1. Introduction
Vitamin D or calciferols are substances from the group of secosteroids. The two bioequivalent forms of calciferol represent vitamin D
2 (ergocalciferol) and vitamin D
3 (cholecalciferol) [
1]. Their main functions are the regulation of calcium homeostasis or gene expression in the parathyroid gland [
2]. In addition, they trigger the expression of genes that regulate the immune system and are responsible for the differentiation of epidermal and hematopoietic cells. A vitamin D deficiency can lead to mineralization disorders of the skeleton (rickets) [
3]. Cholecalciferol is mainly obtained by exposure of the skin to UV-B radiation from sunlight. The B-ring of the sterane backbone of provitamin D
3 is photolytically cleaved at position C9/C10 to form previtamin D
3. Previtamin D
3 is thermodynamically unstable and isomerizes to the more stable form named vitamin D
3. The reaction of 7-dehydrocholesterol to previtamin D
3 is induced in the wavelength range of 280–320 nm [
4]. Cutaneous synthesis can be influenced by various molecules that also absorb radiation (e.g., melanins, proteins, sunscreens, or clothing) [
4,
5,
6]. In an analogous way, ergocalciferol is formed by the interaction of UV-B radiation with ergosterol. Due to the ability of endogenous biosynthesis, the D-vitamin terminology seems to be old-fashioned, and assignment as (pro)-hormones would be more appropriate [
7]. Ergocalciferol and cholecalciferol are not the main biological active metabolites in the organism. Consequently, a transformation occurs. This takes place at different regulation levels. Cholecalciferol is transported via the blood into the liver linked to vitamin D binding protein (DBP). There it gets converted to 25-hydroxycholecalciferol (calcidiol), the major circulating form of vitamin D, which is used to define nutritional vitamin D status. Bound to DBP, calcidiol is transported via the blood to the kidneys where a further hydroxylation occurs. The final activating ligand for the vitamin D receptor is 1α,25-dihydroxycholecalciferol (calcitriol) [
8]. The metabolism from vitamin D to calcitriol is regulated by enzymes of the cytochrome-P450-superfamily. This especially concerns the enzyme 1α-hydroxylase (1α-OHase) [
5]. While cyclic adenosine monophosphate (cAMP) induces the transcription of the enzyme, phosphate and calcium ions as well as calcitriol inhibit them. In addition, regulation via parathyroid hormone (PTH), a hormone that is responsible for calcium homeostasis, is also involved, illustrating the complexity of the whole regulation process. At a low serum calcium level, PTH is secreted by the parathyroid gland. It is taken up by PTH receptors and activates the adenylate cyclase and thus the transcription of 1α-OHase. Furthermore, a calcium sensor protein affects the activity of 1α-OHase [
9].
Vitamin D
2 is mainly taken up with food and present in mushrooms and yeasts. Vitamin D
3-rich foods are, for example, egg yolk or fish with high lipid contents (herring, mackerel) [
10]. However, people with an increased risk of vitamin D deficiency are those with inadequate sun exposure, impaired vitamin D intake or absorption [
11]. According to the German Society for Nutrition (DGE), intake of vitamin D supplements is not sufficient to reach the estimate of adequate intake in the absence of endogenous synthesis, which will provide the desired supply of at least 50 nmol/L 25-hydroxyvitamin D (serum concentration). The difference must be therefore covered by enhanced endogenous synthesis or by the intake of vitamin D supplements [
12]. To gain the advantages of a drug or dietary supplement, absorption, distribution, metabolism, and elimination of the active substance has to be taken into account. Metabolism is an essential component in pharmacokinetics because the excess of a drug needs to be excreted. The conversion of a bioactive compound to a metabolite of different bioactivity significantly influences its effects and sustainability. Phase-I and phase-II metabolism are crucial steps in this process. While phase-I metabolism includes oxidative, reductive, and hydrolytic reactions, phase-II metabolism involves the formation of bonds between the drug and small hydrophilic molecules (conjugation with e.g., glutathione, sulfate, glucuronic acid). The final step of this entire process is elimination/excretion. The hydrophilic molecule is excreted from the organism via different ways [
13].
Due to a high prevalence of vitamin D deficiency in the Northern European population, an intake of vitamin D supplements is conceivable. To ensure goal-oriented and cost-effective research and development, it is therefore of certain importance to elucidate the pharmacokinetics and metabolism of potentially new vitamin D preparations or vitamin D-enriched food matrices at an early stage [
14,
15]. Conventional metabolism studies are based either on in vitro cell culture experiments, usually using hepatic or kidney cells, or cell extracts [
16]. More comprehensively, metabolism is tested with the help of in vivo tests, following interventions (in animal or even human experiments) with the application of foods or drug preparations. With regard to Article 4 of the European Commission Directive 2010/63/EU on the protection of animals used for scientific purposes, it is intended to avoid, reduce, or improve procedures for animals used in animal studies [
17]. Promisingly, the application of electrochemistry directly coupled to mass spectrometry (EC-MS) could be a tool for simulating the endogenous metabolism of vitamin D or yielding metabolites (for follow-up analyses).
EC-MS represents a relatively new approach that allows investigations of endogenous oxidative processes in the organism by simulation. In the past there were many studies that used EC-MS for metabolomics, proteomics, and genomics approaches, as well as in fields of environmental analysis, forensics, and toxicology [
18,
19,
20]. It can be assumed that the approach is capable of mimicking selective redox processes of the cytochrome-P450 enzyme family (CYP). For example, aliphatic, allylic, aromatic, and benzylic hydroxylations,
N-,
O-, and
S-dealkylations,
N- and
S-oxide formation, as well as dehydrogenations have been successfully simulated [
19]. Established methods for in vitro simulations are based on the use of CYP-enzymes of the liver and thus, on animal tissues or animal experiments. In these approaches, reproducibility is limited, because metabolism is dependent on the expression levels and activity of CYP450 enzymes. However, while the most important metabolites have been known for decades, enzyme promiscuity might also lead to further, less studied metabolites [
21]. Other disadvantages of the traditional methods represent the enormous expenditure of time and associated high costs, which usually occur when carrying out intervention studies [
19]. Isolating compounds in appropriate yields from in vivo sample material is laborious and might raise ethical issues.
Therefore, the aim of this study was to investigate the electrochemical behavior of the two D vitamers ergocalciferol and cholecalciferol and to investigate whether it is possible to evaluate phase-I and phase-II metabolism of these essential molecules or if the results are different to the metabolites described in the literature. There are also still a number of metabolites that have not yet been fully elucidated in vivo, as matrix and concentration might have been too low for a comprehensive study. A different approach for obtaining metabolites is evaluated.
3. Materials and Methods
3.1. Chemicals and Materials
Cholecalciferol, ergocalciferol, 25-hydroxyergocalciferol, 1α,25-dihydroxyergocalciferol, and ammonium formate were purchased from Sigma-Aldrich GmbH (Steinheim, Germany). The compound 24,25-dihydroxyergocalciferol was purchased from IsoSciences LLC (Ambler, PA, USA). Methanol and acetonitrile were purchased from Carl Roth GmbH & Co. KG (Karlsruhe, Germany). Formic acid was purchased from VWR International GmbH (Darmstadt, Germany) and D(+)-glucuronic acid sodium salt monohydrate was purchased from Merck Chemicals GmbH (Darmstadt, Germany). All of the chemicals were used at the highest quality available. Water was purified before utilization via the Direct-Q 3 UV-R system (Merck Chemicals GmbH, Darmstadt, Germany).
3.2. Electrochemical Oxidation of Vitamin D by Means of Electrochemistry Directly Coupled to Electrospray Ionization Mass Spectrometry (EC-ESI-MS)
The electrochemical oxidation of vitamin D was performed using an electrochemical system (ROXY™, Antec Leyden B.V., Leiden, The Netherlands), which was equipped with a preparative thin-layer cell (µ-PrepCell, Antec Leyden B.V., Leiden, The Netherlands). The thin-layer cell consisted of a boron-doped diamond working electrode, a titanium counter electrode, and a palladium/hydrogen reference electrode. The electrochemical potential was controlled using a ROXY™ potentiostat (Antec Leyden B.V., Leiden, The Netherlands). A schematic of the instrumental setup is shown in
Figure 9.
A solution containing 100 µM of cholecalciferol or ergocalciferol in methanol/water (90/10, v/v) and 20 mM formic acid was injected into the electrochemical system using an external syringe pump with a set flow rate of 10 µL/min. Moreover, the solvents ethanol (ethanol/water 80/20, v/v) and acetonitrile (acetonitrile/water 50/50, v/v) were tested. Four different solutions of varying pH values were prepared. The solutions were set to pH 2.0, pH 6.0, pH 7.4, and pH 8.0 (with diluted hydrochloric acid and ammonium hydroxide, respectively) to simulate the compartments of the stomach, intestine, blood, and bile. The total volume of µ-PrepCell (depending on an effective spacer thickness of 150 µm) was 11 µL. The temperature of the electrochemical cell during analysis was 23 °C. First, scan mode was applied to determine the electrochemical potential where the conversion rate was the highest (0.0 V to 3.0 V in 0.3 V steps for ergocalciferol and in 0.5 V steps for cholecalciferol). Afterwards, a constant potential was applied. The detection of oxidation products was performed with an ESI-MS ion trap mass analyzer in positive ion mode (amaZon speed ETD, Bruker Daltonik GmbH, Bremen, Germany), with the following mass spectrometer settings: ion spray voltage: 4.5 kV; ion source heater: 350 °C; source gas: 55 psi.
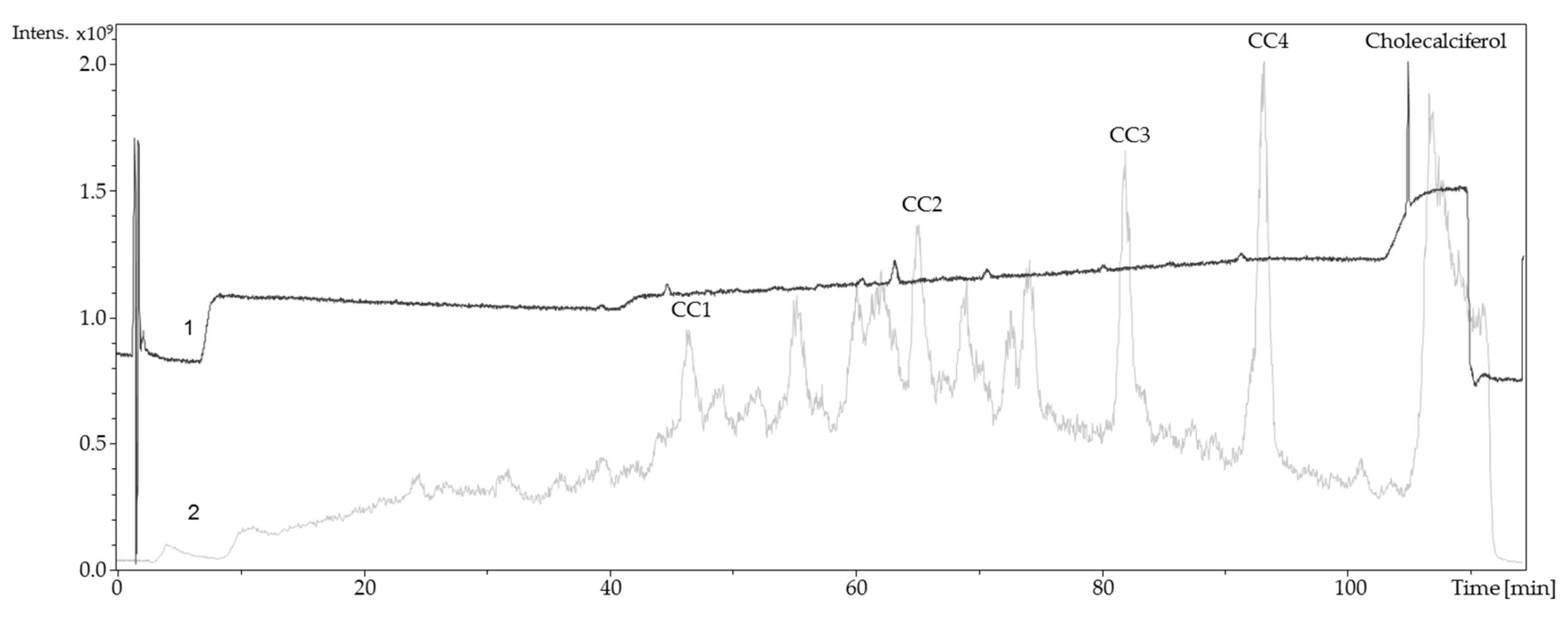
3.3. Identification of Oxidized Metabolites via LC-ESI-MS/DAD
For the determination of the type of oxidized vitamin D derivatives that were generated in the synthesis cell, the solution containing the potentially oxidized species was measured directly with LC-ESI-MS. Chromatographic separation was performed using a Dionex UltiMate™ 3000 ultra-high performance liquid chromatography (UHPLC) system (Thermo Fisher Scientific Inc., Waltham, MA, USA) equipped with a reversed-phase HPLC column (Phenomenex Kinetex
® 2.6 µm RP 18 100 Å, 150 × 2.1 mm) and a Kinetex
® C18 security guard column (Phenomenex Ltd. Deutschland, Aschaffenburg, Germany) using a constant flow of 200 µL/min. Mass spectrometric detection was performed by an ESI-MS ion trap mass analyzer (amaZon speed ETD, Bruker Daltonik GmbH, Bremen, Germany), recording mass spectra in positive ion mode. The injection volume was 5 µL, the column oven temperature was set to 30 °C, and the autosampler was kept at 10 °C. The reversed-phase chromatographic method consisted of a mobile phase system adapted from an existing method with some modifications for the optimized separation of oxidized bile acids [
28]. The mobile phase A was water and B was acetonitrile/methanol (3/1,
v/
v), both containing 0.1% formic acid. The gradient elution started with 70% A for 4 min and then linearly decreased to 35% A within 1 min, which was kept constant for 33 min. Afterwards, composition was decreased to 30% A in 1 min. After a linear decrease to 15% A within 51 min, the percentage of A remained constant at 15% for 10 min. In 1 min, the percentage of A decreased to 0% and held for 5 min. Composition was brought back to an initial ratio of 70% A within 1 min, followed by 7 min of re-equilibration. The LC-MS system was controlled by Bruker Compass HyStar 3.2 (Bruker Daltonik GmbH, Bremen, Germany). Potentially generated oxidation products were identified via retention time, mass spectra and UV/VIS-spectra in comparison to commercially available vitamin D derivatives. For this purpose, solutions of 25-hydroxyergocalciferol, 1α,25-dihydroxyergocalciferol, and 24,25-dihydroxyergocalciferol (each at 0.04 mg/mL in methanol) were injected and analyzed with mass spectrometric and photometric detection.
3.4. Investigation of Adduct Formation of Vitamin D and Glucuronic Acid Using EC-ESI-MS
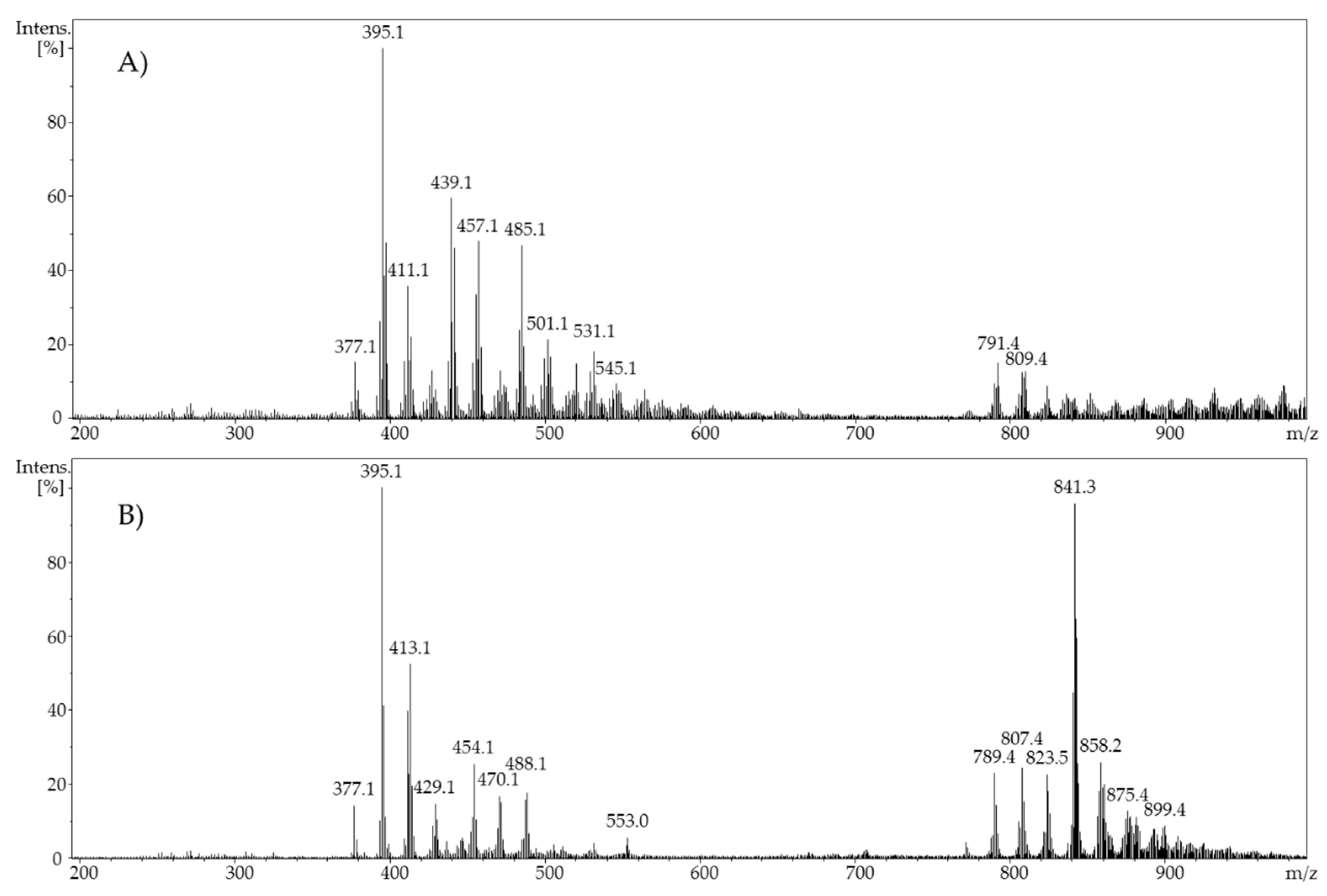
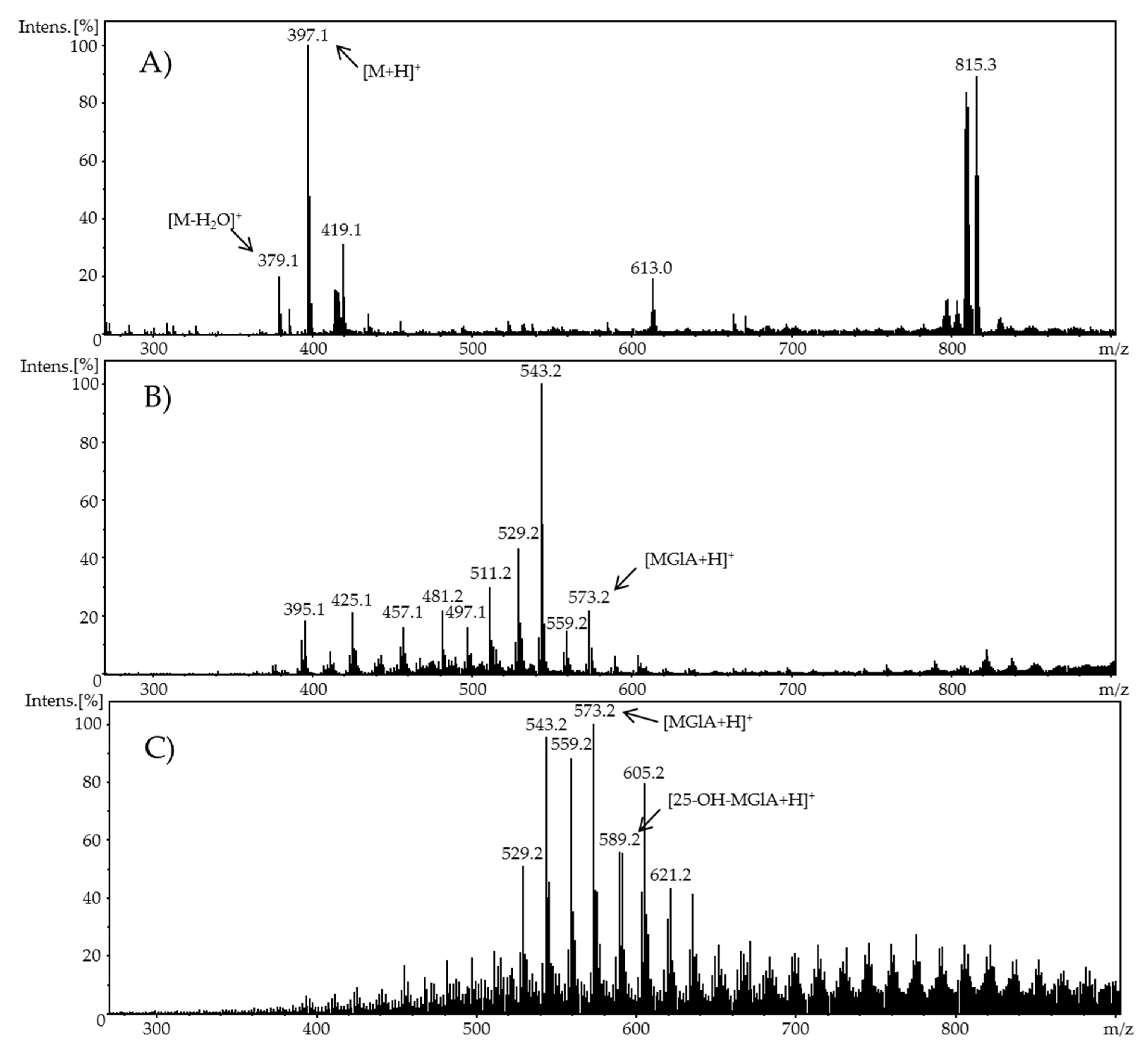
Phase-II metabolism was simulated by the adduct formation of ergocalciferol or cholecalciferol, respectively, and glucuronic acid as an example of phase-II conjugation. For this purpose, a second flow system containing a glucuronic acid solution with a concentration of 1 mM in methanol was added to the oxidized vitamin D solution. The ergocalciferol solution contained 100 µM ergocalciferol in methanol/water (90/10, v/v) and had a pH value of 7.4. Adjusted potentials were either 1.8 V and 3.0 V (ergocalciferol) or 2.0 V and 3.0 V (cholecalciferol). The flow rate of both syringe pumps (glucuronic acid and vitamer) was set to 20 µL/min. The temperature of the electrochemical cell during oxidation was 23 °C. The ratio of both analytes in the reaction coil was 1:1. After a reaction period in a 100 µL reaction coil and an incubation duration of 5 min, the mixture was infused directly into an ESI-MS ion trap mass analyzer (amaZon speed ETD, Bruker Daltonik GmbH, Bremen, Germany) in positive ion mode with the following settings: ion spray voltage: 4.5 kV; ion source heater: 350 °C; source gas: 55 psi.
4. Conclusions
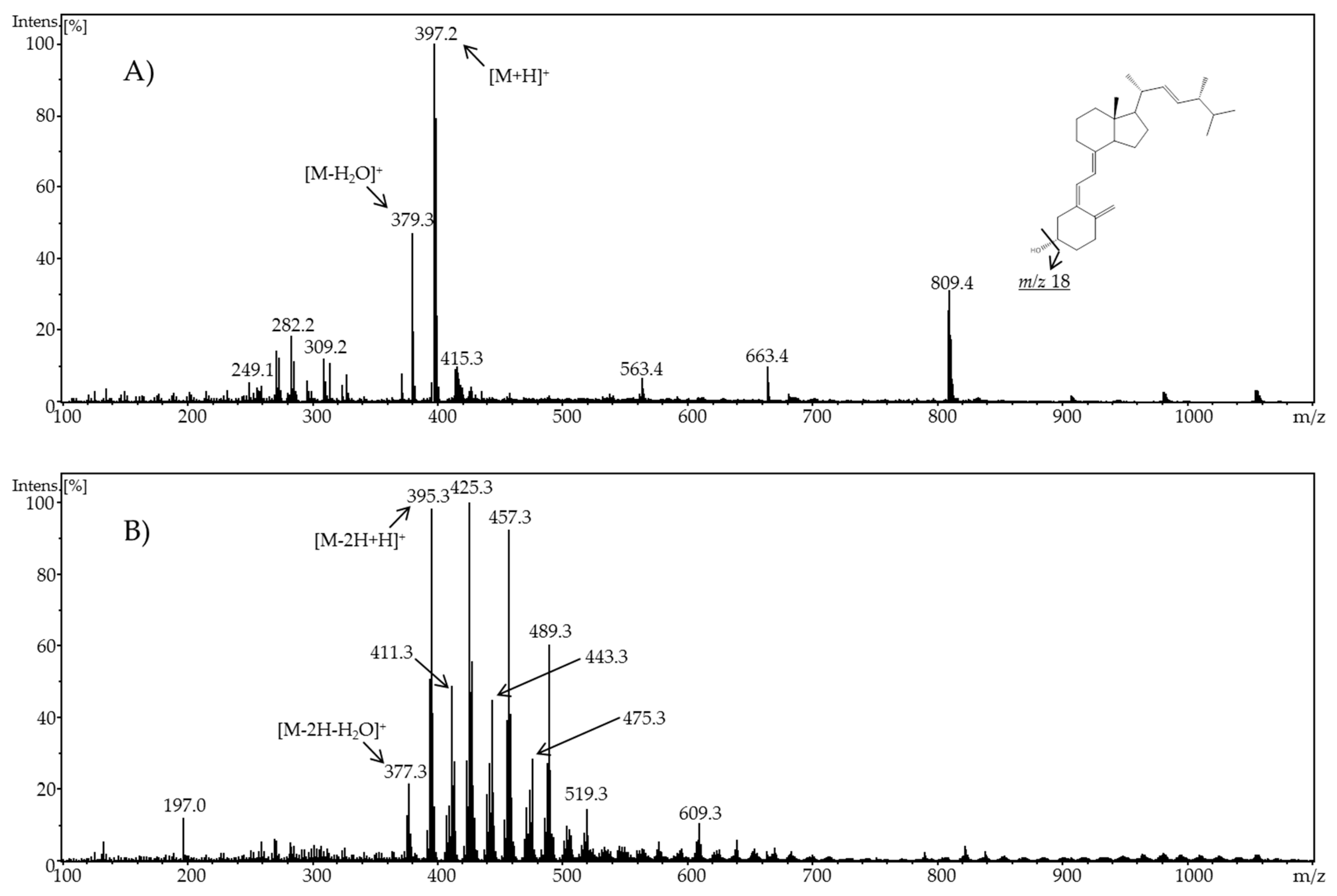
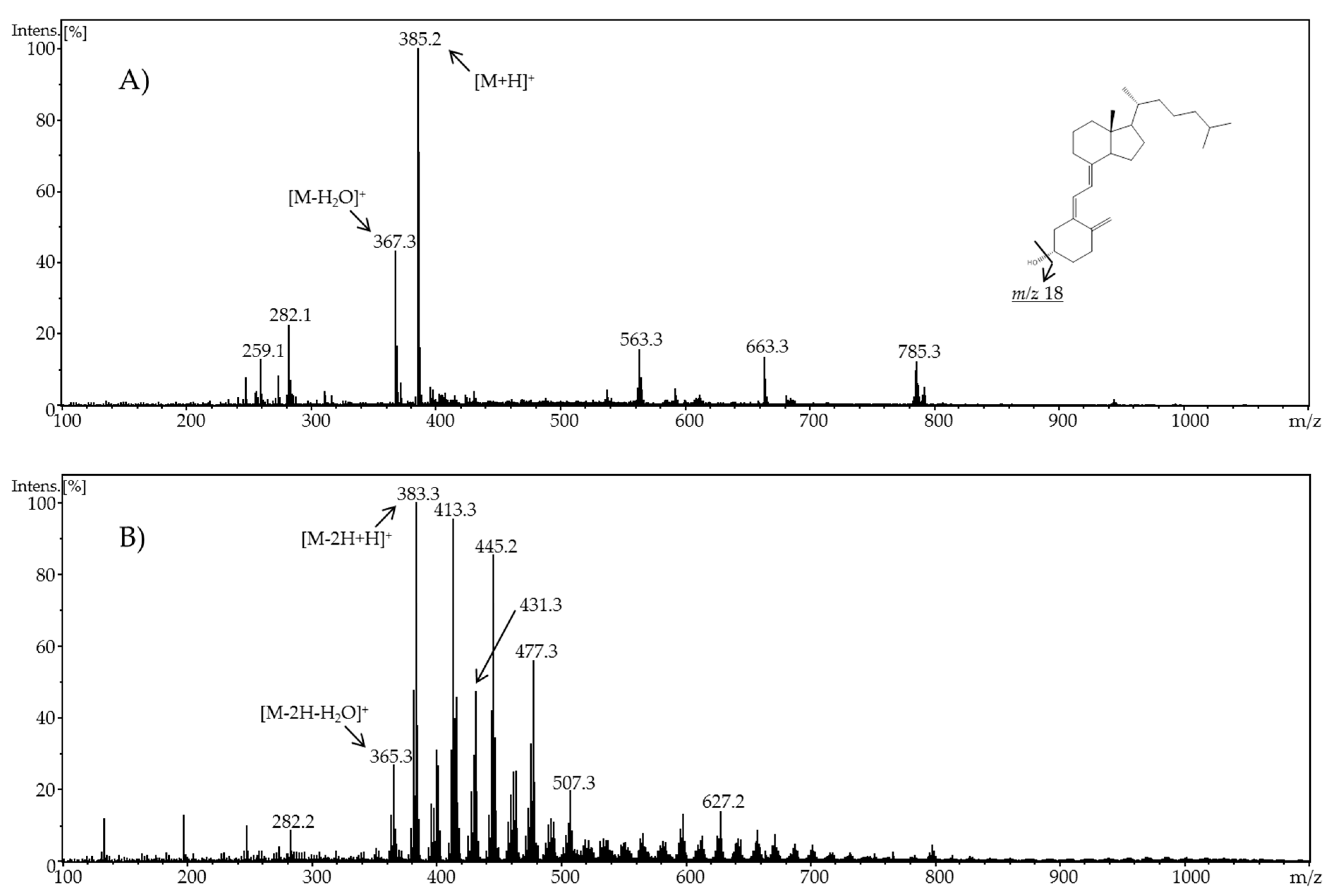
In the present work, the electrochemical behavior of cholecalciferol and ergocalciferol was investigated. Thereby, it was found that both metabolites exhibit a similar electrochemical behavior. For ergocalciferol, a potential of 1.8 V and for cholecalciferol a potential of 2.0 V led to the highest number of oxidation products (metabolites) and an almost quantitative conversion.
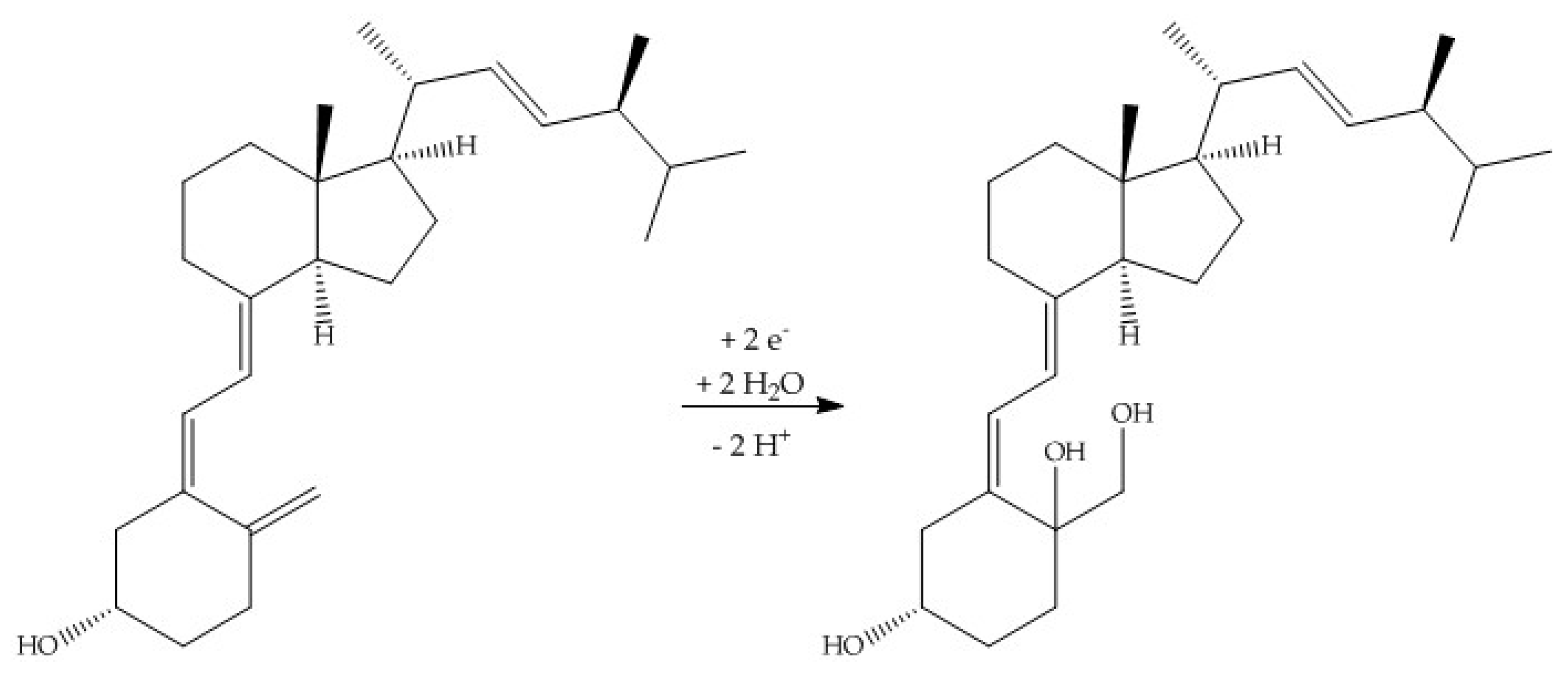
When using methanol or ethanol as part of the solvent, methoxy or ethoxy groups, respectively, were generated. The characterization of the oxidation products should exhibit to which extent the metabolites of the secosteroids can be yielded electrochemically under these conditions. A comparison of retention times, UV signals, and mass spectra indicated that ergocalciferol and cholecalciferol had nearly identical oxidation behaviors. It can be concluded that no reaction took place at the double bond at C22–C23 of ergocalciferol. However, was be determined that the characteristic metabolites such as 25-hydroxyergocalciferol or 1α,25-dihydroxyergocalciferol were not the main products during the electrochemical oxidation. A complete metabolite profile was found. Consequently, it was possible to simulate dehydrations, hydroxylations, and methoxylations or ethoxylations, respectively. Therefore, this approach could be used for the generation of further metabolites that might be of lower concentration in vivo or in vitro. To simulate phase-II metabolism, a binding study with glucuronic acid was performed. Both the educts and the generated oxidation products bind to glucuronic acid. It was possible to generate products that are known from in vivo studies. A detailed clarification of the resulting chemical structures of the oxidation products and adducts was not possible at this time. However, the direct coupling to mass spectrometry and the use of NMR spectroscopy allowed the suggestion of tentative structures. In the future, an enrichment of the oxidation products is recommended so that there is enough yield for further experiments like 2D-NMR.
EC-MS offers numerous advantages. First and foremost, it should be mentioned that it can be a substitute for animal-based studies. Furthermore, in direct comparison to traditional methods, EC-MS requires less time and lower costs and underlies a more precise control of the experimental conditions. It seems to be possible to simulate different oxidation conditions, leading to further metabolites that can be compared to the in vivo formation mechanisms (e.g., with regard to their electrochemical redox potential value).
With regard to food or nutrition applications, this study might provide information for e.g., vitamin D-fortified food products. As vitamin D is added to corresponding food items as a preparation or pure compound(s), the newly identified oxidation products can be used for evaluating the stability of D vitamers under the condition of a “non-original matrix” or presence in complex matrices, in general.
In addition, an EC-MS approach offers the possibility of detecting reactive metabolites and allows targeted follow-up reactions (adduct formation or phase-II metabolism). For example, it could be possible to detect and identify reactive metabolites which would react further in vivo and/or be bound to other molecules that are present. Lohmann and Karst successfully simulated the metabolism of reactive metabolites like amodiaquine [
29]. Furthermore, it was possible to verify the metabolism of tetrazepam based on electrochemical simulation [
16]. All in all, the approach could be a promising tool to support animal studies for the investigation of phase-I and phase-II metabolism.