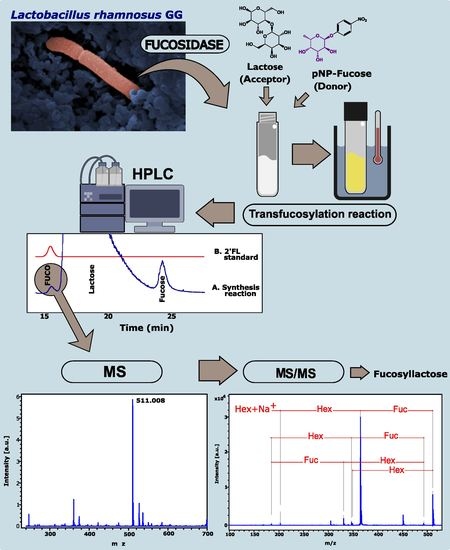
Synthesis of Fucosyl-Oligosaccharides Using α-l-Fucosidase from Lactobacillus rhamnosus GG
Abstract
1. Introduction
2. Results and Discussion
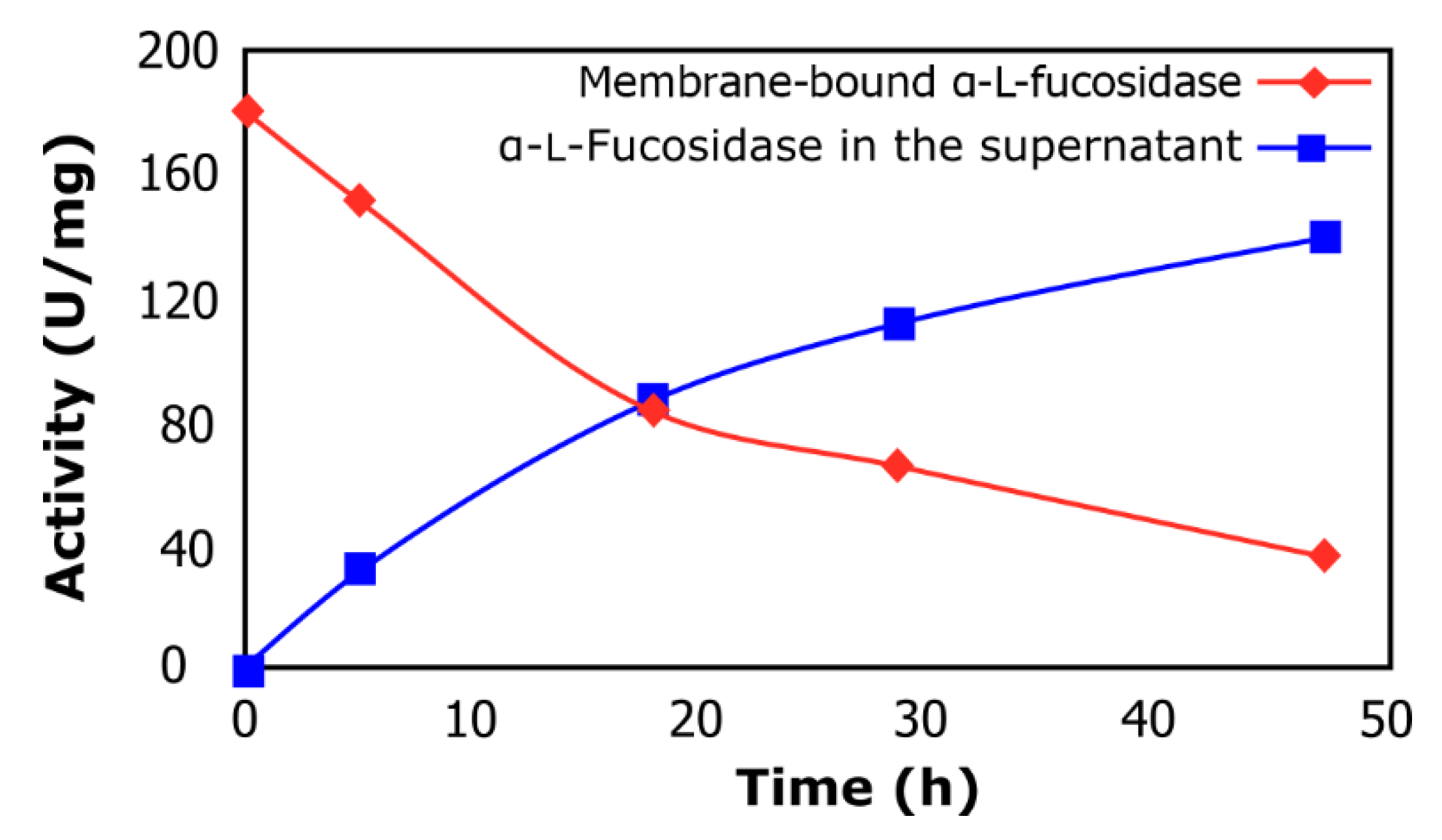
2.1. α-l-Fucosidase Release
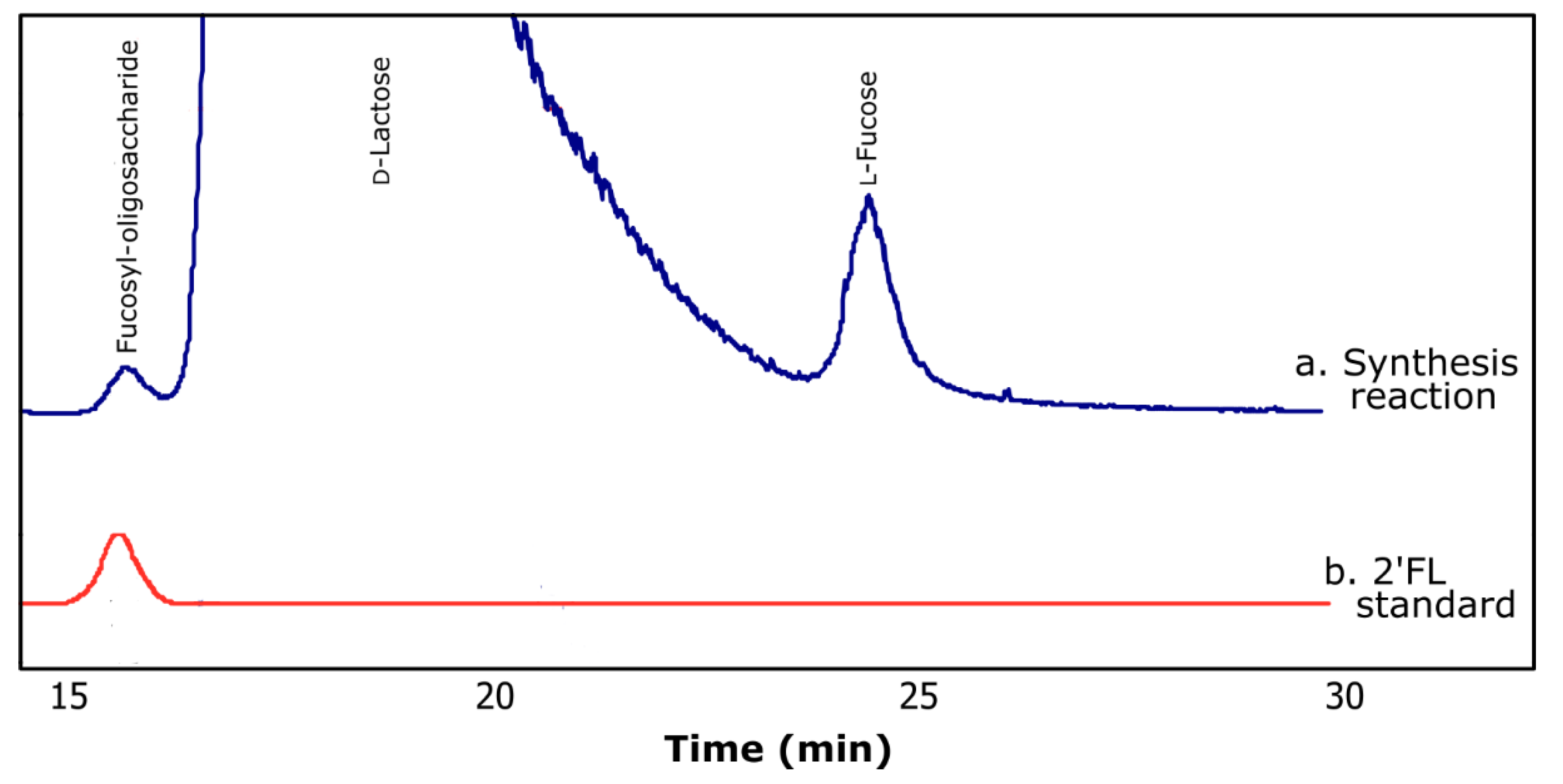
2.2. Synthesis of Fucosyl-Oligosaccharides
2.3. Composition of Synthesized Fucosyl-Oligosaccharide
3. Materials and Methods
3.1. Materials
3.2. Microorganism
3.3. Production of α-lFucosidase
3.4. Release of α-lFucosidase
3.5. Enzyme Activity Assay
3.6. Synthesis of Fucosyl-Oligosaccharides
3.7. Composition of Synthesized Fucosyl-Oligosaccharide
3.8. Carbohydrate Quantification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miñana, I.V. Oligosacáridos en la leche humana. Acta Pediatr. Esp. 2007, 65, 175–179. [Google Scholar]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2009, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J. Anim. Sci. 2009, 87, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Shoaf-Sweeney, K.D.; Hutkins, R.W. Adherence, anti-adherence, and oligosaccharides preventing pathogens from sticking to the host. Adv. Food Nutr. Res. 2009, 55, 101–161. [Google Scholar] [PubMed]
- Smoot, J.T.; Demchenko, A.V. Oligosaccharide synthesis: From conventional methods to modern expeditious strategies. In Advances In Carbohydrate Chemistry And Biochemistry, 1st ed.; Horton, D., Ed.; Elsevier Inc.: Amsterdam, the Netherlands, 2009; Volume 62, pp. 161–250. [Google Scholar]
- Zeuner, B.; Jers, C.; Mikkelsen, J.; Meyer, A. Methods for improving transglycosylation for synthesis of human milk oligosaccharide biomimetics. J. Agric. Food Chem. 2014, 62, 9615–9631. [Google Scholar] [CrossRef] [PubMed]
- Zeuner, B.; Muschiol, J.; Holck, J.; Lezyk, M.; Gedde, M.R.; Jers, C.; Mikkelsen, J.D.; Meyer, A.S. Substrate specificity and transfucosylation activity of GH29 α-l-fucosidases for enzymatic production of human milk oligosaccharides. New Biotechnol. 2018, 41, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Okamoto, K.; Yanase, H. Purification and characterization of extracellular 1,2-alpha-L-fucosidase from Bacillus cereus. J. Biosci. Bioeng. 2005, 99, 629–635. [Google Scholar] [CrossRef]
- Liu, S.W.; Chen, C.S.; Chang, S.S.; Mong, K.K.; Lin, C.H.; Chang, C.W.; Tang, C.Y.; Li, Y.K. Identification of essential residues of human α-lfucosidase and tests of its mechanism. Biochemistry 2009, 48, 110–120. [Google Scholar] [CrossRef]
- Berteau, O.; McCort, I.; Goasdoué, N.; Tissot, B.; Daniel, R. Characterization of a new α-lfucosidase isolated from the marine mollusk Pecten maximus that catalyzes the hydrolysis of α-l-fucose from algal fucoidan (Ascophyllum nodosum). Glycobiology 2002, 12, 273–282. [Google Scholar] [CrossRef]
- Ogata-Arakawa, M.; Muramatsu, T.; Kobata, A. α-lFucosidases from almond emulsin: Characterization of the two enzymes with different specificities. Arch. Biochem. Biophys. 1977, 181, 353–358. [Google Scholar] [CrossRef]
- Khunsook, S.; Bean, B.S.; McGowan, S.R.; Alhadeff, J.A. Purification and characterization of plasma membrane associated human sperm α-l-fucosidase. Biol. Reprod. 2003, 68, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Lozano, Y.; García-Garibay, M.; López-Munguía-Canales, A.; Gómez-Ruiz, L.; Rodríguez-Serrano, G.; Cruz-Guerrero, A. Synthesis of α-lfucosidase in different strains of lactic acid bacteria. Rev. Mex. Ing. Quím. 2015, 14, 623–629. [Google Scholar]
- Rodríguez-Díaz, J.; Monedero, V.; Yebra, M.J. Utilization of natural fucosylated oligosaccharides by three novel alpha-L-fucosidases from a probiotic Lactobacillus casei strain. Appl. Environ. Microbiol. 2011, 77, 703–705. [Google Scholar] [CrossRef]
- Morita, H.; Toh, H.; Oshima, K.; Murakami, M.; Taylor, T.; Igimi, S.; Hattori, M. Complete genome sequence of the probiotic Lactobacillus rhamnosus ATCC 53103. J. Bacteriol. 2009, 191, 7630–7631. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Díaz, J.; Carbajo, R.J.; Pineda-Lucena, A.; Monedero, V.; Yebra, M.J. Synthesis of fucosyl-N-acetylglucosamine disaccharides by transfucosylation using α-lfucosidases from Lactobacillus casei. Appl. Environ. Microbiol. 2013, 79, 3847–3850. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.M.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [PubMed]
- Katayama, T.; Sakuma, A.; Kimura, T.; Makimura, Y.; Hiratake, J.; Sakata, K.; Yamanoi, T.; Kumagai, H.; Yamamoto, K. Molecular cloning and characterization of Bifidobacterium bifidum 1, 2-α-lfucosidase (AfcA), a novel inverting glycosidase (Glycoside Hydrolase Family 95). J. Bacteriol. 2004, 186, 4885–4893. [Google Scholar] [CrossRef]
- Dong, S.; Chang, Y.; Shen, J.; Xue, C.; Chen, F. Purification, expression and characterization of a novel α-l-fucosidase from a marine bacteria Wenyingzhuangia fucanilytica. Protein Expr. Purif. 2017, 129, 9–17. [Google Scholar] [CrossRef]
- Eneyskaya, E.V.; Kulminskaya, A.A.; Kalkkinen, N.; Nifantiev, N.E.; Arbatskii, N.P.; Saenko, A.I.; Chepurnaya, O.V.; Arutyunyan, A.V.; Shabalin, K.A.; Neustroev, K.N. An α-lfucosidase from Thermus sp. with unusually broad specificity. Glycoconjugate J. 2001, 18, 827–834. [Google Scholar] [CrossRef]
- Benešova, E.; Lipovova, P.; Dvorakova, H.; Kralova, B. α-lFucosidase from Paenibacillus thiaminolyticus: Its hydrolytic and transglycosylation abilities. Glycobiology 2013, 23, 1052–1065. [Google Scholar] [CrossRef][Green Version]
- Shvetsova, S.V.; Shabalin, K.A.; Bobrov, K.S.; Ivanen, D.R.; Ustyuzhanina, N.E.; Krylov, V.B.; Nifantiev, N.E.; Naryzhny, S.N.; Zgoda, V.G.; Eneyskaya, E.V.; et al. Characterization of a new α-l-fucosidase isolated from Fusarium proliferatum LE1 that is regioselective to α-(1 → 4)-l-fucosidic linkage in the hydrolysis of α-l-fucobiosides. Biochimie 2017, 132, 54–65. [Google Scholar] [CrossRef]
- Zeng, X.; Murata, T.; Usui, T. Glycosidase-catalyzed synthesis of fucosyl di- and trisaccharide derivatives using α-lfucosidase from Alcaligenes sp. J. Carbohydr. Chem. 2003, 22, 309–316. [Google Scholar] [CrossRef]
- Ajisaka, K.; Shirakabe, M. Regioselective synthesis of α-lfucosyl-containing disaccharides by use of α-lfucosidases of various origins. Carbohydr. Res. 1992, 224, 291–299. [Google Scholar] [CrossRef]
- Farkas, E.; Thiem, J.; Ajisaka, K. Enzymatic synthesis of fucose-containing disaccharides employing the partially purified alpha-L-fucosidase from Penicillium multicolor. Carbohydr. Res. 2000, 328, 293–299. [Google Scholar] [CrossRef]
- Guzmán-Rodríguez, F.; Alatorre-Santamaría, S.; Gómez-Ruiz, L.; Rodríguez-Serrano, G.; García-Garibay, M.; Cruz-Guerrero, A. Synthesis of a fucosylated trisaccharide via transglycosylation by α-lfucosidase from Thermotoga maritima. Appl. Biochem. Biotechnol. 2018, 186, 681–691. [Google Scholar] [CrossRef]
- Petschacher, B.; Nidetzky, B. Biotechnological production of fucosylated human milk oligosaccharides: Prokaryotic fucosyltransferases and their use in biocatalytic cascades or whole cell conversion systems. J. Biotechnol. 2016, 235, 61–83. [Google Scholar] [CrossRef]
- Reglero, A.; Cabezas, J. Glycosidases of molluscs. Eur. J. Biochem. 1976, 66, 379–387. [Google Scholar] [CrossRef]
- Grove, D.S.; Serif, G. Porcine thyroid fucosidase. Biochim. Biophys. Acta 1981, 662, 246–255. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chaves-Munguia, B.; Newburg, D.S. Campylobacter jejuni binds intestinal H(O) antigen (Fucα1,2Galβ1,4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef]
- Weichert, S.; Jennewein, S.; Hufner, E.; Weiss, C.; Borkowski, J.; Putze, J.; Schroten, H. Bioengineered 2’-fucosyllactose and 3-fucosyllactose inhibit the adhesion of Pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr. Res. 2013, 33, 831–838. [Google Scholar] [CrossRef]
- Sotgiu, S.; Arru, G.; Fois, M.L.; Sanna, A.; Musumeci, M.; Rosati, G.; Musumeci, S. Immunomodulation of fucosyl-lactose and lacto-N-fucopentaose on mononuclear cells from multiple sclerosis and healthy subjects. Int. J. Biomed. Sci. 2016, 2, 114–120. [Google Scholar]
- Duska-McEwen, G.; Senft, A.P.; Ruetschilling, T.L.; Barrett, E.G.; Buck, R.H. Human milk oligosaccharides enhance innate immunity to respiratory syncytial virus and influenza in vitro. Food Nutr. Sci. 2014, 5, 1387–1398. [Google Scholar]
- Cruz-Guerrero, A.; Hernández-Sánchez, H.; Figueroa-González, I. Commercial probiotic bacteria and prebiotic carbohydrates: A fundamental study on prebiotics uptake, antimicrobials production and inhibition of pathogens. J. Sci. Food Agric. 2014, 94, 2246–2252. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
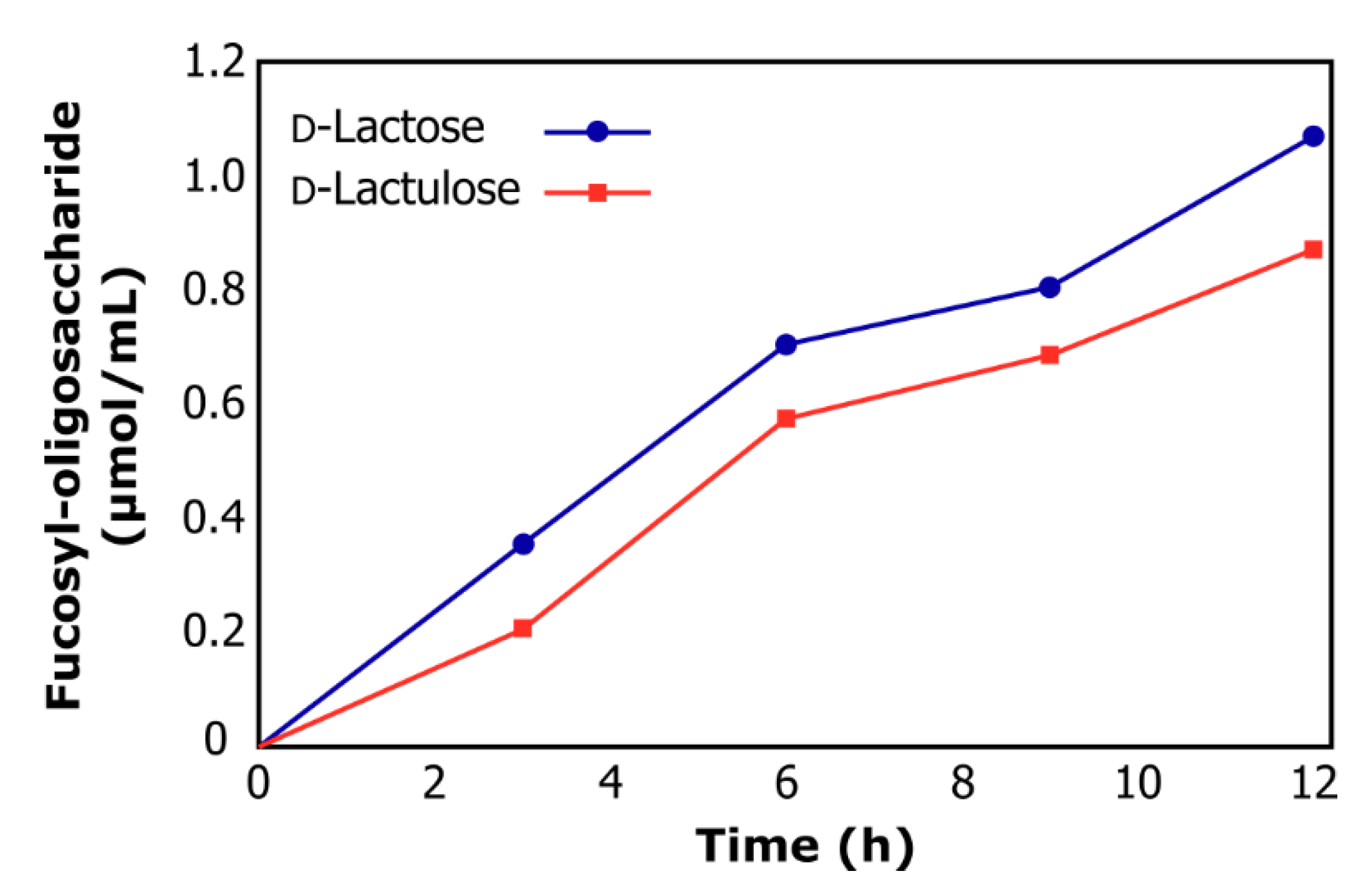
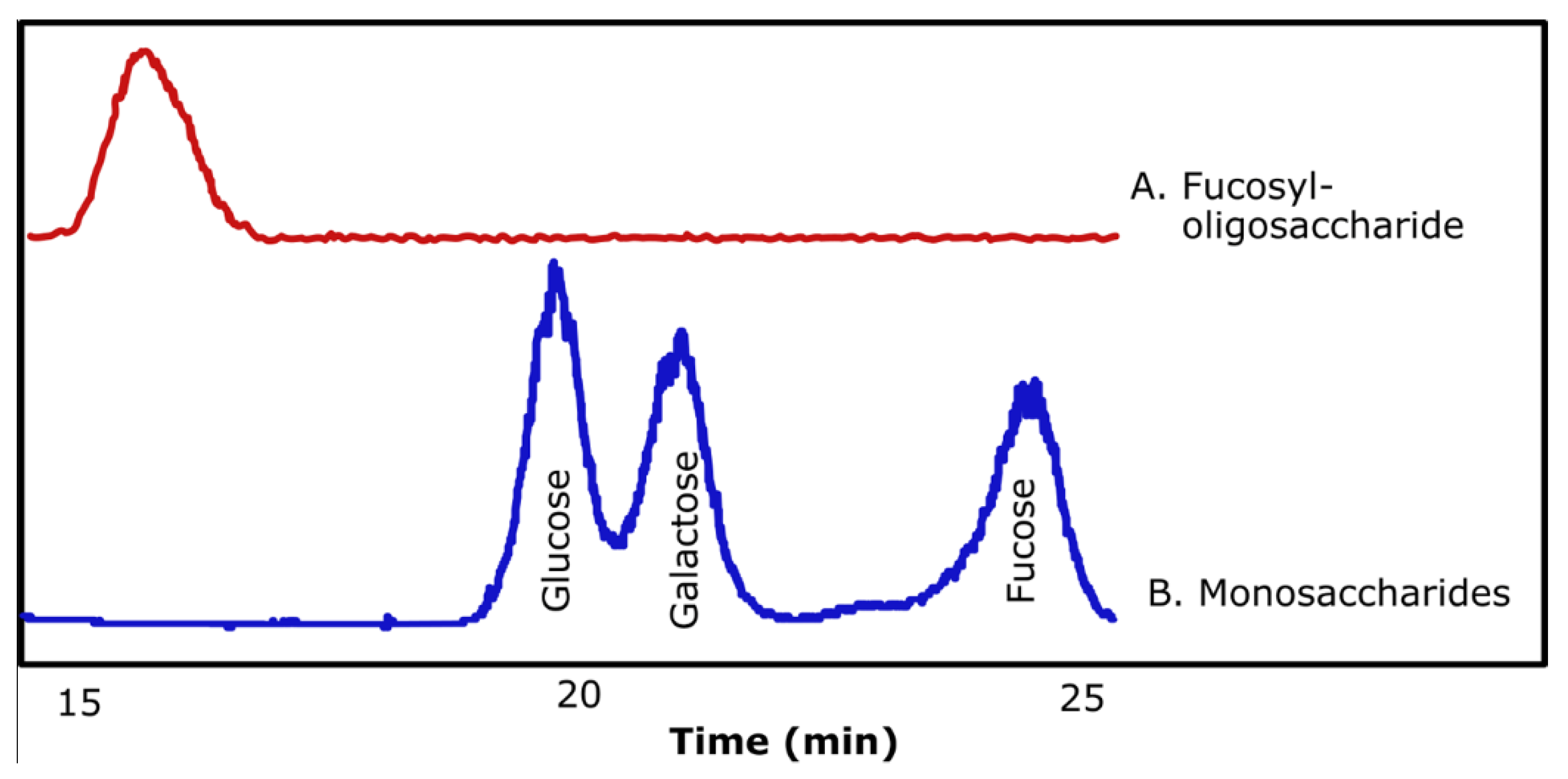
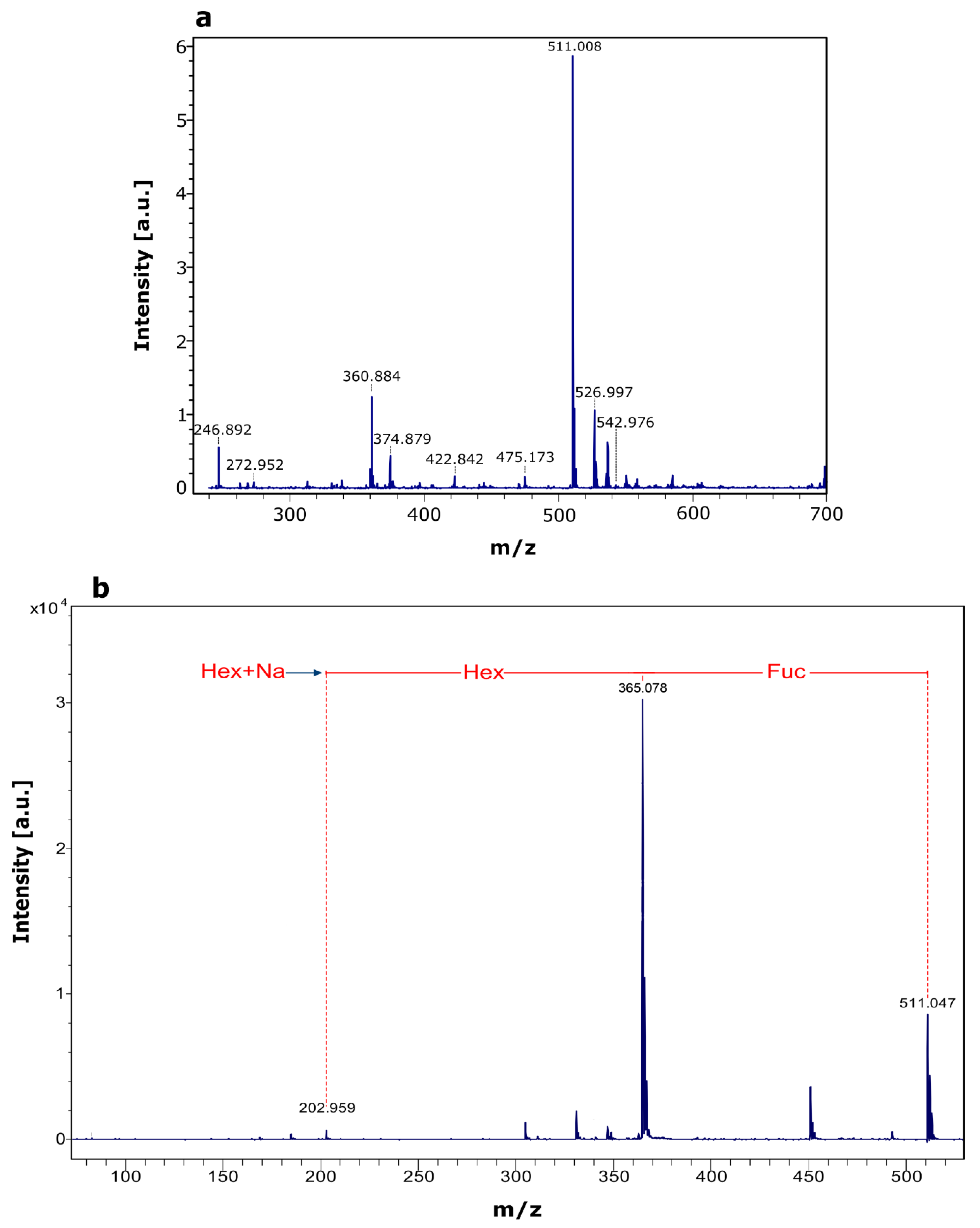
| Acceptor Substrate | Oligosaccharide (µmol/mL) | Yield * (%) |
|---|---|---|
| d-Lactose | 0.75 | 21 |
| d-Lactulose | 1.16 | 25 |
| d-Galactose | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escamilla-Lozano, Y.; Guzmán-Rodríguez, F.; Alatorre-Santamaría, S.; García-Garibay, M.; Gómez-Ruiz, L.; Rodríguez-Serrano, G.; Cruz-Guerrero, A. Synthesis of Fucosyl-Oligosaccharides Using α-l-Fucosidase from Lactobacillus rhamnosus GG. Molecules 2019, 24, 2402. https://doi.org/10.3390/molecules24132402
Escamilla-Lozano Y, Guzmán-Rodríguez F, Alatorre-Santamaría S, García-Garibay M, Gómez-Ruiz L, Rodríguez-Serrano G, Cruz-Guerrero A. Synthesis of Fucosyl-Oligosaccharides Using α-l-Fucosidase from Lactobacillus rhamnosus GG. Molecules. 2019; 24(13):2402. https://doi.org/10.3390/molecules24132402
Chicago/Turabian StyleEscamilla-Lozano, Yolanda, Francisco Guzmán-Rodríguez, Sergio Alatorre-Santamaría, Mariano García-Garibay, Lorena Gómez-Ruiz, Gabriela Rodríguez-Serrano, and Alma Cruz-Guerrero. 2019. "Synthesis of Fucosyl-Oligosaccharides Using α-l-Fucosidase from Lactobacillus rhamnosus GG" Molecules 24, no. 13: 2402. https://doi.org/10.3390/molecules24132402
APA StyleEscamilla-Lozano, Y., Guzmán-Rodríguez, F., Alatorre-Santamaría, S., García-Garibay, M., Gómez-Ruiz, L., Rodríguez-Serrano, G., & Cruz-Guerrero, A. (2019). Synthesis of Fucosyl-Oligosaccharides Using α-l-Fucosidase from Lactobacillus rhamnosus GG. Molecules, 24(13), 2402. https://doi.org/10.3390/molecules24132402