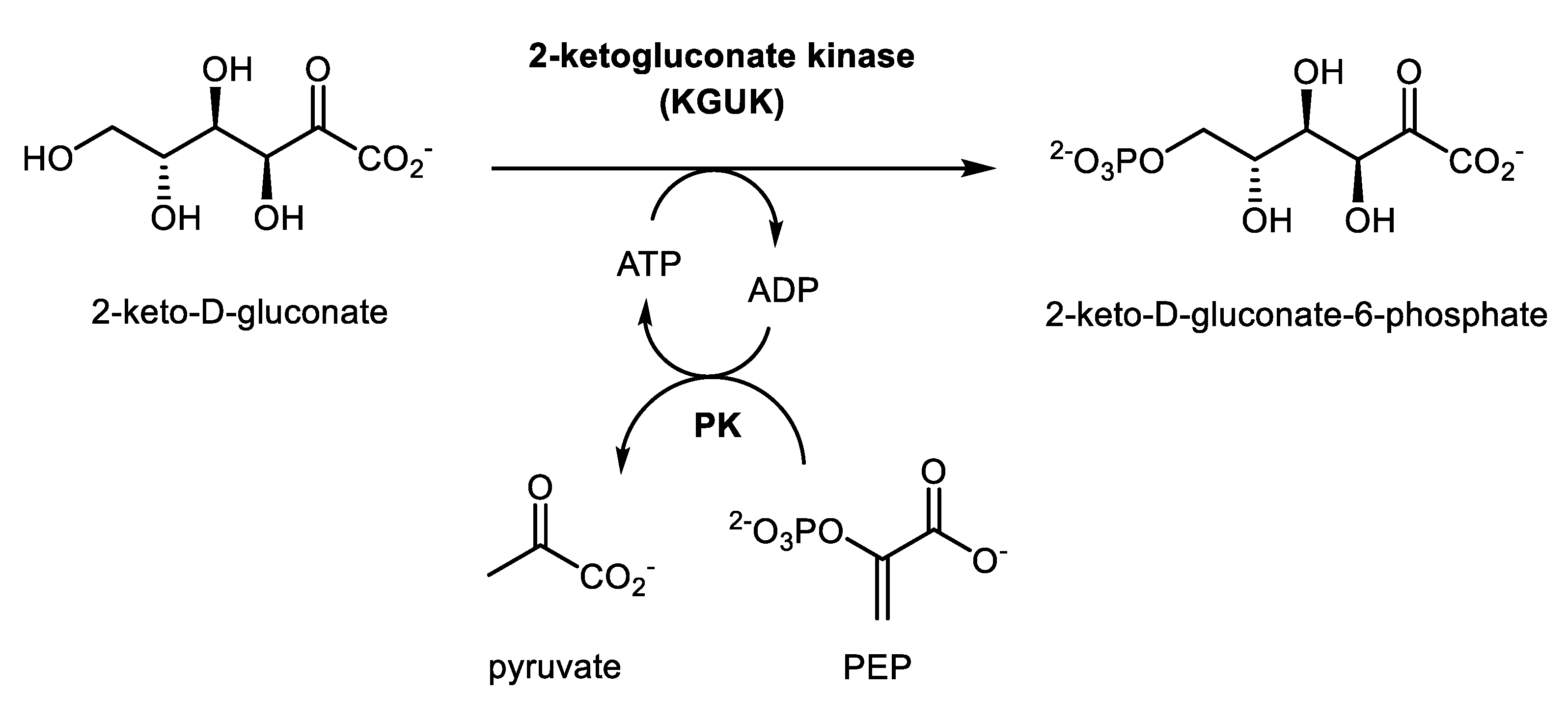
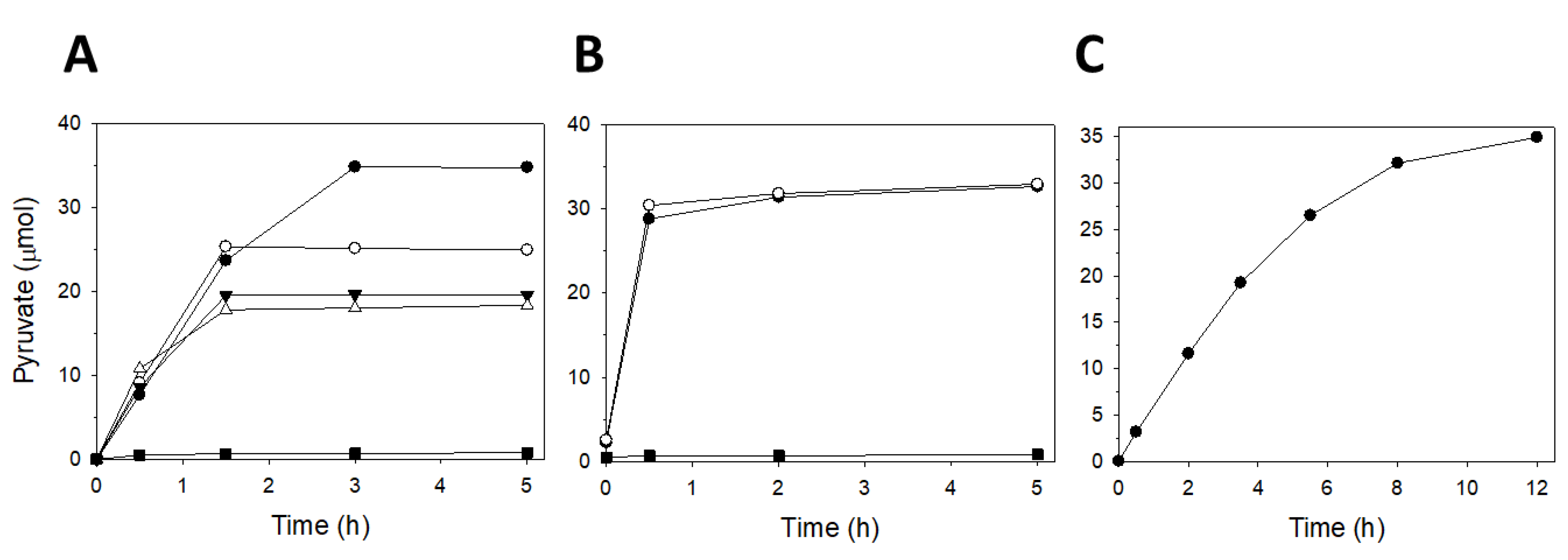
2.2. Enzyme Activity
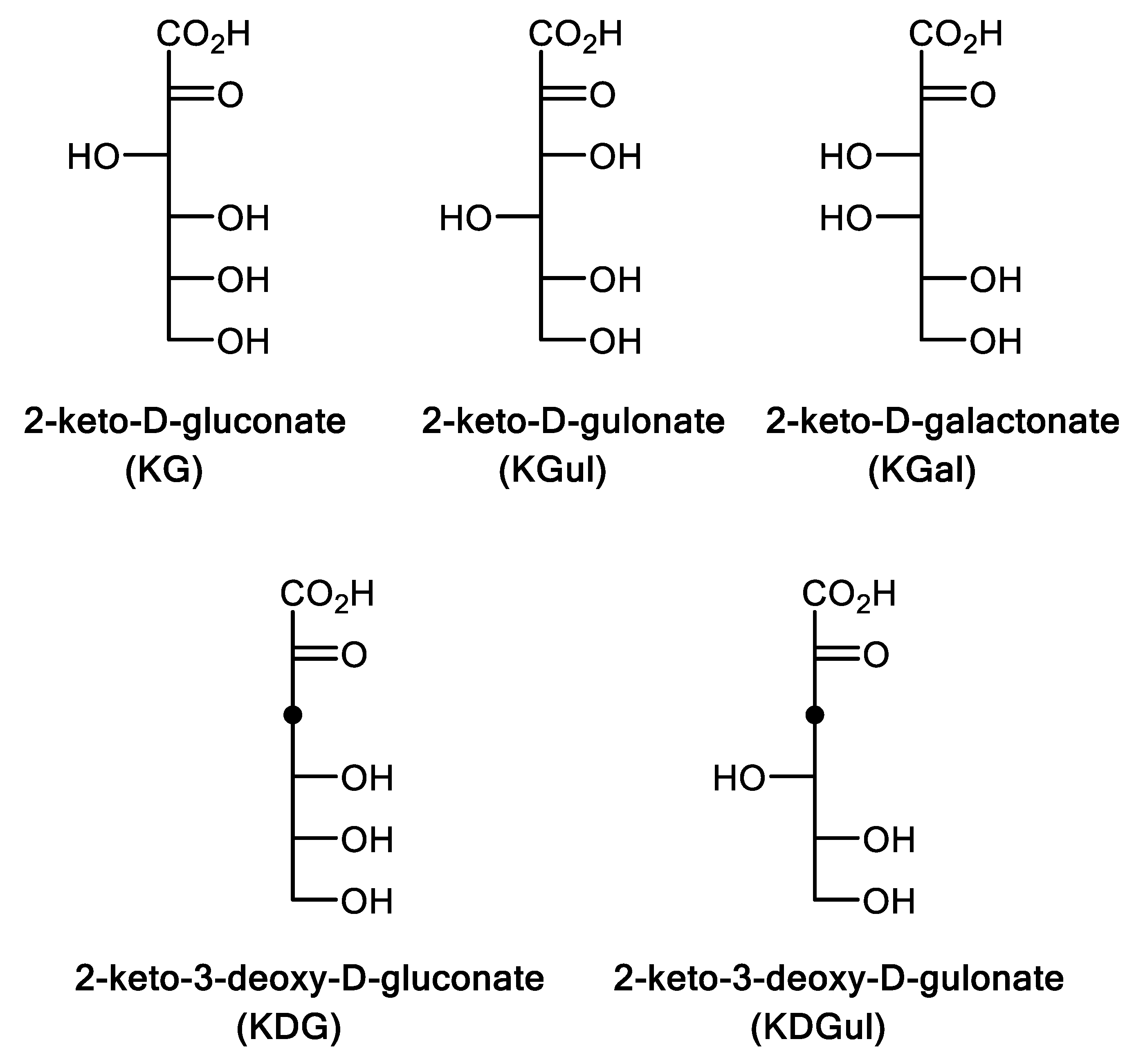
2.3. Substrate Specificity
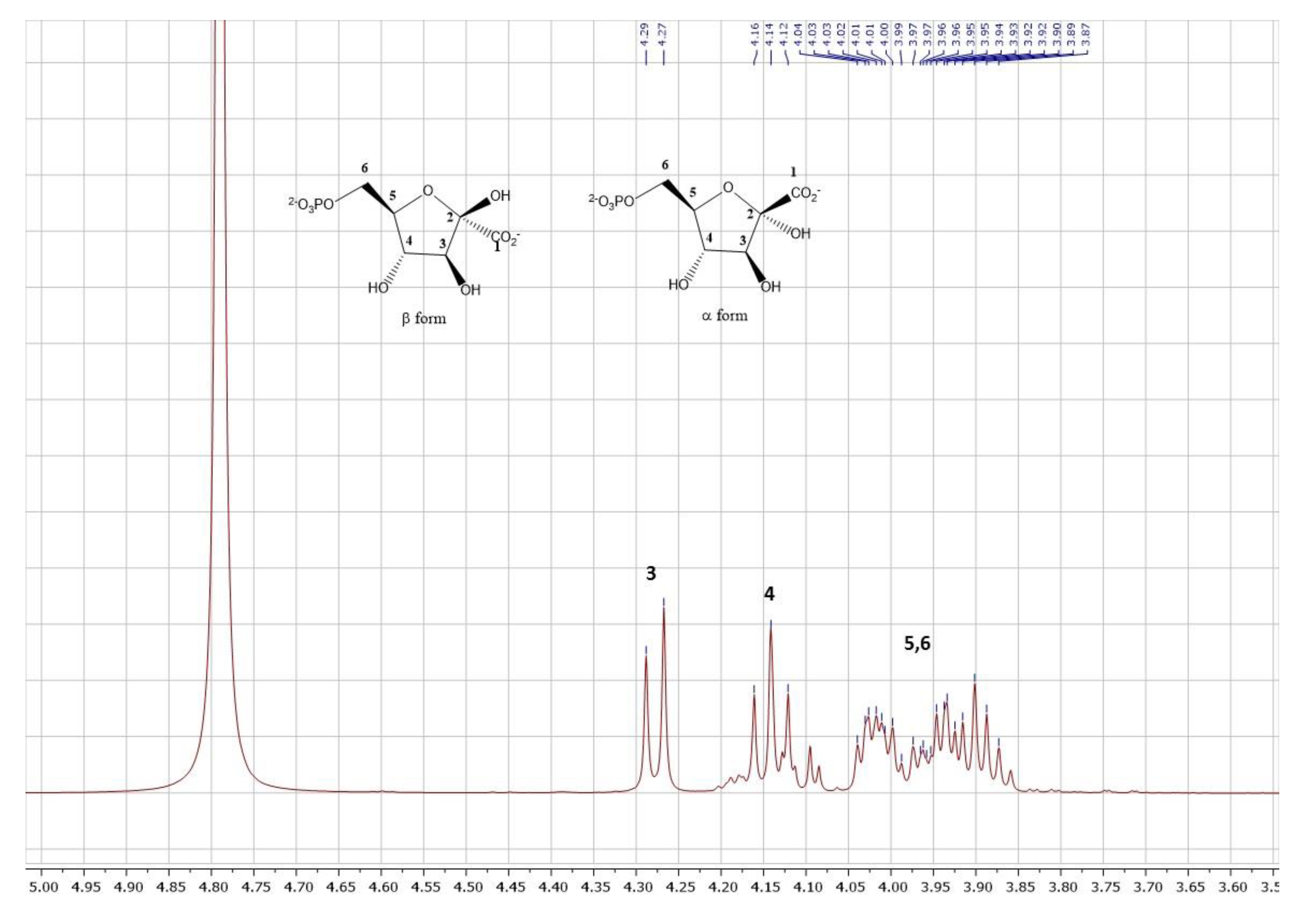
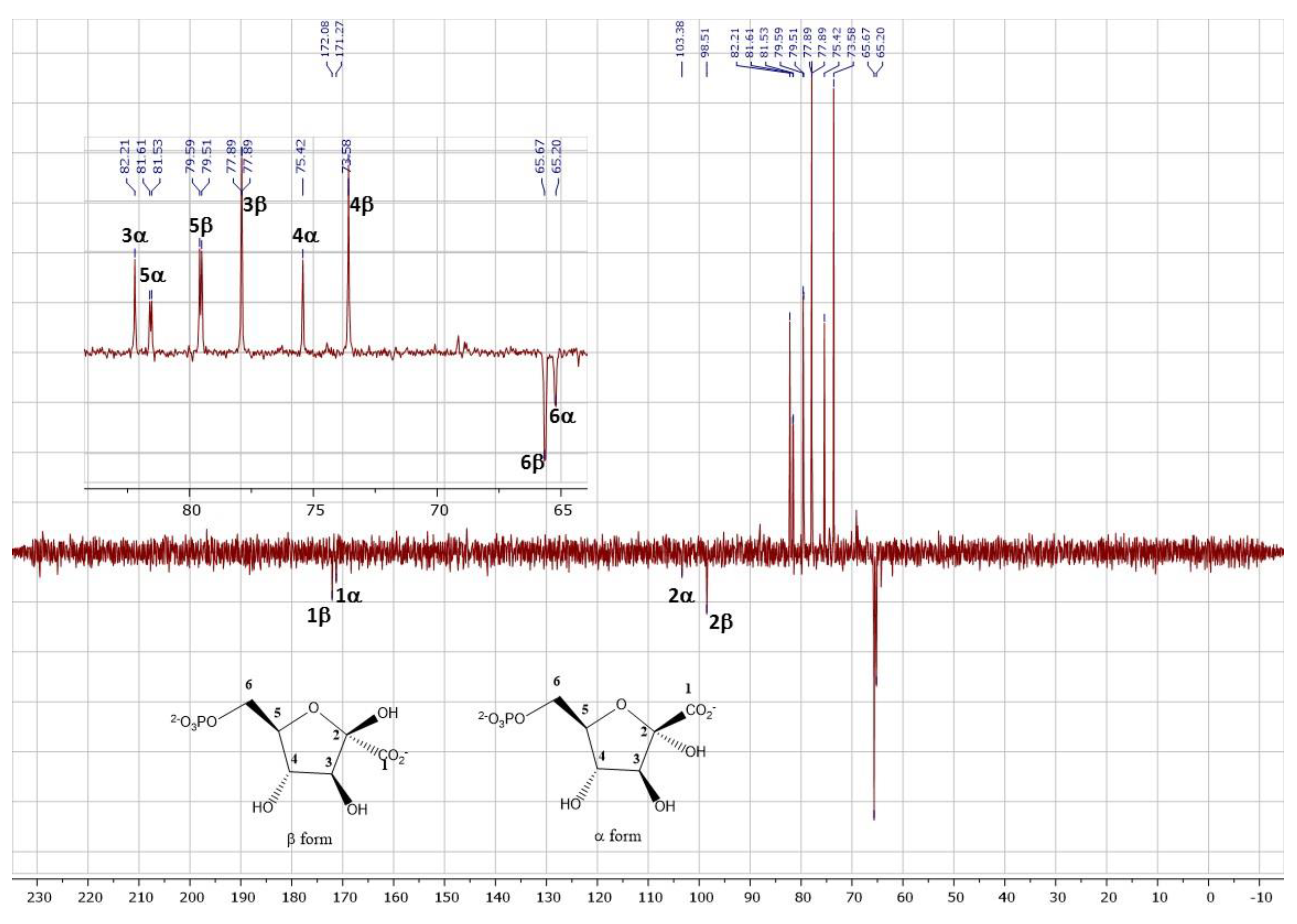
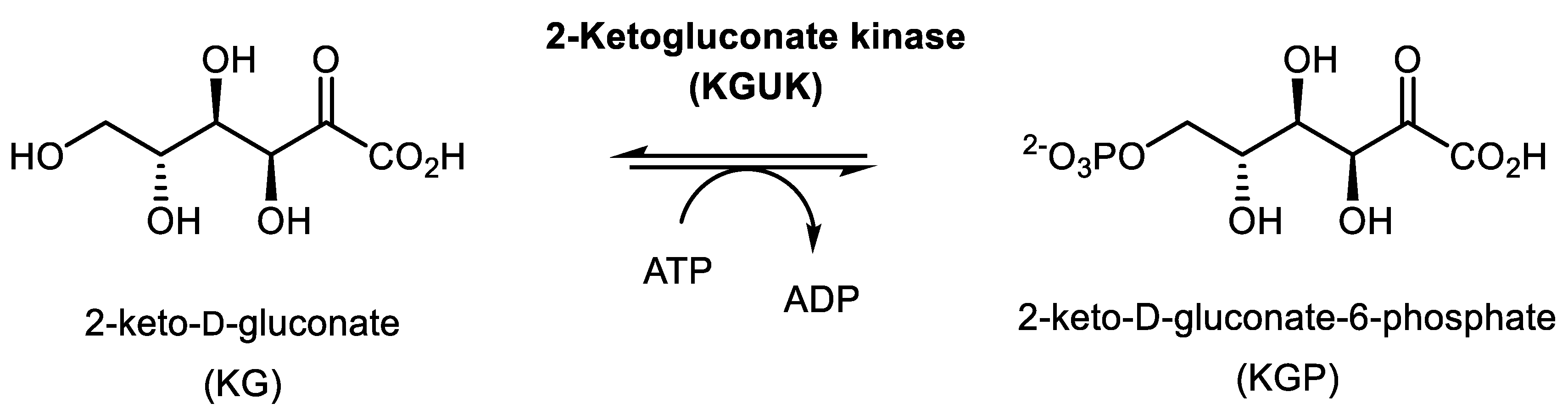
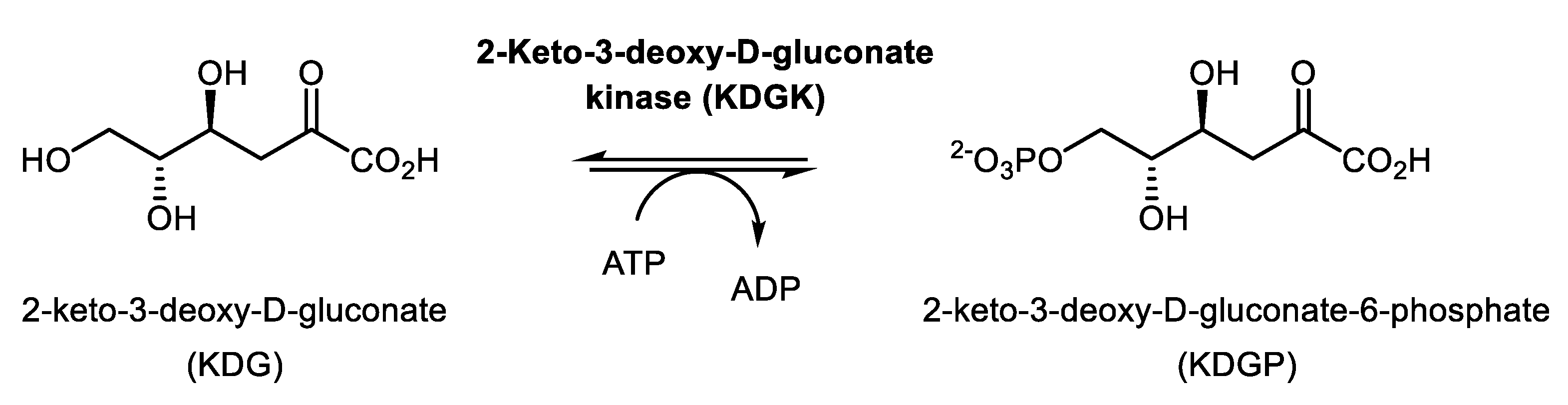
2.4. Synthesis of 2-ketogluconate-6-phosphate
3. Materials and Methods
3.1. General Remarks
3.2. Methods
3.2.1. Cloning
3.2.2. Expression and Purification
3.2.3. Enzyme Activity Assays and Kinetic Studies
3.2.4. Phosphorylation of 2-keto-d-gluconate.
Reaction Progress Monitoring
Preparative Scale Synthesis and Purification of 2-ketogluconate-6-phosphate
3.2.5. Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, L.; Huang, K.; Wei, M.; Meisner, J.; Liu, Y.; Garner, K.; Zang, L.; Wang, X.; Li, X.; Fang, J.; et al. Facile enzymatic synthesis of ketoses. Angew. Chem. Int. Ed. Engl. 2015, 54, 12654–12658. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, R.; Liese, A.; Streit, W. Biocatalytic phosphorylations of metabolites: Past, present, and future. Trends Biotechnol. 2017, 35, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Vergne-Vaxelaire, C.; Mariage, A.; Petit, J.-L.; Fossey-Jouenne, A.; Guérard-Hélaine, C.; Darii, E.; Debard, A.; Nepert, S.; Pellouin, V.; Lemaire, M.; et al. Characterization of a thermotolerant ROK-type mannofructokinase from Streptococcus mitis: Application to the synthesis of phosphorylated sugars. Appl. Microbiol. Biotechnol. 2018, 102, 5569–5583. [Google Scholar] [CrossRef] [PubMed]
- Fessner, W.-D.; Walter, C. “Artificial metabolisms” for the asymmetric one-pot synthesis of branched-chain saccharides. Angew. Chem. Int. Ed. Engl. 1992, 31, 614–616. [Google Scholar] [CrossRef]
- Zimmermann, F.T.; Schneider, A.; Schörken, U.; Sprenger, G.A.; Fessner, W.-D. Efficient multi-enzymatic synthesis of d-xylulose 5-phosphate. Tetrahedron Asymmetry 1999, 10, 1643–1646. [Google Scholar] [CrossRef]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-enzymatic cascade reactions: Overview and perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Sánchez-Moreno, I.; Hélaine, V.; Poupard, N.; Charmantray, F.; Légeret, B.; Hecquet, L.; García-Junceda, E.; Wohlgemuth, R.; Guérard-Hélaine, C.; Lemaire, M. One-pot cascade reactions using fructose-6-phosphate aldolase: Efficient synthesis of D-arabinose 5-phosphate, D-fructose 6-phosphate and analogues. Adv. Synth. Catal. 2012, 354, 1725–1730. [Google Scholar] [CrossRef]
- Guérard-Hélaine, C.; Debacker, M.; Clapés, P.; Szekrenyi, A.; Hélaine, V.; Lemaire, M. Efficient biocatalytic processes for highly valuable terminally phosphorylated C5 to C9 d -ketoses. Green Chem. 2014, 16, 1109–1113. [Google Scholar] [CrossRef]
- Hélaine, V.; Mahdi, R.; Sudhir Babu, G.V.; De Berardinis, V.; Wohlgemuth, R.; Lemaire, M.; Guérard-Hélaine, C. Straightforward synthesis of terminally phosphorylated l-sugars via multienzymatic cascade reactions. Adv. Synth. Catal. 2015, 357, 1703–1708. [Google Scholar] [CrossRef]
- Samland, A.K.; Rale, M.; Sprenger, G.A.; Fessner, W.-D. The transaldolase family: New synthetic opportunities from an ancient enzyme scaffold. ChemBioChem 2011, 12, 1454–1474. [Google Scholar] [CrossRef]
- Schörken, U.; Sprenger, G.A. Thiamin-dependent enzymes as catalysts in chemoenzymatic syntheses. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1998, 1385, 229–243. [Google Scholar] [CrossRef]
- Guérard, C.; Alphand, V.; Archelas, A.; Demuynck, C.; Hecquet, L.; Furstoss, R.; Bolte, J. Transketolase-mediated synthesis of 4-deoxy-d-fructose 6-phosphate by epoxide hydrolase-catalysed resolution of 1,1-diethoxy-3,4-epoxybutane. Eur. J. Org. Chem. 1999, 1999, 3399–3402. [Google Scholar] [CrossRef]
- Shaeri, J.; Wright, I.; Rathbone, E.B.; Wohlgemuth, R.; Woodley, J.M. Characterization of enzymatic D-xylulose 5-phosphate synthesis. Biotechnol. Bioeng. 2008, 101, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Shaeri, J.; Wohlgemuth, R.; Woodley, J.M. Semiquantitative process screening for the biocatalytic synthesis of d-xylulose-5-phosphate. Org. Process. Res. Dev. 2006, 10, 605–610. [Google Scholar] [CrossRef]
- Solovjeva, O.N.; Kochetov, G.A. Enzymatic synthesis of d-xylulose 5-phosphate from hydroxypyruvate and d-glyceraldehyde-3-phosphate. J. Mol. Catal. B Enzym. 2008, 54, 90–92. [Google Scholar] [CrossRef]
- Charmantray, F.; Hélaine, V.; Legeret, B.; Hecquet, L. Preparative scale enzymatic synthesis of d-sedoheptulose-7-phosphate from β-hydroxypyruvate and d-ribose-5-phosphate. J. Mol. Catal. B Enzym. 2009, 57, 6–9. [Google Scholar] [CrossRef]
- Guérard-Hélaine, C.; De Sousa Lopes Moreira, M.; Touisni, N.; Hecquet, L.; Lemaire, M.; Hélaine, V. Transketolase-aldolase symbiosis for the stereoselective preparation of aldoses and ketoses of biological interest. Adv. Synth. Catal. 2017, 359, 2061–2065. [Google Scholar] [CrossRef]
- Trigalo, F.; Szabó, L. The synthesis of D-arabino-hexulosonic acid 6-phosphate and its stability in acid and alkaline medium. Eur. J. Biochem. 1972, 25, 336–340. [Google Scholar] [CrossRef]
- Ciferri, O.; Blakley, E.R.; Simpson, F.J. Purification and properties of the 2-ketogluconokinase of Leuconostoc mesenteroides. Can. J. Microbiol. 1959, 5, 277–291. [Google Scholar] [CrossRef]
- Swanson, B.L.; Hager, P.; Phibbs, P.; Ochsner, U.; Vasil, M.L.; Hamood, A.N. Characterization of the 2-ketogluconate utilization operon in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 2000, 37, 561–573. [Google Scholar] [CrossRef]
- Simons, J.A.; Teixeira de Mattos, M.J.; Neijssel, O.M. Aerobic 2-ketogluconate metabolism of Klebsiella pneumoniae NCTC 418 grown in chemostat culture. J. Gen. Microbiol. 1991, 137, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Yum, D.Y.; Lee, B.Y.; Hahm, D.H.; Pan, J.G. The yiaE gene, located at 80.1 minutes on the Escherichia coli chromosome, encodes a 2-ketoaldonate reductase. J. Bacteriol. 1998, 180, 5984–5988. [Google Scholar] [PubMed]
- De Ley, J. Phospho-2-keto-D-gluconate, an intermediate in the carbohydrate metabolism of Aerobacter cloacae. Biochim. Biophys. Acta 1954, 13, 302. [Google Scholar] [CrossRef]
- Narrod, S.A.; Wood, W.A. Carbohydrate oxidation by Pseudomonas fluorescens. V. Evidence for gluconokinase and 2-ketogluconokinase. J. Biol. Chem. 1956, 220, 45–55. [Google Scholar] [PubMed]
- Frampton, E.W.; Wood, W.A. Purification and properties of 2-ketogluconokinase from Aerobacter aerogenes. J. Biol. Chem. 1961, 236, 2578–2580. [Google Scholar] [PubMed]
- Vicente, M.; Cánovas, J.L. Glucolysis in Pseudomonas putida: Physiological role of alternative routes from the analysis of defective mutants. J. Bacteriol. 1973, 116, 908–914. [Google Scholar]
- De Ley, J.; Vandamme, J. The metabolism of sodium 2-keto-d-gluconate by micro-organisms. Microbiology 1955, 12, 162–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roberts, B.K.; Midgley, M.; Dawes, E.A. The metabolism of 2-oxogluconate by Pseudomonas aeruginosa. J. Gen. Microbiol. 1973, 78, 319–329. [Google Scholar] [CrossRef]
- Lessie, T.G.; Phibbs, P.V. Alternative pathways of carbohydrate utilization in pseudomonads. Annu. Rev. Microbiol. 1984, 38, 359–388. [Google Scholar] [CrossRef]
- del Castillo, T.; Ramos, J.L.; Rodríguez-Herva, J.J.; Fuhrer, T.; Sauer, U.; Duque, E. Convergent peripheral pathways catalyze initial glucose catabolism in Pseudomonas putida: Genomic and flux analysis. J. Bacteriol. 2007, 189, 5142–5152. [Google Scholar] [CrossRef]
- Nikel, P.I.; Chavarría, M.; Fuhrer, T.; Sauer, U.; de Lorenzo, V. Pseudomonas putida KT2440 strain metabolizes glucose through a cycle formed by enzymes of the entner-doudoroff, embden-meyerhof-parnas, and pentose phosphate pathways. J. Biol. Chem. 2015, 290, 25920–25932. [Google Scholar] [CrossRef] [PubMed]
- De Ley, J. The phosphorylation of some carbohydrates, connected with the direct oxidation, by Aerobacter cloacae. Enzymologia 1953, 16, 99–104. [Google Scholar] [PubMed]
- Nandadasa, H.G.; Andreesen, M.; Schlegel, H.G. The utilization of 2-ketogluconate by Hydrogenomonas eutropha H 16. Arch. Microbiol. 1974, 99, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E.; et al. Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, N.; Inagaki, E.; Yasuike, K.; Takio, K.; Tahirov, T.H. Structure of Thermus thermophilus 2-Keto-3-deoxygluconate kinase: Evidence for recognition of an open chain substrate. J. Mol. Biol. 2004, 340, 477–489. [Google Scholar] [CrossRef]
- Ohshima, T.; Kawakami, R.; Kanai, Y.; Goda, S.; Sakuraba, H. Gene expression and characterization of 2-keto-3-deoxygluconate kinase, a key enzyme in the modified Entner-Doudoroff pathway of the aerobic and acidophilic hyperthermophile Sulfolobus tokodaii. Protein Expr. Purif. 2007, 54, 73–78. [Google Scholar] [CrossRef]
- Berardinis, V.; De Guérard-Hélaine, C.; Darii, E.; Bastard, K.; Hélaine, V.; Mariage, A.; Petit, J.-L.; Poupard, N.; Sánchez-Moreno, I.; Stam, M.; et al. Expanding the reaction space of aldolases using hydroxypyruvate as a nucleophilic substrate. Green Chem. 2017, 19, 519–526. [Google Scholar] [CrossRef]
- Lamble, H.J.; Danson, M.J.; Hough, D.W.; Bull, S.D. Engineering stereocontrol into an aldolase-catalysed reaction. Chem. Commun. 2005, 1, 124–126. [Google Scholar] [CrossRef]
- Garland, P.B.; Randle, P.J. A Rapid enzymatic assay for glycerol. Nature 1962, 196, 987. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Sample | Activity (U) | Protein (mg/mL) | Volume (mL) | Activity (U/mg) | Fold Purification | Recovery (%) | |
|---|---|---|---|---|---|---|---|
| 1 | CFE | 96.0 | 13.02 | 20 | 0.38 | — | 100 |
| 2 | IMAC | 40.4 | 0.47 | 10 | 8.70 | 22.8 | 42 |
| Entry | Substrate | Vmax (U/mg) | kcat (s−1) | KM (mM) | kcat/KM (s−1M−1) |
|---|---|---|---|---|---|
| 1 | ATP | 10.2 ± 0.1 | 5.71 ± 0.05 | 0.073 ± 0.002 | 78,220 ± 2500 |
| 2 | KG1 | 10.4 ± 0.4 | 5.8 ± 0.2 | 0.35 ± 0.03 | 16,770 ± 1300 |
| 3 | KGul | 1.9 ± 0.2 | 1.1 ± 0.1 | 4 ± 1 | 246 ± 70 |
| 4 | KDG | 18.2 ± 0.9 | 10.2 ± 0.5 | 1.4 ± 0.2 | 7370 ± 970 |
| 5 | KDGul | 3.7 ± 0.4 | 2.1 ± 0.2 | 25 ± 4 | 83 ± 20 |
| 6 | KGal* | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Moreno, I.; Trachtmann, N.; Ilhan, S.; Hélaine, V.; Lemaire, M.; Guérard-Hélaine, C.; Sprenger, G.A. 2-Ketogluconate Kinase from Cupriavidus necator H16: Purification, Characterization, and Exploration of Its Substrate Specificity. Molecules 2019, 24, 2393. https://doi.org/10.3390/molecules24132393
Sánchez-Moreno I, Trachtmann N, Ilhan S, Hélaine V, Lemaire M, Guérard-Hélaine C, Sprenger GA. 2-Ketogluconate Kinase from Cupriavidus necator H16: Purification, Characterization, and Exploration of Its Substrate Specificity. Molecules. 2019; 24(13):2393. https://doi.org/10.3390/molecules24132393
Chicago/Turabian StyleSánchez-Moreno, Israel, Natalia Trachtmann, Sibel Ilhan, Virgil Hélaine, Marielle Lemaire, Christine Guérard-Hélaine, and Georg A. Sprenger. 2019. "2-Ketogluconate Kinase from Cupriavidus necator H16: Purification, Characterization, and Exploration of Its Substrate Specificity" Molecules 24, no. 13: 2393. https://doi.org/10.3390/molecules24132393
APA StyleSánchez-Moreno, I., Trachtmann, N., Ilhan, S., Hélaine, V., Lemaire, M., Guérard-Hélaine, C., & Sprenger, G. A. (2019). 2-Ketogluconate Kinase from Cupriavidus necator H16: Purification, Characterization, and Exploration of Its Substrate Specificity. Molecules, 24(13), 2393. https://doi.org/10.3390/molecules24132393






