Gas-Phase Fragmentation of Cyclic Oligosaccharides in Tandem Mass Spectrometry
Abstract
1. Introduction
2. First- and Second-Order Mass Spectra of Cyclic Oligosaccharides and Their Derivatives
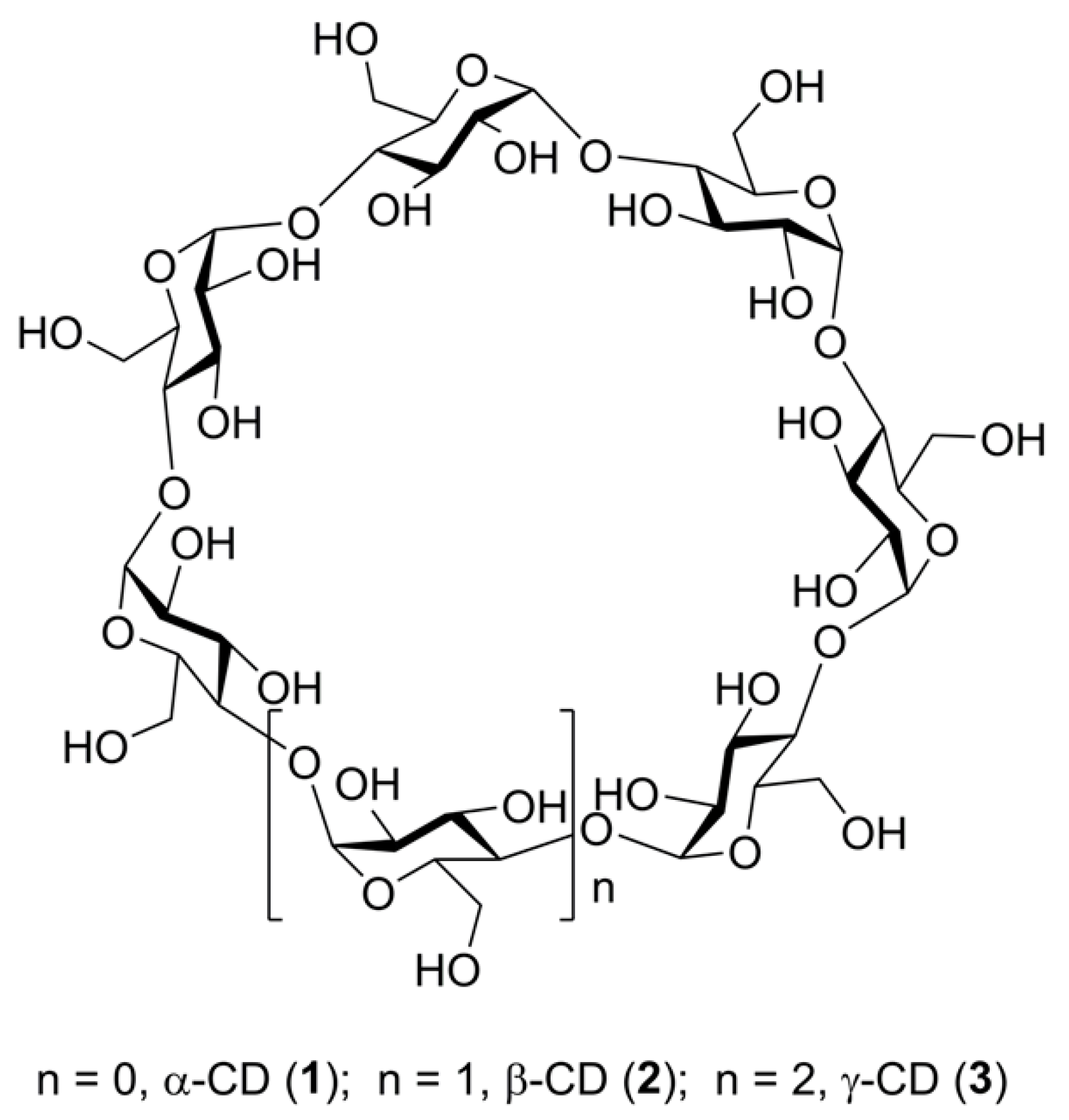
2.1. Mass Spectrometry of Cyclic Oligosaccharides—General Aspects
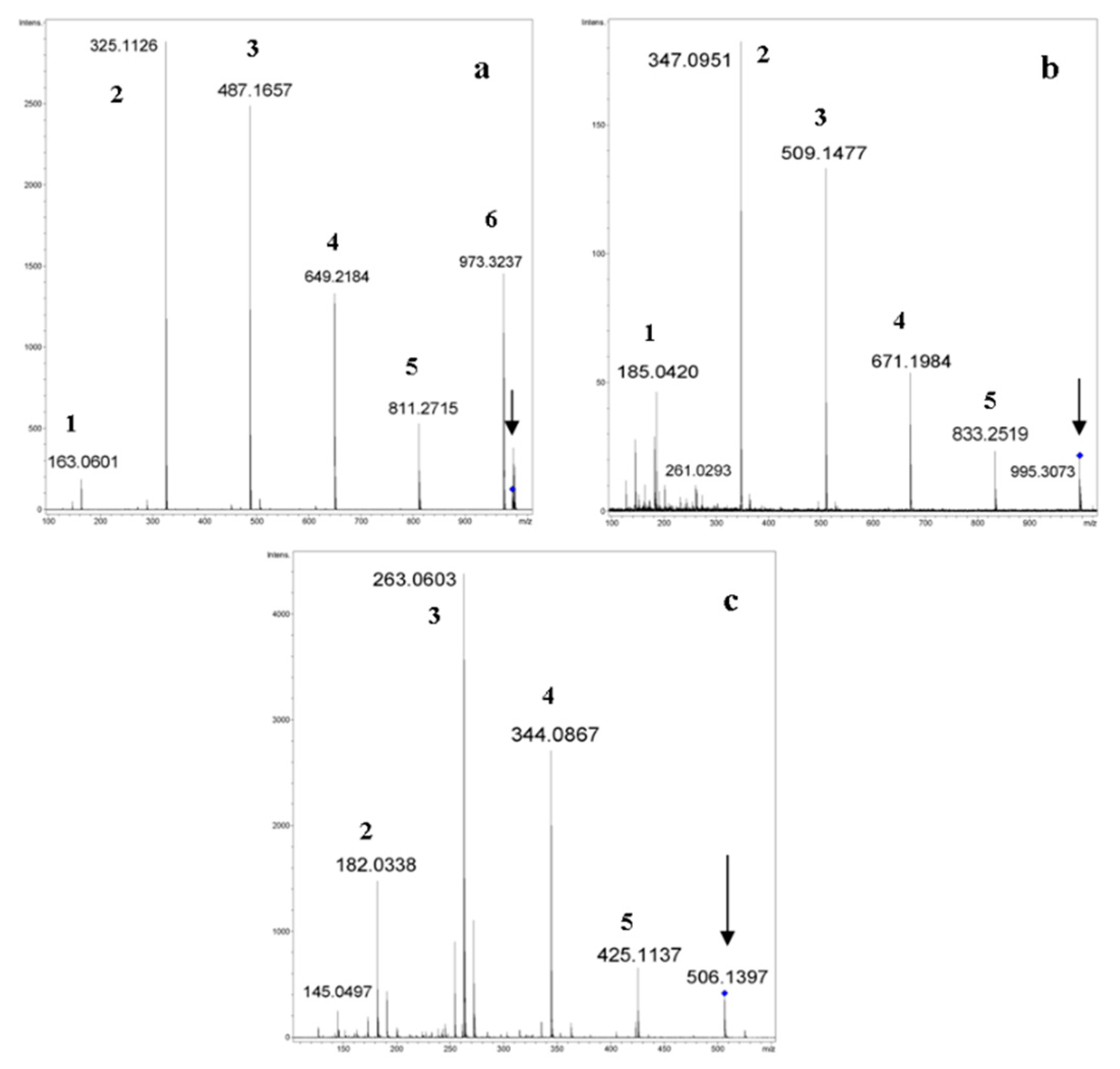
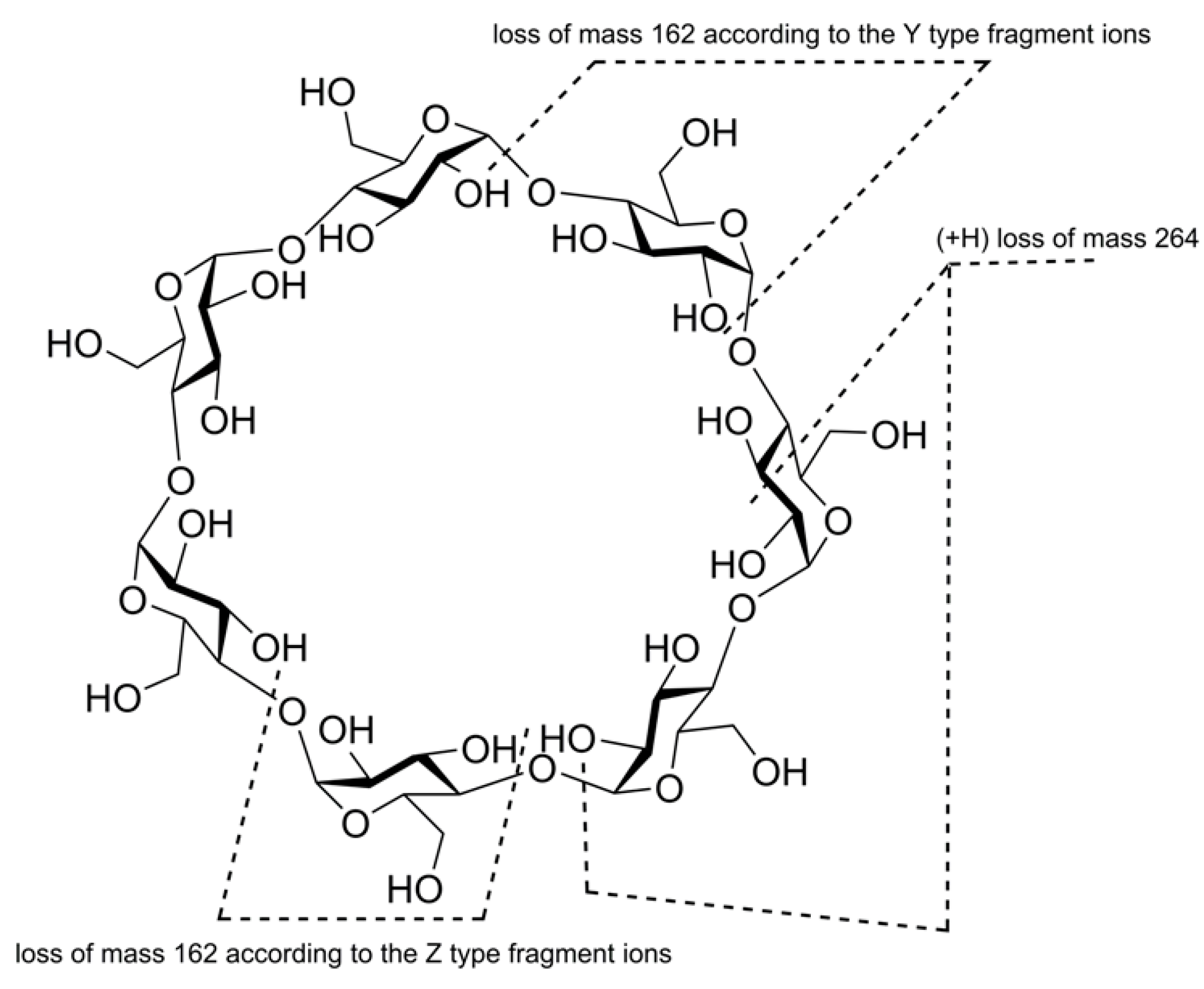
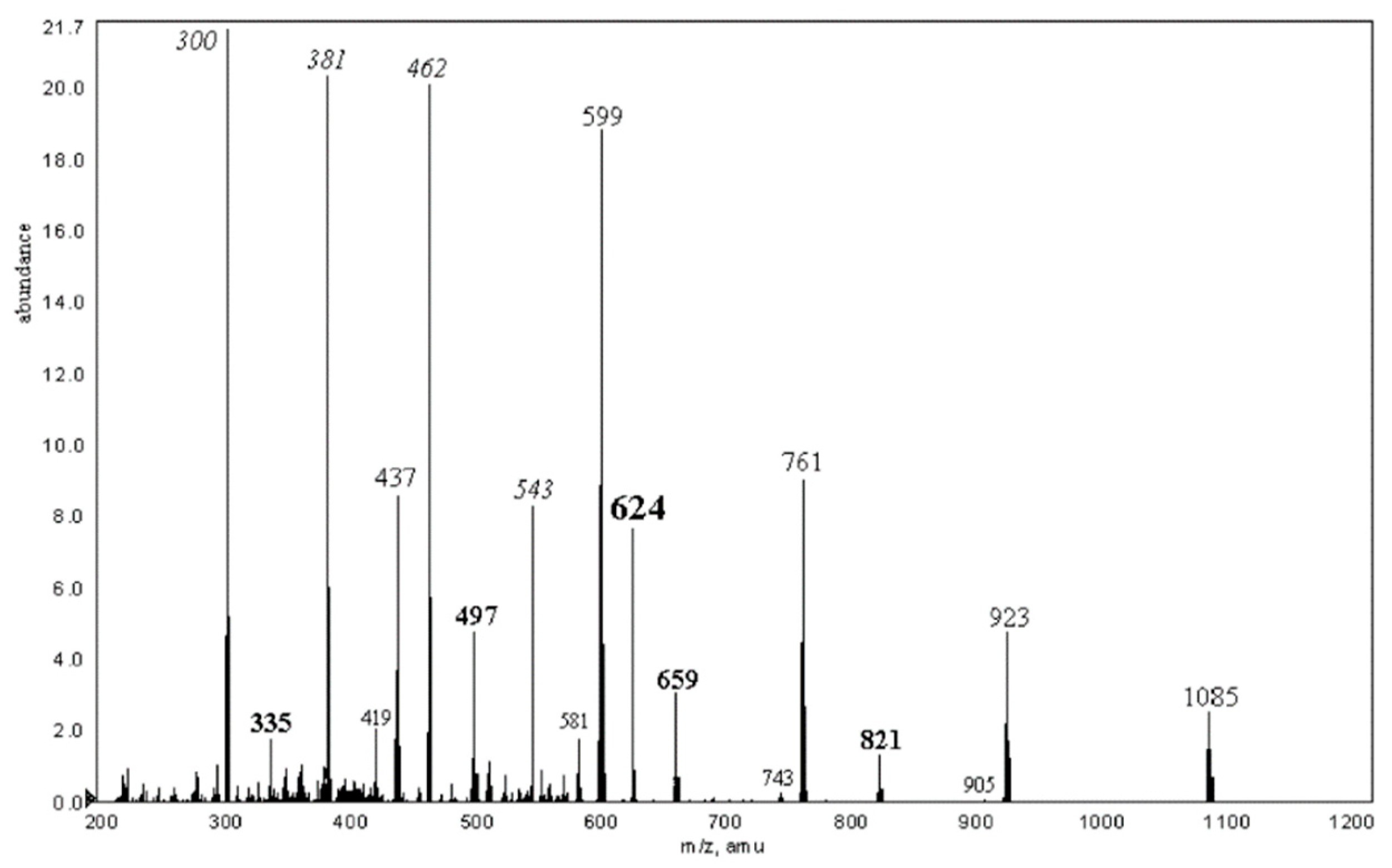
2.2. Tandem Mass Spectra (MSn) of Unsubstituted Cyclooligosaccharides: General Regularities and Attempt of Description
2.3. The Difference in Mass Spectra of Cyclic and Acyclic Oligosaccharides
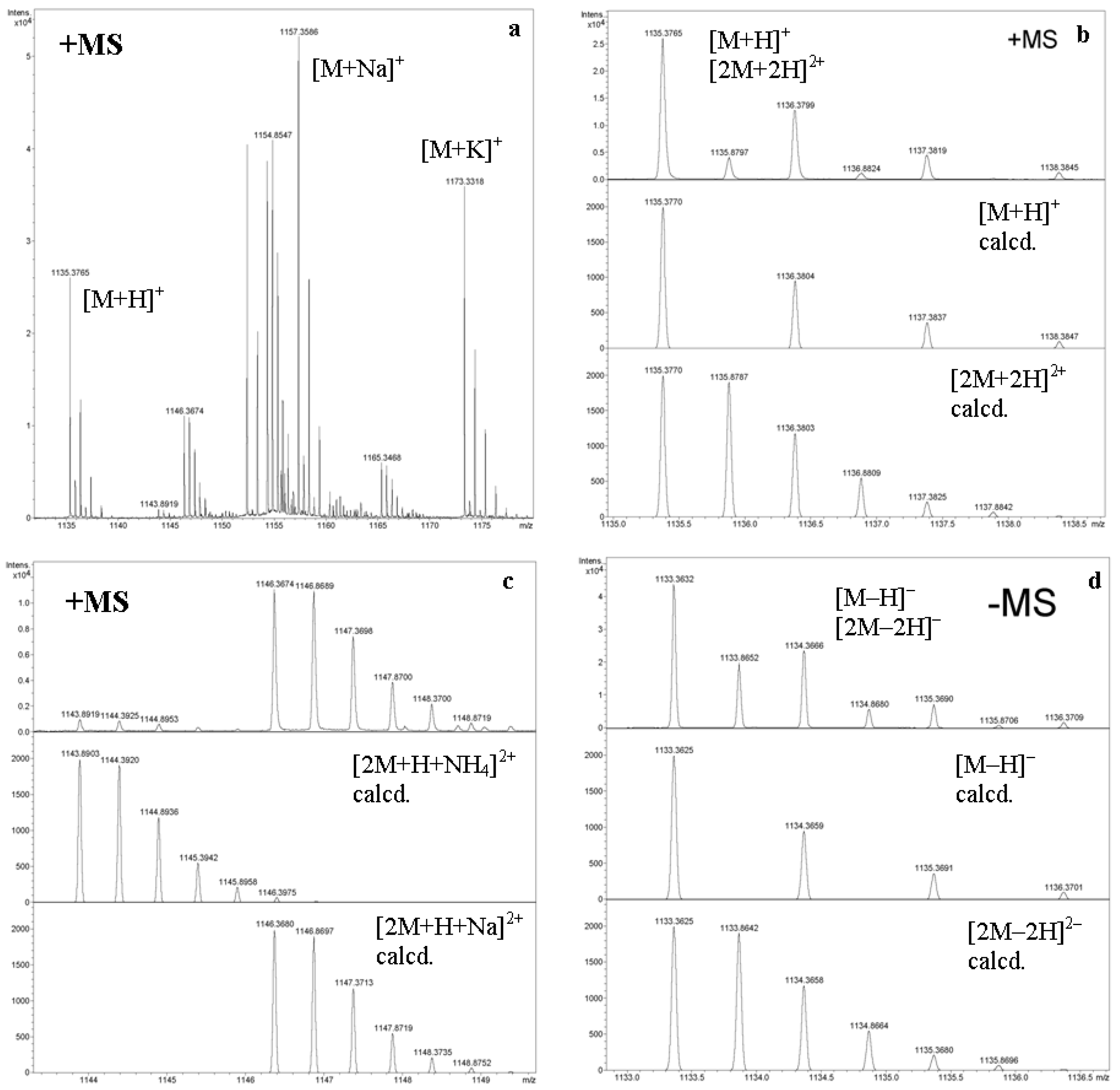
2.3.1. FAB MS, MALDI TOF MS, and ESI MS of Cyclic Oligosaccharides
2.3.2. Cyclic and Acyclic Oligosaccharides: Differences in Affinities to Metal Cations
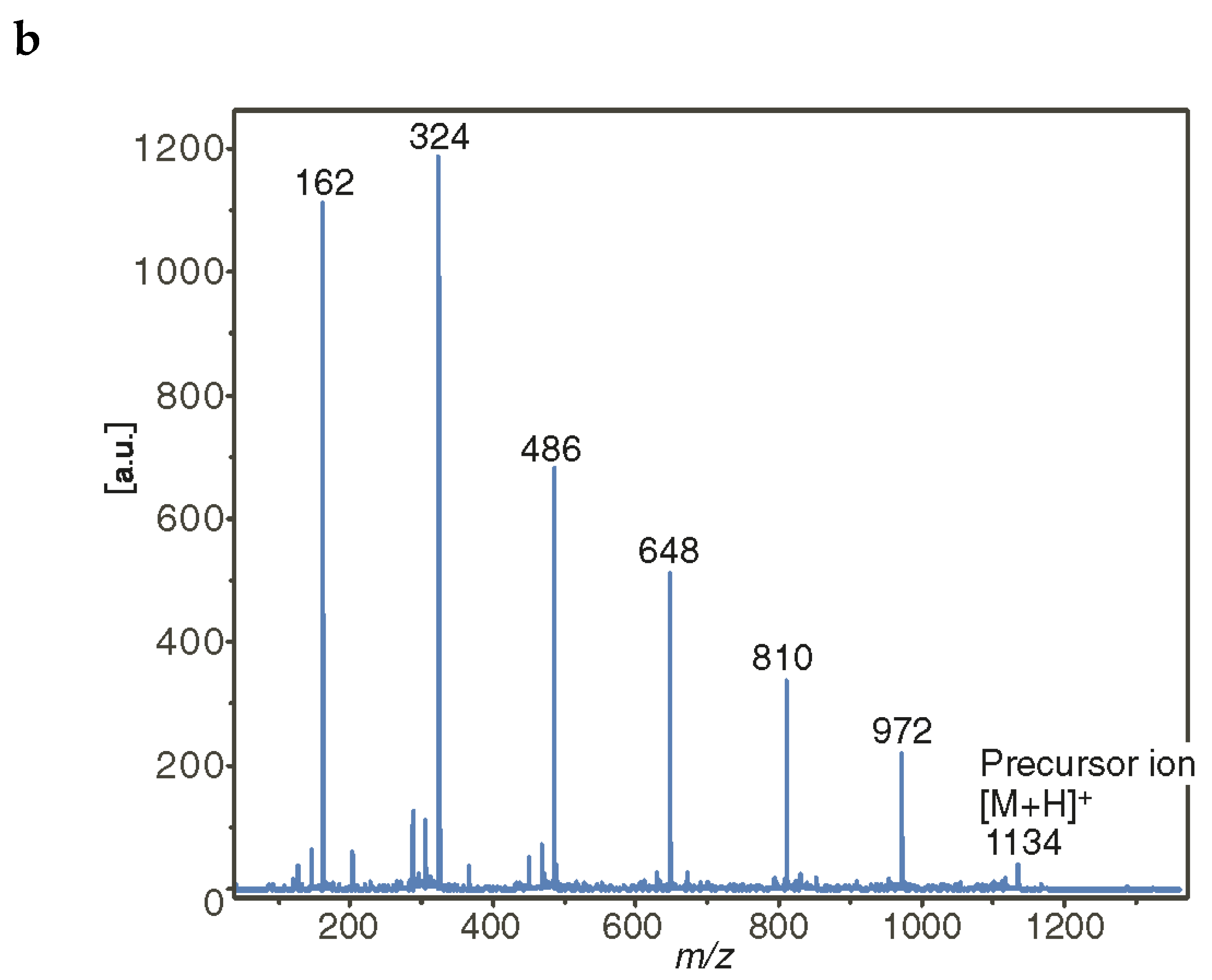
2.3.3. Cyclic and Linear Oligosaccharides: Differences in Tandem Mass Spectra
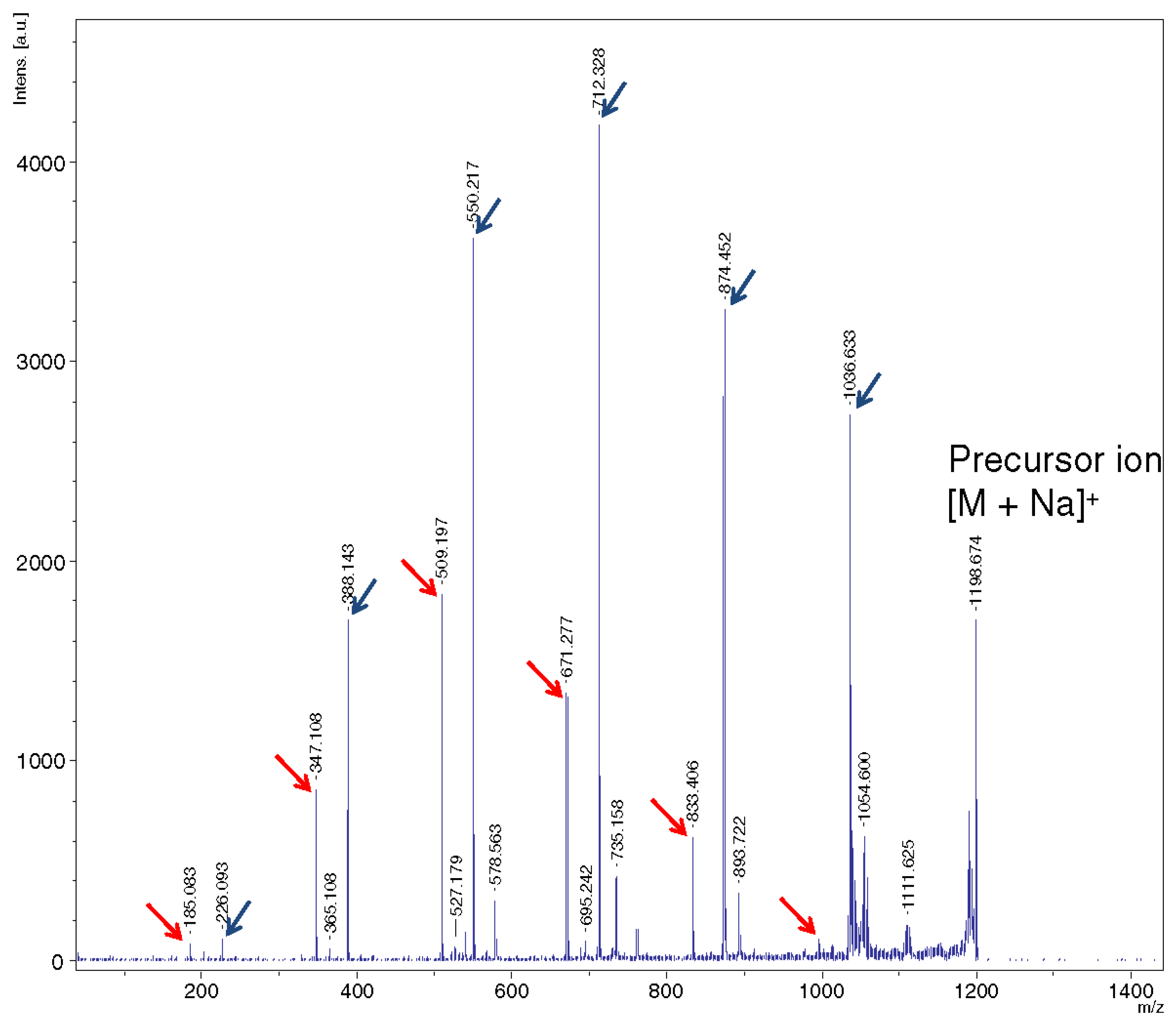
2.4. Complexation of Cyclic OS with Other Molecules And Guests’ Influence on CDs Induced Decay
2.5. Determination of Positions of Substituents in Cyclic Oligosaccharides—General Considerations and Problems
2.5.1. Tandem Mass Spectra of Monosubstituted Cyclic Oligosaccharides: The Effect of a Substituent
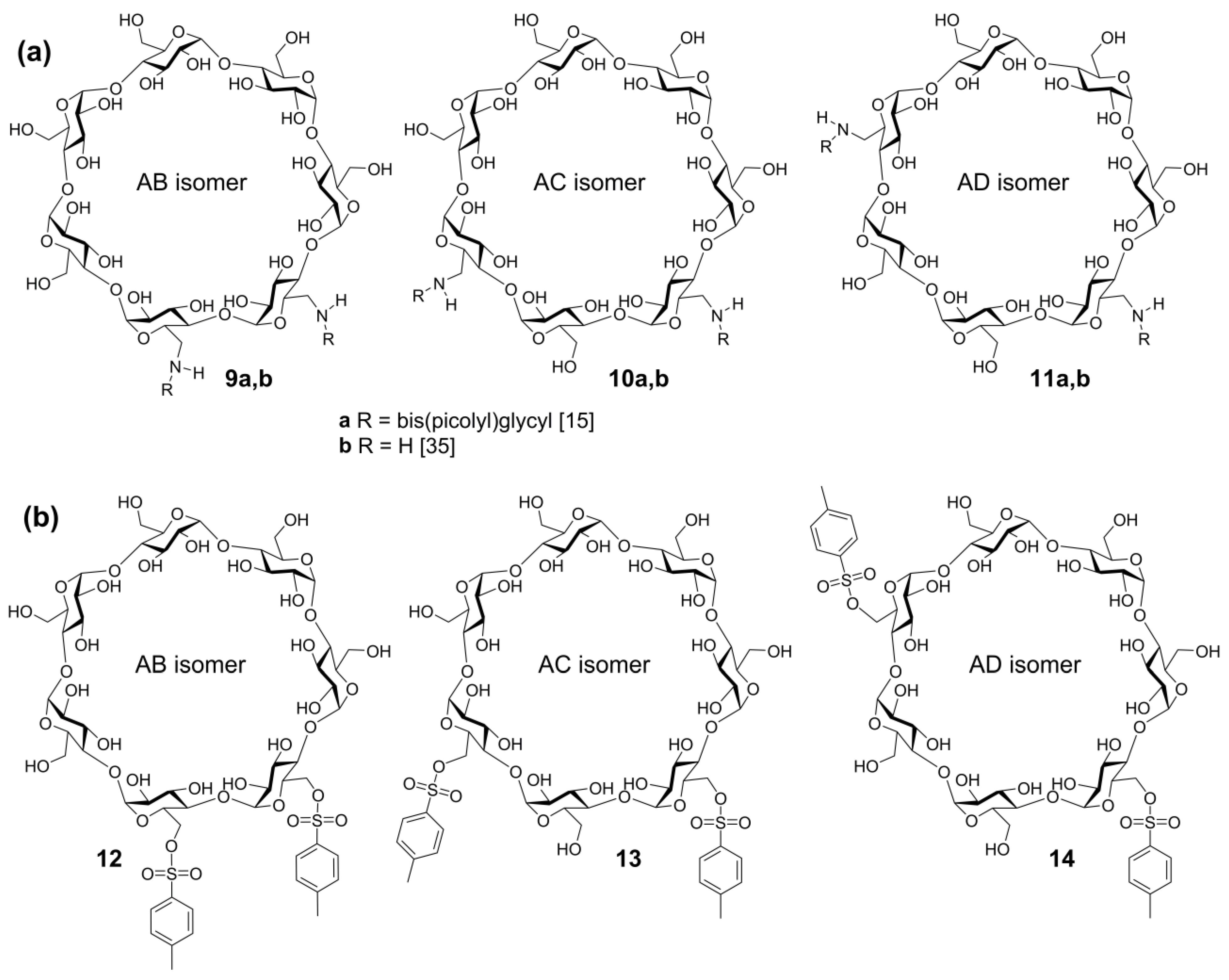
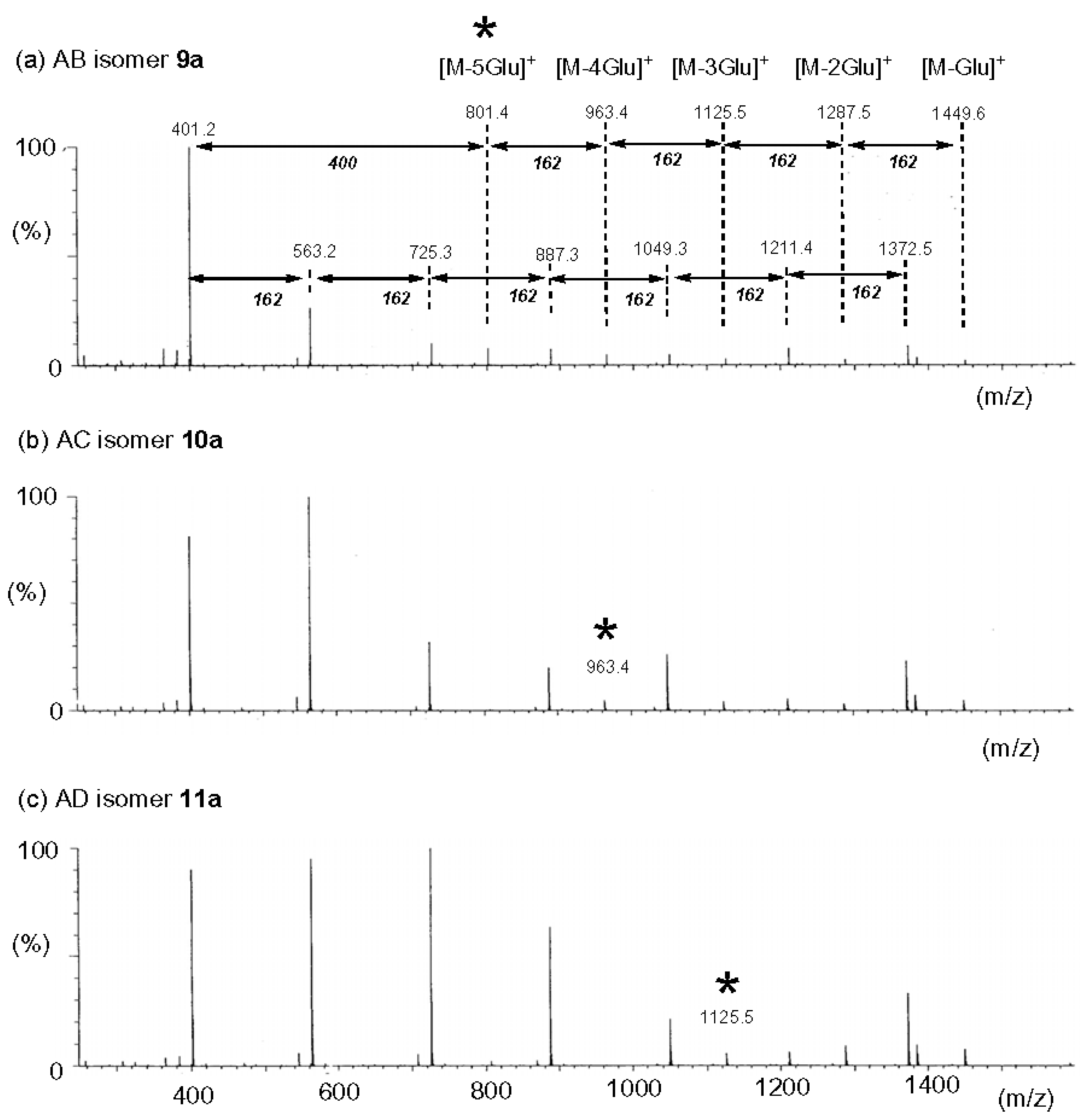
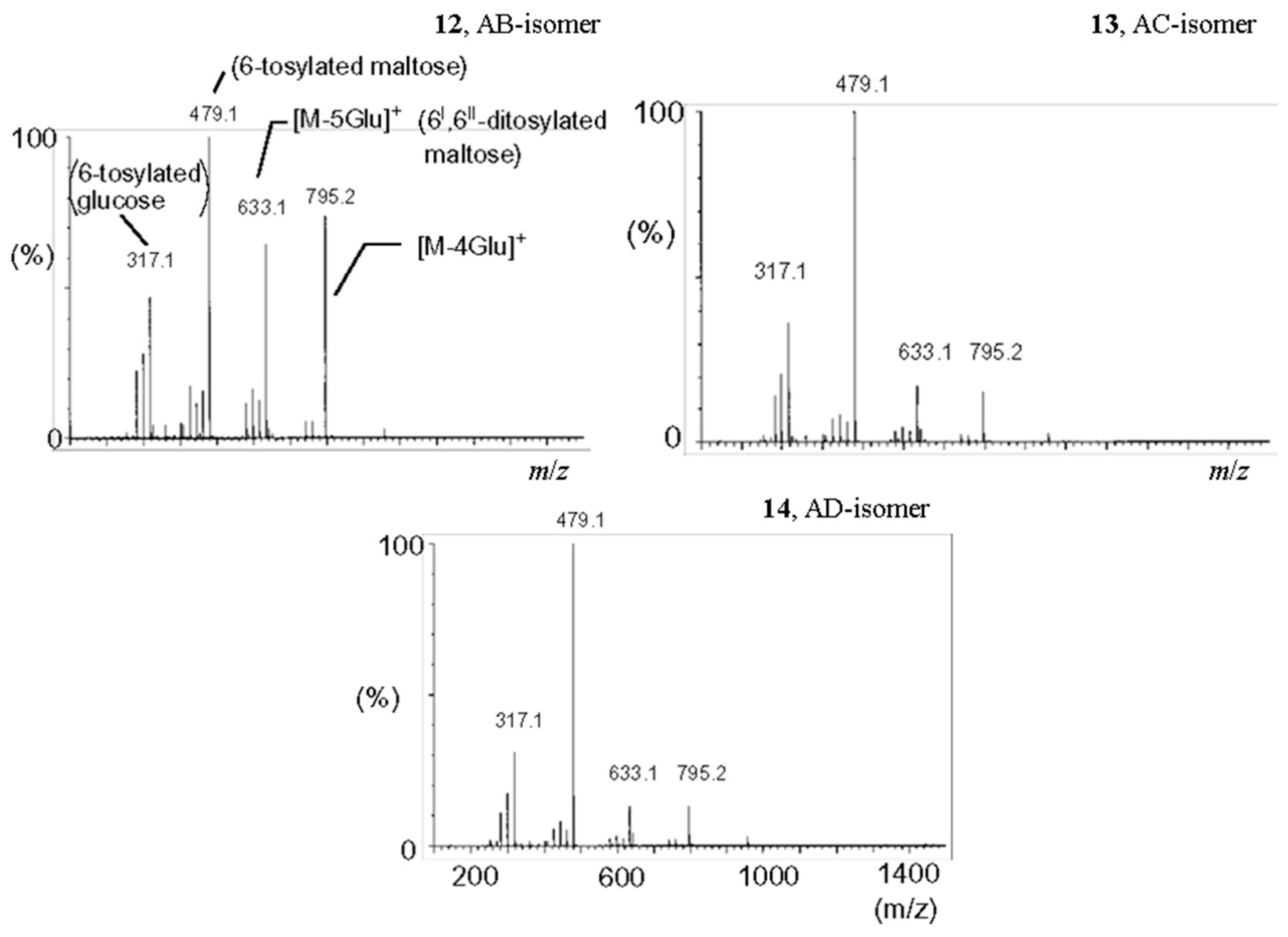
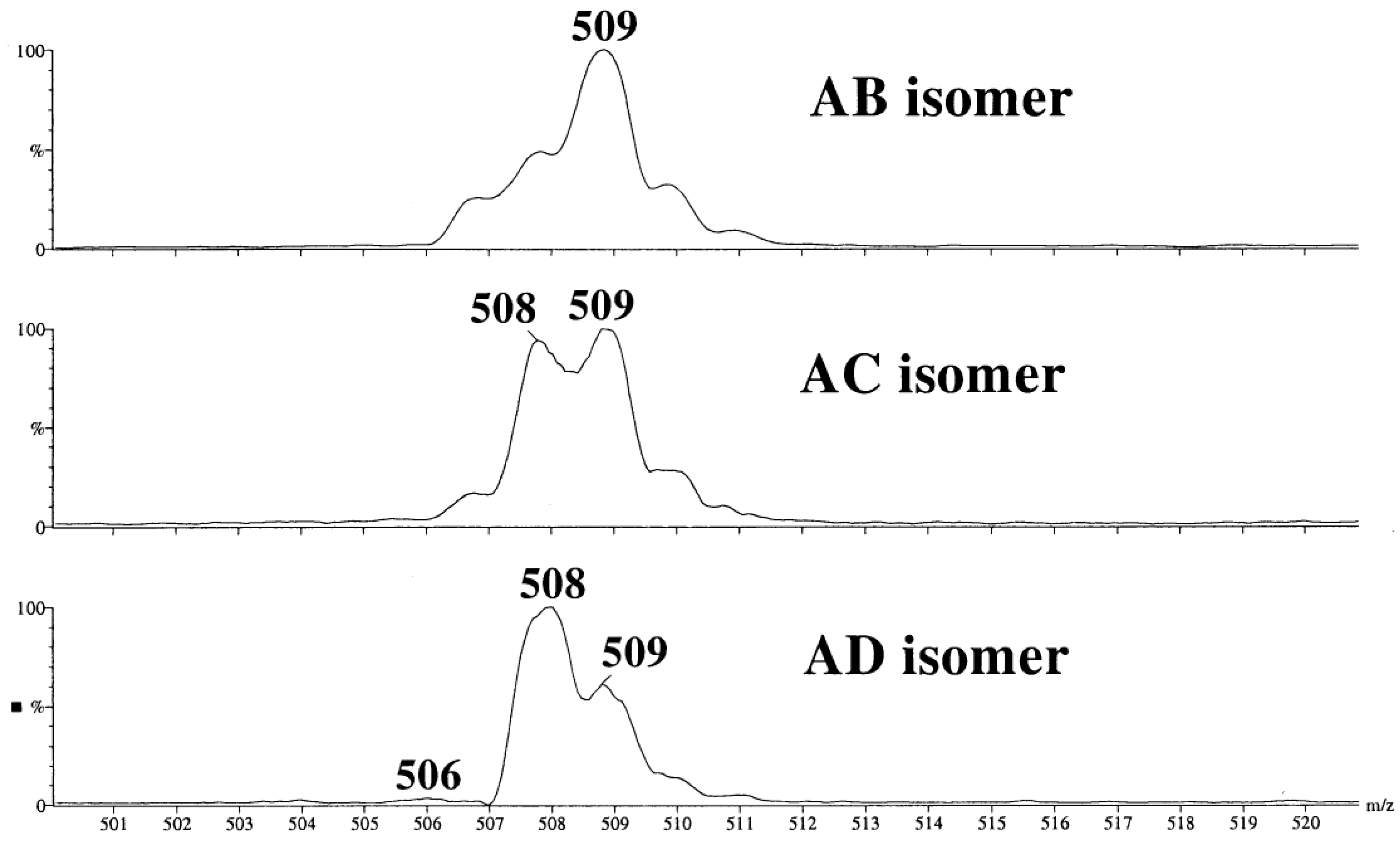
2.5.2. Tandem Mass Spectra of Disubstituted CDs: Effect of Positions of Substituents
- (1)
- Are the bonds between substituents and the carbohydrate units strong enough in comparison to glycosidic bonds, and
- (2)
- Do transitions of substituents between carbohydrate residues (i.e., rearrangements) take place?
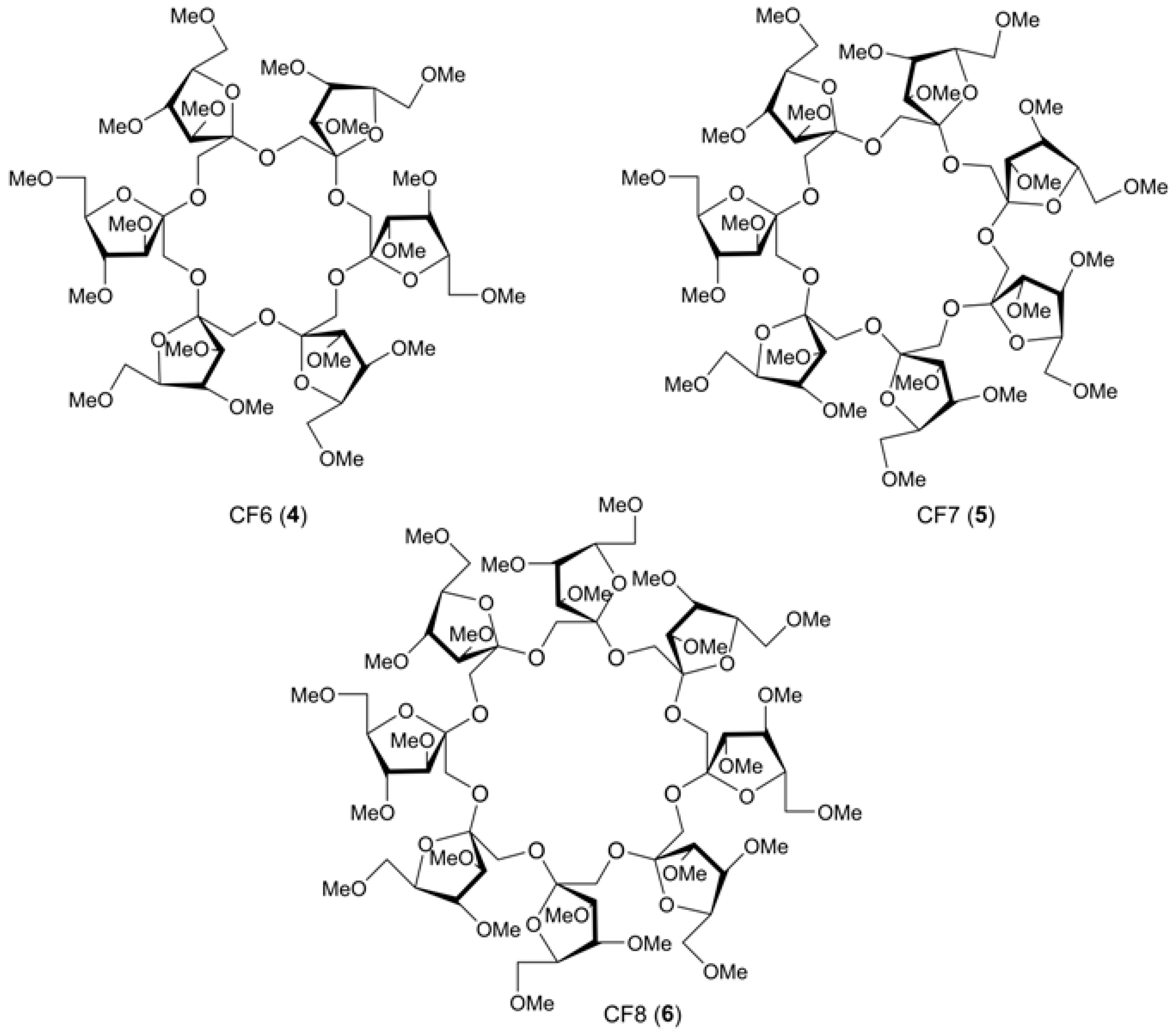
2.6. Tandem Mass Spectra of Complex And Highly Substituted CD Derivatives
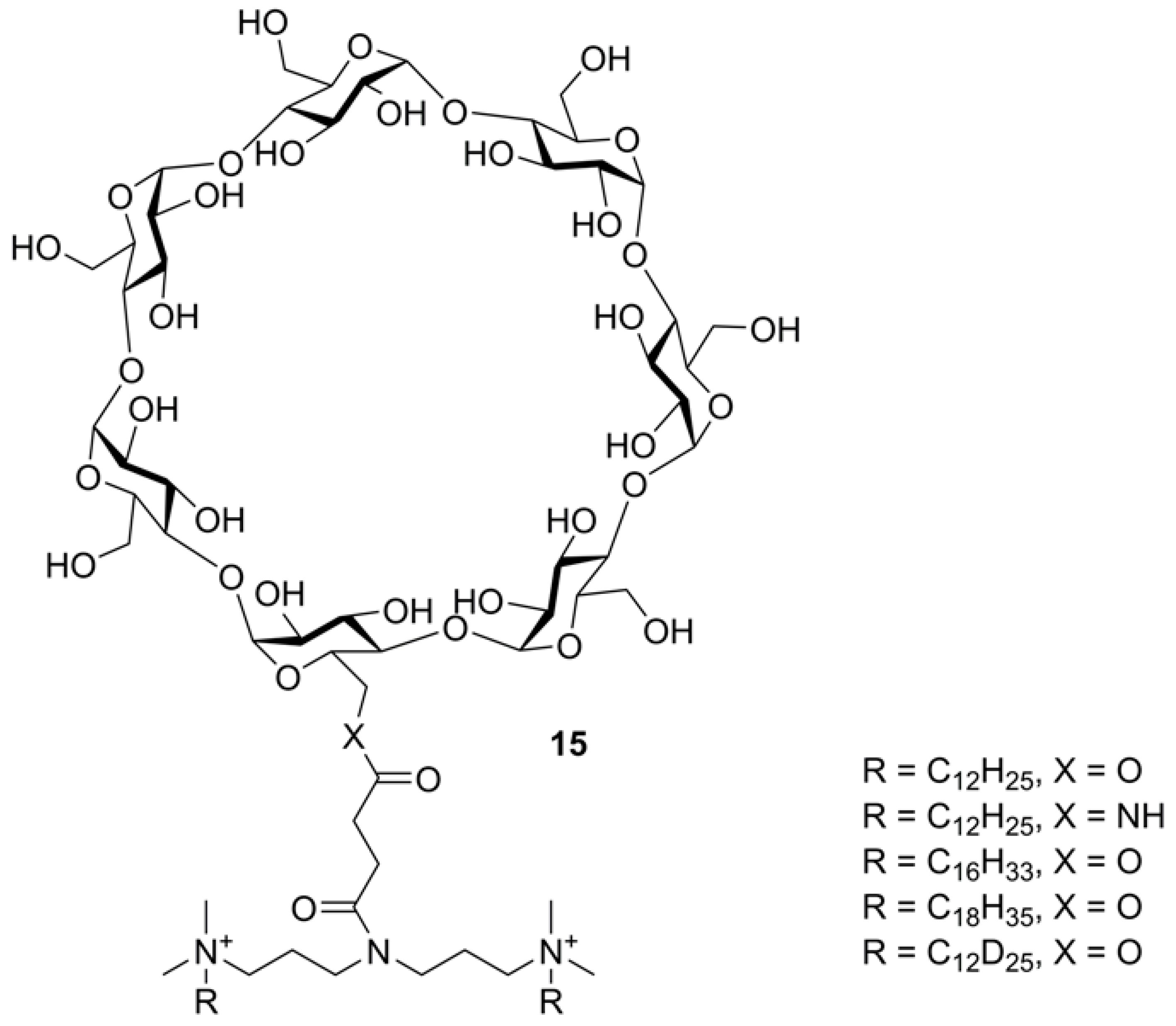
2.6.1. Tandem Mass Spectra of CD Derivatives Possessing Succinyl-Spacered Dicationic Substituent (Gemini Surfactants)
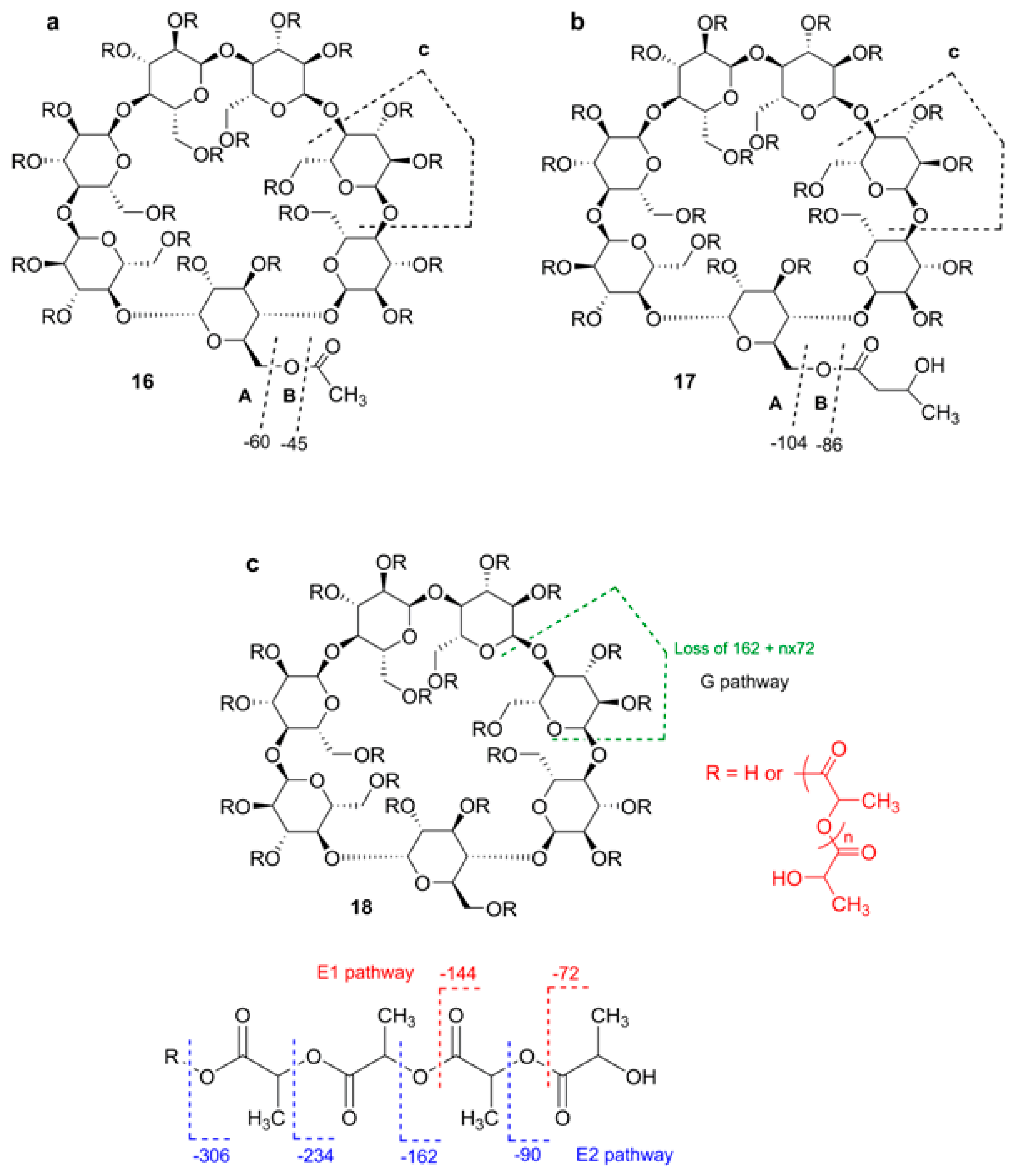
2.6.2. Tandem Mass Spectra of Polysubstituted (Multiply Acylated) CD Derivatives
2.7. Tandem Mass Spectra of Cycloaminooligosaccharides and Mixed Cyclooligosaccharides
2.7.1. General Information
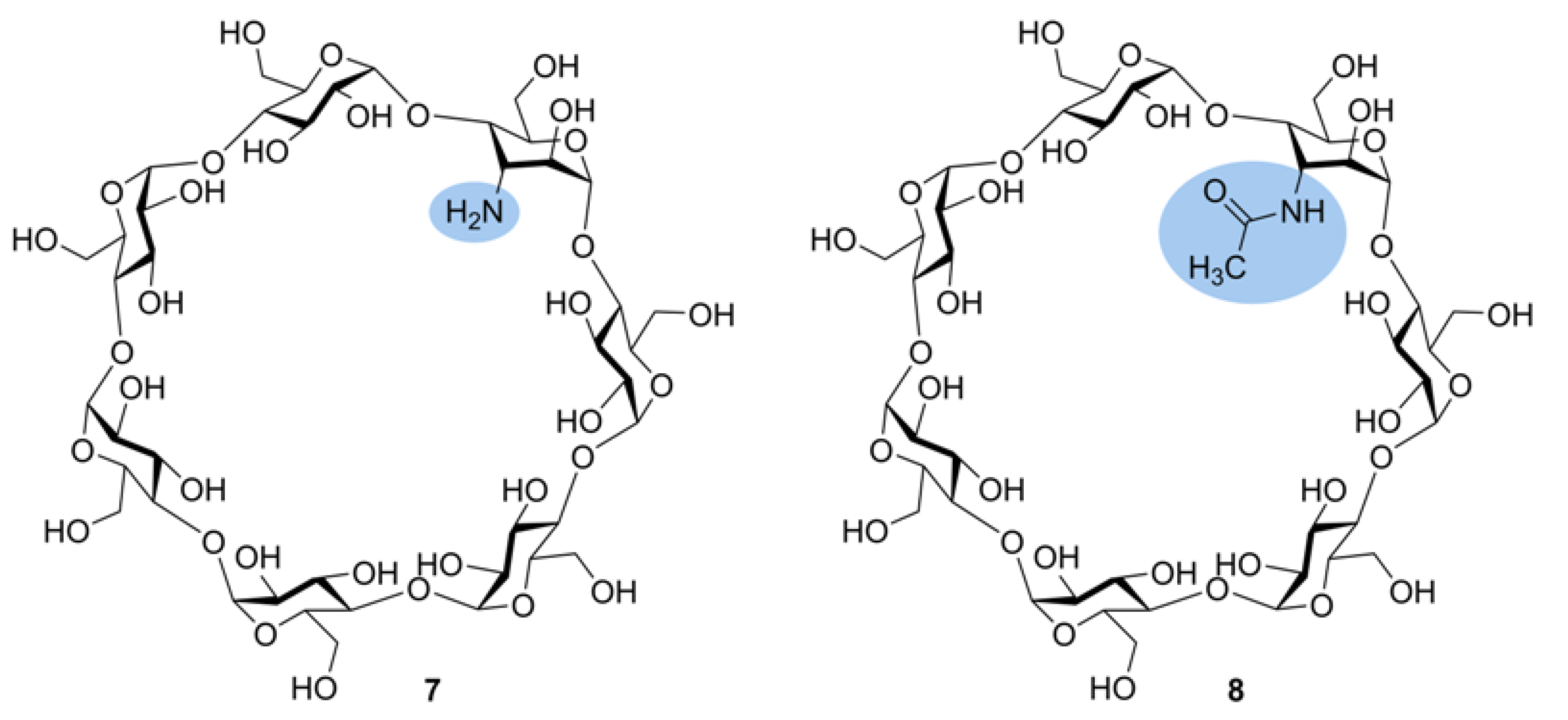
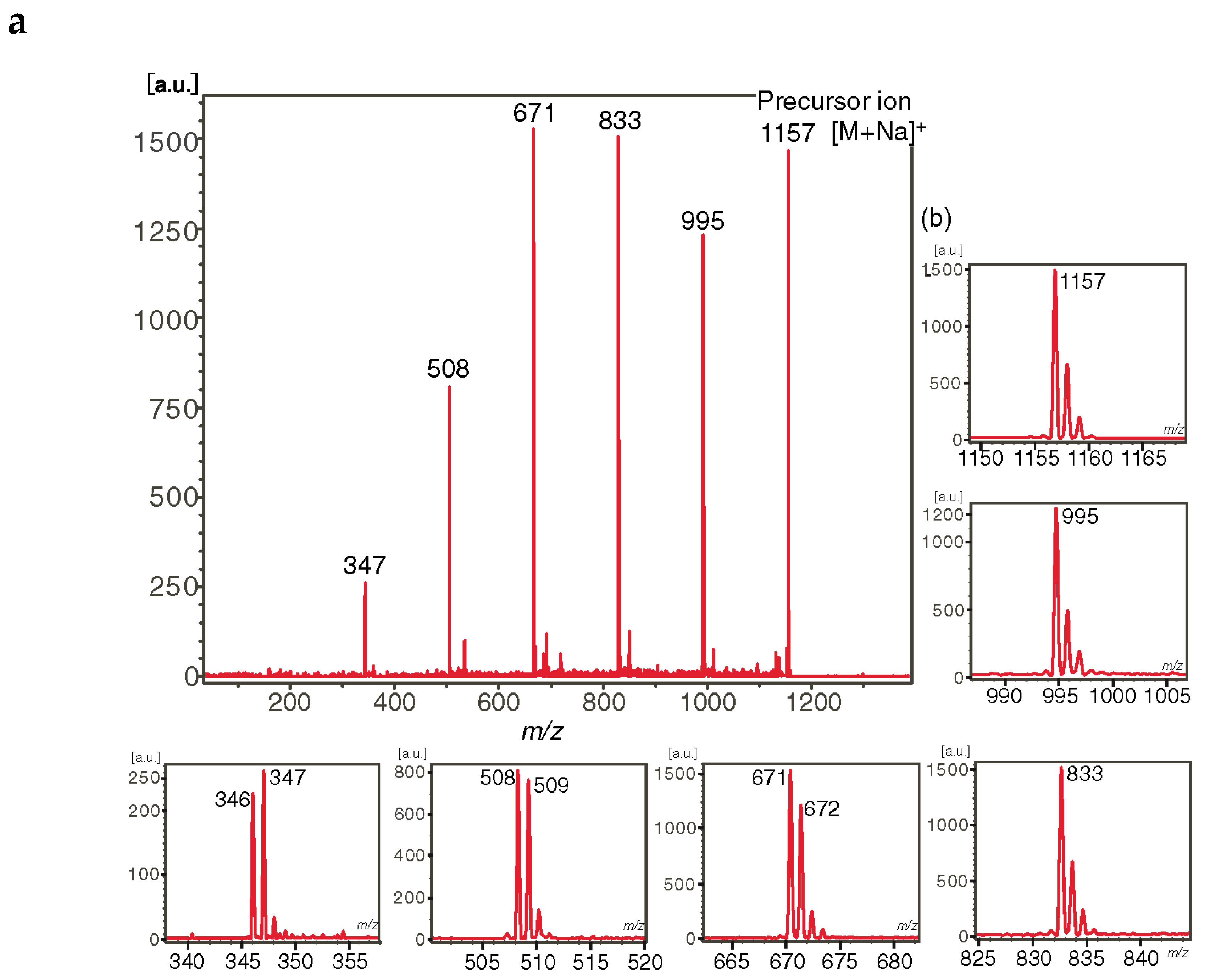
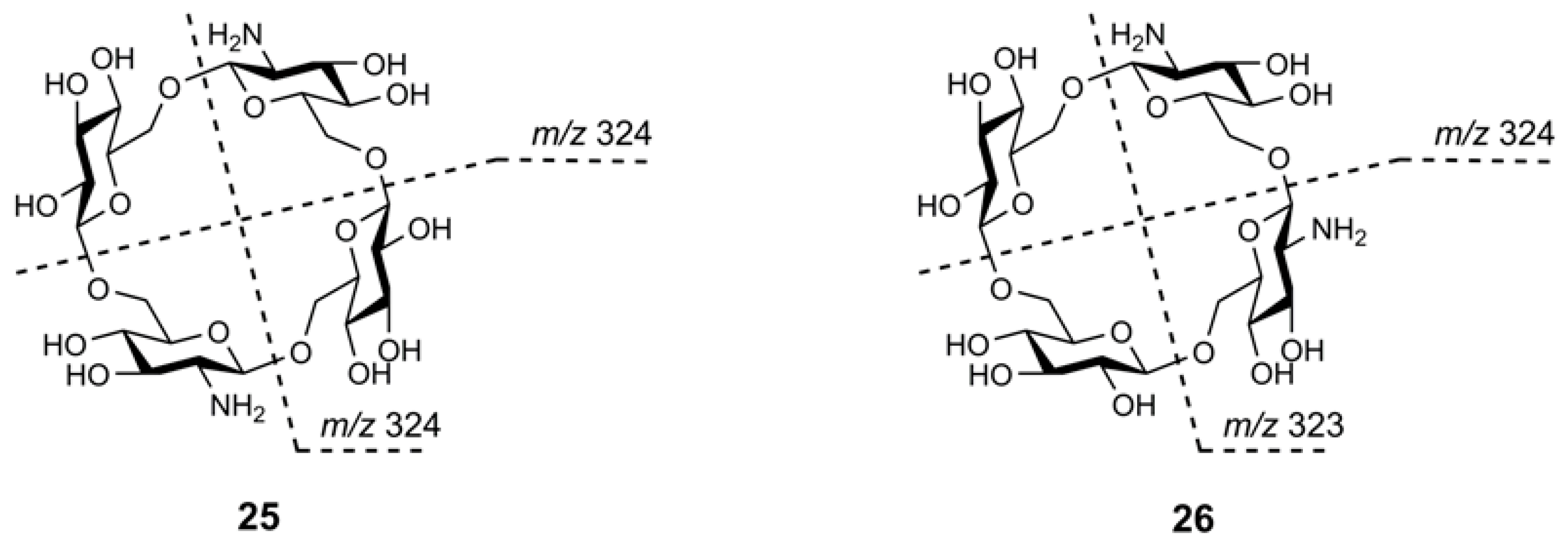
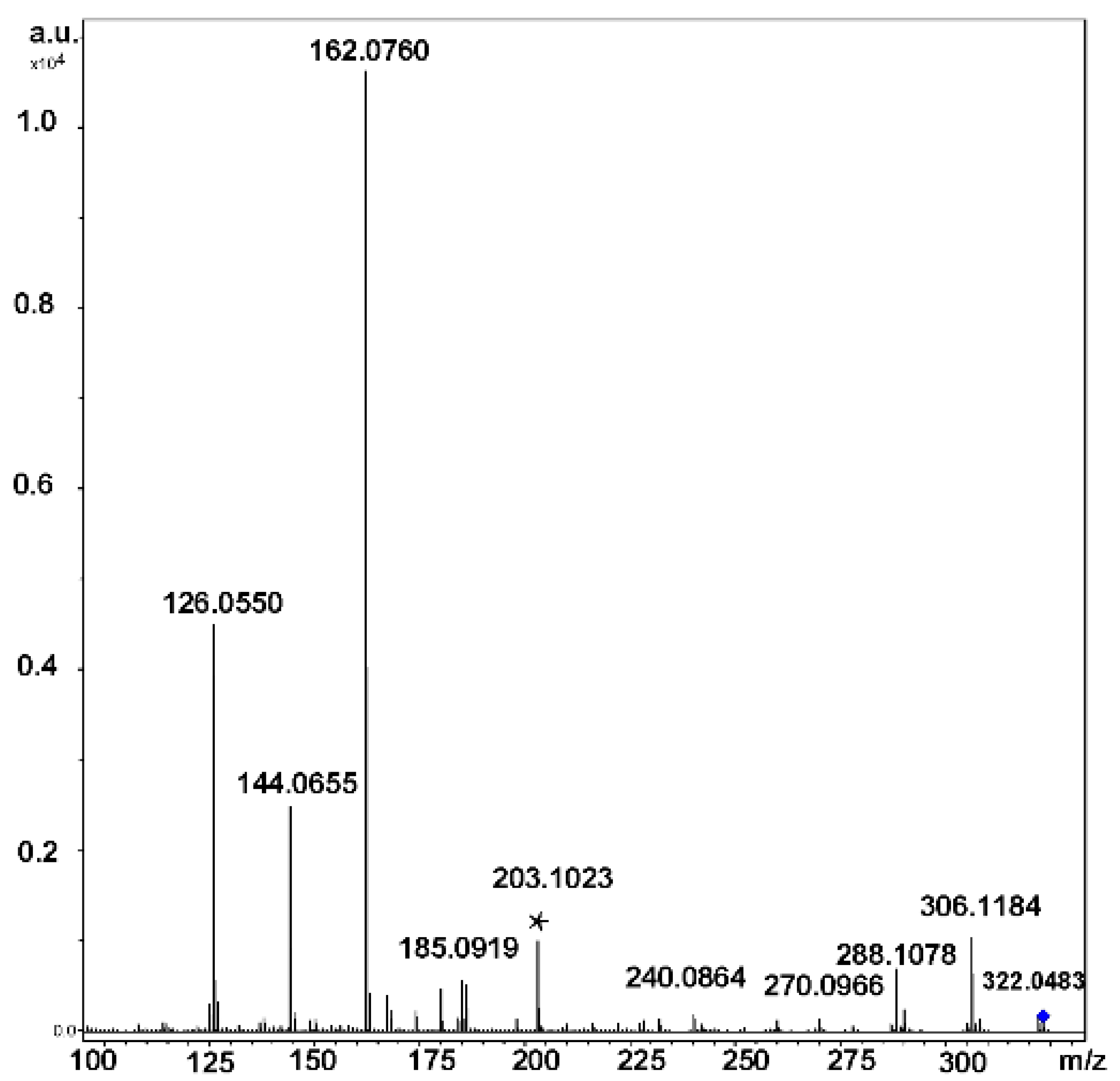
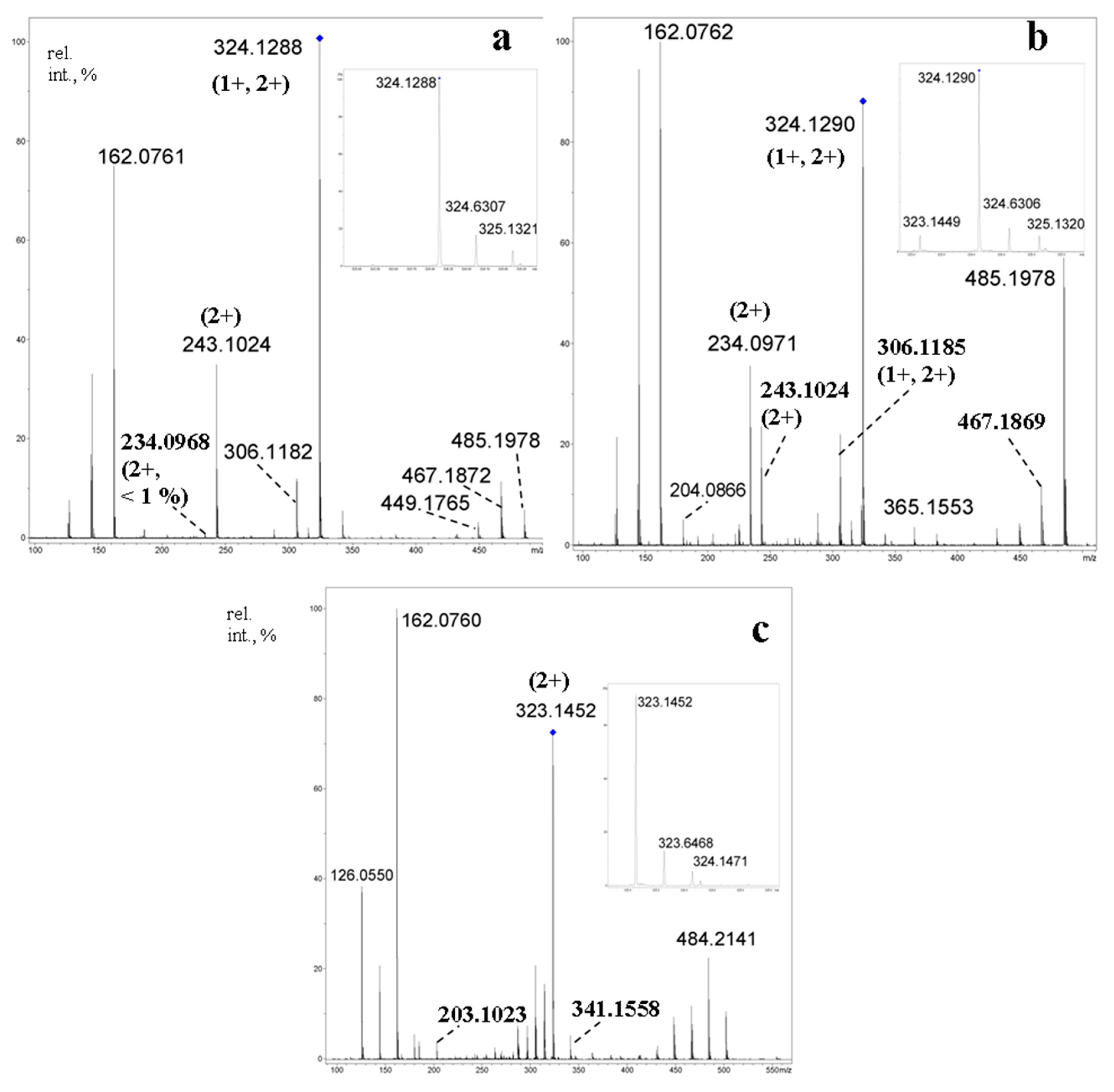
2.7.2. Tandem Mass Spectra of Cycloaminooligosaccharides
2.7.3. Tandem Mass Spectra of Mixed Cyclooligosaccharides
2.8. Miscellaneous
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Nepogodiev, S.A.; Stoddart, J.F. Synthetic cyclic oligosaccharides. Chem. Rev. 1998, 98, 1919–1958. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bogliotti, N. Synthesis and Applications of carbohydrate-derived macrocyclic compounds. Chem. Rev. 2014, 114, 7678–7739. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Analytical techniques for characterization of cyclodextrin compexes in aqueous solution: A review. Journ. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Delivery Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations. I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discovery Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ştefănescu, R.; Stanciu, G.D.; Luca, A.; Caba, I.C.; Tamba, B.I.; Mihai, C.T. Contributions of mass spectrometry to the identification of low molecular weight molecules able to reduce the toxicity of amyloid-β-peptide to cell cultures and transgenic mouse models of Alzheimer’s disease. Molecules 2019, 24, 1167. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.B. (Ed.) Electrospray and MALDI Mass Spectrometry: Fundamentals, Instrumentation, Practicalities, and Biological Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Zaya, J. Mass spectrometry of oligosaccharides. Mass Spectrom. Rev. 2004, 23, 161–227. [Google Scholar] [CrossRef]
- Voyksner, R.; Williams, F.P.; Smith, C.S.; Koble, D.L.; Seltzman, H.H. Analysis of cyclodextrins using fast atom bombardment/mass spectrometry and tandem mass spectrometry. Biol. Mass Spectrom. 1989, 18, 1071–1078. [Google Scholar] [CrossRef]
- Huang, E.C.; Henion, J.D. Characterization of cyclodextrins using ion-evaporation atmospheric-pressure ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1990, 4, 467–471. [Google Scholar] [CrossRef]
- Metzger J., W.; Jung, M.; Schmalzing, D.; Bayer, E.; Schurig, V. Analysis of cyclomalto-oligosaccharides (cyclodextrins) and derivatives thereof by ion-spray mass spectrometry. Carbohydr. Res. 1991, 222, 23–35. [Google Scholar] [CrossRef]
- Wei, W.; Chu, Y.; He, X.; Ding, C. Quantifying non-covalent binding affinity using mass spectrometry: A systematic study on complexes of cyclodextrins with alkali metal cations. Rapid Commun. Mass Spectrom. 2015, 29, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Kellersberger, K.A.; Dejsupa, C.; Liang, Y.; Pope, R.M.; Dearden, D.V. Gas-phase studies of ammonium-cyclodextrin compounds using Fourier transform ion cyclotron resonance. Internat. J. Mass Spectrom. 1999, 193, 181–195. [Google Scholar] [CrossRef]
- Yamamura, H.; Iwata, T.; Kawai, M.; Sato, A. Electrospray ionization tandem mass spectrometry determined regioisomeric structures of disubstituted β-cyclodextrin derivatives. Eur. J. Mass Spectrom. 2006, 12, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kellner, I.D.; Drewello, T. Influence of single skimmer versus dual funnel transfer on the appearance of ESI-generated LiCl cluster/β-cyclodextrin inclusion complexes. J. Am. Soc. Mass Spectrom. 2015, 26, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Berland, K.; Renauld J., B.; Mayer P., M. Utilizing ion mobility and tandem mass spectrometry to evaluate the structure and behaviour of multimeric cyclodextrin complexes. Can. J. Chem. 2015, 93, 1313–1319. [Google Scholar] [CrossRef]
- Kralj, B.; Šmidovnik, A.; Kobe, J. Mass spectrometric investigations of α- and β-cyclodextrin complexes with ortho-, meta-, and para-coumaric acids by negative mode electrospray ionization. Rapid Commun. Mass Spectrom. 2009, 23, 171–180. [Google Scholar] [CrossRef]
- Carroll, J.A.; Penn, S.G.; Fannin, S.T.; Wu, J.; Cancilla, M.T.; Green, M.K.; Lebrilla, C.B. A dual vacuum chamber Fourier transform mass spectrometer with rapidly interchangeable LSIMS, MALDI, and ESI sources: Initial results with LSIMS and MALDI. Anal. Chem. 1996, 68, 1798–1804. [Google Scholar] [CrossRef]
- Mele, A.; Malpezzi, L. Noncovalent association phenomena of 2,3-dihydroxybenzoic acid with cyclic and linear oligosaccharides. A matrix-assisted laser desorption/ionization time-of-flight mass spectrometric and X-ray crystallographic study. J. Am. Soc. Mass Spectrom. 2000, 11, 228–236. [Google Scholar] [CrossRef]
- Shizuma, M.; Takai, Y.; Kawamura, M.; Takeda, T.; Sawada, M. Complexation characteristics of permethylated cycloinulohexaose, cycloinuloheptaose, and cycloinulooctaose with metal cations. J. Chem. Soc. Perkin Trans. 2001, 2, 1306–1314. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.H.; Wang, J.; Kroll, P.; Shug, K.A.; Armstrong, D.W. Study of complexation between cyclofructans and alkali metal cations by electrospray ionization mass spectrometry and density functional theory calculations. Int. J. Mass Spectrom. 2010, 291, 118–127. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yao, L.; Sun, C.; Zheng, S.; Pan, Y. Evaluation and determination of the cyclofructans–amino acid complexes binding pattern by electrospray ionizatiom mass spectrometry. J. Mass Spectrom. 2014, 49, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cui, M.; Liu, S.; Song, F.; Elkin, Y.N. Electrospray ionization mass spectrometry of cyclodextrin complexes with amino acids in incubated solution and in eluates of gel permeation chromatography. Rapid Commun. Mass Spectrom. 1998, 12, 2016–2022. [Google Scholar] [CrossRef]
- Wei, W.; Chu, Y.; Ding, C. Gas-phase binding of noncovalent complexes between α-cyclodextrin and amino acids investigated by mass spectrometry. Analyt. Lett. 2014, 47, 2221–2237. [Google Scholar] [CrossRef]
- Chen, Y.; Zuo, Z.; Dai, X.; Xiao, P.; Fang, X.; Wang, X.; Wang, W.; Ding, C.-F. Gas-phase complexation of α-/β-cyclodextrin with amino acids studied by ion-mobility-mass spectrometry and molecular dynamics simulations. Talanta 2018, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barylyuk, K.; Balabin, R.M.; Grűnstein, D.; Kikkeri, R.; Frankevich, V.; Seeberger, P.H.; Zenobi, R. What happens to hydrophobic interactions during transfer from the solution to the gas phase? The case of electrospray-based soft ionization methods. J. Am. Soc. Mass Spectrom. 2011, 22, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Lebrilla, C.B. The gas-phase chemistry of cyclodextrin inclusion complexes. Acc. Chem. Res. 2001, 34, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yao, Z.-P. Chiral recognition and determination of enantiomeric excess by mass spectrometry: A review. Anal. Chim. Acta 2017, 968, 1–20. [Google Scholar] [CrossRef]
- Przybylski, C.; Bonnet, V.; Cėzard, C. Probing the common alkali metal affinity of native and variously methylated β-cyclodextrins by combining electrospray tandem mass spectrometry and molecular modeling. Phys. Chem. Chem. Phys. 2015, 17, 19288–19305. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Endresz, G.; Blaschke, G.; Juza, M.; Jakubetz, H.-J.; Schurig, V. Analysis of charged cyclomaltooligosaccharide (cyclodextrin) derivatives by ion-spray, matrix-assisted laser desorption/ionization time-of-flight and fast atom bombardment mass spectrometry, and by capillary electrophoresis. Carbohydr. Res. 1996, 287, 139–155. [Google Scholar] [CrossRef]
- Madhusudanan, K.P. Multiple lithium exchange under lithium cationization of cyclodextrins. J. Mass Spectrom. 2003, 38, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Franski, R.; Gierczyc, B.; Schroeder, G.; Beck, S.; Springer, A.; Linscheid, M. Mass Spectrometric decompositions of cationized β-cyclodextrin. Carbohydr. Res. 2005, 340, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wei, W.; Chu, Y.; Liu, Z.; Ding, C. Investigation of non-covalent complexes of cyclodextrins with Li+ in gas phase by mass spectrometry. Chin. J. Chem. Phys. 2013, 26, 287–294. [Google Scholar] [CrossRef][Green Version]
- Kostyukevich, Y.T.; Kononikhin, A.S.; Bugrova, A.E.; Starodubtseva, N.L.; Popov, I.; Nikolaev, E. Investigation of ion-molecular complexes of beta-cyclodextrin with proteins and metals in gas phase. Macroheterocycles 2017, 10, 110–116. [Google Scholar] [CrossRef]
- Sforza, S.; Galaverna, G.; Corradini, R.; Dossena, A.; Marchelli, R. ESI-mass spectrometry analysis of unsubstituted and disubstituted β-cyclodextrins: Fragmentation mode and identification of the AB, AC, AD regioisomers. J. Am. Soc. Mass Spectrom. 2003, 14, 124–135. [Google Scholar] [CrossRef]
- Jacquet, R.; Elfakir, C.; Lafosse, M. Characterization of new methylated beta-cyclodextrin with low degree of substitution by electrospray ionization mass spectrometry and liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 3097–3102. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.A.; Ngoka, L.; Beggs, C.G.; Lebrilla, C.B. Liquid secondary ion mass spectrometry/Fourier transform mass spectrometry of oligosaccharide anions. Anal. Chem. 1993, 65, 1582–1587. [Google Scholar] [CrossRef]
- Wang, L.; Chai, Y.; Sun, C.; Armstrong, D.W. Complexation of cyclofructans with transition metal ions studied by electrospray ionization mass spectrometry and collision-induced dissociation. Int. J. Mass Spectrom. 2012, 323–324, 21–27. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjugate J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Osman, S.F.; Hotchkiss, A.; Bhagwat, A.A.; Keister, D.L.; Valentine, K.M. Cyclolaminarinose. A new biologically active β-(1→3) cyclic glucan. Carbohydr. Res. 1996, 296, 23–37. [Google Scholar] [CrossRef]
- Bhagwat, A.A.; Mithőfer, A.; Pfeffer, P.E.; Kraus, C.; Spickers, N.; Hotchkiss, A.; Ebel, J.; Keister, D.L. Further studies of the role of cyclic β-glucans in symbiosis. An ndvC mutant of Bradyrhizobium japonicum synthesizes cyclodekakis-(1→3)-β-glucosyl. Plant. Physiol. 1999, 119, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Reason, A.J.; Haslam, S.M.; McDowell, R.A.; Chizhov, O.S.; Usov, A.I. Structural analysis of laminarans by MALDI and FAB mass spectrometry. Carbohydr. Res. 1998, 310, 203–210. [Google Scholar] [CrossRef]
- Dell, A.; York, S.; McNeil, M.; Darvill, A.G.; Albersheim, P. The cyclic structure of β-d-(1→2)-linked D-glucans secreted by Rhizobia and Agrobacteria. Carbohydr. Res. 1983, 117, 185–200. [Google Scholar] [CrossRef]
- Talaga, P.; Stahl, B.; Wieruszeski, J.-M.; Hillenkamp, F.; Tsuyumu, S.; Lippens, G.; Bohin, J.-P. Cell-associated glucans of Bukholderia solanacearum and Xantomonas campestris pv. citri: A new family of periplasmic glucans. J. Bacteriol. 1996, 178, 2263–2271. [Google Scholar]
- Jung, Y.; Park, H.; Cho, E.; Jung, S. Strucural analyses of novel glycerophosphorylated α-cyclosophorohexadecaoses isolated from X. campestris pv. campestris. Carbohydr. Res. 2005, 340, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Kwon, C.; Lee, S.; Tahir, M.N.; Park, S.; Jung, S. Biotinylation of the rhizobial cyclic β-glucans and succinoglycans crucial for symbiosis with legumes. Carbohydr. Res. 2014, 389, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Lee, H.M.; Jang, S.; Shin, J. Comparison of ionization behaviors of ring and linear carbohydrates in MALDI-TOF MS. Internat. J. Mass Spectrom. 2009, 279, 53–58. [Google Scholar] [CrossRef]
- Bashir, S.; Giannakopulos, A.E.; Derrick, P.J.; Critchley, P.; Bottrill, A.; Padley, H.D. Matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. A comparison of fragmentation patterns of linear dextran obtained by in-source decay, post-source decay and collision-induced dissociation and stability of linear and cyclic glucans studied by in-source decay. Eur. J. Mass Spectrom. 2004, 10, 109–120. [Google Scholar]
- Przybylski, C.; Bonnet, V. Discrimination of cyclic and linear oligosaccharides by tandem mass spectrometry using collision-induced dissociation (CID), pulsed-Q-dissociation, and the higher energy C-trap dissociation modes. Rapid Comm. Mass Spectrom. 2013, 27, 75–87. [Google Scholar] [CrossRef]
- Vrkic, A.K.; O’Hair, A.J.; Lebrilla, C.B. Unusual covalent bond-breaking reactions of β-cyclodextrin inclusion complexes of nucleobases/nucleosides and related guest molecules. Eur. J. Mass Spectrom. 2003, 9, 563–577. [Google Scholar] [CrossRef]
- Mele, A.; Panzeri, W.; Selva, A.; Mauri, P. Fast atom bombardment, electrospray, ionspray and tandem mass spectrometry of 1 : 1 β-cyclodextrin/5-methoxytryptamine hydrochloride host-guest complex: Host protonation and fragmentation due to guest deamination. Eur. J. Mass Spectrom. 2000, 6, 169–174. [Google Scholar] [CrossRef]
- Cai, Y.; Tarr, M.A.; Xu, G.; Yalcin, T.; Cole, R.B. Dication induced stabilization of gas-phase ternary beta-cyclodextrin inclusion complexes observed by electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 449–459. [Google Scholar] [CrossRef]
- Tripodo, G.; Wischke, C.; Neffe, A.T.; Lendlein, A. Efficient synthesis of pure monotosylated beta-cyclodextrin. Carbohydr. Res. 2013, 381, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Yamagaki, T.; Sugahara, K.; Watanabe, T. Amino and acetamide functional group effects on the ionization and fragmentation of sugar chains in positive-ion mass spectrometry. J. Am. Soc. Mass Spectrom. 2014, 25, 95–103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kovačik, V.; Pātoprsty, V.; Havliček, V.; Kovač, P. Matrix-assisted laser desorpton/ionization and electrospray ionization mass spectrometry: Sodium cationized oligosaccharides do not exhibit "internal-residue" loss rearrangement. Eur. Mass Spectrom. 1998, 4, 417–420. [Google Scholar] [CrossRef]
- Wuhrer, M.; Deelder, A.M.; van der Burgt, Y.E.M. Mass spectrometric glycan rearrangements. Mass Spectrom. Rev. 2011, 30, 664–680. [Google Scholar] [CrossRef] [PubMed]
- Donkuru, M.; Chitanda, J.M.; Verrall, R.E.; El Aneed, A. Multi-stage tandem mass spectrometric analysis of novel β-cyclodextrin-substituted and novel bis-pyridinium gemini surfactants designed as nanomedical drug delivery agents. Rapid Comm. Mass Spectrom. 2014, 28, 757–772. [Google Scholar] [CrossRef]
- Takashima, Y.; Osaki, M.; Harada, A. Cyclodextrin-initiated polymerization of cyclic esters in bulk: Formation of polyester-tethered cyclodextrins. J. Am. Chem. Soc. 2004, 126, 13588–13589. [Google Scholar] [CrossRef]
- Shen, J.; Hao, A.Y.; Du, G.Y.; Zhang, H.C.; Sun, H.Y. A convenient preparation of 6-oligo(lactic acid) cyclomaltohexaose as kinetically degradable derivative for controlled release of Amoxicillin. Carbohydr. Res. 2008, 343, 2517–2522. [Google Scholar] [CrossRef]
- Peptu, C.; Nicolescu, A.; Peptu, C.A.; Harabagiu, V.; Simionescu, B.C.; Kowalczuk, M. Mass spectrometry characterization of 3-OH butyrated β-cyclodextrin. J. Polym. Sci., Part. A: Polym. Chem. 2010, 48, 5581–5592. [Google Scholar] [CrossRef]
- Miao, Y.; Rousseau, C.; Mortreux, A.; Martin, P.; Zinck, P. Access to new carbohydrate-functionalized polylactides via organocatalyzed ring-opening polymerization. Polymer 2011, 52, 5018–5026. [Google Scholar] [CrossRef]
- Peptu, C.; Balan-Porcarasu, M.; Šiškova, A.M.; Škultėty, Ľ.; Mosnaček, J. Cyclodextrin tethered with oligolactides—Green synthesis and structural assessment. Beilstein J. Org. Chem. 2017, 13, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, S.; Liu, H.; Yang, C.; Kang, Y.; Wang, M. Unimolecular micelles of amphiphilic cyclodextrin-core star-like block copolymers for anticancer drug delivery. Chem. Commun. 2010, 51, 15568–15771. [Google Scholar] [CrossRef] [PubMed]
- Peptu, C.; Harabagiu, V. Tandem mass spectrometry characterization of esterified cyclodextrins. Dig. J. Nanomater. Biostruct. 2013, 8, 1551–1561. [Google Scholar]
- Peptu, C.; Danchenko, M.; Škultėty, Ľ.; Mosnaček, J. Structural architectural features of cyclodextrin oligoesters revealed by fragmentation mass spectrometry analysis. Molecules 2018, 23, 2259. [Google Scholar] [CrossRef] [PubMed]
- Peptu, C.; Kwiecien, I.; Harabagiu, V.; Simionescu, B.C.; Kowalczuk, M. Modification of β-cyclodextrin through solution ring-opening oligomerization of β-butyrolactone. Cellulose Chem. Technol. 2014, 48, 1–10. [Google Scholar]
- Kieken, F.; West, C.; Keddadouche, K.; Elfakir, C.; Choisnard, L.; Gese, A.; Wouessidjewe, D. Characterization of complex amphiphilic cyclodextrin mixturtes by high-performance liquid chromatography and mass spectrometry. J. Chromatogr. A 2008, 1189, 385–391. [Google Scholar] [CrossRef]
- Normand, M.; Kirillov, E.; Carpentier, J.-F.; Guillaume, S.M. Cyclodextrin-centered polyesters. Controlled ring-opening polymerization of cyclic esters from β-cyclodextrin-diol. Macromolecules 2012, 45, 1122–1130. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Gening, M.L.; Pinsker, O.A.; Yudina, O.N.; Tsvetkov, Y.E.; Nifantiev, N.E. Gas-phase fragmentation studies of cyclic oligo-(1→6)-β-d-glucosamines by electrospray mass spectrometry using a hybrid high-resolution mass spectrometer. Russ. Chem. Bull. 2018, 67, 144–149. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Gening, M.L.; Pinsker, O.A.; Tsvetkov, Y.E.; Nifantiev, N.E. Isomeric effects in collisionally-induced dissociation of β-(1→6)-linked cyclic tetrasaccharides of the Glcp2GlcpN2 composition. Mass-Spectrometria 2018, 15, 262–266, (in Russ., Engl. Transl. in J. Analyt. Chem., in press). [Google Scholar]
- Gening, M.L.; Titov, D.V.; Grachev, A.A.; Gerbst, A.G.; Yudina, O.N.; Chizhov, A.O.; Tsvetkov, Y.E.; Nifantiev, N.E. Synthesis, NMR and conformational studies of cyclic oligo-(1→6)-β-d-glucosamines. Eur. J. Org. Chem. 2010, 13, 2465–2475. [Google Scholar] [CrossRef]
- Gening, M.L.; Tsvetkov, Y.E.; Pier, G.B.; Nifantiev, N.E. Synthesis of oligo-β-(1→6)-glucosamines corresponding to the fragments of the surface polysaccharide of Staphylococcus aureus. Carbohydr. Res. 2007, 342, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Grachev, A.A.; Gerbst, A.G.; Gening, M.L.; Titov, D.V.; Yudina, O.N.; Tsvetkov, Y.E.; Shashkov, A.S.; Pier, G.B.; Nifantiev, N.E. NMR and conformational studies of linear and cyclic oligo-(1→6)-β-d-Glucosamines. Carbohydr. Res. 2011, 346, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Gening, M.L.; Titov, D.V.; Cecioni, S.; Audfray, A.; Gerbst, A.G.; Tsvetkov, Y.E.; Krylov, V.B.; Imberty, A.; Nifantiev, N.E.; Vidal, S. Synthesis of multivalent carbohydrate-centered glycoclusters as nanomolar ligands of the bacterial lectin LecA from Pseudomonas aeruginosa. Chem. Eur. J. 2013, 19, 9272–9275. [Google Scholar] [CrossRef] [PubMed]
- Gening, M.L.; Tsvetkov, Y.E.; Titov, D.V.; Gerbst, A.G.; Yudina, O.N.; Grachev, A.A.; Shashkov, A.S.; Vidal, S.; Imberty, A.; Saha, T.; et al. Linear and cyclic oligo-β-(1→6)-d-glucosamines: Synthesis, conformations and applications for design of a vaccine and oligodentate glycoconjugates. Pure Appl. Chem. 2013, 85, 1879–1891. [Google Scholar] [CrossRef]
- Saha, T.; Roy, A.; Gening, M.L.; Titov, D.V.; Gerbst, A.G.; Tsvetkov, Y.E.; Nifantiev, N.E.; Talukdar, P. Cyclo-oligo-(1→6)-β-d-glucosamine based artificial channels for tunable transmembrane ion transport. Chem. Commun. 2014, 50, 5514–5516. [Google Scholar] [CrossRef]
- Roy, A.; Saha, T.; Gening, M.L.; Titov, D.V.; Gerbst, A.G.; Tsvetkov, Y.E.; Nifantiev, N.E.; Talukdar, P. Trimodal control of ion transport activity on cyclo-oligo-(1→6)-β-d-glucosamine based artificial ion transport systems. Chem. Eur. J. 2015, 21, 17445–17452. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szeman, J.; Sohajda, T. Analytical characterization of cyclodextrins: History, official methods and recommended new techniques. Journ. Pharm. Biomed. Anal. 2016, 130, 347–365. [Google Scholar] [CrossRef]
- Moutard, S.; Djedaїni-Pilard, F.; Meudal, S.; Luijen, W.; Perly, B.; Pilard, S. Structural identification of new glycolipids based on cyclodextrin using high-resolution positive and negative electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2535–2540. [Google Scholar] [CrossRef]
- Lesur, D.; Gassama, A.; Moreau, V.; Djedani-Pilard, F.; Brique, A.; Pilard, S. Electrospray ionization mass spectrometry: A key analytical tool for the characterization of regioselectively derivatized maltooligosaccharides obtained starting from natural β-cyclodextrin. Rapid Commun. Mass Spectrom. 2006, 20, 747–754. [Google Scholar] [CrossRef]
- Greisch, J.-F.; Kritsoglou, S.; Leyh, B.; De Pauw, E. Mass spectrometric study of the ionized C60: (γ-cyclodextrin)2 inclusion complex by collision induced dissociation. J. Mass Spectrom. 2008, 43, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, C.; Jarroux, N. Analysis of polydisperse polyrotaxane based on poly(ethylene oxide) and α-cyclodextrins using nanoelectrospray and LTQ-orbitrap. Analyt. Chem. 2011, 83, 8460–8467. [Google Scholar] [CrossRef] [PubMed]
- Banoub, J.; Tibault, P.; Gouėth, P.Y.; Ronco, G.; Villa, P. Electrospray tandem mass spectrometry of a novel series of amphipathic functionalized ether-linked di- and trisaccharides and cyclic oligosaccharides. J. Mass Spectrom. 1997, 32, 109–121. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chizhov, A.O.; Tsvetkov, Y.E.; Nifantiev, N.E. Gas-Phase Fragmentation of Cyclic Oligosaccharides in Tandem Mass Spectrometry. Molecules 2019, 24, 2226. https://doi.org/10.3390/molecules24122226
Chizhov AO, Tsvetkov YE, Nifantiev NE. Gas-Phase Fragmentation of Cyclic Oligosaccharides in Tandem Mass Spectrometry. Molecules. 2019; 24(12):2226. https://doi.org/10.3390/molecules24122226
Chicago/Turabian StyleChizhov, Alexander O., Yury E. Tsvetkov, and Nikolay E. Nifantiev. 2019. "Gas-Phase Fragmentation of Cyclic Oligosaccharides in Tandem Mass Spectrometry" Molecules 24, no. 12: 2226. https://doi.org/10.3390/molecules24122226
APA StyleChizhov, A. O., Tsvetkov, Y. E., & Nifantiev, N. E. (2019). Gas-Phase Fragmentation of Cyclic Oligosaccharides in Tandem Mass Spectrometry. Molecules, 24(12), 2226. https://doi.org/10.3390/molecules24122226





