Abstract
An efficient approach towards the synthesis of 6-aryl-4-azidocinnolines was developed with the aim of exploring the photophysical properties of 6-aryl-4-azidocinnolines and their click reaction products with alkynes, 6-aryl-4-(1,2,3-1H-triazol-1-yl)cinnolines. The synthetic route is based on the Richter-type cyclization of 2-ethynyl-4-aryltriazenes with the formation of 4-bromo-6-arylcinnolines and nucleophilic substitution of a bromine atom with an azide functional group. The developed synthetic approach is tolerant to variations of functional groups on the aryl moiety. The resulting azidocinnolines were found to be reactive in both CuAAC with terminal alkynes and SPAAC with diazacyclononyne, yielding 4-triazolylcinnolines. It was found that 4-azido-6-arylcinnolines possess weak fluorescent properties, while conversion of the azido function into a triazole ring led to complete fluorescence quenching. The lack of fluorescence in triazoles could be explained by the non-planar structure of triazolylcinnolines and a possible photoinduced electron transfer (PET) mechanism. Among the series of 4-triazolylcinnoline derivatives a compound bearing hydroxyalkyl substituent at triazole ring was found to be cytotoxic to HeLa cells.
Keywords:
azides; cinnolines; triazoles; CuAAC; alkynes; cycloalkynes; Richter cyclization; Suzuki coupling; fluorescence; cytotoxicity 1. Introduction
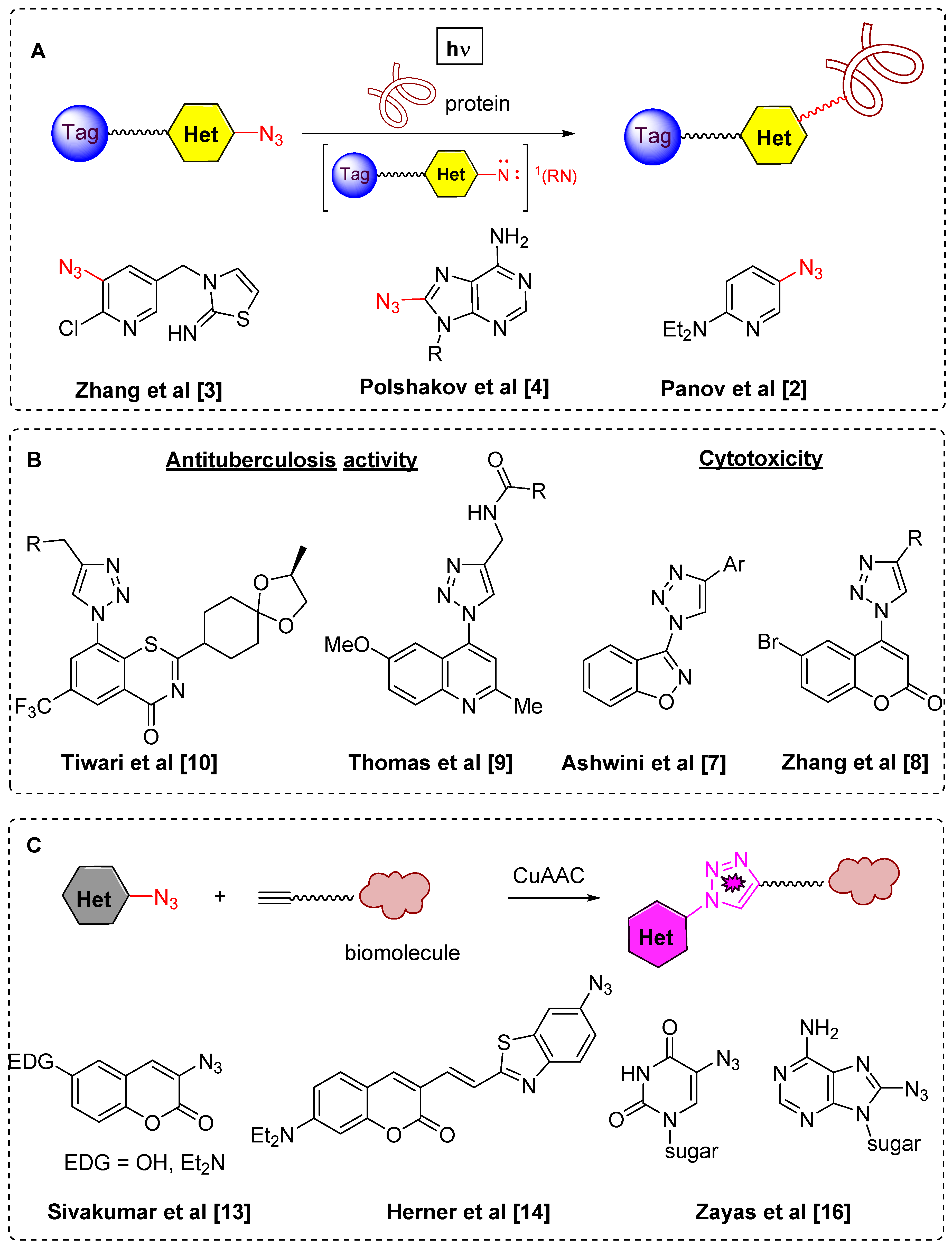
During last decade azido-substituted heterocycles have been broadly studied in several areas. The first one is photoaffinity labeling [1] which is an important biological tool for the investigation of different biological processes by specific modification of proteins using their interaction with singlet nitrenes generated from azidoheterocyles under UV irradiation (Figure 1A) [2,3,4]. The second approach involves use of azidoheterocycles in the synthesis of triazolylheterocycles, mainly by Cu-catalyzed alkyne-azide cycloaddition (CuAAC) [5,6]. This methodology allowed a great variety of biologically active triazolyl substituted heterocycles to be synthesized and tested in numerous biological assays (Figure 1B) [7,8,9,10]. The third main field where azidoheterocycles are in great demand is as azide-based fluorophore tags for biological imaging (Figure 1C) [11,12,13,14,15,16].
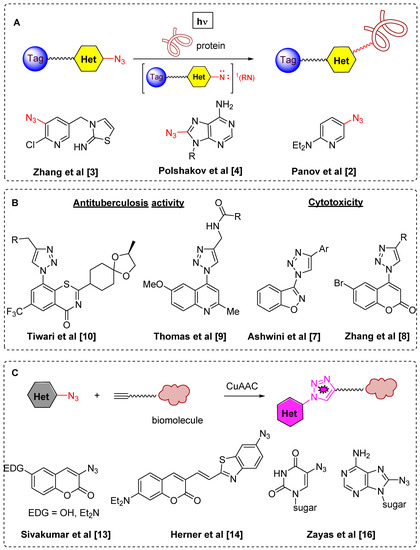
Figure 1.
Recent areas of azidoheterocycles and triazolylheterocycles application–photoaffinity labeling (A); biologically-active compounds (B); fluorogenic azides (C).
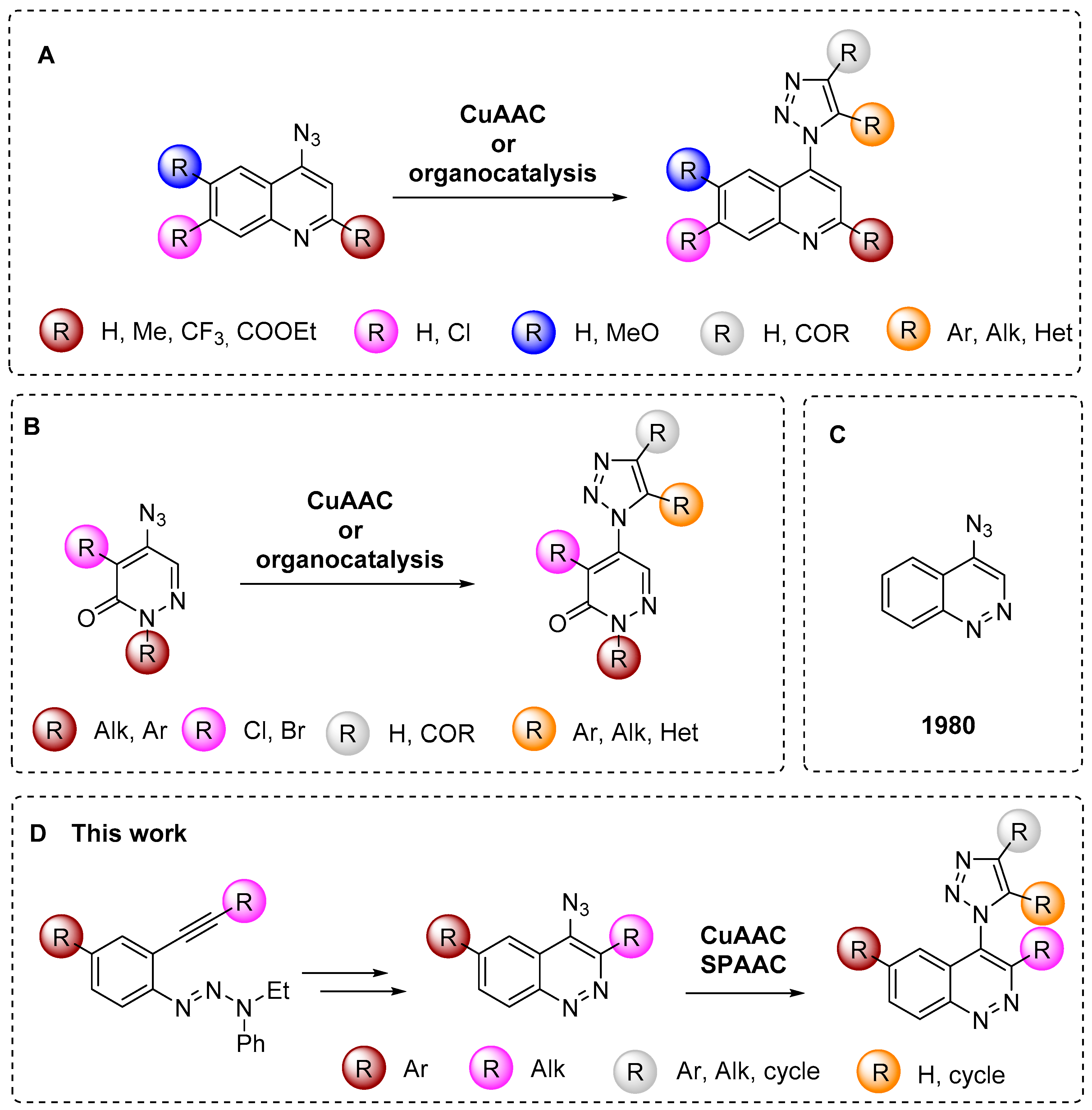
Among azidoheterocycles, azidoquinolines (Figure 2A) [17,18,19,20,21,22,23] and azidopyridazines (Figure 2B) [24,25,26,27,28] are well known, however only one known example of an azidocinnloline has been reported to date (Figure 2C) [29], while triazolylcinnolines still remain unknown. Taking into account that the cinnoline core can be found both in biologically active compounds with different types of activities [30,31,32,33,34] and in fluorescent materials [35,36,37,38,39,40], the development of a synthetic route towards azidocinnolines and triazolylcinnolines could lead to new biologically active molecules and interesting fluorescent probes.
Figure 2.
Known examples of azidoquinolines, azidopyridazines and the corresponding triazoles compared to azidocinnoline derivatives (A–D).
2. Results and Discussion
2.1. Synthesis of 6-aryl-4-azidocinnolines
Several routes have been established for introduction of an azido group into a heterocyclic ring [41]. The most common techniques are azidodediazoniation of arenediazonium salts, synthesis of azides from the corresponding heteroarylboronic acids and nucleophilic substitution of activated halogens. In most cases involving 4-azidoquinolines nucleophilic substitution of a chlorine atom at the C4 position of a quinoline ring has been employed [42,43,44,45]. A few examples are known of bromine substitution [46,47] along with azidodediazoniation [48] and conversion of the corresponding quinolones to azidoquinolines employing diphenylphospharylazide [49].
The synthetic routes towards cinnolines include different cyclization techniques and named reactions [33,50,51]. One of them, which allows 4-halocinnolines to be obtained in high yields, is the Richter-type cyclization [52] of ortho-ethynylarenediazonium salts [53] and ortho-ethynylaryltriazenes [54,55,56]. The halogen atom at C4 position can be substituted with various nucleophilic reagents, i.e., water [53], alcoholates [57,58,59], sulfides [56,58,60] and amines [58,61,62,63,64]. The only mentioned example of a 4-azidocinnoline molecule was also obtained from the corresponding chlorocinnoline by nucleophilic substitution with sodium azide in ethanol [29].
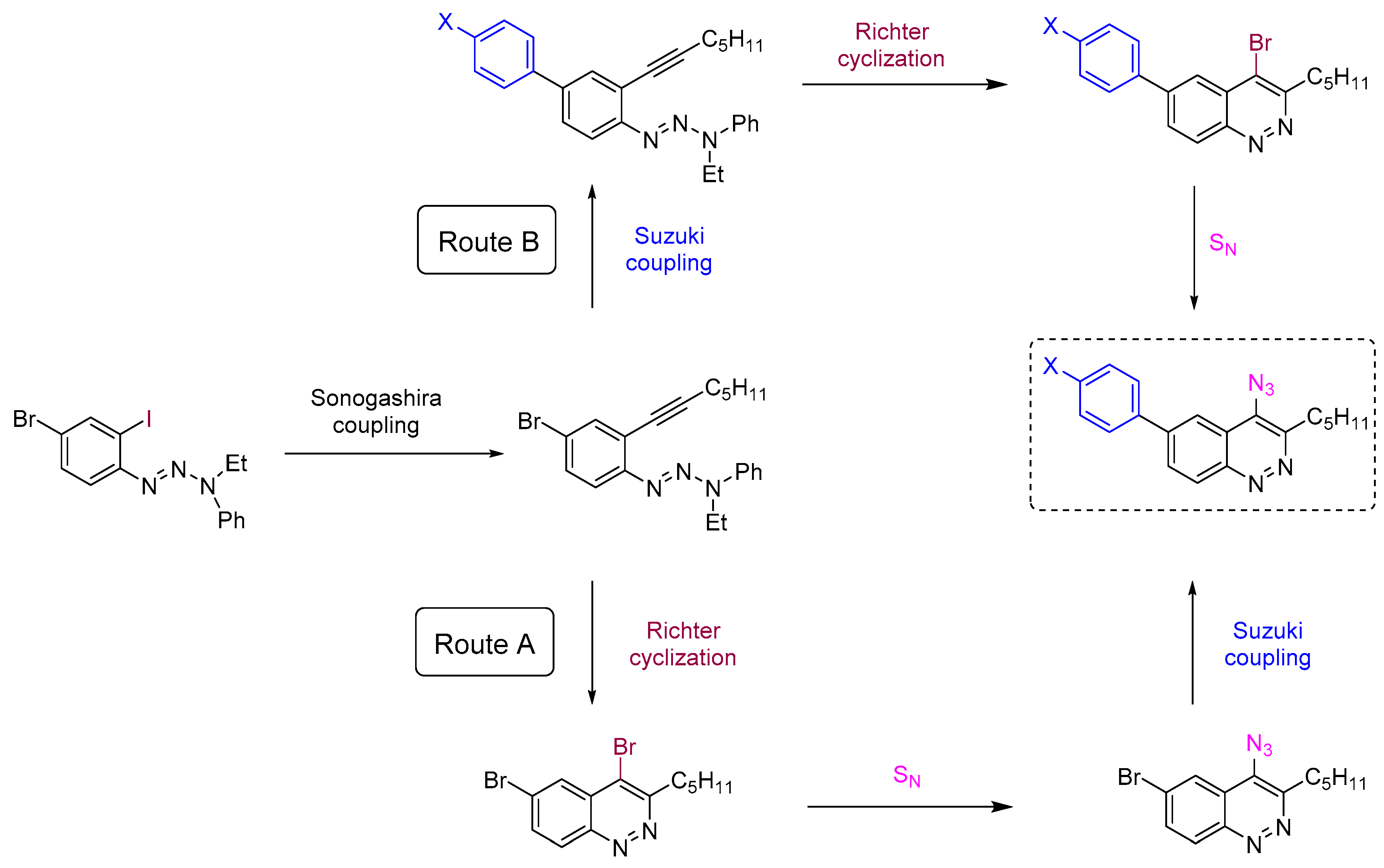
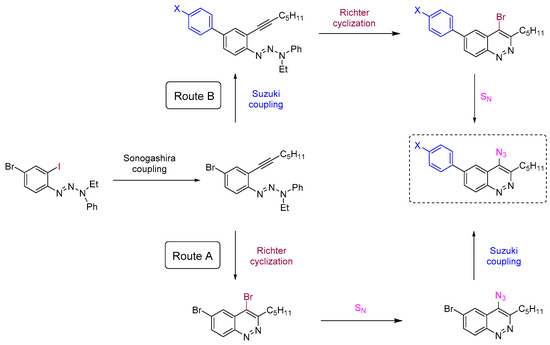
To reach the target 4-azido-6-arylcinnolines compounds we decided to use 4-bromocinnolines, which are synthetically accessible by the Richter cyclization in higher yields compared to corresponding chloro derivatives [54], while the bromine atom at the C4 position should be still reactive towards nucleophilic substitution. Firstly, two synthetic routes (A and B) have been proposed for target molecules (Scheme 1).
Scheme 1.
Proposed synthetic “Routes A“ and “B” towards 6-aryl-4-azidicinnolines.
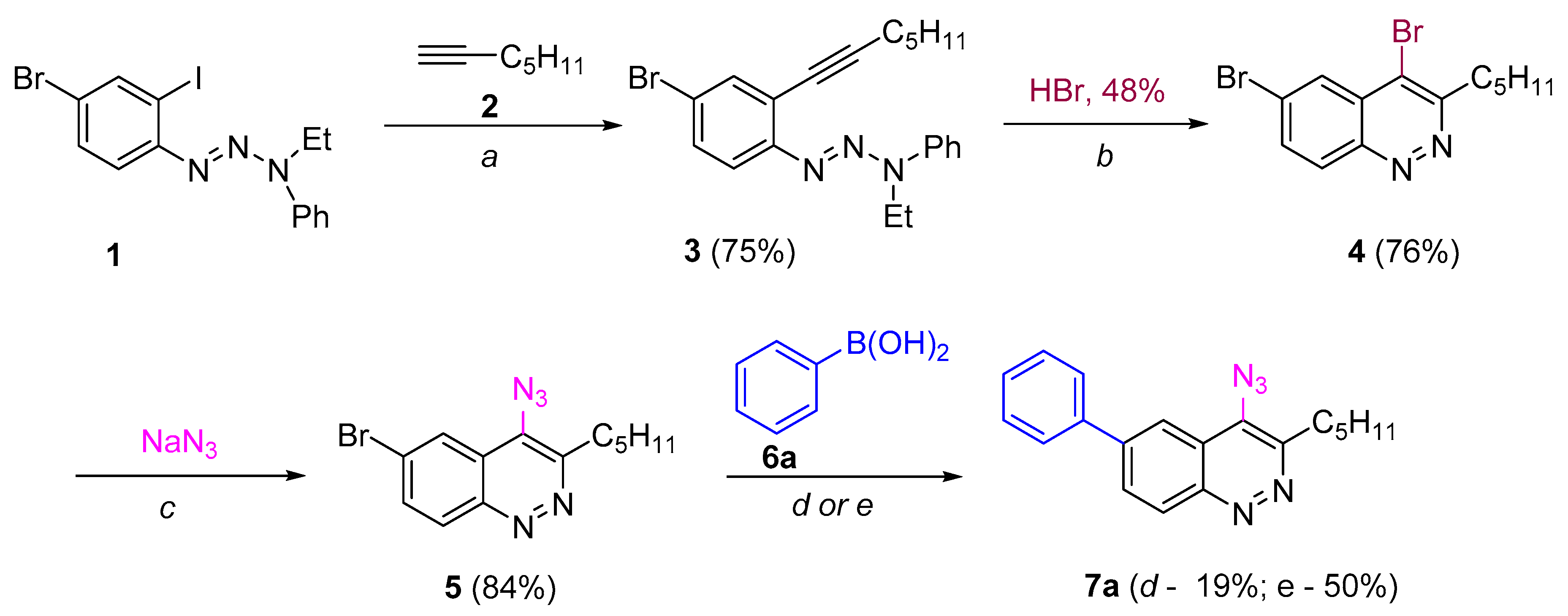
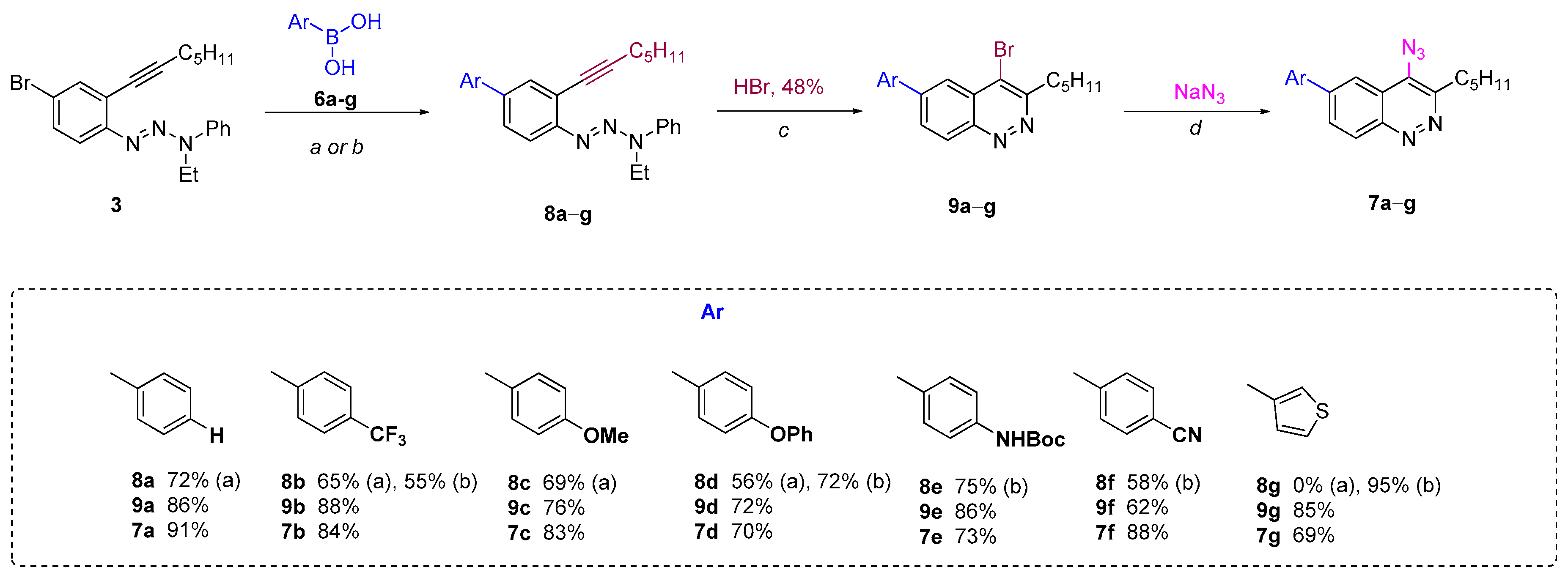
The first step for both routes is the chemoselective Sonogashira coupling of the C-I group of 4-bromo-2-iodophenyltriazene with hept-1-yne (Scheme 1), which has been used previously for the synthesis of poly(arylene ethynylene)s [40].
“Route A” seems to be more divergent and rational than “Route B”, because it allows for variations of the aromatic substituent during the last synthetic step. Therefore we chose “Route A” to start with. The Richter-type cyclization followed by the regioselective substitution of bromine atom with sodium azide in absolute DMF at the activated C4 position proceeded smoothly and did not affect the bromine atom at C6. Hence 4-azido-6-bromocinnoline 5 was obtained in good yield (Scheme 2). Next we tried to carry out the Suzuki coupling. Despite the fact that this transformation of substrates bearing aromatic azido groups is known, yields usually are low, because the whole process is accompanied by several side reactions that are common for both Suzuki coupling (homocoupling, reductive dehalogenation) and for azides (denitrogenative decomposition through nitrene intermediates and through the Staudinger reaction if phosphine ligands are present in the reaction mixture) [65]. Two types of conditions tested differed in the base (Na2CO3 or K3PO4) and solvents (toluene/dioxane/H2O or only dioxane, respectively) used (Scheme 2). The formation of the desired 4-azido-6-phenylcinnoline 7a as the main product was observed in both cases. However the isolated yields of the product 7a were found to be between low (conditions d, Scheme 2) to moderate (conditions e, Scheme 2). Moreover the Suzuki coupling proceeded with the formation of many byproducts that required time-consuming purification of the target azide by column chromatography. Therefore we turned to “Route B”.
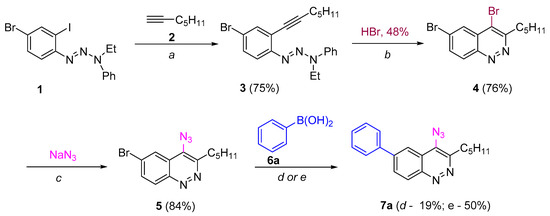
Scheme 2.
Synthesis 6-aryl-4-azidocinnolines through “Route A”. Reagents and conditions: (a) Pd(PPh3)4, CuI, Et3N, THF, 40 °C, 5 h; (b) HBr (20 eqiuv), acetone, 0.01 M, rt, 10 min; (c) NaN3 (5 equiv), 50 °C, 24 h; (d, Method A) − Pd(PPh3)4, Na2CO3, toluene/dioxane/H2O, 80 °C, 1 h, (e, Method B) Pd(PPh3)4, K3PO4, dioxane, 80 °C, 1 h.
The Suzuki coupling of aromatic halotriazenes has been employed recently in the initial steps of the synthesis of hexadehydrotribenzo[12]annulene [66]. Similar conditions (Na2CO3, toluene/dioxane/H2O, 100 °C) for our substrates gave 4-phenyltriazene 8a in 72% yield. Applying next the Richter-type cyclization and nucleophilic substitution of bromine with sodium azide under the same conditions as for “Route A” enabled us to produce the final 4-azido-6-phenylcinnoline 7a in better overall yield (42%, “Route B”) (Scheme 3) compared to “Route A” (24%) (Scheme 2). Same routes?
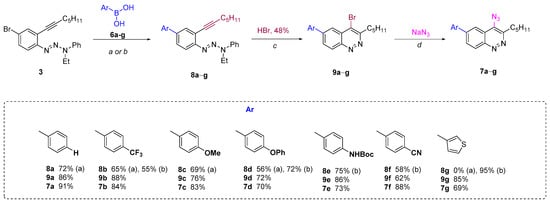
Scheme 3.
Synthesis of 6-aryl-4-azidicinnolines 7a−g through “Route B”. Reagents and conditions: (a, Method A) Pd(PPh3)4, Na2CO3, toluene/dioxane/H2O, 100 °C, 24 h; (b, Method B) Pd(PPh3)4, K3PO4, dioxane, 100 °C, 2−5 h; (c) HBr (20 eqiuv), acetone, 0.01M, rt, 10 min; (d) NaN3 (5 equiv), 50 °C, 24 h.
The scope of “Route B” was then checked with different aryl- and heteroarylboronic acids 6b–g. “Conditions a” worked well for the CF3 (b), MeO (c) and PhO (d) series. However, these Suzuki coupling conditions failed when applied to other boronic acids bearing NHBoc (6e), CN (6f) and 3-thienyl (6g) groups. Thus, either complete or partial decomposition of the starting triazenes occurred when a mixture of organic solvent with water in the presence of sodium carbonate as a base was used. Excluding water from the reaction mixture and changing the base to potassium phosphate allowed all three triazenes 8e–g to be obtained in high yields. The Richter cyclization and the subsequent nucleophilic substitution of bromine by an azido group for all compounds proceeded smoothly under the same conditions as for the unsubstituted phenyltriazene 8a, providing a series of key 6-aryl(heteroaryl)-4-azidocinnolines 7 in high yields (Scheme 3).
2.2. Study of 6-aryl-4-azidocinnoline’s Reactivity in the Sythesis of 6-aryl-4-triazolylcinnolines
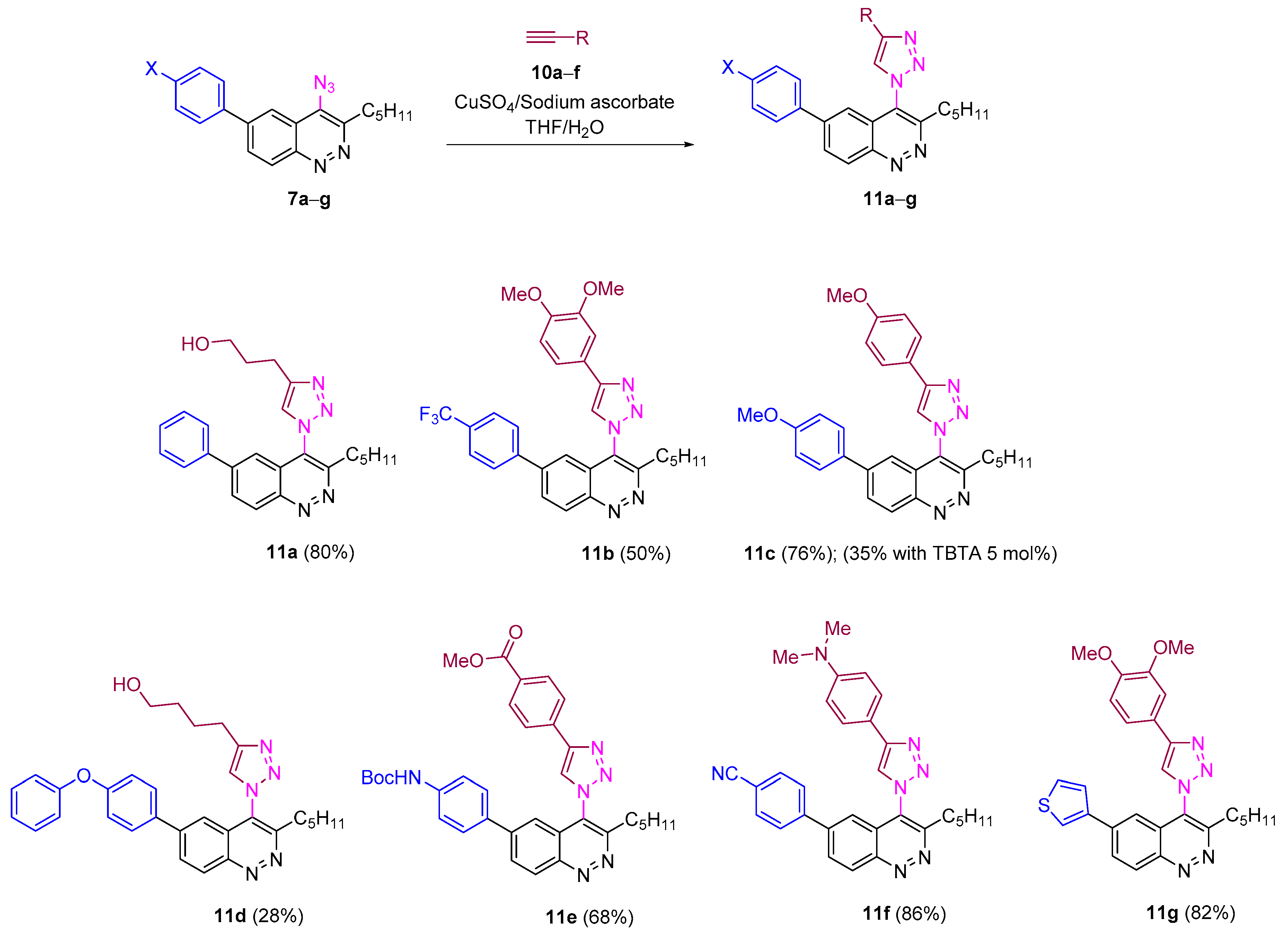
All azidocinnolines were found to be stable crystalline compounds when stored at −18 °C and slowly decomposed in solution. Thus complete decomposition of azidocinnoline 7a in acetone-d6 was detected after a week. Despite this fact, all azides were stable under conditions used for CuAAC. Thus carrying out CuAAC of azidocinnolines 7a–g both with terminal aromatic alkynes bearing EWG, EDG and aliphatic alkynes in the mixture of THF/H2O using the copper (II) sulfate/sodium ascorbate catalytic system afforded the corresponding 4-triazolylcinnolines mostly in good yields (Scheme 4).
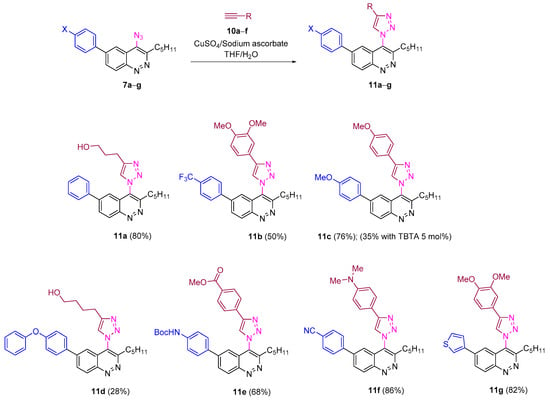
Scheme 4.
Reactivity of 4-azidocinnolines 7a−g in CuAAC.
One modification of the well-studied CuAAC is to carry out the reaction in the presence of tris(triazolyl) ligands, which improves the yields, shortens the reaction time and improves the synthetic accessibility of triazole derivatives that are not available when the common Cu(II)/ascorbate catalytic system is used [67]. Surprisingly, when the CuAAC of azidocinnoline 7c with 3,4-dimethoxyphenylacetylene was run in the presence of the TBTA ligand (tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine), the reaction proceeded very slowly and full conversion was not achieved even after two days. Therefore triazolylcinnoline 11c was isolated in only 35 % yield (the yield of 11c without TBTA ligand was 76%).
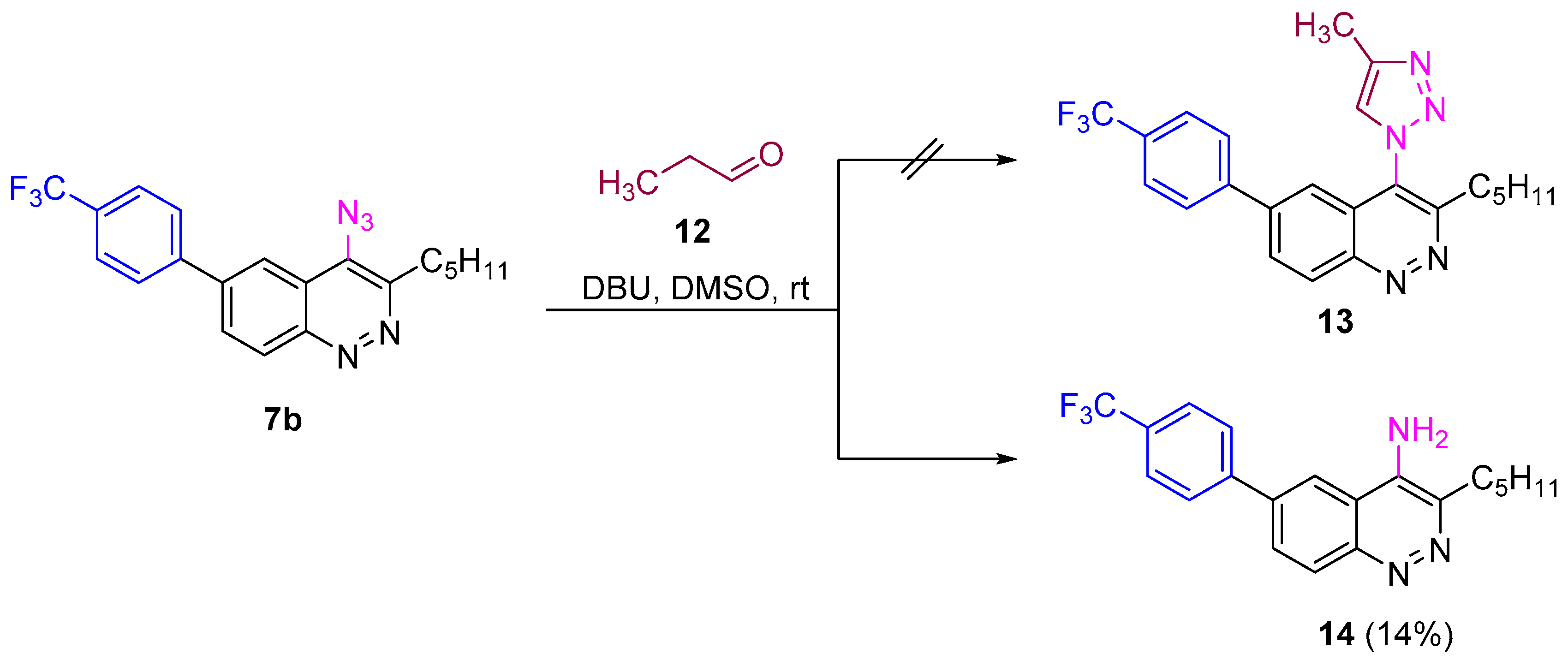
Next we checked the reactivity of azidocinnolines synthesized in reactions other than CuAAC used for the synthesis of 1H-1,2,3-triazole derivatives, like the enol-mediated organocatalytic synthesis of triazoles and strain-promoted alkyne-azide cycloaddition (SPAAC). Unfortunately, enol-mediated reaction [68] of azide 7b with propionic aldehyde catalysed by 1,8-diazabicyclo-[5.4.0]undec-7-ene (DBU) did not go as expected. The only isolated product was 4-aminocinnoline, which was presumably formed from the corresponding nitrene as an intermediate of azide decomposition (Scheme 5).
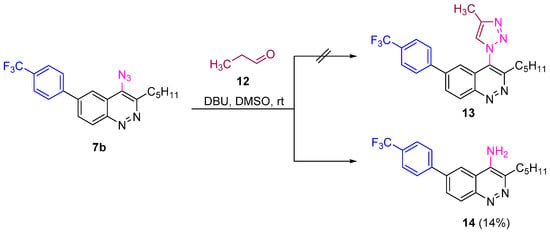
Scheme 5.
Reactivity of azidocinnoline 7b in enol-mediated organocatalytic triazole synthesis.
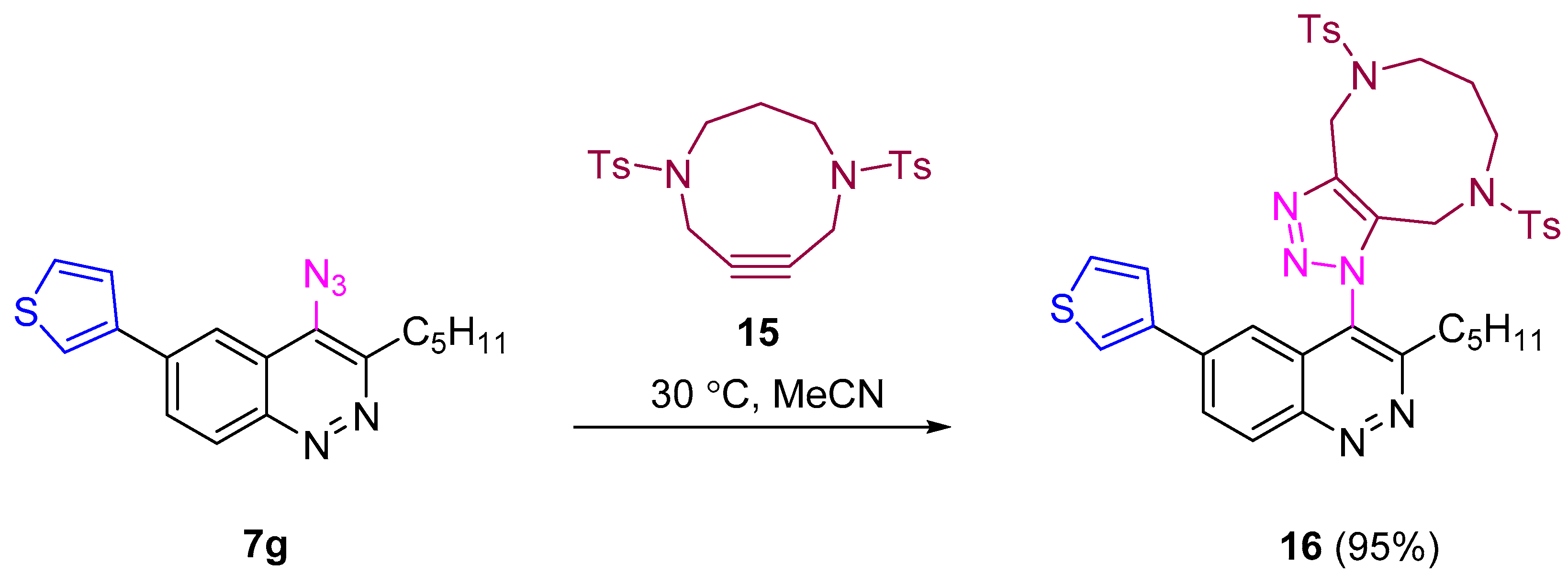
SPAAC of thienylazidocinnoline 7g with tosylated diazacyclononyne [69] proceeded smoothly at 30 °C and afforded corresponding triazole in excellent yield (95%, Scheme 6). Thus synthesized 6-aryl-4-azidocinnolines were found to be suitable substrates for the synthesis of 6-aryl-4-triazolylcinnolines through both CuAAC and SPAAC.
Scheme 6.
Reactivity of 4-azidocinnoline 7g in SPAAC.
2.3. Photophysical Properties of 6-aryl-4-azidocinnolines and 6-aryl-4-triazolylcinnolines
Having both azidocinnolines and triazolylcinnolines in hand, we next studied their photophysical properties. Fluorogenic azides represent a class of fluorescent dyes which are only weakly fluorescent without conversion into triazoles. However, when a triazole moiety is introduced into the molecule, it imparts fluorescent properties to the entire molecular scaffold. Fluorogenic azides are of special interest for bioimaging, because they allow avoiding a series of steps to wash the excess of dye from the labeled materials and thus they eliminate the problem of background fluorescence. Syntheses and applications of various fluorogenic heterocyclic azides have been reported recently and discussed in the Introduction section [11] (Figure 1). Taking into account that the aryl substituent, azide group and azo-group of a cinnoline core are conjugated, we decided to investigate whether 6-aryl-4-azidocinnolines could be used as fluorogenic azide dyes.
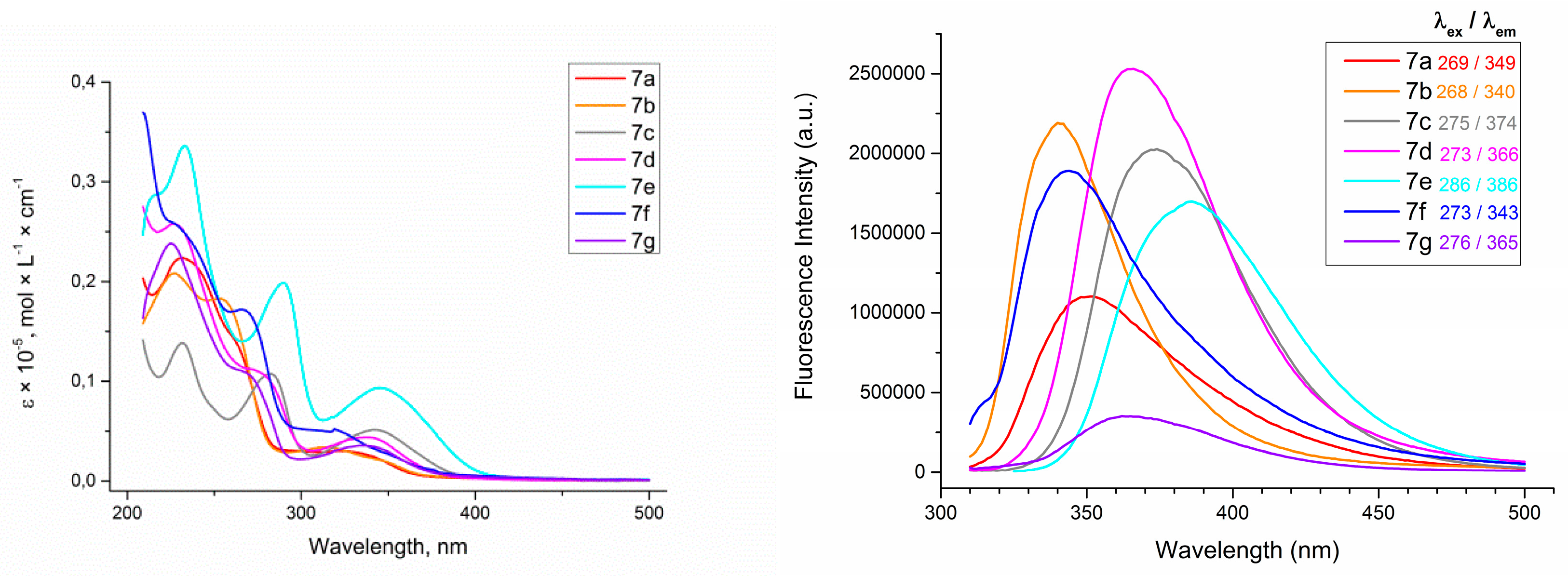
Firstly, the absorption and emission spectra of azidocinnoline solutions in THF were measured (Figure 3). The obtained data revealed that both EWG and EDG groups at the para-position of an aryl ring attached to C6 atom provided an increase in fluorescence brightness. To quantify the observed fluorescence, the absolute quantum yields of fluorescence (QY) of azidocinnolines 7 were measured (Table 1).
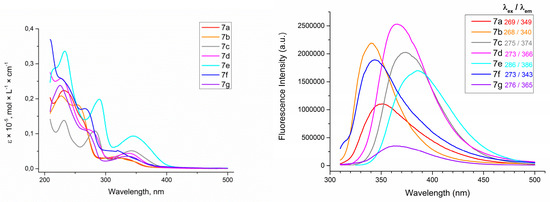
Figure 3.
Absorption spectra of 4-azidocinnolines 7a−g solutions in THF, C = 1 × 10−5 mol/L (left) and emission spectra of 4-azidocinnolines 7a−g solutions in THF, C = 0.33 × 10−5 mol/L (right).

Table 1.
Absolute QY for THF solutions of 6-aryl-4-azifocinnolines 7a−g.
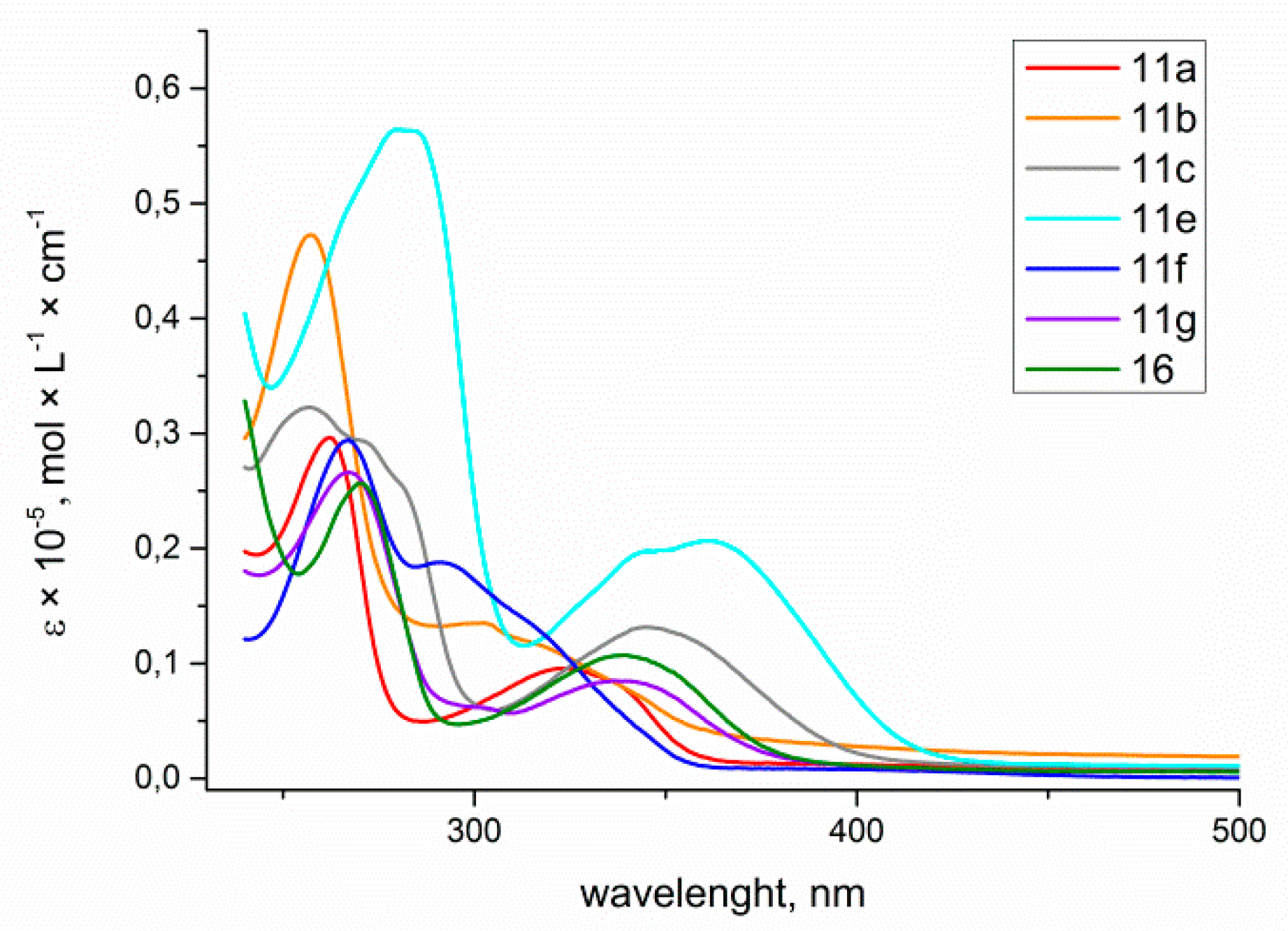
The obtained data revealed that all 6-aryl-4-azidocinnolines 7 possess weak fluorescence with quantum yield values of less than 1%, with the highest QY being observed for 4-azido-6-(4-methoxyphenyl)cinnoline. Hoping to observe fluorescent properties in triazole derivatives 11, 16 the absorption and emission spectra for the series of triazolylcinnolines 11a−c,e−g, 16 were measured (Figure 4 and Figure 5).
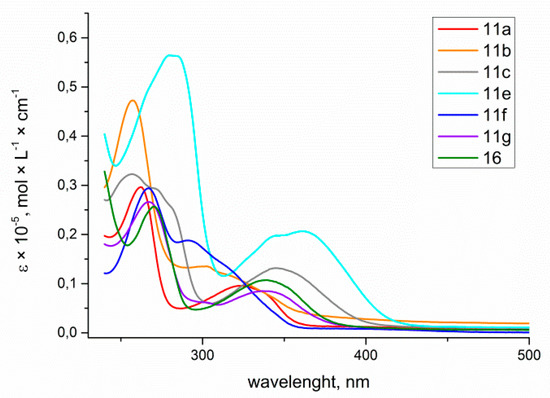
Figure 4.
Absorption spectra of 4-triazolylcinnolines 11a−c, e−g, 16 solutions in THF, C = 1 × 10−5 mol/L.
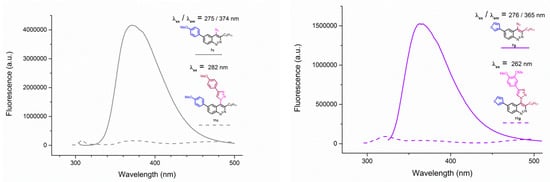
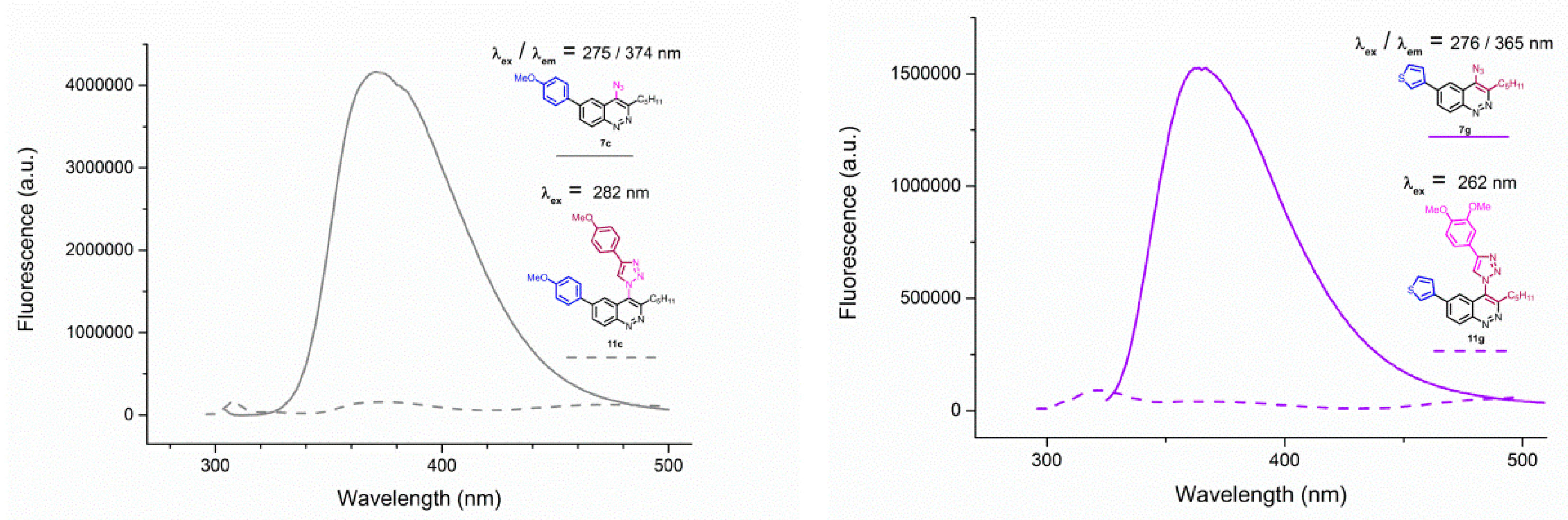
Figure 5.
Emission spectra of 4-azidocinnolines/4-triazolylcinnolnes 7c/11c (left) and 7g/11g (right) solutions in THF, C = 1 × 10−5 mol/L.
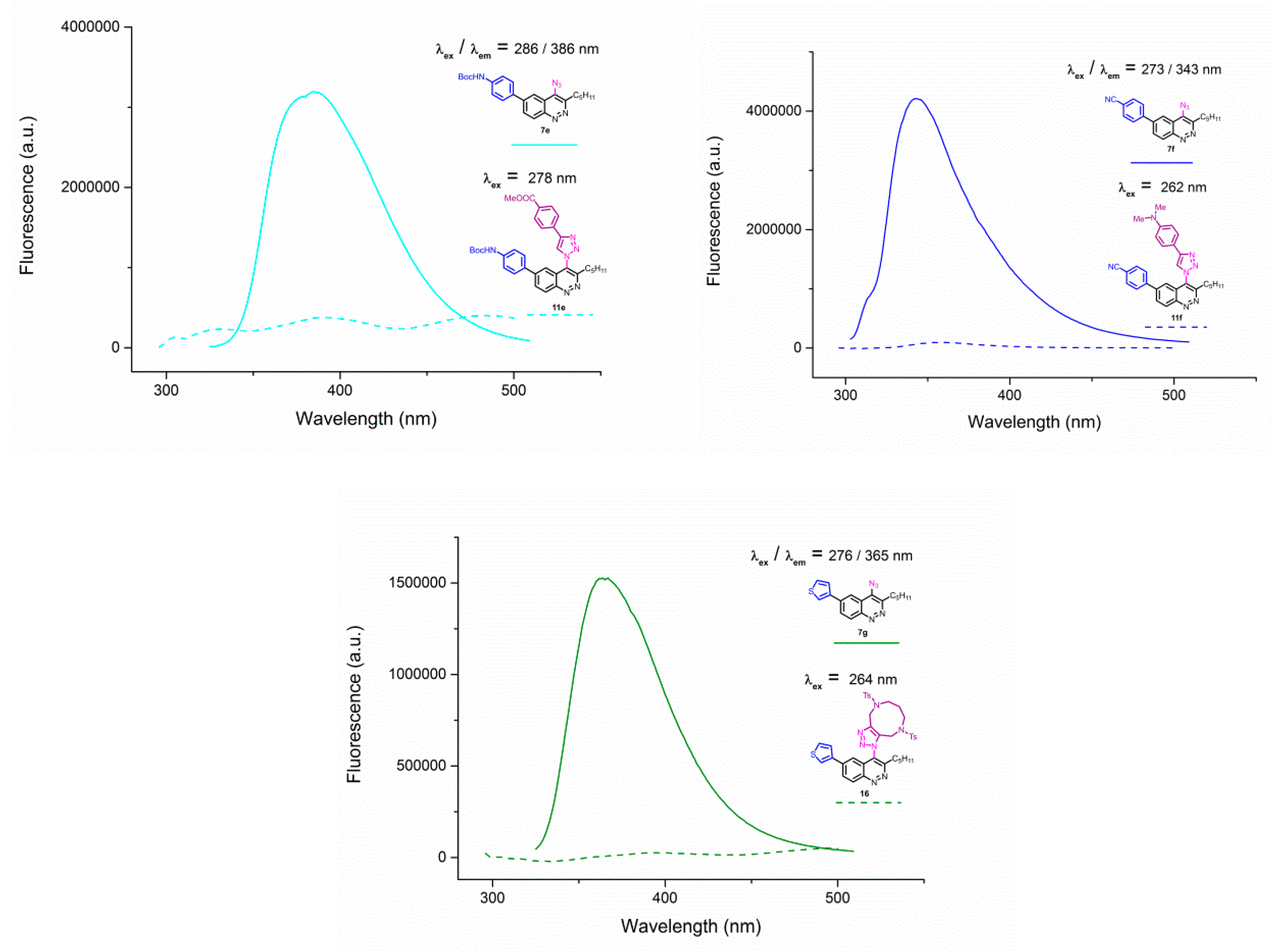
Unfortunately, all triazoles were found to be devoid of any fluorescence. Emission spectra of azide/triazole pairs with MeO group 7c/11c and 3-thienyl group 7f/11f are presented on Figure 5 (for the spectra of the whole series see Appendix A–Figure A1 and Figure A2).
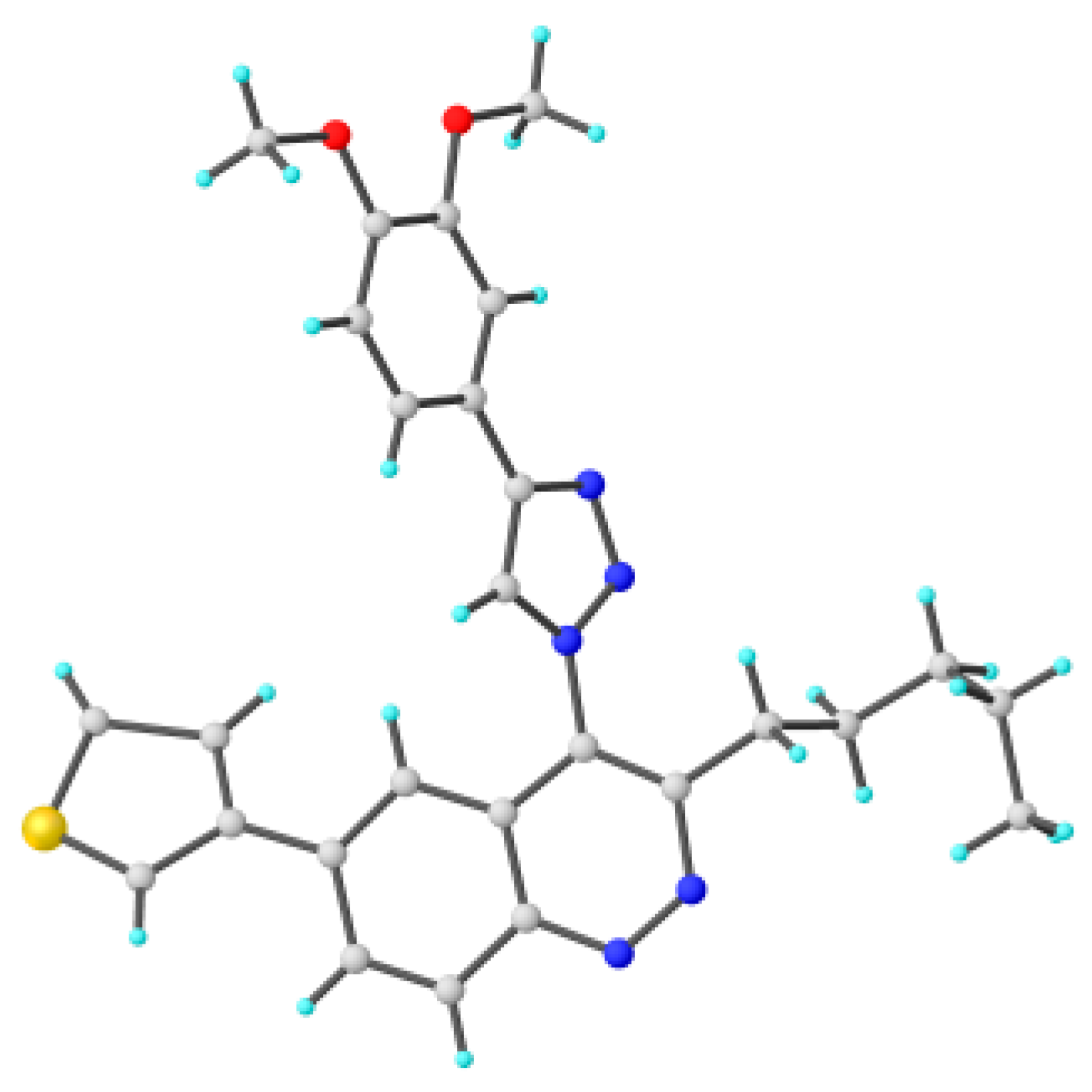
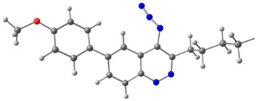
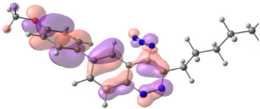
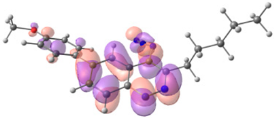
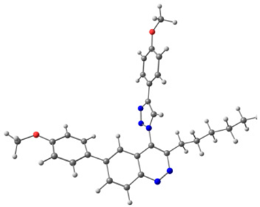
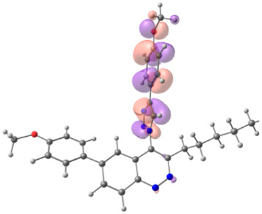
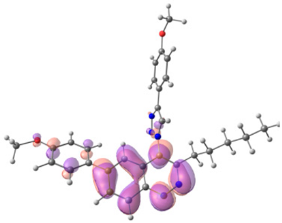
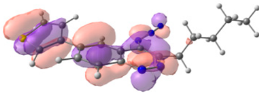
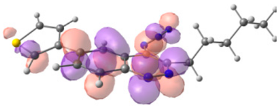
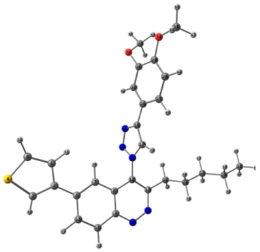
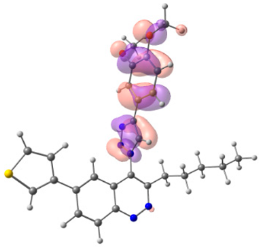
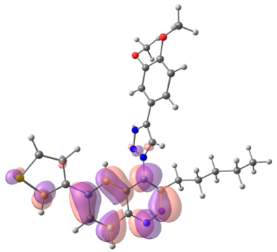
To explain the observed loss of fluorescence for triazoles, quantum chemical calculations for S0 states of the azide/triazoles pairs 7c/11c and 7f/11f were carried out. Geometry optimization was achieved using DFT calculations (B3LYP 6-311 + G(2d,2p)). The obtained data revealed that triazoles 11c and 11f are non-planar molecules: the triazole rings of both compounds lay out of the corresponding cinnoline plane (Table 2). X-Ray studies confirmed the non-planar geometry of triazole 11f in the solid state (Figure 6). The dihedral angle between the triazole and cinnoline rings was found to be 64.8°. Therefore, despite the extension of the conjugated chain, the non-planar geometry of 4-triazolylcinnolines could be one of reasons for the observed absence of fluorescence.

Table 2.
Optimized geometries of azidocinnolines, triazolylcinnolines and their frontier molecular orbitals.
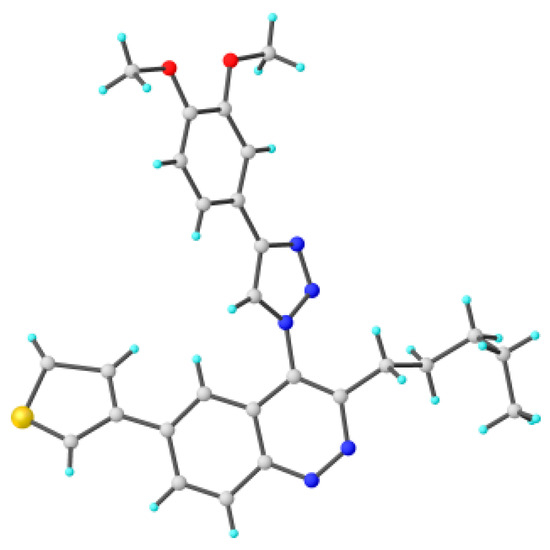
Figure 6.
Molecular structures of triazolylcinnoline 11g obtained from X-Ray analysis.
Analysis of the frontier molecular orbitals of triazolylcinnolines 11c and 11f showed that the HOMO is associated with the donor part of the molecule (triazole) whereas the LUMO is concentrated in the acceptor cinnoline part. On the other hand the frontier molecular orbitals of azidocinnolines 7c and 7f are evenly distributed over the entire molecule (Table 2).
Therefore, another reason for the lack of fluorescence of triazolylcinnolines compared to azidocinnolines could be intramolecular photoinduced electron transfer (PET) that is known to be responsible for fluorescence quenching [70]. For example, 1,4-bis(2-hydrohyphenyl)-1,2,3-triazole with a similar frontier molecular orbital separation between both 2-hydrohyphenyl substituents exhibited very weak fluorescence [71].
2.4. Biological Studies of 6-aryl-4-triazolylcinnolines
Next we turned to biological activity screening tests. Both the triazole fragment and cinnoline moiety can be found in variety of compounds with antibacterial, antifungal and anticancer properties. Therefore we studied the antibacterial and antifungal activities of triazoles 11a,c,e−g, 16 against Escherichia coli and Saccharomyces cerevisiae, respectively. The cytotoxicity of triazolylcinnolines and their activity as DNA-cleavage agents were also tested.
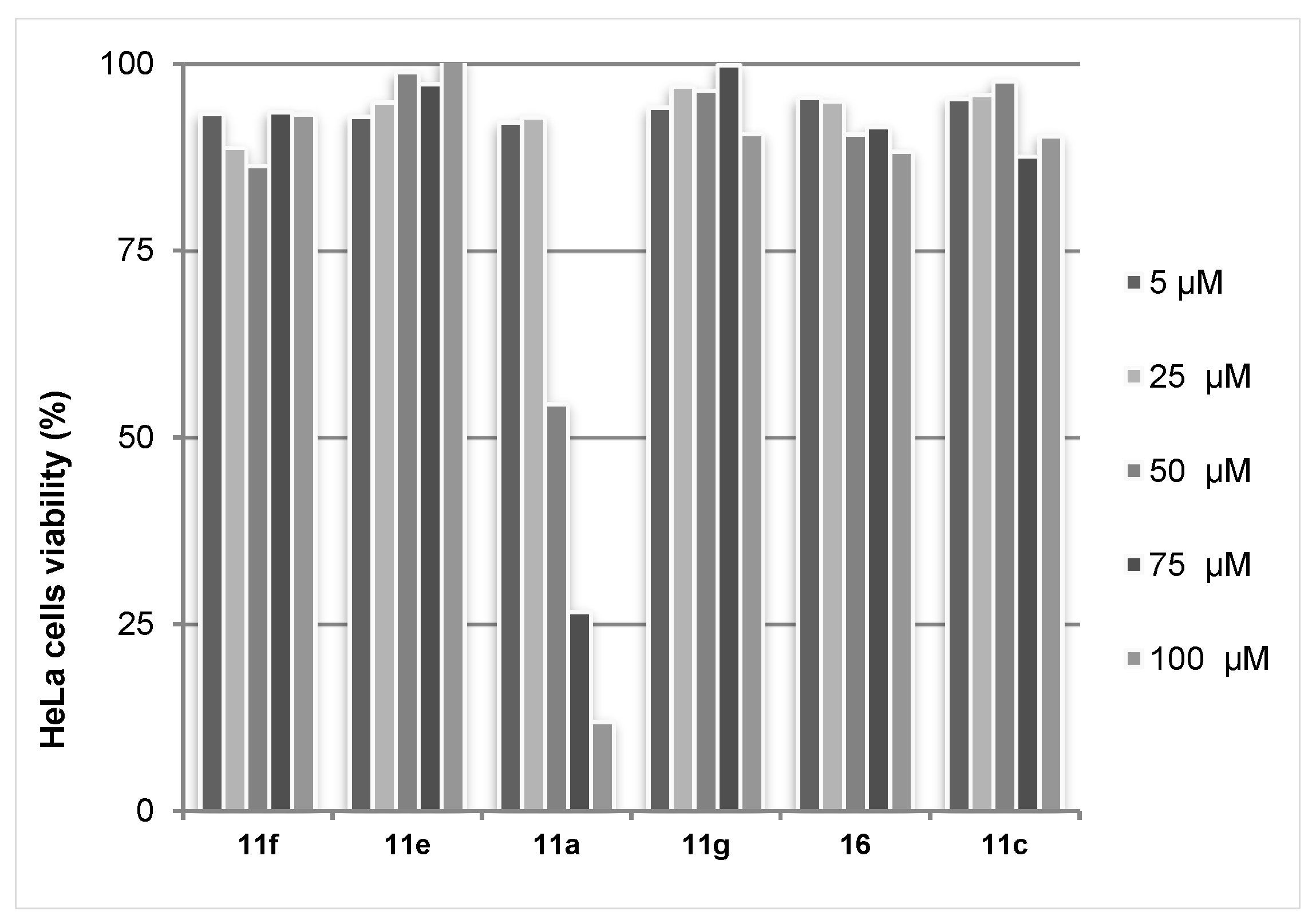
The obtained data revealed that the studied triazolylcinnolines 11a,c,e−g, 16 are inactive towards the Gram-negative bacterium Escherichia coli and fungus Saccharomyces cerevisiae. On the other hand the MTT test for screening of cytotoxicity allowed identifying triazolylcinnoline 11a as being active. Despite the fact that other triazolylcinnolines did not display reduced HeLA cell viability, the IC50 for compound 11a bearing hydroxyalkyl substituent was found to be 56.5 μM (Figure 7). Triazolylcinnoline 11a possess a flat heteroaromatic moiety−6-arylcinnoline, which might have DNA intercalating activity. Therefore we tested the ability of triazolylcinnolines to cleave DNA. None of the compounds affected the DNA plasmid pBR322, indicating a different mechanism of cytotoxicity.
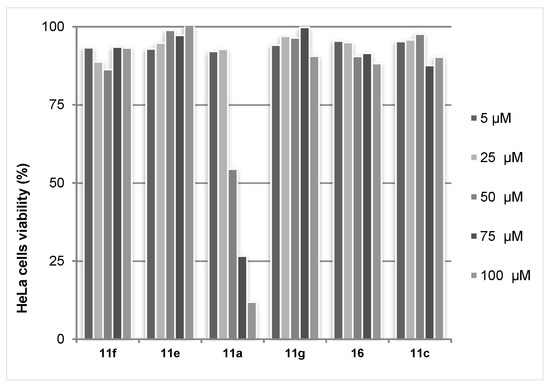
Figure 7.
MTT test results with HeLa cell line for triazolylcinnolines 11,16.
3. Materials and Methods
3.1. General Information
Solvents and reagents used for reactions were purchased from commercial suppliers. Catalyst Pd(PPh3)4 was purchased from Sigma-Aldrich (München, Germany). Solvents were dried under standard conditions; chemicals were used without further purification. 4-Bromo-2-iodoaniline [72], 1-(4-bromo-2-iodophenyl)-3-ethyl-3-phenyltriaz-1-ene (1) [40], Co2(CO)6-complex of diazacyclononyne 15 [69] and TBTA [67] were synthesized by known procedures without any modifications. Evaporation of solvents and concentration of reaction mixtures were performed in vacuum at 35 °C on a rotary evaporator. Thin-layer chromatography (TLC) was carried out on silica gel plates (Silica gel 60, F254, Merck (Darmstadt, Germany) with detection by UV or staining with a basic aqueous solution of KMnO4. Melting points (mp) determined are uncorrected. 1H and 13CNMR spectra were recorded at 400 and 100 MHz, respectively, at 25 °C in acetone-d6 without the internal standard using a 400 МHz Avance spectrometer (Bruker, Billerica, MA, USA). The 1H-NMR data are reported as chemical shifts (δ), multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad), coupling constants (J, given in Hz), and number of protons. The 13C NMR data are reported as the chemical shifts (δ) with coupling constant J(C−F) for F-containing compounds. Chemical shifts for 1H and 13C are reported as δ values (ppm) and referenced to residual solvent (δ = 2.05 ppm for 1H; δ = 29.84 for 13C–for spectra in acetone-d6, δ = 7.26 ppm for 1H; δ = 77.16 ppm for 13C–for spectra in CDCl3 and δ = 2.50 ppm for 1H; δ = 39.52 ppm for 13C–for spectra in DMSO-d6). High resolution mass spectra (HRMS) were determined using electrospray ionization (ESI) in the mode of positive ion registration with a Bruker microTOF mass analyzer (Billerica, MA, USA). UV–vis spectra for solutions of all compounds in THF were recorded on a UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan) at 20 °C. Fluorescence spectra for the same solutions were recorded on a FluoroMax-4 spectrofluorometer (Horiba Scientific, Glasgow, Scotland) at 20 °C. IR spectra were measured using a Nicolet 8700 spectrometer (Thermo Scientific, Madison, WI, USA) equipped with a Thermo Scientific Smart iTR™ as an Attenuated Total Reflectance (ATR) sampling accessory. Data for 11f were collected using an XtaLAB SuperNova diffractometer (Rigaku Oxford Diffraction, Tokio, Japan) equipped with an HyPix3000 CCD area detector operated with monochromated microfocused CuKα radiation (λ[CuKα] = 1.54184 Å). All the data were integrated and corrected for background, Lorentz, and polarization effects by means of the CrysAlisPro (Tokyo, Japan) [73] program complex. Absorption correction was applied using the empirical spherical model within the CrysAlisPro program complex using spherical harmonics, implemented in the SCALE3 ABSPACK scaling algorithm. The unit-cell parameters were refined by the least-squares techniques. The structures were solved by direct methods and refined using the SHELX [74] program incorporated in the OLEX2 [75] program package.
3.2. Synthetic Methods
3.2.1. The Richter-Type Cyclization Protocol of Triazenes 3, 8
To a solution of triazene 3 or 8 (1 equiv) in acetone (C = 0.1 M) HBr (48% aqueous solution, 20 equiv) was quickly added dropwise while maintaining the reaction mixture temperature of 20 °C by cooling the reaction mixture with a water bath. The resulting mixture was stirred for 10 minutes. Upon completion of the reaction, the reaction mixture was diluted with an aqueous solution of triethylamine (21 equiv). The resulting mixture was extracted with ethyl acetate, the combined organic layers were washed with water, brine and dried over anhydrous Na2SO4. The solvent was removed in vacuum and the crude 4-bromocinnoline was purified by column chromatography.
3.2.2. General Procure for the Suzuki Coupling (Method A)
To a solution of triazene 3 or azidocinnoline 5 (1 equiv) in a mixture of toluene/1,4-dioxane/water (1:2:2) (C = 0.1 M) in a vial was added arylboronic acid 6 (1.5 equiv), Pd(PPh3)4 (5 mol%) and Na2CO3 (2 equiv). The vial was sealed; the reaction mixture was evacuated and flushed with Ar several times. The vial with the reaction mixture was placed in a preheated oil bath (80−100 °C) and stirred for 1−24 h (TLC control). After completion the reaction, the mixture was cooled and poured into a saturated aqueous solution of NH4Cl and extracted with ethyl acetate. The combined organic layers were washed with saturated aqueous solutions of NH4Cl and brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure to yield the crude product, which was purified by column chromatography on silica gel.
3.2.3. General Procure for the Suzuki Coupling (Method B)
Triazene 3 or azidocinnoline 5 (1 equiv), ArB(OH)2 6 (1.5 equiv), K3PO4 (2 equiv), and Pd(PPh3)4 (5 mol %) were placed in a vial. The vial was sealed, and the mixture was evacuated and flushed with Ar several times. 1,4-Dioxane (C = 0.1 M) was added, and the vial with the reaction mixture was placed in a preheated oil bath (80−100 °С) and stirred for 1−20 h (TLC control). After cooling to rt, the reaction mixture was filtered through a pad silica gel and washed with ethyl acetate. Solvents were removed under reduced pressure, and the crude product was purified by column chromatography on silica gel.
3.2.4. General Procure for the Nucleophilic Substitution
Sodium azide (2–5 equiv) was added to a solution of bromocinnoline (1 equiv) in absolute DMF (C = 0.1 M). The mixture was degassed and stirred under argon at 50 °C for 24 h (TLC control). Upon completion the reaction, the reaction mixture was poured into water, extracted with ethyl acetate; the combined organic layers were washed three times with water and two times with brine, dried over anhydrous Na2SO4. The solvent was removed in vacuum to yield the crude product, which was purified by column chromatography on silica gel.
3.2.5. General Procure for CuAAC Synthesis of 4-triazolylcinnolines
To a stirred mixture of a terminal alkynes (1 equiv) and 4-azidocinnolines (1 equiv) in a mixture of water/THF (1:1) were added sodium ascorbate (10 mol%) and CuSO4 × 5H2O (5 mol%). The reaction mixture was vigorously stirred at 30 °C until the completion of the reaction (TLC control). The resulting mixture was diluted with saturated aqueous solution of NH4Cl (10 mL) and extracted with ethyl acetate (3 × 15 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure to yield the crude product, which was purified by column chromatography on silica gel.
3.2.6. Detailed Synthetic Procedures
1-[4-Bromo-2-(hept-1-yn-1-yl)phenyl]-3-ethyl-3-phenyl-triaz-1-ene (3). To a solution of 1-(4-bromo-2-iodophenyl)-3-ethyl-3-phenyltriaz-1-ene (1) (1 equiv, 860 mg, 2 mmol) in a mixture of triethylamine (8 mL) and THF (4 mL) degassed by freeze-pump-thaw cycling were added Pd(PPh3)4 (5 mol.%, 116 mg, 0.1 mmol), CuI (15 mol.%, 57.0 mg, 0.3 mmol). The reaction mixture was degassed once again. Then hept-1-yne 2 (1.5 equiv, 289 mg, 0.39 mL, 3 mmol) was added via a syringe and the reaction mixture was stirred at 40 °C for 5 hours (TLC control). Upon completion of the reaction, the reaction mixture was poured into a saturated aqueous solution of NH4Cl, extracted with ethyl acetate (3 х 50 mL); the combined organic layers were washed with saturated aqueous solutions of NH4Cl, brine and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the crude product was purified by column chromatography, using hexane/EtOAc/Et3N (90:1:0.01) as the eluent to give 3 (600 mg, 75%) as an orange oil. 1Н-NMR (acetone-d6) δ 7.58 – 7.60 (m, 3 H), 7.52 – 7.41 (m, 4 H), 7.17 (t, J = 7.4 Hz, 1H), 4.43 (q, J = 7.0 Hz, 2H), 2.48 (t, J = 7.0 Hz, 2H), 1.62 (p, J = 7.1 Hz, 2H), 1.51 − 1.44 (m, 2H), 1.41 − 1.32 (m, 5H), 0.91 (t, J = 7.3 Hz, 3H). 13С-NMR (acetone-d6) 151.6, 144.9, 135.9, 132.2, 130.2, 124.8, 123.0, 120.3, 119.4, 118.0, 97.3, 77.9, 41.2, 31.9, 29.2, 22.9, 20.1, 14.3, 11.2. HRMS (ESI): calcd. for C21H25BrN3 [M + H]+, 398.1232; found 398.1240.
4,6-Dibromo-3-pentylcinnoline (4). This compound was synthesized according to the general procedure for the Richter cyclization from triazene 3 (219 mg, 0.55 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (40:1) as the eluent gave 150 mg (76%) of the title compound. 1H-NMR (acetone-d6) δ 8.42 (d, J = 9.0 Hz, 1H), 8.36 (d, J = 1.9 Hz, 1H), 8.08 (dd, J = 9.0, 2.0 Hz, 1H), 3.45 – 3.36 (m, 2H), 1.98 – 1.86 (m, 2H), 1.52 – 1.38 (m, 4H), 0.92 (t, J = 7.1 Hz, 3H). 13C-NMR (acetone-d6) δ 158.6, 149.0, 135.0, 132.9, 128.8, 128.4, 128.1, 125.5, 36.8, 32.3, 29.4, 23.1, 14.3. HRMS (ESI): calcd. for C13H14N2Br2Na [M + Na]+, 378.9416; found 378.9426.
4-Azido-6-bromo-3-pentylcinnoline (5). This compound was synthesized according to the general procedure for the nucleophilic substitution from bromocinnoline 4 (804 mg, 2.25 mmol) and sodium azide (730 mg, 11.25 mmol) in absolute DMF (22.3 mL). Purification of the crude product by column chromatography using hexane/EtOAc (10:1) as the eluent gave azidocinnoline 5 (603 mg, 84%). 1H- NMR (CDCl3) δ 8.36 (d, J = 9.1 Hz, 1H), 8.32 (d, J = 2.1 Hz, 1H), 7.87 (dd, J = 9.0, 2.0 Hz, 1H), 3.40–3.32 (m, 2H), 1.96–1.84 (m, 2H), 1.54–1.35 (m, 4H), 0.93 (t, J = 7.1 Hz, 3H). 13C-NMR (CDCl3) δ 151.4, 148.4, 134.2, 131.7, 131.4, 126.3, 123.9, 121.8, 32.5, 31.7, 29.7, 22.6, 14.1. HRMS (ESI): calcd. for C13H14N5BrNa [M + Na]+, 342.0325; found 342.0336.
3-Ethyl-1-[3-(hept-1-yn-1-yl)-[1,1′-biphenyl]-4-yl]-3-phenyltriaz-1-ene (8a). (A) This compound was synthesized according to the general procedure for the Suzuki coupling (Method A) from triazene 3 (230 mg, 0.57 mmol) and phenylboronic acid 6a (105 mg, 0.86 mmol) at 100 °C; reaction time − 24. Purification of the crude product by column chromatography using hexane/EtOAc (70:1) as the eluent gave 8a (166 mg, 73%) as an orange oil. 1Н-NMR (acetone-d6) δ 7.73−7.60 (m, 7H), 7.50–7.36 (m, 5 H), 7.5 –7.37 (m, 5H), 7.16 (t, J = 7.4 Hz, 1H), 4.46 (q, J = 7.0 Hz, 2H), 2.50 (t, J = 7.0 Hz, 2H), 1.6–1.61 (m, 2H), 1.53–1.46 (m, 2H), 1.42–1.33 (m, 5H), 0.92 (t, J = 7.3 Hz, 3H). 13С-NMR (acetone-d6) δ 150.6, 144.1, 139.8, 138.8, 131.0, 129.3, 128.9, 127.5, 126.8, 126.6, 123.6, 120.7, 118.1, 116.8, 94.8, 78.4, 40.0, 31.0, 28.5, 22.0, 19.2, 13.4, 10.4. HRMS (ESI): calcd. for C27H30N3 [M + H]+, 396.2434; found: 396.2433.
3-Ethyl-1-[3-(hept-1-yn-1-yl)-4′-(trifluoromethyl)-[1,1′-biphenyl]-4-yl]-3-phenyltriaz-1-ene (8b). (A) This compound was synthesized by the general procedure for the Suzuki coupling (Method A) from triazene 3 (100 mg, 0.25 mmol) and 4-trifluoromethylphenylboronic acid 6b (72 mg, 0.38 mmol) at 100 °C; reaction time–24 h. Purification of the crude product by column chromatography using hexane/EtOAc (80:1) as the eluent gave 8b (75.0 mg, 65%) as an orange oil.
(B) This compound was synthesized by the general procedure for the Suzuki coupling (method B) from triazene 3 (300 mg, 0.754 mmol) and 4-trifluoromethylphenylboronic acid 6b (72 mg, 0.38 mmol) 6b (190 mg, 1.50 mmol) at 100 °C; reaction time – 24 h. Purification of the crude product by column chromatography using hexane/EtOAc (80:1) as the eluent gave 8b (205 mg, 55%) as an orange oil. 1Н-NMR (acetone-d6) δ 7.93 (d, J = 8.2 Hz, 2H), 7.84−7.80 (m, 3H), 7.75–7.65 (m, 2H), 7.64–7.58 (m, 2H), 7.49–7.40 (m, 2H), 7.22–7.13 (m, 1H), 4.47 (q, J = 7.0 Hz, 2H), 2.51 (t, J = 7.0 Hz, 2H), 1.71 – 1.59 (m, 2H), 1.56−1.44 (m, 2H), 1.43–1.31 (m, 5H), 0.92 (t, J = 7.3 Hz, 3H). 13С-NMR (acetone-d6) δ 152.3, 145.0, 144.6, 138.0, 132.3, 130.2, 129.7 (q, 2JC-F = 32.3 Hz), 128.2, 128.0, 126.7 (q, 3JC-F = 3.8 Hz), 125.5 (q, 1JC-F = 270.5 Hz), 124.7, 121.8, 119.3, 117.9, 96.1, 79.1, 41.1, 31.9, 29.4, 22.9, 20.1, 14.3, 11.3. HRMS (ESI): calcd. for C28H29F3N3 [M + H]+, 464.2313; found: 464.2308.
3-Ethyl-1-[3-(hept-1-yn-1-yl)-4'-methoxy-[1,1′-biphenyl]-4-yl]-3-phenyltriaz-1-ene (8c). This compound was synthesized by the general procedure for the Suzuki coupling (method A) from triazene 3 (100 mg, 0.25 mmol) and 4-methoxyphenylboronic acid 6c (58 mg, 0.38 mmol) at 100 °C; reaction time – 24 h. Purification of the crude product by column chromatography using hexane/EtOAc (80:1) as the eluent gave 8c (66 mg, 62%) as a brown oil. 1Н-NMR (acetone-d6) δ 7.68 (d, J = 2.0 Hz, 1H), 7.66−7.54 (m, 6H), 7.48–7.38 (m, 2H), 7.17–7.13 (m, 1H), 7.07–6.99 (m, 2H), 4.45 (q, J = 7.0 Hz, 2H), 3.85 (s, 3H), 2.50 (t, J = 7.0 Hz, 2H), 1.70–1.58 (m, 2H), 1.55−1.43 (m, 2H), 1.43 – 1.30 (m, 5H), 0.91 (t, J = 7.3 Hz, 3H). 13С-NMR (acetone-d6) δ 160.5, 150.9, 145.1, 139.5, 133.0, 131.4, 130.2, 128.6, 127.3, 124.4, 121.6, 119.0, 117.7, 115.2, 95.5, 79.4, 55.7, 40.8, 31.9, 29.4, 22.9, 20.2, 14.3, 11.3. HRMS (ESI): calcd. for C28H32N3O [M + H]+, 426.2540; found 426.2525.
3-Ethyl-1-[3-(hept-1-yn-1-yl)-4′-phenoxy-[1,1′-biphenyl]-4-yl]-3-phenyltriaz-1-ene (8d). (A) This compound was synthesized by the general procedure for the Suzuki coupling (method A) from triazene 3 (100 mg, 0.25 mmol) and 4-phenoxyphenylboronic acid 6d (81 mg, 0.38 mmol) at 100 °C; reaction time – 24 h. Purification of the crude product by column chromatography using hexane/EtOAc (70:1) as the eluent gave 8d (68 mg, 56%) as a brown oil.
(B) This compound was synthesized by the general procedure for the Suzuki coupling (method B) from triazene 3 (300 mg, 0.75 mmol) and 4-phenoxyphenylboronic acid 6d (323 mg, 1.51 mmol) at 100 °C; reaction time – 4 h. Purification of the crude product by column chromatography using hexane/EtOAc (80:1) as the eluent gave 8d (265 mg, 72%) as a brown oil. 1Н-NMR (acetone-d6) δ 7.76–7.68 (m, 3H), 7.67−7.58 (m, 4H), 7.48–7.38 (m, 4H), 7.22–7.14 (m, 2H), 7.12–7.04 (m, 4H), 4.46 (q, J = 7.0 Hz, 2H), 2.50 (t, J = 7.0 Hz, 2H), 1.70–1.59 (m, 2H), 1.56−1.44 (m, 2H), 1.42–1.33 (m, 5H), 0.91 (t, J = 7.3 Hz, 3H). 13С-NMR (acetone-d6) δ 158.1, 158.0, 151.3, 145.1, 139.1, 135.8, 131.7, 130.9, 130.2, 129.1, 127.5, 124.5, 124.5, 121.7, 119.9, 119.9, 119.1, 117.7, 95.7, 79.3, 40.9, 31.9, 23.0, 20.2, 14.3, 11.3. HRMS (ESI): calcd. for C33H33N3ONa [M + Na]+, 510.2516; found 510.2521.
tret-Butyl [4′-(3-ethyl-3-phenyltriaz-1-en-1-yl)-3′-(hept-1-yn-1-yl)-[1,1′-biphenyl]-4-yl]carbamate (8e). This compound was synthesized by the general procedure for the Suzuki coupling (method B) from triazene 3 (360 mg, 0.905 mmol) and {4-[(tert-butoxycarbonyl)amino]phenyl}boronic acid 6e (430 mg, 1.8 mmol) at 100 °C; reaction time – 2.5 h. Purification of the crude product by column chromatography using hexane/EtOAc (15:1) as the eluent gave 8e (345 mg, 75%) as an yellow oil. 1Н-NMR (acetone-d6) δ 8.49 (s, 1H), 7.72–7.56 (m, 9H), 7.48–7.39 (m, 2H), 7.20–7.10 (m, 1H), 4.45 (q, J = 7.0 Hz, 2H), 2.50 (t, J = 7.0 Hz, 2H), 1.69–1.59 (m, 2H), 1.54−1.45 (m, 11H), 1.42–1.32 (m, 5H), 0.91 (t, J = 7.3 Hz, 3H). 13С-NMR (acetone-d6) δ 153.7, 151.0, 145.1, 140.3, 139.4, 134.5, 131.4, 130.2, 127.8, 127.2, 124.4, 121.6, 119.5, 119.0, 117.7, 95.5, 80.1, 79.4, 40.8, 31.9, 29.4, 28.6, 22.9, 20.1, 14.3, 11.3. HRMS (ESI): calcd. for C32H38N4O2 [M + H]+, 511.3068; found 511.3086.
4′-(3-Ethyl-3-phenyltriaz-1-en-1-yl)-3′-(hept-1-yn-1-yl)-[1,1′-biphenyl]-4-carbonitrile (8f). This compound was synthesized by the general procedure for the Suzuki coupling (method B) from triazene 3 (340 mg, 0.854 mmol) and 4-cyanophenylboronic acid 6f (251 mg, 1.7 mmol) at 80 °C; reaction time – 10 h. Purification of the crude product by column chromatography using hexane/EtOAc (30:1) as the eluent gave 8f (190 mg, 53%) as an yellow oil. 1H-NMR (acetone-d6) δ 7.96–7.83 (m, 4H), 7.79 (d, J = 2.0 Hz, 1H), 7.76–7.63 (m, 2H), 7.62–7.56 (m, 2H), 7.50–7.38 (m, 2H), 7.21–7.15 (m, 1H), 4.45 (q, J = 7.1 Hz, 2H), 2.49 (t, J = 7.0 Hz, 2H), 1.66–1.59 (m, 2H), 1.55–1.41 (m, 2H), 1.40–1.31 (m, 5H), 0.89 (t, J = 7.3 Hz, 3H). 13C-NMR (acetone-d6) δ 152.5, 145.1, 145.0, 137.6, 133.6, 132.4, 130.2, 128.4, 128.0, 124.8, 121.9, 119.4, 119.3, 117.9, 111.8, 96.2, 79.0, 41.1, 31.9, 22.9, 20.1, 14.3, 11.3. HRMS (ESI): calcd. for C28H28N4Na [M + Na]+, 443.2206; found 443.2191.
3-Ethyl-1-(2-(hept-1-yn-1-yl)-4-(thiophen-3-yl)phenyl)-3-phenyltriaz-1-ene (8g). This compound was synthesized by the general procedure for the Suzuki coupling (method B) from triazene 3 (330 mg, 0.83 mmol) and thiophen-3-ylboronic acid 6g (286 mg, 1.66 mmol) at 100 °C; reaction time – 4 h. Purification of the crude product by column chromatography using hexane/EtOAc (30:1) as the eluent gave 8g (316 mg, 95%) as a yellow oil. 1H-NMR (acetone-d6) δ 7.79–7.78 (m, 2H), 7.68 (dd, J = 8.5, 2.1 Hz, 1H), 7.64–7.51 (m, 5H), 7.46–7.37 (m, 2H), 7.17–7.13 (m, 1H), 4.44 (q, J = 7.0 Hz, 2H), 2.49 (t, J = 7.0 Hz, 2H), 1.70–1.58 (m, 2H), 1.56–1.43 (m, 2H), 1.41–1.32 (m, 5H), 0.91 (t, J = 7.3 Hz, 3H). 13C-NMR (acetone-d6) δ 151.2, 145.0, 141.9, 134.7, 131.2, 130.2, 127.5, 127.1, 126.9, 124.5, 121.7, 121.5, 119.0, 117.7, 95.7, 79.3, 40.9, 31.9, 29.4, 22.9, 20.2, 14.3, 11.3. HRMS (ESI): calcd. for C25H27N3SCu [M + Cu]+, 464.1216; found 464.1194.
4-Bromo-3-pentyl-6-phenylcinoline (9a). This compound was synthesized by the general method for the Richter cyclization from triazene 8a (200 mg, 0.505 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (10:1→7:1) as the eluent gave 9a (153 mg, 86%) as a yellow oil. 1H-NMR (acetone-d6) δ 8.51 (d, J = 8.8 Hz, 1H), 8.30–8.21 (m, 2H), 7.89–7.84 (m, 2H), 7.60–7.47 (m, 3H), 3.40–3.35 (m, 2H), 1.95–1.87 (m, 2H), 1.51–1.36 (m, 4H), 0.92 (t, J = 7.1 Hz, 3H). 13C-NMR (acetone-d6) δ 158.0, 149.8, 145.6, 139.8, 131.4, 131.1, 130.2, 129.8, 128.6, 127.5, 127.1, 123.5, 36.8, 32.3, 29.4, 23.1, 14.3. HRMS (ESI): calcd. for C19H20N2Br [M + H]+, 355.0804; found 355.0802.
4-Bromo-3-pentyl-6-[4-(trifluoromethyl)phenyl]cinnoline (9b). This compound was synthesized by the general method for the Richter cyclization from triazene 8b (50 mg, 0.108 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (20:1) as the eluent gave 9b (40 mg, 88%), m.p. 88–89 °C. 1H-NMR (acetone-d6) δ 8.58 (d, J = 8.8 Hz, 1H), 8.39 (d, J = 1.6 Hz, 1H), 8.30 (dd, J = 8.8, 1.9 Hz, 1H), 8.12 (d, J = 8.1 Hz, 2H), 7.92 (d, J = 8.2 Hz, 2H), 3.42–3.39 (m, 2H), 2.00–1.86 (m, 2H), 1.53–1.36 (m, 4H), 0.93 (t, J = 7.1 Hz, 3H). 13C-NMR (100 MHz, acetone-d6) δ 158.3, 149.9, 144.1, 143.8, 131.7, 131.2 (q, 2JC-F = 32.3 Hz), 131.0, 129.5, 127.4, 127.3, 127.0 (q, 3JC-F = 3.4 Hz), 126.7 (q, 1JC-F = 271.5 Hz), 124.6, 36.9, 32.3, 29.4, 23.1, 14.3. HRMS (ESI): calcd. for C20H18BrF3N2Na [M + Na]+, 445.0498; found 445.0480.
4-Bromo-6-(4-methoxyphenyl)-3-pentylcinnoline (9c). This compound was synthesized by the general method for the Richter cyclization from triazene 8c (228 mg, 0.536 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (10:1→5:1) as the eluent gave 9c (157 mg, 76%), m.p. 85–87 °C. 1H-NMR (acetone-d6) δ 8.52–8.45 (m, 1H), 8.25–8.21 (m, 2H), 7.88–7.82 (m, 2H), 7.17–7.09 (m, 2H), 3.90 (s, 3H), 3.43–3.30 (m, 2H), 1.99–1.86 (m, 2H), 1.54–1.36 (m, 4H), 0.93 (t, J = 7.1 Hz, 3H). 13C-NMR (acetone-d6) δ 161.7, 157.9, 149.7, 145.3, 132.0, 131.3, 130.9, 129.9, 127.7, 126.9, 122.3, 115.7, 55.8, 36.9, 32.3, 23.2, 14.3. HRMS (ESI): calcd. for C20H21BrN2ONa [M + Na]+, 407.0729; found 407.0708.
4-Bromo-3-pentyl-6-(4-phenoxyphenyl)cinnoline (9d). This compound was synthesized by the general method for the Richter cyclization from triazene 8d (180 mg, 0.37 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (10:1→5:1) as the eluent gave 9d (121 mg, 72%), m.p. 77–78 °C. 1H-NMR (400 MHz, acetone-d6) δ = 8.51 (dd, J = 8.8, 0.7 Hz, 1H), 8.31–8.21 (m, 2H), 7.95–7.87 (m, 2H), 7.50–7.40 (m, 2H), 7.26–7.08 (m, 5H), 3.43–3.34 (m, 2H), 1.99–1.86 (m, 2H), 1.54–1.35 (m, 4H), 0.92 (t, J = 7.1 Hz, 3H). 13C-NMR (100 MHz, acetone-d6) δ 159.5, 158.0, 157.5, 149.7, 144.9, 134.6, 131.4, 131.0, 130.9, 130.3, 127.6, 127.0, 124.9, 122.9, 120.3, 119.8, 36.8, 32.3, 23.1, 14.3. HRMS (ESI): calcd. for C25H23BrN2ONa [M + Na]+, 469.0886; found 469.0897.
tert-Butyl [4-(4-bromo-3-pentylcinnolin-6-yl)phenyl]carbamate (9e). This compound was synthesized by the general method for the Richter cyclization from triazene 8e (170 mg, 0.333 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (5:1) as the eluent gave 9e (135 mg, 86%). 1H-NMR (acetone-d6) δ 8.66 (s, 1H), 8.53–8.45 (m, 1H), 8.31–8.22 (m, 2H), 7.89–7.74 (m, 4H), 3.43–3.34 (m, 2H), 1.99–1.86 (m, 2H), 1.52 (s, 9H), 1.51–1.37 (m, 5H), 0.93 (t, J = 7.0 Hz, 3H). 13C NMR (acetone-d6) δ 158.0, 153.7, 149.8, 145.3, 141.8, 133.3, 131.3, 130.8, 129.0, 127.7, 126.9, 122.3, 119.6, 80.4, 36.8, 32.3, 28.5, 23.1, 14.3. HRMS (ESI): calcd. for C24H28BrN3O2Na [M + Na]+, 492.1257; found 492.1262.
4-(4-Bromo-3-pentylcinnolin-6-yl)benzonitrile (9f). This compound was synthesized by the general method for Richter cyclization from triazene 8f (190 mg, 0.452 mmol). Purification of the crude product by recrystallization from hexane/EtOAc (1:1) gave 9f (107 mg, 62%) as a brown solid. 1H-NMR (acetone-d6) δ 8.60 (d, J = 8.8 Hz, 1H), 8.44–8.40 (m, 1H), 8.32 (dd, J = 8.8, 1.9 Hz, 1H), 8.15–8.10 (m, 2 H), 8.02–7.97 (m, 2H), 3.45–3.38 (m, 2H), 1.98–1.87 (m, 2H), 1.54–1.34 (m, 4H), 0.93 (t, J = 7.0 Hz, 3H).13C-NMR (acetone-d6) δ 152.4, 149.9, 144.2, 143.8, 133.9, 133.7, 131.8, 130.9, 129.6, 127.3, 124.9, 119.3, 113.4, 36.8, 32.3, 29.4, 23.1, 14.3. HRMS (ESI): calcd. for C20H18BrN3Na [M + Na]+, 402.0576; found 402.0583.
4-Bromo-3-pentyl-6-(thiophen-3-yl)cinnoline (9g). This compound was synthesized by the general method for the Richter cyclization from triazene 8g (280 mg, 0.698 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (10:1) as the eluent gave 9g (214 mg, 85%). 1H-NMR (acetone-d6) δ 8.47–8.40 (m, 1H), 8.33–8.25 (m, 2H), 8.14 (dd, J = 2.9, 1.4 Hz, 1H), 7.74 (dd, J = 5.1, 1.4 Hz, 1H), 7.68 (dd, J = 5.1, 2.9 Hz, 1H), 3.39–3.31 (m, 2H), 1.95–1.85 (m, 2H), 1.50–1.36 (m, 4H), 0.91 (t, J = 7.1 Hz, 3H). 13C-NMR (acetone-d6) δ 158.0, 149.7, 141.0, 140.1, 131.3, 130.6, 128.5, 127.8, 127.1, 126.9, 125.2, 122.0, 36.8, 32.3, 29.4, 23.1, 14.3. HRMS (ESI): calcd. for C17H17BrN2SNa [M + Na]+, 383.0188; found 383.0188.
4-Azido-3-pentyl-6-phenylcinoline (7a). (A) This compound was synthesized by the general procedure for the Suzuki coupling (method A) from 4-azido-6-bromocinnoline 5 (50 mg, 0.156 mmol) and phenylboronic acid 6a (38 mg, 0.312 mmol) at 80 °C; reaction time – 1 h. Purification of the crude product by column chromatography using hexane/EtOAc (5:1→2:1→1:2) as the eluent gave 7a (9,5 mg, 19%). (B) Azidocinnoline 7a was obtained by the general procedure for the Suzuki coupling (method B) from 4-azido-6-bromcinnoline 5 (50 mg, 0.156 mmol), phenylboronic acid 6a (38 mg, 0.312 mmol) and K3PO4 (99 mg, 0.468 mmol, 3 equiv) at 80 °C; reaction time–1 h. Purification of the crude product by column chromatography using hexane/EtOAc (5:1→2:1→1:2) as the eluent gave 7a (25 mg, 50%). С) Azidocinnoline 7a was prepared by the general procure for the nucleophilic substitution from bromocinnoline 9a (650 mg, 1.83 mmol) and sodium azide (594 mg, 9.15 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (8:1) as the eluent gave 7a (528 mg, 91%). 1H-NMR (acetone-d6) δ 8.49 (d, J = 8.9 Hz, 1H), 8.41 (d, J = 1.6 Hz, 1H), 8.23 (dd, J = 8.9, 1.6 Hz, 1H), 7.91–7.84 (m, 2H), 7.63–7.53 (m, 2H), 7.52–7.48 (m, 1H), 3.41–3.28 (m, 2H), 1.97–1.90 (m, 2H), 1.57–1.35 (m, 4H), 0.93 (t, J = 7.1 Hz, 3H). 13C-NMR (acetone-d6) δ 151.6, 150.3, 144.1, 140.2, 133.2, 131.2, 130.8, 130.2, 129.7, 128.5, 121.8, 119.3, 32.9, 32.4, 23.2, 14.3. HRMS (ESI): calcd. for C19H20N5 [M + H]+, 318.1713; found 318.1714. IR, cm−1, ν: 2117 (N3).
4-Azido-3-pentyl-6-(4-(trifluoromethyl)phenyl)cinnoline (7b). This compound was synthesized by the general procure for the nucleophilic substitution from bromocinnoline 9b (130 mg, 0.308 mmol) and sodium azide (100 mg, 1.54 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (3:1) as the eluent gave 7b (100 mg, 84%). 1H-NMR (acetone-d6) δ 8.57–8.48 (m, 2H), 8.27 (dd, J = 8.8 Hz, 2.0 Hz, 1H), 8.11 (d, J = 8.1 Hz, 2H), 7.92 (d, J = 8.1 Hz, 2H), 3.42–3.33 (m, 2H), 2.00–1.88 (m, 2H), 1.54–1.38 (m, 4H), 0.93 (t, J = 7.0 Hz, 3H). 13C-NMR (acetone-d6) δ 151.8, 150.3, 144.1, 142.5, 133.4, 131.5, 130.9 (q, 2JC–F = 32.4 Hz), 130.6, 129.3, 127.0 (q, 3JC–F = 3.8 Hz), 125.4 (q, 1JC–F = 271.1 Hz), 121.6, 120.5, 32.9, 32.4, 30.0, 23.2, 14.3 ppm. HRMS (ESI): calcd. for C20H18F3N5Na [M + Na]+, 408.1407; found 408.1403. IR, cm−1, ν: 2122 (N3).
4-Azido-6-(4-methoxyphenyl) -3-pentylcinnoline (7c). This compound was synthesized by the general procure for the nucleophilic substitution from bromocinnoline 9c (155 mg, 0.404 mmol) and sodium azide (65.7 mg, 1.01 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (5:1→3:1) as the eluent gave 7c (116 mg, 83%). 1H-NMR (acetone-d6) δ 8.44 (d, J = 8.9 Hz, 1H), 8.33 (d, J = 2.0 Hz, 1H), 8.20 (dd, J = 8.9, 1.9 Hz, 1H), 7.88–7.77 (m, 2H), 7.22–7.08 (m, 2H), 3.89 (s, 3H), 3.38–3.21 (m, 2H), 1.96–1.88 (m, 2H), 1.53–1.40 (m, 4H), 0.93 (t, J = 7.1 Hz, 3H). 13C-NMR (acetone-d6) δ 161.6, 151.5, 150.2, 143.8, 132.9, 132.2, 131.1, 130.5, 129.7, 121.9, 118.0, 115.6, 55.8, 32.9, 32.4, 30.0, 23.2, 14.3. HRMS (ESI): calcd. for C20H21N5ONa [M + Na]+, 370.1638; found 370.1626. IR, cm−1, ν: 2116 (N3).
4-Azido-3-pentyl-6-(4-phenoxyphenyl)cinnoline (7d). This compound was synthesized by the general procure for the nucleophilic substitution from bromocinnoline 9d (120 mg, 0.269 mmol) and sodium azide (87 mg, 1.35 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (5:1→ 3:1) as the eluent gave 7d (77 mg, 70%). 1H-NMR (acetone-d6) δ 8.47 (d, J = 8.9 Hz, 1H), 8.38 (d, J = 1.8 Hz, 1H), 8.22 (dd, J = 8.9, 1.9 Hz, 1H), 7.94–7.86 (m, 2H), 7.49 – 7.40 (m, 2H), 7.25–7.13 (m, 3H), 7.16–7.08 (m, 2H), 3.39–3.30 (m, 2H), 1.99–1.87 (m, 2H), 1.53–1.39 (m, 4H), 0.93 (t, J = 7.0 Hz, 3H). 13C-NMR (acetone-d6) δ 159.4, 157.6, 151.6, 150.2, 143.4, 134.9, 133.1, 131.2, 131.0, 130.9, 130.6, 130.1, 124.9, 121.8, 120.3, 119.8, 119.7, 118.7, 32.9, 32.4, 30.0, 23.2, 14.3. HRMS (ESI): calcd. for C25H23N5ONa [M + Na]+, 432.1795; found 432.1797.
tert-Butyl (4-(4-azido-3-pentylcinnolin-6-yl)phenyl)carbamate (7e). This compound was synthesized by the general procure for the nucleophilic substitution from bromocinnoline 9e (88 mg, 0.188 mmol) and sodium azide (61 mg, 0.94 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (2:1→ 1:1) as the eluent gave 7e (59 mg, 73%). 1H-NMR (acetone-d6) δ 8.65 (s, 1H), 8.46 (d, J = 8.9 Hz, 1H), 8.38 (d, J = 1.7 Hz, 1H), 8.23 (dd, J = 8.9, 1.8 Hz, 1H), 7.86–7.73 (m, 4H), 3.39–3.29 (m, 2H), 1.98–1.87 (m, 2H), 1.52 (s, 9H), 1.49–1.40 (m, 4H), 0.93 (t, J = 7.0 Hz, 3H). 13C-NMR (acetone-d6) δ = 153.7, 153.6, 151.5, 150.2, 143.7, 141.7, 133.6, 133.0, 131.1, 130.5, 128.9, 121.9, 119.6, 119.5, 118.1, 80.4, 32.9, 32.4, 29.8, 28.5, 23.2, 14.3. HRMS (ESI): calcd. for C24H29N6O2 [M + H]+, 433.2347; found 433.2342.
4-(4-Azido-3-pentylcinnolin-6-yl)benzonitrile (7f). This compound was synthesized by the general procure for the nucleophilic substitution from bromocinnoline 9f (100 mg, 0.264 mmol) and sodium azide (86 mg, 1.32 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (5:1) as the eluent gave 7f (80 mg, 88%). 1H-NMR (acetone-d6) δ 8.57–8.47 (m, 2H), 8.27 (dd, J = 8.9, 2.0 Hz, 1H), 8.15–8.04 (m, 2H), 8.02–7.92 (m, 2H), 3.46–3.22 (m, 2H), 2.00–1.88 (m, 2H), 1.56–1.36 (m, 4H), 0.93 (t, J = 7.1 Hz, 3H). 13C-NMR (acetone-d6) δ 151.9, 150.3, 144.5, 142.1, 133.9, 133.5, 131.5, 130.4, 129.5, 121.6, 120.7, 119.1, 113.2, 32.9, 32.4, 29.1, 23.2, 14.3. HRMS (ESI): calcd. for C20H18N6Na [M + Na]+, 365.1485; found 365.1472.
4-Azido-3-pentyl-6-(thiophen-3-yl)cinnoline (7g). This compound was synthesized by the general procure for the nucleophilic substitution from bromocinnoline 9g (185 mg, 0.514 mmol) and sodium azide (167 mg, 2.57 mmol). Purification of the crude product by column chromatography using hexane/EtOAc (7:1→3:1) as the eluent gave 7g (115 mg, 69%), m.p. 100–102 °C. 1H-NMR (acetone-d6) δ 8.46–8.39 (m, 2H), 8.29 (dd, J = 8.9, 1.9 Hz, 1H), 8.13 (dd, J = 2.9, 1.4 Hz, 1H), 7.76 (dd, J = 5.1, 1.4 Hz, 1H), 7.70 (dd, J = 5.1, 2.9 Hz, 1H), 3.37–3.29 (m, 2H), 1.98–1.86 (m, 2H), 1.54–1.37 (m, 4H), 0.93 (t, J = 7.1 Hz, 3H). 13C-NMR (acetone-d6) δ 151.6, 150.2, 141.3, 138.7, 133.0, 131.2, 130.3, 128.4, 127.1, 124.7, 122.0, 117.9, 32.9, 32.4, 30.0, 23.2, 14.3. HRMS (ESI): calcd. for C17H17N5SNa [M + Na]+, 346.1097; found 346.1085.
3-[1-(3-Pentyl-6-phenylcinnolin-4-yl)-1H-1,2,3-triazol-4-yl]propan-1-ol (11a). This compound was synthesized in accordance with the general procedure from pent-4-yn-1-ol 10a (47.8 mg, 0.568 mmol), 4-azidocinnoline 7a (90.0 mg, 0.284 mmol), CuSO4∙5H2O (3.5 mg, 0.014 mmol), and sodium ascorbate (5.60 mg, 0.028 mmol) in a mixture of THF/H2O (5.6 mL); reaction time – 5 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 2:1) to afford triazole 11a as a yellowish solid (100 mg, 88%), m.p. 89−91 °C. 1H-NMR (acetone-d6) δ 8.67 (d, J = 8.9 Hz, 1H), 8.35 (s, 1H), 8.30 (dd, J = 8.9, 1.9 Hz, 1H), 7.75–7.68 (m, 2H), 7.56–7.42 (m, 4H), 3.74–3.67 (m, 3H), 3.04–2.95 (m, 4H), 2.03–1.98 (m, 2H), 1.83–1.71 (m, 2H), 1.34–1.24 (m, 4H), 0.88–0.80 (m, 3H). 13C-NMR (acetone-d6) δ 154.3, 150.8, 149.2, 146.0, 139.7, 131.3, 131.2, 130.4, 130.1, 129.9, 128.5, 125.8, 123.6, 119.1, 61.6, 33.3, 32.5, 32.2, 30.0, 22.9, 22.7, 14.2. HRMS ESI: [M + H]+ calcd. for C24H27N5O+: 402.2288; found: 402.2293.
4-[4-(3,4-Dimethoxyphenyl)-1H-1,2,3-triazol-1-yl]-3-pentyl-6-[4-(trifluoromethyl)phenyl]cinnoline (11b). This compound was synthesized in accordance with the general procedure from 4-ethynyl-1,2-dimethoxybenzene 10b (21.0 mg, 0.130 mmol), 4-azidocinnoline 7b (50.0 mg, 0.130 mmol), CuSO4∙5H2O (1.7 mg, 0.007 mmol), and sodium ascorbate (2.6 mg, 0.013 mmol) in a mixture of THF/H2O (5 mL); reaction time – 3 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 2:1) to afford triazole 11b as a yellowish solid (36 mg, 50%). 1H-NMR (acetone-d6) δ 8.92 (s, 1H), 8.76 (d, J = 8.9 Hz, 1H), 8.37 (dd, J = 8.9, 1.7 Hz, 1H), 7.98 (d, J = 8.5 Hz, 2H), 7.86–7.79 (m, 3H), 7.66 (d, J = 2.0 Hz, 1H), 7.58 (dd, J = 8.3, 2.0 Hz, 1H), 7.08 (d, J = 8.3 Hz, 1H), 3.90 (s, 3H), 3.87 (s, 3H), 3.11–3.03 (m, 2H), 1.83 (p, J = 7.2 Hz, 2H), 1.36–1.23 (m, 4H), 0.83 (t, J = 7.0 Hz, 3H). 13C-NMR (acetone-d6) δ 154.5, 150.88, 150.87, 150.82, 149.0, 144.6, 143.6 (q, 5JC–F = 1.2 Hz), 131.6, 131.32, 131.1 (q, 2JC–F = 32.4 Hz), 130.3, 129.5, 126.9 (q, 3JC–F = 3.8 Hz), 125.2 (q, 1JC–F = 271.4 Hz), 124.06, 124.05, 123.3, 120.4, 119.4, 113.2, 110.6, 56.25, 56.24, 32.6, 32.2, 30.0, 22.9, 14.2. HRMS (ESI): calcd. for C30H28N5O2F3Na [M + Na]+; 570.2087; found: 570.2081.
6-(4-Methoxyphenyl)-4-[4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl]-3-pentylcinnoline (11c). (A) This compound was prepared in accordance with the general procedure (method A) from 4-ethynyl-1-methoxybenzene 10c (13.8 mg, 0.104 mmol), 4-azidocinnoline 7c (40.0 mg, 0.104 mmol), CuSO4∙5H2O (1.3 mg, 0.005 mmol), and sodium ascorbate (2.10 mg, 0.010 mmol) in a mixture of THF/H2O (4 mL); reaction time − 2 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 3:1) to afford triazole 11c as a yellowish solid (35 mg, 76%). (B) This compound was prepared in accordance with the general procedure (method A) from mixture 4-ethynyl-1-methoxybenzene 10c (24.0 mg, 0.177 mmol), 4-azidocinnoline 7c (68.0 mg, 0.177 mmol), CuSO4∙5H2O (2.5 mg, 0.009 mmol), and sodium ascorbate (3.6 mg, 0.009 mmol) in the mixture of THF/H2O (4 mL) with the addition of TBTA (5.0 mg, 0.009 mmol); reaction time − 50 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 3:1) to afford triazole 11c as a solid (30 mg, 35%).1H-NMR (CDCl3) δ 8.68 (d, J = 8.9 Hz, 1H), 8.11 (dd, J = 8.9, 1.9 Hz, 1H), 8.01 (s, 1H), 7.94–7.88 (m, 2H), 7.58–7.52 (m, 2H), 7.44 (d, J = 1.8 Hz, 1H), 7.07–7.03 (m, 2H), 7.00–6.95 (m, 2H), 3.89 (s, 3H), 3.84 (s, 3H), 3.07–2.98 (m, 2H), 1.90–1.82 (m, 2H), 1.35–1.28 (m, 4H), 0.85 (t, J = 6.8 Hz, 4H). 13C-NMR (CDCl3) δ 160.8, 160.3, 153.7, 150.0, 148.5, 145.6, 131.2, 130.7, 130.5, 129.1, 127.5, 123.2, 122.4, 121.5, 117.0, 114.9, 114.7, 55.6, 32.0, 31.7, 29.8, 22.4, 14.0. HRMS (ESI): calcd. for C29H29N5O2Na [M + Na]+; 502.2213; found: 502.2227.
4{1-[3-Pentyl-6-(4-phenoxyphenyl)cinnolin-4-yl]-1H-1,2,3-triazol-4-yl}butan-1-ol (11d). This compound was prepared in accordance with the general procedure from hex-5-yn-1-ol 10d (3.60 mg, 0.037 mmol), 4-azido-3-pentyl-6-(4-phenoxyphenyl)cinnoline 7d (15.0 mg, 0.037 mmol), CuSO4∙5H2O (0.46 mg, 0.0018 mmol), and sodium ascorbate (0.67 mg, 0.0037 mmol) in a mixture of THF/H2O (1.5 mL); reaction time − 21 h. The crude product was purified by column chromatography (eluent: hexane/acetone = 2:1) to afford triazole 11d as a brown oil (5 mg, 28%). 1H-NMR (DMSO-d6) δ 8.71 (d, J = 8.9 Hz, 1H), 8.55 (s, 1H), 8.35 (dd, J = 8.9, 1.9 Hz, 1H), 7.78–7.72 (m, 2H), 7.48–7.40 (m, 2H), 7.38 (d, J = 1.9 Hz, 1H), 7.23–7.17 (m, 1H), 7.15–7.04 (m, 4H), 4.41 (t, J = 5.1 Hz, 1H, OH), 3.45 (td, J = 6.4, 5.1 Hz, 2H), 2.94–2.90 (m, 2H), 2.84 (t, J = 7.4 Hz, 2H), 1.81–1.72 (m, 2H), 1.64 (m, 2H), 1.53 (m, 2H), 1.22 (m, 4H), 0.81 (m, 3H). 13C-NMR (DMSO-d6) δ 158.0, 155.9, 153.0, 149.3, 147.9, 143.9, 132.8, 130.5, 130.2, 130.1, 129.3, 129.2, 125.4, 124.1, 122.2, 119.2, 118.9, 117.1, 60.3, 31.8, 31.2, 30.8, 28.5, 25.4, 24.7, 21.6, 13.6. HRMS (ESI): calcd. for C31H33N5O2Na [M + Na]+; 530.2526; found: 530.2510.
Methyl 4-{1-[6-(4-{[tert-butoxycarbonyl]amino}phenyl)-3-pentylcinnolin-4-yl]-1H-1,2,3-triazol-4-yl} benzoate (11e). This compound was prepared in accordance with the general procedure from methyl 4-ethynylbenzoate 10e (10.7 mg, 0.067 mmol), 4-azidocinnoline 7e (29.0 mg, 0.067 mmol), CuSO4∙5H2O (0.84 mg, 0.0034 mmol), and sodium ascorbate (1.22 mg, 0.067 mmol) in a mixture of THF/H2O (3 mL); reaction time – 2 h. The crude product was purified by column chromatography (eluent: hexane/acetone = 3:1) to afford triazole 11e as a yellow solid (27 mg, 68%). 1H-NMR (DMSO-d6) δ 9.56 (s, 1H), 9.40 (s, 1 H), 8.70 (d, J = 9.0 Hz, 1H), 8.36 (dd, J = 9.0, 1.9 Hz, 1H), 8.19–8.15 (m, 2H), 8.13–8.09 (m, 2H), 7.72–7.67 (m, 2H), 7.62–7.56 (m, 2H), 7.54 (d, J = 1.9 Hz, 1H), 3.89 (s, 3H), 2.97–2.93 (m, 2H), 1.77–1.64 (m, 2H), 1.47 (s, 9H), 1.26–1.15 (m, 4H), 0.80–0.72 (m, 3H). 13C-NMR (DMSO-d6) δ 165.9, 152.8, 152.6, 149.4, 146.2, 144.4, 140.5, 134.4, 131.1, 130.5, 130.0, 129.2, 128.7, 128.1, 126.0, 125.7, 122.0, 118.4, 116.3, 79.4, 52.2, 31.2, 30.7, 28.5, 28.0, 21.6, 13.6. HRMS (ESI): calcd. for C34H36N6O4Na [M + Na]+; 615.2690; found: 615.2683.
4-{4-[4-(4-{Dimethylamino}phenyl)-1H-1,2,3-triazol-1-yl]-3-pentylcinnolin-6-yl}benzonitrile (11f). This compound was prepared in accordance with the general procedure from 4-ethynyl-N,N-dimethylaniline 10f (25.4 mg, 0.175 mmol), 4-azidocinnoline 7f (60.0 mg, 0.175 mmol), CuSO4∙5H2O (2.19 mg, 0.0088 mmol), and sodium ascorbate (3.19 mg, 0.017 mmol) in the mixture of THF/H2O (6 mL); reaction time − 4 h. The crude product was purified by column chromatography (eluent: hexane/acetone = 3:1) to afford triazole 11f as a yellow solid (74 mg, 86%). 1H-NMR (DMSO-d6) δ 9.04 (s, 1H), 8.79–8.76 (m, 1H), 8.41–8.37 (m, 1H), 7.96–7.95 (m, 4H), 7.85–7.75 (m, 2H), 7.69–7.68 (m, 1H), 6.84 (d, J = 8.4 Hz, 2H), 3.00 (t, J = 7.6 Hz, 2H), 2.96 (s, 6H), 1.82–1.57 (m, 2H), 1.23–1.17 (m, 4H), 0.86–0.57 (m, 3H). 13C-NMR (DMSO-d6) δ 153.1, 150.4, 149.5, 147.9, 142.8, 142.5, 133.1, 130.6, 129.2, 128.6, 126.5, 122.8, 121.7, 119.4, 118.5, 117.4, 112.3, 111.6, 31.3, 30.7, 28.5, 21.6, 13.6. HRMS (ESI): calcd. for C30H30N7 [M + H]+; 488.2557; found: 488.2558.
4-(4-(3,4-Dimethoxyphenyl)-1H-1,2,3-triazol-1-yl)-3-pentyl-6-(thiophen-3-yl)cinnoline (11g). This compound was prepared in accordance with the general procedure from 4-ethynyl-1,2-dimethoxybenzene 10b (27.6 mg, 0.170 mmol), 4-azidocinnoline 7g (55.0 mg, 0.170 mmol), CuSO4∙5H2O (2.12 mg, 0.0085 mmol), and sodium ascorbate (3.70 mg, 0.017 mmol) in the mixture of THF/H2O (6 mL); reaction time − 4 h. The crude product was purified by column chromatography (eluent: hexane/EtOAc = 2:1) to afford triazole 11g as a yellow solid (68 mg, 82%). 1H-NMR (DMSO-d6) δ 9.17 (s, 1H), 8.68 (dd, J = 9.0, 0.6 Hz, 1H), 8.44 (dd, J = 9.0, 1.9 Hz, 1H), 8.23 (dd, J = 2.9, 1.4 Hz, 1H), 7.69 (dd, J = 5.1, 2.9 Hz, 1H),7.61–7.60 (m, 3H), 7.56 (dd, J = 8.2, 2.0 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 3.86 (s, 3H), 3.82 (s, 3H), 2.97–2.93 (m, 2H), 1.74–1.67 (m, 2H), 1.24–1.20 (m, 4H), 0.82–0.74 (m, 3H). 13C-NMR (DMSO-d6) δ 153.0, 149.4, 149.2, 149.1, 147.4, 139.4, 139.3, 130.4, 130.1, 128.9, 128.2, 126.4, 125.6, 124.0, 122.6, 122.3, 118.2, 116.2, 112.2, 109.3, 55.61, 55.60, 31.2, 30.8, 28.6, 21.6, 13.6. HRMS (ESI): calcd. for C27H27N5O2SNa [M + Na]+; 508.1778; found: 508.1790. Single crystals of 11g were grown from the solution in chloroform by the vapor exchange with hexane. Crystal data for 11g C27H27N5O2S (M = 485.59 g/mol): triclinic, space group P (no. 2), a = 8.2511(5) Å, b = 10.9441(6) Å, c = 14.6090(6) Å, α = 105.898(5)°, β = 94.236(4)°, γ = 107.263(5)° V = 1194.17(12) Å3, Z = 2, T = 115(3) K, μ(CuKα) = 1.489 mm−1, Dcalc = 1.350 g/cm3, 9946 reflections measured (6.382° ≤ 2Θ ≤ 139.978°), 4507 unique (Rint = 0.0378, Rsigma = 0.0505) which were used in all calculations. The final R1 was 0.0516 (I > 2σ(I)) and wR2 was 0.1510. Crystallographic data (excluding structure factors) for the structures reported in this work have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 1902675 (Supplementary Materials).
3-Pentyl-6-(4-(trifluoromethyl)phenyl)cinnolin-4-amine (14). To a stirred solution of 4-azidocinnolines 7b (50.0 mg, 0.13 mmol) in DMSO (0.3 mL) at room temperature were propanal 12 (22.0 mg, 30.0 μL, 0.39 mmol) followed by 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (6.0 mg, 5.8 μL, 0.039 mmol). The reaction mixture was stirred at room temperature for 3 hours (TLC control). The resulting mixture was diluted with water (10 mL) and extracted with ethyl acetate (3 × 8 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure to yield the crude product, which was purified by column chromatography on silica gel. 1H-NMR (acetone-d6) δ 8.60 (d, J = 1.9 Hz, 1H), 8.24 (d, J = 8.9 Hz, 1H), 8.08 (d, J = 8.7 Hz, 3H), 7.85 (d, J = 8.2 Hz, 2H), 6.51 (s, 2H), 3.23–3.04 (m, 2H), 1.93–1.79 (m, 2H), 1.49–1.35 (m, 4H), 0.90 (t, J = 7.0 Hz, 3H). 13C-NMR (acetone-d6) δ 149.0, 144.9 (5JC–F = 1.2 Hz), 143.4, 139.5, 138.7, 130.7, 130.1 (2JC–F = 32.3 Hz), 129.0, 128.9, 126.7 (3JC–F = 3.8 Hz), 125.4 (1JC–F = 271.2 Hz), 120.6, 116.1, 32.6, 32.3, 28.3, 23.3, 14.4.HRMS (ESI): calcd. for C20H21F3N3 [M + H]+; 360.1682; found: 360.1674.
1,5-Ditosyl-7,8-didehydro-2,3,4,5,6,9-hexahydro-1H-1,5-diazonine (15). To a stirred at 0 °C solution of Co2(CO)6-complex of 15 (100.0 mg, 0.139 mmol) [69] in acetone (3 mL) tetrabutylammonium fluoride hydrate (195 mg, 0.696 mmol, calculated for TBAF monohydrate) was added. The reaction mixture was allowed to warm to room temperature and then was stirred at this temperature for 1.5 hours (TLC control). After completion of the reaction the mixture was diluted with water (10 mL) and extracted with ethyl acetate (3 × 15 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure to yield the crude product, which was purified by column chromatography on silica gel (eluent: hexane/acetone = 3:1) to afford diazacyclononyne 15 as a white solid (56 mg, 93%). Spectral data were identical to those reported previously [69]. 1H-NMR (CDCl3) δ 7.69–7.62 (m, 4H), 7.37–7.30 (m, 4H), 3.79 (s, 4H), 3.31–3.24 (m, 4H), 2.44 (s, 6H), 2.14–2.09 (m, 2H).
1-[3-Pentyl-6-(thiophen-3-yl)cinnolin-4-yl]-5,9-ditosyl-1,4,5,6,7,8,9,10-octahydro-[1,2,3]triazolo-[4,5-g][1,5]- diazonine (16). A mixture of diazacyclononyne 15 (58 mg, 0.133 mmol) and 4-azidocinnoline 7g (43.0 mg, 0.133 mmol) in acetonitrile (2 mL) was stirred at 30 °C for 5 h. The solvent was removed under reduced pressure and the crude product was purified by column chromatography on silicagel usin hexane/acetone (3:1) as the eluent to give triazole 16 as a yellowish solid (95 mg, 95%). 1H-NMR (DMSO-d6) δ 8.71 (d, J = 9.0 Hz, 1H), 8.48 (dd, J = 9.0, 1.9 Hz, 1H), 8.22 (dd, J = 2.9, 1.4 Hz, 1H), 7.84 – 7.77 (m, 2H), 7.69 (dd, J = 5.2, 2.9 Hz, 1H), 7.59 (dd, J = 5.1, 1.4 Hz, 1H), 7.49 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 2.0 Hz, 1H), 7.18–7.09 (m, 4H), 4.78 (d, J = 16.2 Hz, 1H), 4.62 (d, J = 16.3 Hz, 1H), 4.18 (s, 2H), 3.24 (dt, J = 7.3, 3.6 Hz, 2H), 3.22–3.11 (m, 1H), 2.98–2.90 (m, 1H), 2.68–2.61 (m, 1H), 2.43 (s, 3H), 2.27 (s, 3H), 1.91–1.74 (m, 4H), 1.30–1.22 (m, 4H), 0.86–0.78 (m, 3H). 13C-NMR (DMSO-d6) δ 154.2, 149.3, 143.8, 143.7, 143.2, 139.6, 139.2, 134.6, 134.5, 133.9, 130.4, 130.3, 130.0, 129.7, 128.2, 127.3, 126.8, 126.6, 126.2, 125.6, 122.8, 115.6, 48.7, 48.6, 45.3, 42.1, 31.0, 31.0, 29.4, 28.5, 21.7, 21.0, 20.8, 13.7. HRMS (ESI): calcd. for C38H42N7O4S3 [M + H]+: 756.2455; found: 756.2466.
3.3. DFT Calculations
All computations were carried out at the DFT/HF hybrid level of theory using Becke’s three-parameter hybrid exchange functional in combination with the gradient-corrected correlation functional of Lee, Yang, and Parr (B3LYP) by using the GAUSSIAN 2003 program packages [76]. The geometries optimization was performed using the 6-311+G(2d,2p) basis set (standard 6–311 basis set added with polarization (d, p) and diffuse functions). Optimizations were performed on all degrees of freedom, and optimized structures were verified as true minima with no imaginary frequencies. The Hessian matrix was calculated analytically for the optimized structures in order to prove the location of correct minima and to estimate the thermodynamic parameters.
3.4. The Absolute Fluorescence Quantum Yield Measurements
The absolute fluorescence quantum yield was measured on a Horiba Fluorolog-3 spectrometer (Edison, NJ, USA) equipped with an integrating sphere. A xenon lamp coupled to a double monochromator was used as excitation light source. The sample (1 cm quartz cuvette cell with molecular solution in THF) or blank (pure THF) were directly illuminated in the center of the integrating sphere. The optical density of all investigated sample solutions in THF did not exceed 0.1 at the luminescence excitation wavelength. Under the same conditions (e.g., excitation wavelength, spectral resolution, temperature), the luminescence spectrum of the sample Ec, the luminescence spectrum of the blank Ea, the Rayleigh scattering spectrum of the sample Lc, and the Rayleigh scattering spectrum of the solvent La were measured. The absolute fluorescence quantum yield was determined according to the formula:
3.5. Cell Culture Cultivation and Cytotoxicity Studies
The HeLa cells were obtained from the Russian Collection of Cell cultures (Institute of Cytology RAS, Saint Petersburg, Russia). Cells were cultivated in DMEM medium containing 10% FBS (fetal bovine serum) at 37 °C and 5% CO2. Compounds were diluted with DMSO to concentrations of 7.5; 5; 2.5; 0.5 and 0.1 mM. These solutions were diluted 100 x times with culture medium to the final concentrations of 100, 75, 50, 25, 5 and 1 μM/L (1% DMSO). Cytotoxicity assays were made using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) [77]. Cells were placed in 96-well plates (5000 of cells per well in 100 μL of DMEM + FBS without compounds) and preincubated for 24 hours. Subsequently culture medium was aspirated and 100 μL volumes of media with compounds were added to the wells. After 24 h of drug exposure, cytotoxicity assays were performed using MTT. Twenty μL of MTT solution (5 mg/mL) was added to the cell cultures. During the following 3 h of incubation mitochondrial dehydrogenase activity of vital cells caused the production of formazan crystals from MTT. Culture medium was aspirated and formazan was dissolved in 100 μL of DMSO. The optical density at 570 nm, which is directly proportional to the number of surviving cells, was measured using microplate reader Uniplan AIFR-01 (Pikon, Moscow, Russia).
4. Conclusions
In summary, a convenient synthetic route towards 4-azido-6-arylcinnolines based on Suzuki coupling, Richter-type cyclization and nucleophilic substitution of activated aromatic bromine atom with azido function was developed. The crucial point in the synthetic sequences is to carry out the Suzuki coupling as the initial step and the introduction of an azido group as the final step. Despite the possibility of chemoselective substitution of the bromine atom at C4 in 4,6-dibromocinnoline with the formation of 4-azido-6-bromocinnoline, the azide moiety at C4 of cinnoline ring was found to be unstable under Suzuki reaction conditions. The synthetic route developed here is functional group tolerant to various substituents on the aryl moiety. All 4-azidocinnolines underwent CuAAC with terminal alkynes providing 4-triazolylcinnolines in moderate to high yields. The reactivity of 4-azidocinnolines in SPAAC was also confirmed. Whereas 4-azidocinnolines display fluorescent properties under excitation at their low wavelength absorption maximum, the corresponding 4-triazolylcinnolines were devoid of any fluorescence, probably due to their non-planar geometry and PET mechanism. Among the series of 4-triazolylcinnolines synthesized the compound 11a bearing a hydroxyalkyl-substituted triazole ring was found to be cytotoxic toward HeLa cells at micromolar concentrations.
Supplementary Materials
The following are available online, copies of 1H, 13C{1H}, and DEPT NMR spectra for all newly synthesized compounds; Cartesian coordinates and energies and isosurfaces of MO for optimized geometries of 7c,f and 11c,f; cif file with X-ray data for compound 11g.
Author Contributions
Conceptualization, N.A.D. and I.A.B.*; methodology, N.A.D.; validation, A.A.V, A.I.G.; formal analysis, I.V.K., A.V.P., I.A.B; investigation, N.S.B., A.I.G., A.A.V., A.M.R., A.A.V., N.I.S., I.V.K., A.V.P., I.A.B, P.V.T.; writing—N.A.D., A.I.G, A.M.R., A.V.P.; writing—review and editing, N.A.D., I.A.B.*; visualization, N.A.D., A.I.G., I.A.B.; supervision, N.A.D.; project administration, N.A.D.; I.A.B.*; funding acquisition, A.A.V.
Funding
This research was funded by Russian Foundation for Basic Research (RFBR), grant number 18-33-01265.
Acknowledgments
We are grateful to all staff members of the Research Park of SPSU, who carried out analysis for current research. The research was carried out by using the equipment of the SPSU Resource Centres: Magnetic Resonance Research Centre, Chemical Analysis and Materials Research Centre, Centre for Optical and Laser Materials Research, Centre for X-ray Diffraction Studies, Chemistry Educational Centre. The authors are grateful to N. Samusik (Stanford University) for the helpful comments and additions to the text.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
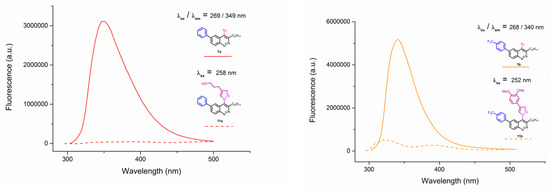
Figure A1.
Emission spectra of 4-azidocinnolines/4-triazolylcinnolnes 7a/11a; 7b/11b; 7e/11e; 7f/11f.
Figure A1.
Emission spectra of 4-azidocinnolines/4-triazolylcinnolnes 7a/11a; 7b/11b; 7e/11e; 7f/11f.
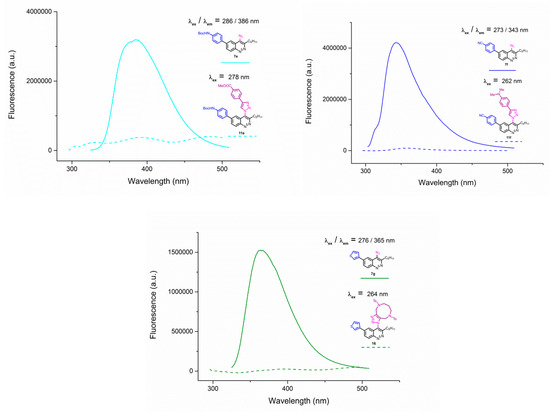
Figure A2.
Emission spectra of 4-azidocinnolines/4-triazolylcinnolnes 7a/11a; 7b/11b; 7e/11e; 7f/11f; 7g/16 solutions in THF, C = 1 × 10−5 mol/L.
Figure A2.
Emission spectra of 4-azidocinnolines/4-triazolylcinnolnes 7a/11a; 7b/11b; 7e/11e; 7f/11f; 7g/16 solutions in THF, C = 1 × 10−5 mol/L.
References
- Vodovozova, E.L. Photoaffinity labeling and its application in structural biology. Biochemisty 2007, 72, 1–20. [Google Scholar] [CrossRef]
- Panov, M.S.; Voskresenska, V.D.; Ryazantsev, M.N.; Tarnovsky, A.N.; Wilson, R.M. 5-azido-2-aminopyridine, a new nitrene/nitrenium ion photoaffinity labeling agent that exhibits reversible intersystem crossing between singlet and triplet nitrenes. J. Am. Chem. Soc. 2013, 135, 19167–19179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, N.; Tomizawa, M.; Casida, J.E. Structural features of azidopyridinyl neonicotinoid probes conferring high affinity and selectivity for mammalian α4β2 and Drosophila nicotinic receptors. J. Med. Chem. 2002, 45, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Polshakov, D.; Rai, S.; Wilson, R.M.; Mack, E.T.; Vogel, M.; Krause, J.A.; Burdzinski, G.; Platz, M.S. Photoaffinity labeling with 8-azidoadenosine and its derivatives: Chemistry of closed and opened adenosine diazaquinodimethanes. Biochemistry 2005, 44, 11241–11253. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Ashwini, N.; Garg, M.; Mohan, C.D.; Fuchs, J.E.; Rangappa, S.; Anusha, S.; Swaroop, T.R.; Rakesh, K.S.; Kanojia, D.; Madan, V.; et al. Synthesis of 1,2-benzisoxazole tethered 1,2,3-triazoles that exhibit anticancer activity in acute myeloid leukemia cell lines by inhibiting histone deacetylases, and inducing p21 and tubulin acetylation. Bioorg. Med. Chem. 2015, 23, 6157–6165. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.; Luo, H.; Liu, H.; Han, X.; Yan, G.; Ding, Z.; et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2014, 24, 799–807. [Google Scholar] [CrossRef]
- Thomas, K.D.; Adhikari, A.V.; Chowdhury, I.H.; Sumesh, E.; Pal, N.K. New quinolin-4-yl-1,2,3-triazoles carrying amides, sulphonamides and amidopiperazines as potential antitubercular agents. Eur. J. Med. Chem. 2011, 46, 2503–2512. [Google Scholar] [CrossRef]
- Tiwari, R.; Miller, P.A.; Chiarelli, L.R.; Mori, G.; Šarkan, M.; Centárová, I.; Cho, S.; Mikušová, K.; Franzblau, S.G.; Oliver, A.G.; et al. Design, syntheses, and anti-TB activity of 1,3-benzothiazinone azide and click chemistry products inspired by BTZ043. ACS Med. Chem. Lett. 2016, 7, 266–270. [Google Scholar] [CrossRef]
- Cserép, G.B.; Herner, A.; Kele, P. Bioorthogonal fluorescent labels: A review on combined forces. Methods Appl. Fluoresc. 2015, 3, 042001. [Google Scholar] [CrossRef] [PubMed]
- Beatty, K.E.; Liu, J.C.; Xie, F.; Dieterich, D.C.; Schuman, E.M.; Wang, Q.; Tirrell, D.A. Fluorescence visualization of newly synthesized proteins in mammalian cells. Angew. Chem. Int. Ed. 2006, 45, 7364–7367. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, K.; Xie, F.; Cash, B.M.; Long, S.; Barnhill, H.N.; Wang, Q. A fluorogenic 1,3-dipolar cycloaddition reaction of 3-azidocoumarins and acetylenes. Org. Lett. 2004, 6, 4603–4606. [Google Scholar] [CrossRef]
- Herner, A.; Estrada Girona, G.; Nikić, I.; Kállay, M.; Lemke, E.A.; Kele, P. New generation of bioorthogonally applicable fluorogenic dyes with visible excitations and large stokes shifts. Bioconjugate Chem. 2014, 25, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Neef, A.B.; Schultz, C. Selective fluorescence labeling of lipids in living cells. Angew. Chem. Int. Ed. 2009, 48, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Zayas, J.; Annoual, M.; Das, J.K.; Felty, Q.; Gonzalez, W.G.; Miksovska, J.; Sharifai, N.; Chiba, A.; Wnuk, S.F. Strain promoted click chemistry of 2- or 8-azidopurine and 5-azidopyrimidine nucleosides and 8-azidoadenosine triphosphate with cyclooctynes. Application to living cell fluorescent imaging. Bioconjugate Chem. 2015, 26, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Brandão, G.C.; Rocha Missias, F.C.; Arantes, L.M.; Soares, L.F.; Roy, K.K.; Doerksen, R.J.; Braga de Oliveira, A.; Pereira, G.R. Antimalarial naphthoquinones. Synthesis via click chemistry, in vitro activity, docking to Pf DHODH and SAR of lapachol-based compounds. Eur. J. Med. Chem. 2018, 145, 191–205. [Google Scholar] [CrossRef]
- Ellanki, A.R.; Islam, A.; Rama, V.S.; Pulipati, R.P.; Rambabu, D.; Rama Krishna, G.; Malla Reddy, C.; Mukkanti, K.; Vanaja, G.R.; Kalle, A.M.; et al. Solvent effect on copper-catalyzed azide–alkyne cycloaddition (CuAAC): Synthesis of novel triazolyl substituted quinolines as potential anticancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 3455–3459. [Google Scholar] [CrossRef]
- Leite, D.I.; de Fontes, F.V.; Bastos, M.M.; Hoelz, L.V.B.; Bianco, M.D.C.A.D.; de Oliveira, A.P.; da Silva, P.B.; da Silva, C.F.; Batista, D.D.G.J.; da Gama, A.N.S.; et al. New 1,2,3-triazole-based analogues of benznidazole for use against Trypanosoma cruzi infection: In vitro and in vivo evaluations. Chem. Biol. Drug Des. 2018, 92, 1670–1682. [Google Scholar] [CrossRef]
- Chopra, R.; Chibale, K.; Singh, K. Pyrimidine-chloroquinoline hybrids: Synthesis and antiplasmodial activity. Eur. J. Med. Chem. 2018, 148, 39–53. [Google Scholar] [CrossRef]
- Holla, B.S.; Mahalinga, M.; Karthikeyan, M.S.; Poojary, B.; Akberali, P.M.; Kumari, N.S. Synthesis, characterization and antimicrobial activity of some substituted 1,2,3-triazoles. Eur. J. Med. Chem. 2005, 40, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Jin, H.; Fan, J.; Flores, H.; Perlmutter, J.S.; Tu, Z. Synthesis of fluorine-containing phosphodiesterase 10A (PDE10A) inhibitors and the in vivo evaluation of F-18 labeled PDE10A PET tracers in rodent and nonhuman primate. J. Med. Chem. 2015, 58, 8584–8600. [Google Scholar] [CrossRef] [PubMed]
- Hamann, A.R.; De Kock, C.; Smith, P.J.; Van Otterlo, W.A.L.; Blackie, M.A.L. Synthesis of novel triazole-linked mefloquine derivatives: Biological evaluation against Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2014, 24, 5466–5469. [Google Scholar] [CrossRef] [PubMed]
- Brooke, D.G.; van Dam, E.M.; Watts, C.K.W.; Khoury, A.; Dziadek, M.A.; Brooks, H.; Graham, L.K.; Flanagan, J.U.; Denny, W.A. Targeting the Warburg Effect in cancer; relationships for 2-arylpyridazinones as inhibitors of the key glycolytic enzyme 6-phosphofructo-2-kinase/2,6-bisphosphatase 3 (PFKFB3). Bioorg. Med. Chem. 2014, 22, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Janosi, T.Z.; Makkai, G.; Kegl, T.; Matyus, P.; Kollar, L.; Erostyak, J. Light-enhanced fluorescence of multi-level cavitands possessing pyridazine upper rim. J. Fluoresc. 2016, 26, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wang, H.; Bartberger, M.D. Accelerating effect of triazolyl and related heteroaryl substituents on SNAr reactions: Evidence of hydrogen-bond stabilized transition states. J. Am. Chem. Soc. 2015, 137, 12261–12268. [Google Scholar] [CrossRef]
- Qian, W.; Winternheimer, D.; Allen, J. A “Click and Activate” approach in one-pot synthesis of a triazolyl-pyridazinone library. Org. Lett. 2011, 13, 1682–1685. [Google Scholar] [CrossRef]
- Qian, W.; Winternheimer, D.; Amegadzie, A.; Allen, J. One-pot synthesis of [1,2,3]triazole-fused pyrazinopyridazindione tricycles by a ‘click and activate’ approach. Tetrahedron Lett. 2012, 53, 271–274. [Google Scholar] [CrossRef]
- Kamiya, S.; Sueyoshi, S.; Miayhara, M.; Yanagimachi, K.; Nakashima, T. Synthesis of 4-azidoquinoline 1-oxides and related compounds. Chem. Pharm. Bull. (Tokyo) 1980, 28, 1485–1490. [Google Scholar] [CrossRef][Green Version]
- Hu, E.; Kunz, R.K.; Rumfelt, S.; Chen, N.; Bürli, R.; Li, C.; Andrews, K.L.; Zhang, J.; Chmait, S.; Kogan, J.; et al. Discovery of potent, selective, and metabolically stable 4-(pyridin-3-yl)cinnolines as novel phosphodiesterase 10A (PDE10A) inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 2262–2265. [Google Scholar] [CrossRef]
- Awad, E.D.; El-Abadelah, M.M.; Matar, S.; Zihlif, M.A.; Naffa, R.G.; Al-Momani, E.Q.; Mubarak, M.S. Synthesis and biological activity of some 3-(4-(substituted)-piperazin-1-yl)cinnolines. Molecules 2011, 17, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Mondal, C.; Halder, A.K.; Adhikari, N.; Jha, T. Structural findings of cinnolines as anti-schizophrenic PDE10A inhibitors through comparative chemometric modeling. Mol. Divers. 2014, 18, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.T.; Jung, J.-W.; Kim, N.-J. Recent advances in the synthesis of biologically active cinnoline, phthalazine and quinoxaline derivatives. Curr. Org. Chem. 2017, 21, 1265–1291. [Google Scholar] [CrossRef]
- Al-Qtaitat, M.A.; El-Abadelah, M.M.; Sabri, S.S.; Matar, S.A.; Hammad, H.M.; Mubarak, M.S. Synthesis, Characterization, and Bioactivity of Novel Bicinnolines Having 1-Piperazinyl Moieties. J. Heterocycl. Chem. 2019, 56, 158–164. [Google Scholar] [CrossRef]
- Mitsumori, T.; Bendikov, M.; Sedó, J.; Wudl, F. Synthesis and properties of novel highly fluorescent pyrrolopyridazine derivatives. Chem. Mater. 2003, 15, 3759–3768. [Google Scholar] [CrossRef]
- Shen, Y.; Shang, Z.; Yang, Y.; Zhu, S.; Qian, X.; Shi, P.; Zheng, J.; Yang, Y. Structurally rigid 9-amino-benzo[c]cinnoliniums make up a class of compact and large stokes-shift fluorescent dyes for cell-based imaging applications. J. Org. Chem. 2015, 80, 5906–5911. [Google Scholar] [CrossRef]
- Galenko, E.E.; Galenko, A.V.; Khlebnikov, A.F.; Novikov, M.S.; Shakirova, J.R. Synthesis and intramolecular azo coupling of 4-diazopyrrole-2-carboxylates: Selective approach to benzo and hetero [c]-fused 6H-pyrrolo[3,4-c]pyridazine-5-carboxylates. J. Org. Chem. 2016, 81, 8495–8507. [Google Scholar] [CrossRef]
- Mayakrishnan, S.; Arun, Y.; Balachandran, C.; Emi, N.; Muralidharan, D.; Perumal, P.T. Synthesis of cinnolines via Rh(III)-catalysed dehydrogenative C–H/N–H functionalization: Aggregation induced emission and cell imaging. Org. Biomol. Chem. 2016, 14, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, J.-C.; Chen, L.-D.; Li, J.; An, Y.; Lü, C.-W.; Tian, Y.-Q. Efficient synthesis of diethyl benzo[c]cinoline-3,8-dicarboxylate for fluorescence quenching materials. New J. Chem. 2017, 41, 2786–2792. [Google Scholar] [CrossRef]
- Danilkina, N.A.; Vlasov, P.S.; Vodianik, S.M.; Kruchinin, A.A.; Vlasov, Y.G.; Balova, I.A. Synthesis and chemosensing properties of cinnoline-containing poly(arylene ethynylene)s. Beilstein J. Org. Chem. 2015, 11, 373–384. [Google Scholar] [CrossRef]
- Pinho e Melo, T.M.V.D. Synthesis of Azides. In Organic Azides; Bräse, S., Banert, K., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2010; Volume 75, pp. 53–94. [Google Scholar]
- De Souza, M.V.N.; Pais, K.C.; Kaiser, C.R.; Peralta, M.A.; de, L.; Ferreira, M.; Lourenço, M.C.S. Synthesis and in vitro antitubercular activity of a series of quinoline derivatives. Bioorg. Med. Chem. 2009, 17, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Guantai, E.M.; Ncokazi, K.; Egan, T.J.; Gut, J.; Rosenthal, P.J.; Smith, P.J.; Chibale, K. Design, synthesis and in vitro antimalarial evaluation of triazole-linked chalcone and dienone hybrid compounds. Bioorg. Med. Chem. 2010, 18, 8243–8256. [Google Scholar] [CrossRef] [PubMed]
- Mantoani, S.; Chierrito, T.; Vilela, A.; Cardoso, C.; Martínez, A.; Carvalho, I. Novel Triazole-Quinoline Derivatives as Selective Dual Binding Site Acetylcholinesterase Inhibitors. Molecules 2016, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Gao, Y.; Kang, D.; Huang, B.; Huo, Z.; Liu, H.; Poongavanam, V.; Zhan, P.; Liu, X. Design, synthesis and biological evaluation of tacrine-1,2,3-triazole derivatives as potent cholinesterase inhibitors. MedChemComm 2018, 9, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Sumangala, V.; Poojary, B.; Chidananda, N.; Fernandes, J.; Kumari, N.S. Synthesis and antimicrobial activity of 1,2,3-triazoles containing quinoline moiety. Arch. Pharmacal Res. 2010, 33, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Abass, M.; Hassan, M.M. Chemistry of substituted quinolinones. v. synthesis and use of quinolinylphosphazenes in amination of 8-methylquinoline. Phosphorus Sulfur Silicon Relat. Elem. 2000, 167, 275–288. [Google Scholar] [CrossRef]
- Stadlbauer, W. Methoden zur Darstellung von 4-Azido-2(1H)-chinolonen. Monatsh. Chem. 1986, 117, 1305–1323. [Google Scholar] [CrossRef]
- Aizikovich, A.; Kuznetsov, V.; Gorohovsky, S.; Levy, A.; Meir, S.; Byk, G.; Gellerman, G. A new application of diphenylphosphorylazide (DPPA) reagent: convenient transformations of quinolin-4-one, pyridin-4-one and quinazolin-4-one derivatives into the 4-azido and 4-amino counterparts. Tetrahedron Lett. 2004, 45, 4241–4243. [Google Scholar] [CrossRef]
- Vinogradova, O.V.; Balova, I.A. Methods for the synthesis of cinnolines (review). Chem. Heterocycl. Compd. 2008, 44, 501–522. [Google Scholar] [CrossRef]
- Mathew, T.; Papp, A.Á.; Paknia, F.; Fustero, S.; Surya Prakash, G.K. Benzodiazines: Recent synthetic advances. Chem. Soc. Rev. 2017, 46, 3060–3094. [Google Scholar] [CrossRef]
- Richter, V. Ueber Cinnolinderivate. Ber. Dtsch. Chem. Ges. 1883, 16, 677–683. [Google Scholar] [CrossRef]
- Vasilevsky, S.F.; Tretyakov, E.V. Cinnolines and pyrazolopyridazines.—Novel synthetic and mechanistic aspects of the Richter reaction. Liebigs Ann. 1995, 775–779. [Google Scholar] [CrossRef]
- Bräse, S.; Dahmen, S.; Heuts, J. Solid-phase synthesis of substituted cinnolines by a Richter type cleavage protocol. Tetrahedron Lett. 1999, 40, 6201–6203. [Google Scholar] [CrossRef]
- Goeminne, A.; Scammells, P.J.; Devine, S.M.; Flynn, B.L. Richter cyclization and co-cyclization reactions of triazene-masked diazonium ions. Tetrahedron Lett. 2010, 51, 6882–6885. [Google Scholar] [CrossRef]
- Vinogradova, O.V.; Sorokoumov, V.N.; Balova, I.A. A short route to 3-alkynyl-4-bromo(chloro)cinnolines by Richter-type cyclization of ortho-(dodeca-1,3-diynyl)aryltriaz-1-enes. Tetrahedron Lett. 2009, 50, 6358–6360. [Google Scholar] [CrossRef]
- Holzer, W. On the Synthesis and Reactivity of 4-(oxiran-2-ylmethoxy)cinnoline: Targeting a cinnoline analogue of propranolol. Sci. Pharm. 2008, 76, 19–32. [Google Scholar] [CrossRef]
- Holzer, W.; Eller, G.A.; Schönberger, S. On the tautomerism of cinnolin-4-ol, cinnolin-4-thiol, and cinnolin-4-amine. Heterocycles 2008, 75, 77–86. [Google Scholar] [CrossRef]
- Hoegenauer, K.; Soldermann, N.; Hebach, C.; Hollingworth, G.J.; Lewis, I.; von Matt, A.; Smith, A.B.; Wolf, R.M.; Wilcken, R.; Haasen, D.; et al. Discovery of novel pyrrolidineoxy-substituted heteroaromatics as potent and selective PI3K delta inhibitors with improved physicochemical properties. Bioorg. Med. Chem. Lett. 2016, 26, 5657–5662. [Google Scholar] [CrossRef]
- Danilkina, N.A.; Gorbunova, E.G.; Sorokoumov, V.N.; Balova, I.A. Study of cyclyzation of o-(1-Alkynyl)- and o-(1,3-Butadiynyl)aryltriazenes under the action of acids. Russ. J. Org. Chem. 2012, 48, 1424–1434. [Google Scholar] [CrossRef]
- Satyanarayana, M.; Feng, W.; Cheng, L.; Liu, A.A.; Tsai, Y.-C.; Liu, L.F.; LaVoie, E.J. Syntheses and biological evaluation of topoisomerase I-targeting agents related to 11-[2-(N,N-dimethylamino)ethyl]-2,3-dimethoxy-8,9-methylenedioxy-11H-isoquino[4,3-c]cinnolin-12-one (ARC-31). Bioorg. Med. Chem. 2008, 16, 7824–7831. [Google Scholar] [CrossRef]
- Devine, W.; Woodring, J.L.; Swaminathan, U.; Amata, E.; Patel, G.; Erath, J.; Roncal, N.E.; Lee, P.J.; Leed, S.E.; Rodriguez, A.; et al. Protozoan parasite growth inhibitors discovered by cross-screening yield potent scaffolds for lead discovery. J. Med. Chem. 2015, 58, 5522–5537. [Google Scholar] [CrossRef] [PubMed]
- Barlaam, B.; Cadogan, E.; Campbell, A.; Colclough, N.; Dishington, A.; Durant, S.; Goldberg, K.; Hassall, L.A.; Hughes, G.D.; MacFaul, P.A.; et al. Discovery of a series of 3-cinnoline carboxamides as orally bioavailable, highly potent, and selective ATM inhibitors. ACS Med. Chem. Lett. 2018, 9, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Dakin, L.A.; Del Valle, D.J.; Bruce Diebold, R.; Drew, L.; Gero, T.W.; Ogoe, C.A.; Omer, C.A.; Repik, G.; Thakur, K.; et al. 3-Amido-4-anilinocinnolines as a novel class of CSF-1R inhibitor. Bioorg. Med. Chem. Lett. 2011, 21, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, M.J.; Mphahlele, M.M. Direct one-pot synthesis of primary 4-amino-2,3-diarylquinolines via Suzuki-Miyaura cross-coupling of 2-aryl-4-azido-3-iodoquinolines with arylboronic acids. Molecules 2011, 16, 8958–8972. [Google Scholar] [CrossRef] [PubMed]
- Hisaki, I.; Senga, H.; Shigemitsu, H.; Tohnai, N.; Miyata, M. Construction of 1D π-stacked superstructures with inclusion channels through symmetry-decreasing crystallization of discotic molecules of C3 cymmetry. Chem. Eur. J. 2011, 17, 14348–14353. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Krasnova, L.B.; Iwasaki, M.; Fokin, V.V. Cu-Catalyzed azide-alkyne aycloaddition: preparation of tris((1-benzyl-1H-1,2,3-triazolyl)methyl)amine. Org. Synth. 2011, 88, 238–246. [Google Scholar]
- Ramachary, D.B.; Shashank, A.B.; Karthik, S. An organocatalytic azide-aldehyde [3 + 2] cycloaddition: high-yielding regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles. Angew. Chem. Int. Ed. 2014, 53, 10420–10424. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Mitsuda, N.; Kashiwagi, T.; Igawa, K.; Tomooka, K. Heteroatom-embedded Medium-Sized Cycloalkynes: Concise Synthesis, Structural Analysis, and Reactions. Angew. Chem. Int. Ed. 2015, 54, 1190–1194. [Google Scholar] [CrossRef]
- Batat, P.; Vives, G.; Bofinger, R.; Chang, R.-W.; Kauffmann, B.; Oda, R.; Jonusauskas, G.; McClenaghan, N.D. Dynamics of ion-regulated photoinduced electron transfer in BODIPY-BAPTA conjugates. Photochem. Photobiol. Sci. 2012, 11, 1666–1674. [Google Scholar] [CrossRef]
- Meisner, Q.J.; Accardo, J.V.; Hu, G.; Clark, R.J.; Jiang, D.E.; Zhu, L. Fluorescence of hydroxyphenyl-substituted “click” triazoles. J. Phys. Chem. A 2018, 122, 2956–2973. [Google Scholar] [CrossRef]
- Fra, L.; Millán, A.; Souto, J.A.; Muñiz, K. Indole synthesis based on a modified koser reagent. Angew. Chem. Int. Ed. 2014, 53, 7349–7353. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro; Version 1.171.39.35a; Rigaku Oxford Diffraction; Rigaku Corporation: Tokyo, Japan, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision D.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cancer Cell Culture. In Cancer Cell Culture: Methods and Protocols, Second Edition, Methods in Molecular Biology; Langdon, S.P., Ed.; Humana Press: Totowa, NJ, USA, 2003; Volume 88, pp. 237–245. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).