Investigation of the Binding Affinity of a Broad Array of l-Fucosides with Six Fucose-Specific Lectins of Bacterial and Fungal Origin
Abstract
1. Introduction
2. Results and Discussion
3. Experiment
3.1. General Methods
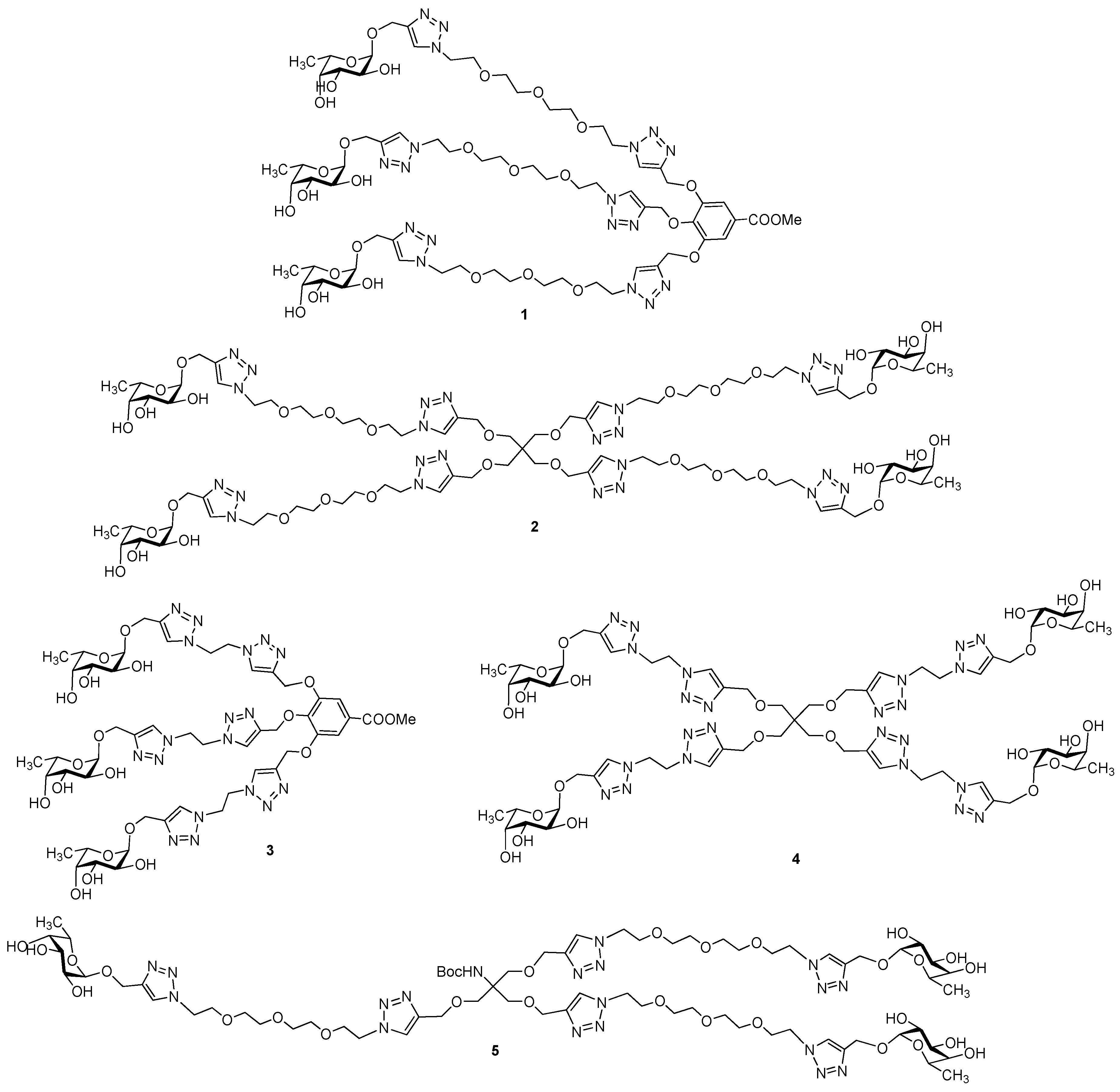
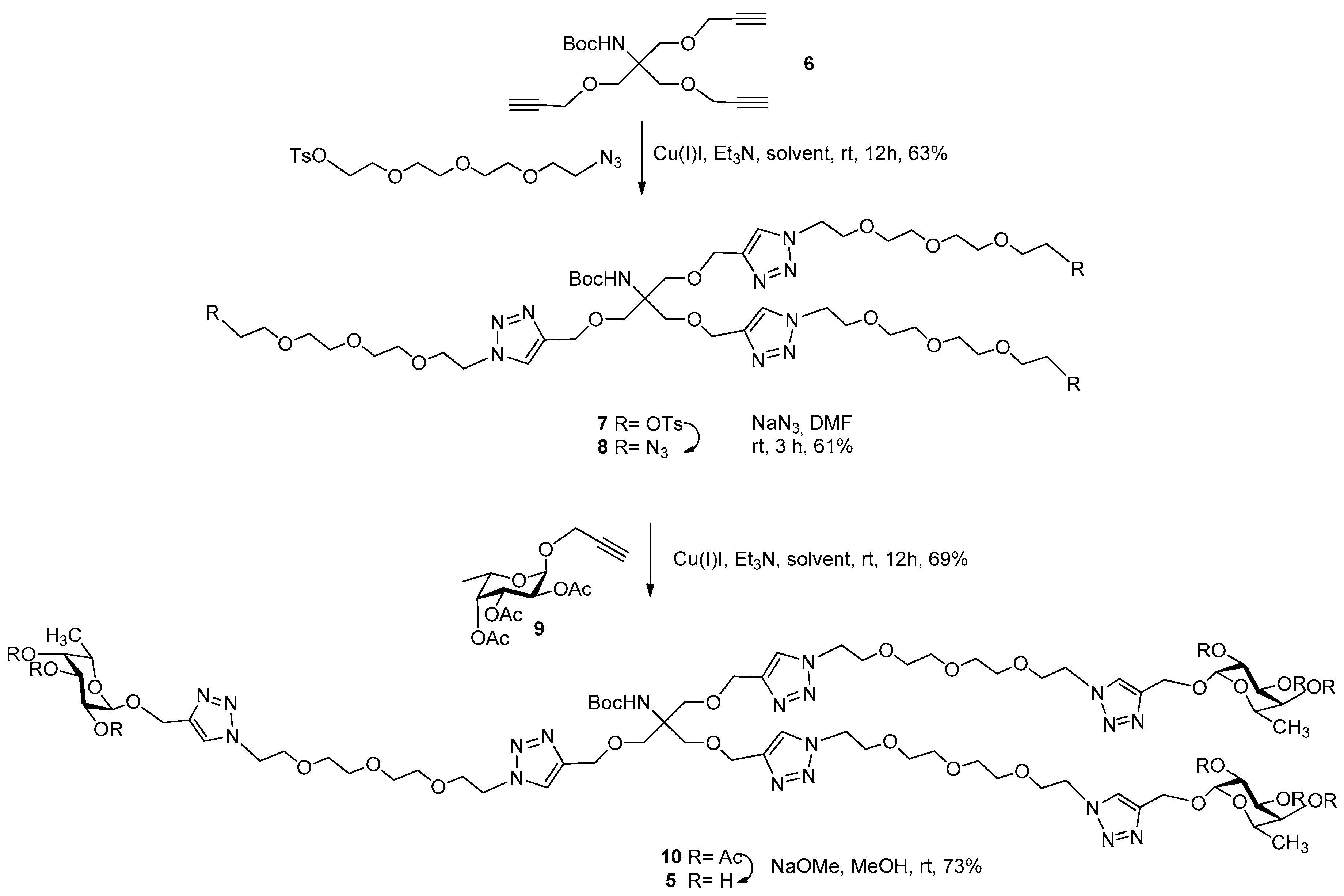
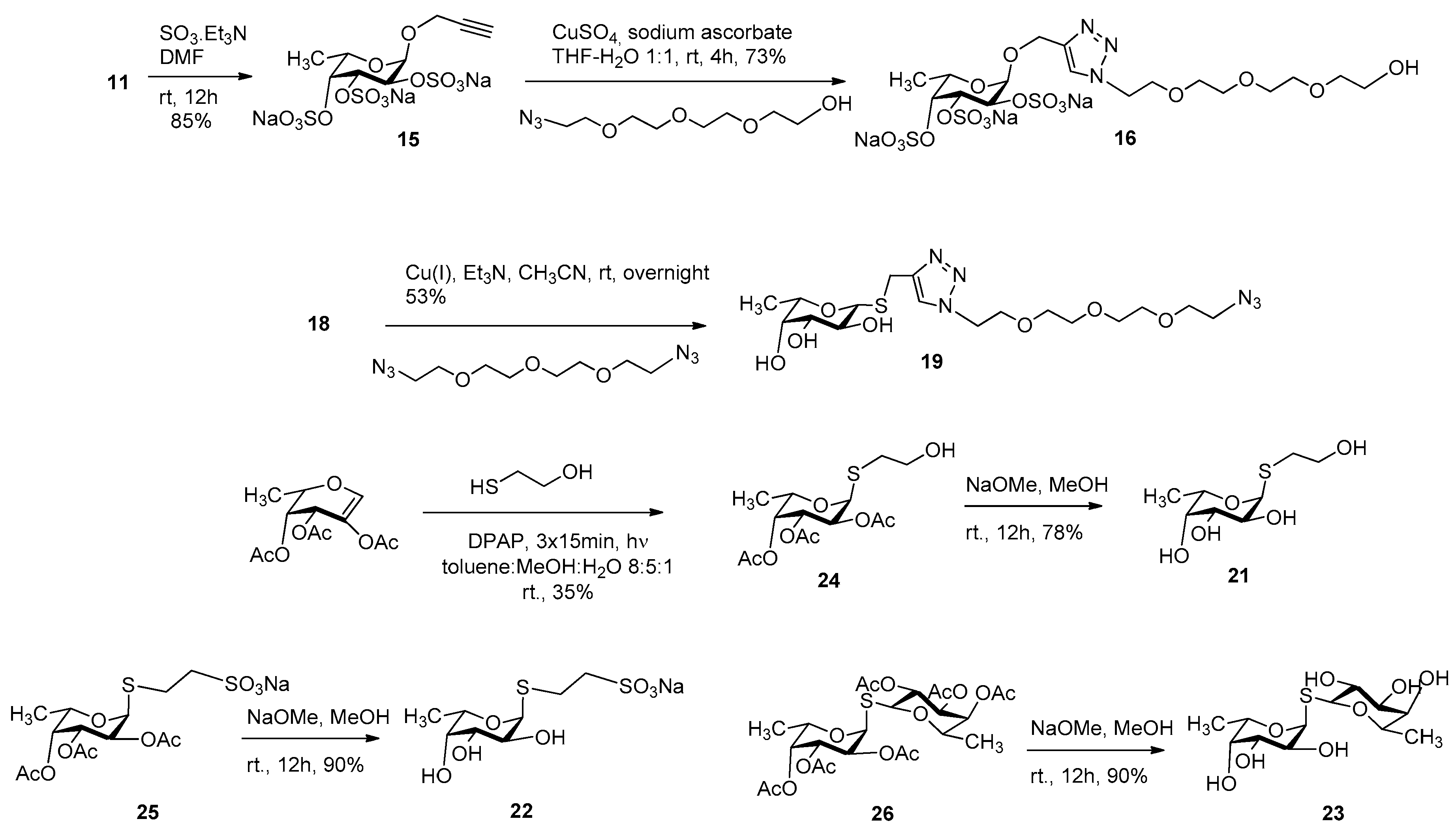
3.2. Synthesis
3.3. Lectins Production and Purification
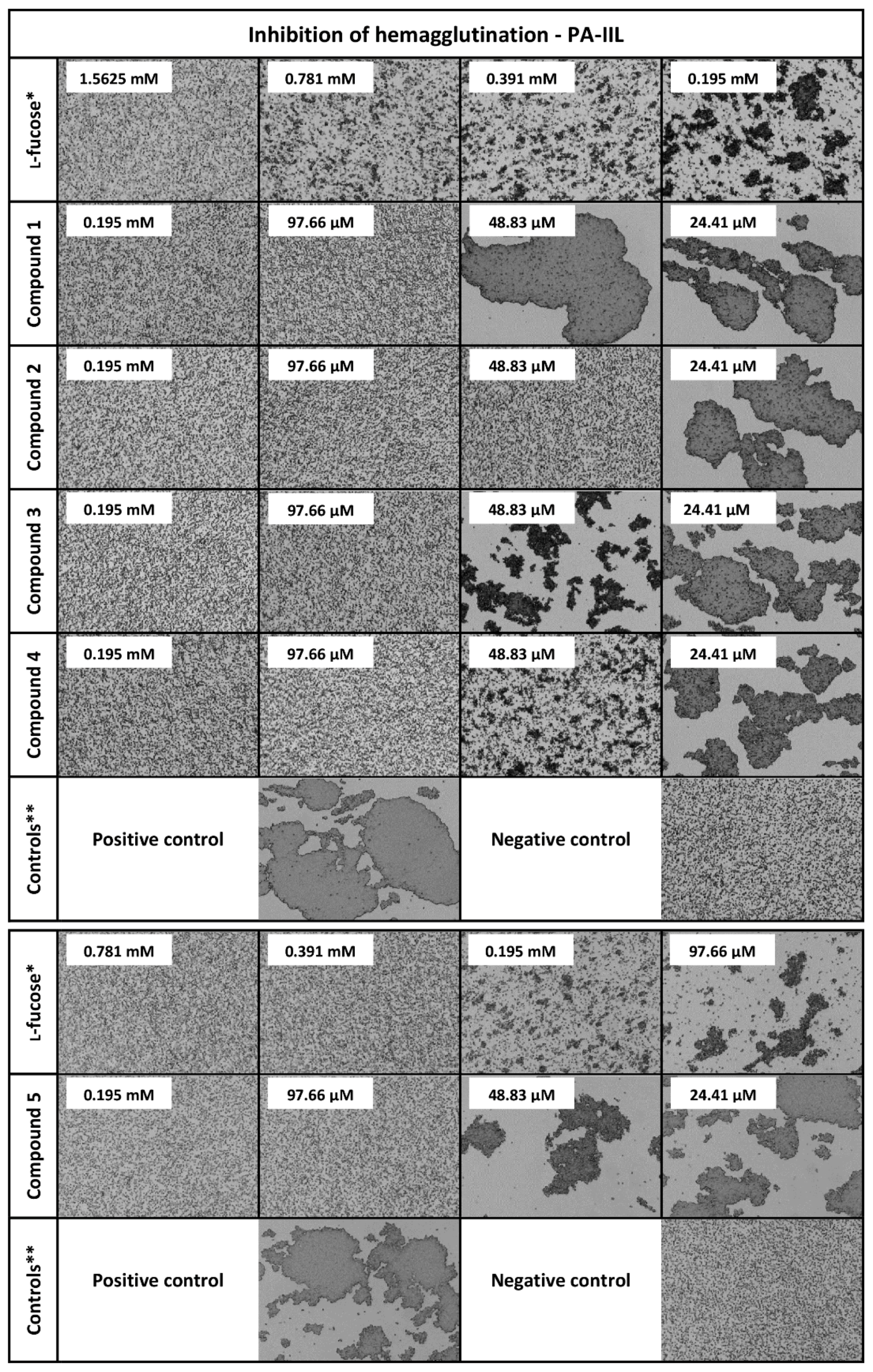
3.4. Hemagglutination Inhibition Assay
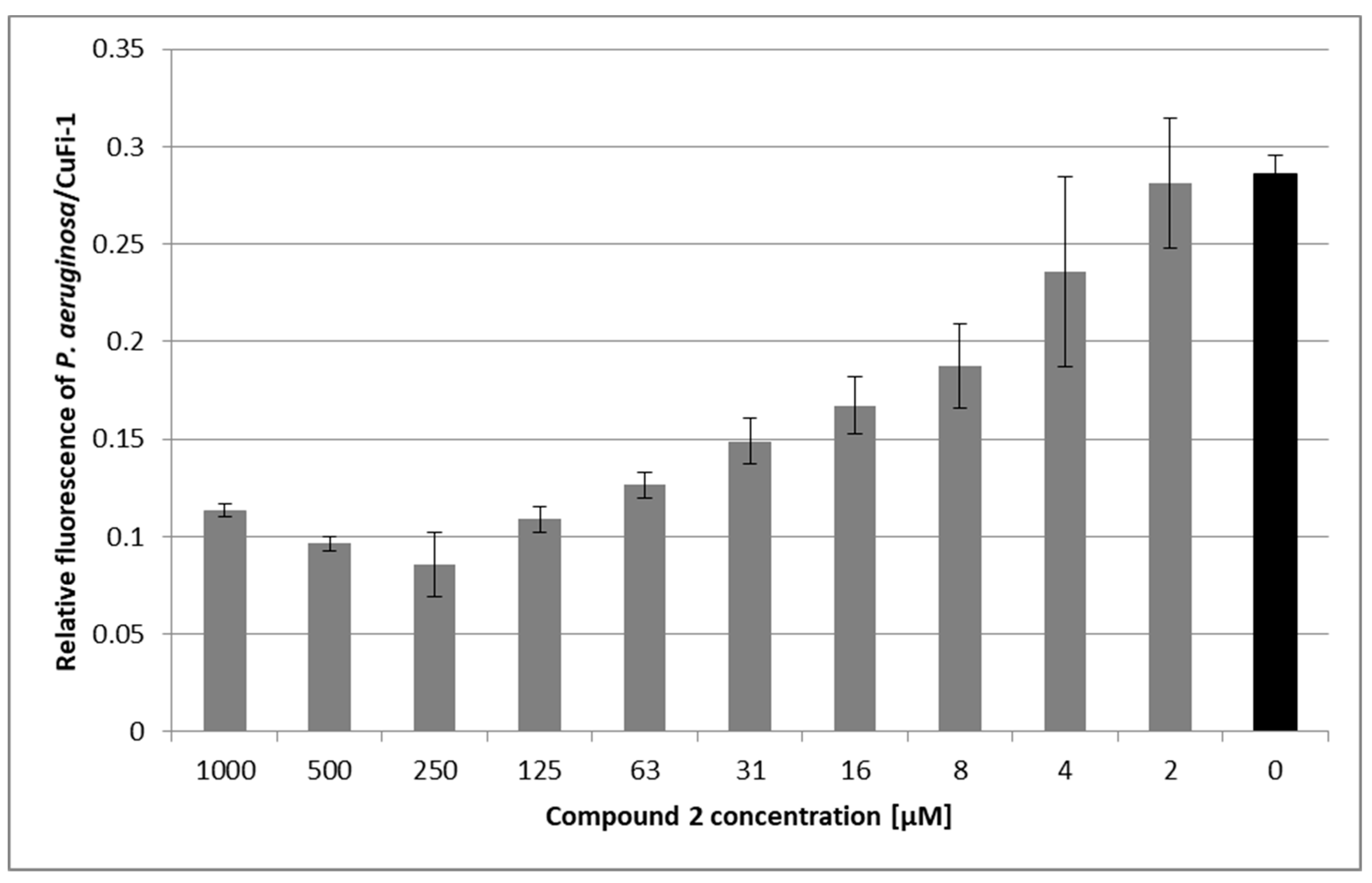
3.5. Pseudomonas Aeruginosa Adherence Assay
3.5.1. Cell Labelling
3.5.2. Inhibition of Pseudomonas aeruginosa Adhesion on Epithelial Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef]
- Scanlin, T.F.; Glick, M.C. Terminal glycosylation in cystic fibrosis. Biochim. Biophys. Acta 1999, 1455, 241–253. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef]
- Imberty, A.; Wimmerová, M.; Mitchell, E.P.; Gilboa-Garber, N. Structures of the lectins from Pseudomonas aeruginosa: Insight into the molecular basis for host glycan recognition. Microbes Infect. 2004, 6, 221–228. [Google Scholar] [CrossRef]
- Chemani, C.; Imberty, A.; de Bentzmann, S.; Pierre, M.; Wimmerová, M.; Guery, B.P.; Faure, K. Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infect. Immun. 2009, 77, 2065–2075. [Google Scholar] [CrossRef]
- Šulák, O.; Cioci, G.; Lameignère, E.; Balloy, V.; Round, A.; Gutsche, I.; Malinovská, L.; Chignard, M.; Kosma, P.; Aubert, D.F.; et al. Burkholderia cenocepacia BC2L-C is a super lectin with dual specificity and proinflammatory activity. PLoS Pathog. 2011, 7. [Google Scholar] [CrossRef]
- Houser, J.; Komarek, J.; Kostlanova, N.; Cioci, G.; Varrot, A.; Kerr, S.C.; Lahmann, M.; Balloy, V.; Fahy, J.V.; Chignard, M.; et al. A soluble fucose-specific lectin from Aspergillus fumigatus conidia-structure, specificity and possible role in fungal pathogenicity. PLoS ONE 2013, 8, 1–15. [Google Scholar] [CrossRef]
- Mitchell, E.P.; Sabin, C.; Šnajdrová, L.; Pokorná, M.; Perret, S.; Gautier, C.; Hofr, C.; Gilboa-Garber, N.; Koča, J.; Wimmerová, M.; et al. High affinity fucose binding of Pseudomonas aeruginosa lectin PA-IIL: 1.0 A resolution crystal structure of the complex combined with thermodynamics and computational chemistry approaches. Proteins 2005, 58, 735–746. [Google Scholar] [CrossRef]
- Houser, J.; Komarek, J.; Cioci, G.; Varrot, A.; Imberty, A.; Wimmerova, M. Structural insights into Aspergillus fumigatus lectin specificity: AFL binding sites are functionally non-equivalent. Acta Cryst. 2015, D71, 442–453. [Google Scholar] [CrossRef]
- Yamaki, K.; Yoshino, S. Aspergillus oryzae lectin induces anaphylactoid oedema and mast cell activation through its interaction with fucose of mast cell–bound non-specific IgE. Scand. J. Immun. 2011, 74, 445–453. [Google Scholar] [CrossRef]
- Wimmerova, M.; Mitchell, E.; Sanchez, J.F.; Gautier, C.; Imberty, A. Crystal structure of fungal lectin: Six-bladed beta-propeller fold and novel fucose recognition mode for Aleuria aurantia lectin. J. Biol. Chem. 2003, 278, 27059–27067. [Google Scholar] [CrossRef]
- Denny, T.P. Ralstonia solanacearum—A plant pathogen in touch with its host. Trends Microbiol. 2000, 8, 486–489. [Google Scholar] [CrossRef]
- Kostlánová, N.; Mitchell, E.P.; Lortat-Jacob, H.; Oscarson, S.; Lahmann, M.; Gilboa-Garber, N.; Chambat, G.; Wimmerová, M.; Imberty, A. The fucose-binding lectin from Ralstonia solanacearum. A new type of beta-propeller architecture formed by oligomerization and interacting with fucoside, fucosyllactose, and plant xyloglucan. J. Biol. Chem. 2005, 280, 27839–27849. [Google Scholar] [CrossRef]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus Multivalent Glycoconjugates for the Design of High Affinity Lectin Ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef]
- Goyard, D.; Baldoneschi, V.; Varrot, A.; Fiore, M.; Imberty, A.; Richichi, B.; Renaudet, O.; Nativi, C. Multivalent Glycomimetics with Affinity and Selectivity toward Fucose-Binding Receptors from Emerging Pathogens. Bioconjug. Chem. 2018, 29, 83–88. [Google Scholar] [CrossRef]
- Lehot, V.; Brissonnet, Y.; Dussouy, C.; Brument, S.; Cabanettes, A.; Gillon, E.; Deniaud, D.; Varrot, A.; Le Pape, P.; Gouin, S.G. Multivalent fucosides with nanomolar affinity for the aspergillus fumigatus lectin flea prevent spore adhesion to pneumocytes. Chemistry 2018, 24, 19243–19249. [Google Scholar] [CrossRef]
- Kašaková, M.; Malinovská, L.; Klejch, T.; Hlaváčková, M.; Dvořáková, H.; Fujdiarová, E.; Rottnerová, Z.; Maťátková, O.; Lhoták, P.; Wimmerová, M.; et al. Selectivity of original C-Hexopyranosyl Calix[4]arene conjugates towards lectins of different origin. Carbohydr. Res. 2018, 469, 60–72. [Google Scholar] [CrossRef]
- Sommer, R.; Wagner, S.; Rox, K.; Varrot, A.; Hauck, D.; Wamhoff, E.C.; Schreiber, J.; Ryckmans, T.; Brunner, T.; Rademacher, C.; et al. Glycomimetic, orally bioavailable lecb inhibitors block biofilm formation of Pseudomonas aeruginosa. J. Am. Chem. Soc. 2018, 140, 2537–2545. [Google Scholar] [CrossRef]
- Hauck, D.; Joachim, I.; Frommeyer, B.; Varrot, A.; Philipp, B.; Möller, H.M.; Imberty, A.; Exner, T.E.; Titz, A. Discovery of two classes of potent glycomimetic inhibitors of pseudomonas aeruginosa lecb with distinct binding modes. ACS Chem. Biol. 2013, 8, 1775–1784. [Google Scholar] [CrossRef]
- Buffet, K.; Gillon, E.; Holler, M.; Nierengarten, J.F.; Imberty, A.; Vincent, S.P. Fucofullerenes as tight ligands of RSL and LecB, two bacterial lectins. Org. Biomol. Chem. 2015, 13, 6482. [Google Scholar] [CrossRef]
- Reymond, J.L.; Bergmann, M.; Darbre, T. Glycopeptide dendrimers as Pseudomonas aeruginosa biofilm inhibitors. Chem. Soc. Rev. 2013, 42, 4814. [Google Scholar] [CrossRef]
- Galanos, N.; Gillon, E.; Imberty, A.; Matthews, S.E.; Vidal, S. Pentavalent pillar[5]arene-based glycoclusters and their multivalent binding to pathogenic bacterial lectins. Org. Biomol. Chem. 2016, 14, 3476. [Google Scholar] [CrossRef]
- Donnier-Maréchal, M.; Galanos, N.; Grandjean, T.; Pascal, Y.; Ji, D.; Dong, L.; Gillon, E.; He, X.; Imberty, A.; Kipnis, E.; et al. Perylenediimide-based glycoclusters as high affinity ligands of bacterial lectins: Synthesis, binding studies and anti-adhesive properties. Org. Biomol. Chem. 2017, 15, 10037. [Google Scholar] [CrossRef]
- Hua, Y.; Beshr, G.; Garvey, C.J.; Tabor, R.F.; Titz, A.; Wilkinson, B.L. Photoswitchable Janus glycodendrimer micelles as multivalent inhibitors of LecA and LecB from Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces. 2017, 159, 605–612. [Google Scholar] [CrossRef]
- Adamová, L.; Malinovská, L.; Wimmerová, M. New sensitive detection method for lectin hemagglutination using microscopy. Microsc. Res. Tech. 2014, 77, 841–849. [Google Scholar] [CrossRef]
- Jančaříková, G.; Herczeg, M.; Fujdiarová, E.; Houser, J.; Kövér, K.E.; Borbás, A.; Wimmerová, M.; Csávás, M. Synthesis of α-L-fucopyranoside-presenting glycoclusters and investigation of their interaction with recombinant Photorhabdus asymbiotica lectin (PHL). Chem. Eur. J. 2018, 24, 4055–4068. [Google Scholar] [CrossRef]
- Herczeg, M.; Mező, E.; Molnár, N.; Ng, S.-K.; Lee, Y.-C.; Dah-Tsyr Chang, M.; Borbás, A. Inhibitory effect of multivalent rhamnobiosides on recombinant horseshoe crab plasma lectin interactions with Pseudomonas aeruginosa PAO1. Chem. Asian J. 2016, 11, 3398–3413. [Google Scholar] [CrossRef]
- Chabre, Y.M.; Contino-Pepin, C.; Placide, V.; Shiao, T.C.; Roy, R. Expeditive synthesis of glycodendrimer scaffolds based on versatile TRIS and mannoside derivatives. J. Org. Chem. 2008, 73, 5602–5605. [Google Scholar] [CrossRef]
- Leaver, D.J.; Dawson, R.M.; White, J.M.; Polyzos, A.; Hughes, A.B. Synthesis of 1,2,3-triazole linked galactopyranosides and evaluation of cholera toxin inhibition. Org. Biomol. Chem. 2011, 21, 8465–8474. [Google Scholar] [CrossRef]
- Deguise, I.; Lagnoux, D.; Roy, R. Synthesis of glycodendrimers containing both fucoside and galactoside residues and their binding properties to Pa-IL and PA-IIL lectins from Pseudomonas aeruginosa. New J. Chem. 2007, 31, 1321–1331. [Google Scholar] [CrossRef]
- Guchhait, G.; Misra, A.K. Efficient glycosylation of unprotected sugars using sulfamic acid: A mild eco-friendly catalyst. Catal. Commun. 2011, 14, 52–57. [Google Scholar] [CrossRef]
- Matta, K.L.; Girotra, R.N.; Barlow, J.J. Synthesis of p-nitrobenzyl and p-nitrophenyl 1-thioglycopyranosides. Carbohydr. Res. 1975, 43, 101–109. [Google Scholar] [CrossRef]
- Adinolfi, M.; Capasso, D.; Di Gaetano, S.; Iadonisi, A.; Leone, L.; Pastore, A. A straightforward synthetic access to symmetrical glycosyl disulfides and biological evaluation thereof. Org. Biomol. Chem. 2011, 9, 6278–6283. [Google Scholar] [CrossRef]
- Eszenyi, D.; Kelemen, V.; Balogh, F.; Bege, M.; Csávás, M.; Herczegh, P.; Borbás, A. Promotion of a reaction by cooling—Stereoselective 1,2-cis-alpha-Thioglycoconjugation by Thiol-Ene coupling at −80 °C. Chem. Eur. J. 2018, 24, 18–4532. [Google Scholar] [CrossRef]
- Lázár, L.; Csávás, M.; Herczeg, M.; Herczegh, P.; Borbás, A. Synthesis of S-linked glycoconjugates and S-disaccharides by thiol-ene coupling reaction of enoses. Org. Lett. 2012, 14, 4650–4653. [Google Scholar] [CrossRef]
- Varela, O.; De Fina, G.M.; De Lederkremer, R.M. The reaction of 2-hydroxyglycal esters with alcohols in the presence of N-iodosuccinimide, stereoselective synthesis of α anomers of alkyl 3-deoxyhex-2-enopyranosides and 3,4-dideoxyhex-3-enopyranosid-2-uloses. Carbohydr. Res. 1987, 167, 187–196. [Google Scholar] [CrossRef]
- Cecioni, S.; Praly, J.-P.; Matthews, S.E.; Wimmerová, M.; Imberty, A.; Vidal, S. Rational design and synthesis of optimized glycoclusters for multivalent lectin–carbohydrate interactions: influence of the linker arm. Chem. Eur. J. 2012, 18, 6250–6263. [Google Scholar] [CrossRef]
- Deniaud, D.; Julienne, K.; Gouin, S.G. Insights in the rational design of synthetic multivalent glycoconjugates as lectin ligands. Org. Biomol. Chem. 2011, 9, 966–979. [Google Scholar] [CrossRef]
- Tielker, D.; Hacker, S.; Loris, R.; Strathmann, M.; Wingender, J.; Wilhelm, S.; Rosenau, F.; Jaeger, K.-E. Pseudomonas aeruginosa LecB is located in the outer membrane and is involved in biofilm formation. Microbiology 2005, 151, 1313–1323. [Google Scholar] [CrossRef]
- Cott, C.; Thuenauer, R.; Landi, A.; Kühn, K.; Juillot, S.; Imberty, A.; Madl, J.; Eierhoff, T.; Römer, W. Pseudomonas aeruginosa lectin LecB inhibits tissue repair processes by triggering β-catenin degradation. Biochim. Biophys. Acta 2016, 1863, 1106–1118. [Google Scholar] [CrossRef]
- Nilsson, E.; Kollberg, H.; Johannesson, M.; Wejåker, P.E.; Carlander, D.; Larsson, A. More than 10 years’ continuous oral treatment with specific immunoglobulin Y for the prevention of Pseudomonas aeruginosa infections: A case report. J. Med. Food 2007, 10, 375–378. [Google Scholar] [CrossRef]
- Herrmann, G.; Yang, L.; Wu, H.; Song, Z.; Wang, H.; Høiby, N.; Ulrich, M.; Molin, S.; Riethmüller, J.; Döring, G. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J. Infect. Dis. 2010, 202, 1585–1592. [Google Scholar] [CrossRef]
- Hauber, H.P.; Schulz, M.; Pforte, A.; Mack, D.; Zabel, P.; Schumacher, U. Inhalation with fucose and galactose for treatment of Pseudomonas aeruginosa in cystic fibrosis patients. Int. J. Med. Sci. 2008, 5, 371–376. [Google Scholar] [CrossRef]
- Kubíčková, B.; Hadrabová, J.; Vašková, L.; Mandys, V.; Stiborová, M.; Hodek, P. Susceptibility of airways to Pseudomonas aeruginosa infection: Mouse neuraminidase model. Monatsh. Chem. 2017, 148, 1993–2002. [Google Scholar] [CrossRef]
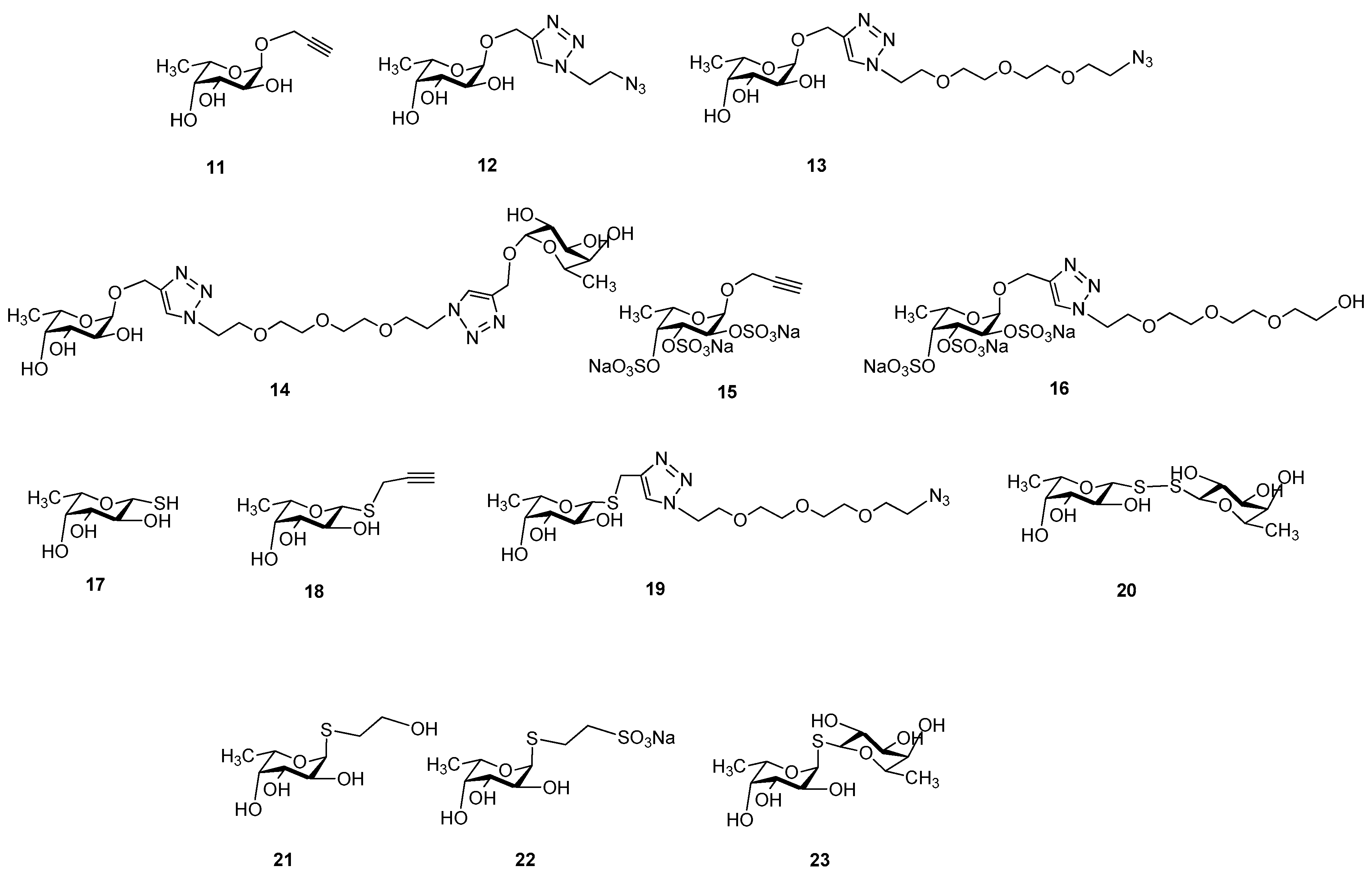
Sample Availability: Samples of the compounds 1, 2, 3, 4, 5, 11–23 are available from the authors. |
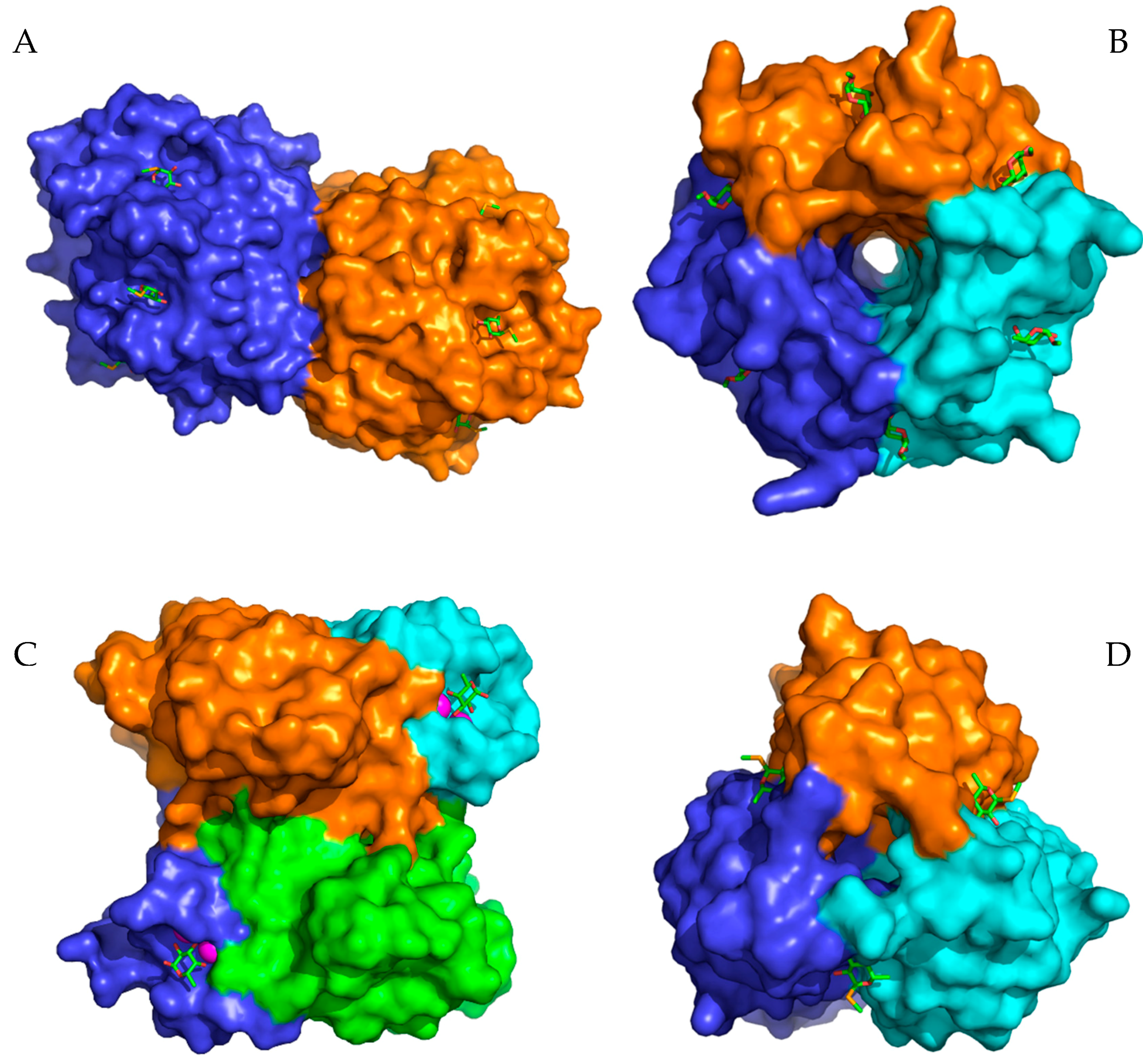
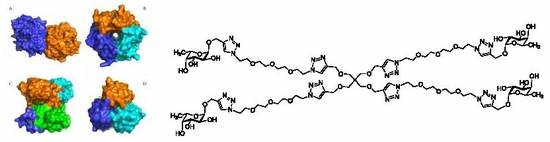
| AFL | RSL | AAL | AOL | PA-IIL | BC2L-C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor | Valency | Potency | β# | Potency | β# | Potency | β# | Potency | β# | Potency | β# | Potency | β# |
| l-fucose* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Compound 1 | 3 | 64 | 21.3 | 512 | 170.7 | 32 | 10.7 | 64 | 21.3 | 16 | 5.3 | 8 | 2.7 |
| Compound 2 | 4 | 128 | 32 | 1024 | 256 | 128 | 32 | 256 | 64 | 32 | 8 | 16 | 4 |
| Compound 3 | 3 | 16 | 5.3 | 512 | 170.7 | 16 | 5.3 | 8 | 2.7 | 16 | 5.3 | 4 | 1.3 |
| Compound 4 | 4 | 16 | 4 | 256 | 64 | 16 | 4 | 8 | 2 | 16 | 4 | 4 | 1 |
| Compound 5 | 3 | 32 | 10.7 | 128 | 42.7 | 64 | 21.3 | 128 | 42.7 | 4 | 1.3 | 8 | 2.7 |
| Compound 11 | 1 | 4 | 4 | 8 | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 1 | 1 |
| Compound 12 | 1 | 8 | 8 | 32 | 32 | 8 | 8 | 8 | 8 | 4 | 4 | 2 | 2 |
| Compound 13 | 1 | 4 | 4 | 8 | 8 | 4 | 4 | 4 | 4 | 4 | 4 | 2 | 2 |
| Compound 14 | 2 | 4 | 2 | 128 | 64 | 8 | 4 | 4 | 2 | 8 | 4 | 2 | 1 |
| Compound 15 | 1 | 0.125 | 0.125 | ND | - | ND | - | 0.5 | 0.5 | 0.5 | 0.5 | ND | - |
| Compound 16 | 1 | 0.125 | 0.125 | ND | - | ND | - | ND | - | 0.125 | 0.125 | 0.25 | 0.25 |
| Compound 17 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | 0.25 | ND | - |
| Compound 18 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0.5 | 0.03125 | 0.03125 | 0.5 | 0.5 |
| Compound 19 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 0.0625 | 0.0625 | 0.5 | 0.5 |
| Compound 20 | 2 | 0.5 | 0.25 | 2 | 1 | 1 | 0.5 | 1 | 0.5 | 0.125 | 0.0625 | 1 | 0.5 |
| Compound 21 | 1 | 2 | 2 | 4 | 4 | 2 | 2 | 4 | 4 | 4 | 4 | 4 | 4 |
| Compound 22 | 1 | 2 | 2 | 4 | 4 | 2 | 2 | 2 | 2 | 4 | 4 | 4 | 4 |
| Compound 23 | 2 | 8 | 4 | 8 | 4 | 4 | 2 | 8 | 4 | 4 | 2 | 8 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thai Le, S.; Malinovska, L.; Vašková, M.; Mező, E.; Kelemen, V.; Borbás, A.; Hodek, P.; Wimmerová, M.; Csávás, M. Investigation of the Binding Affinity of a Broad Array of l-Fucosides with Six Fucose-Specific Lectins of Bacterial and Fungal Origin. Molecules 2019, 24, 2262. https://doi.org/10.3390/molecules24122262
Thai Le S, Malinovska L, Vašková M, Mező E, Kelemen V, Borbás A, Hodek P, Wimmerová M, Csávás M. Investigation of the Binding Affinity of a Broad Array of l-Fucosides with Six Fucose-Specific Lectins of Bacterial and Fungal Origin. Molecules. 2019; 24(12):2262. https://doi.org/10.3390/molecules24122262
Chicago/Turabian StyleThai Le, Son, Lenka Malinovska, Michaela Vašková, Erika Mező, Viktor Kelemen, Anikó Borbás, Petr Hodek, Michaela Wimmerová, and Magdolna Csávás. 2019. "Investigation of the Binding Affinity of a Broad Array of l-Fucosides with Six Fucose-Specific Lectins of Bacterial and Fungal Origin" Molecules 24, no. 12: 2262. https://doi.org/10.3390/molecules24122262
APA StyleThai Le, S., Malinovska, L., Vašková, M., Mező, E., Kelemen, V., Borbás, A., Hodek, P., Wimmerová, M., & Csávás, M. (2019). Investigation of the Binding Affinity of a Broad Array of l-Fucosides with Six Fucose-Specific Lectins of Bacterial and Fungal Origin. Molecules, 24(12), 2262. https://doi.org/10.3390/molecules24122262