Optimization of Alkali Extraction and Properties of Polysaccharides from Ziziphus jujuba cv. Residue
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experimental Evaluation
2.2. Optimization of Extracting Conditions by the Box–Behnken Design
2.3. Response Surface Analysis
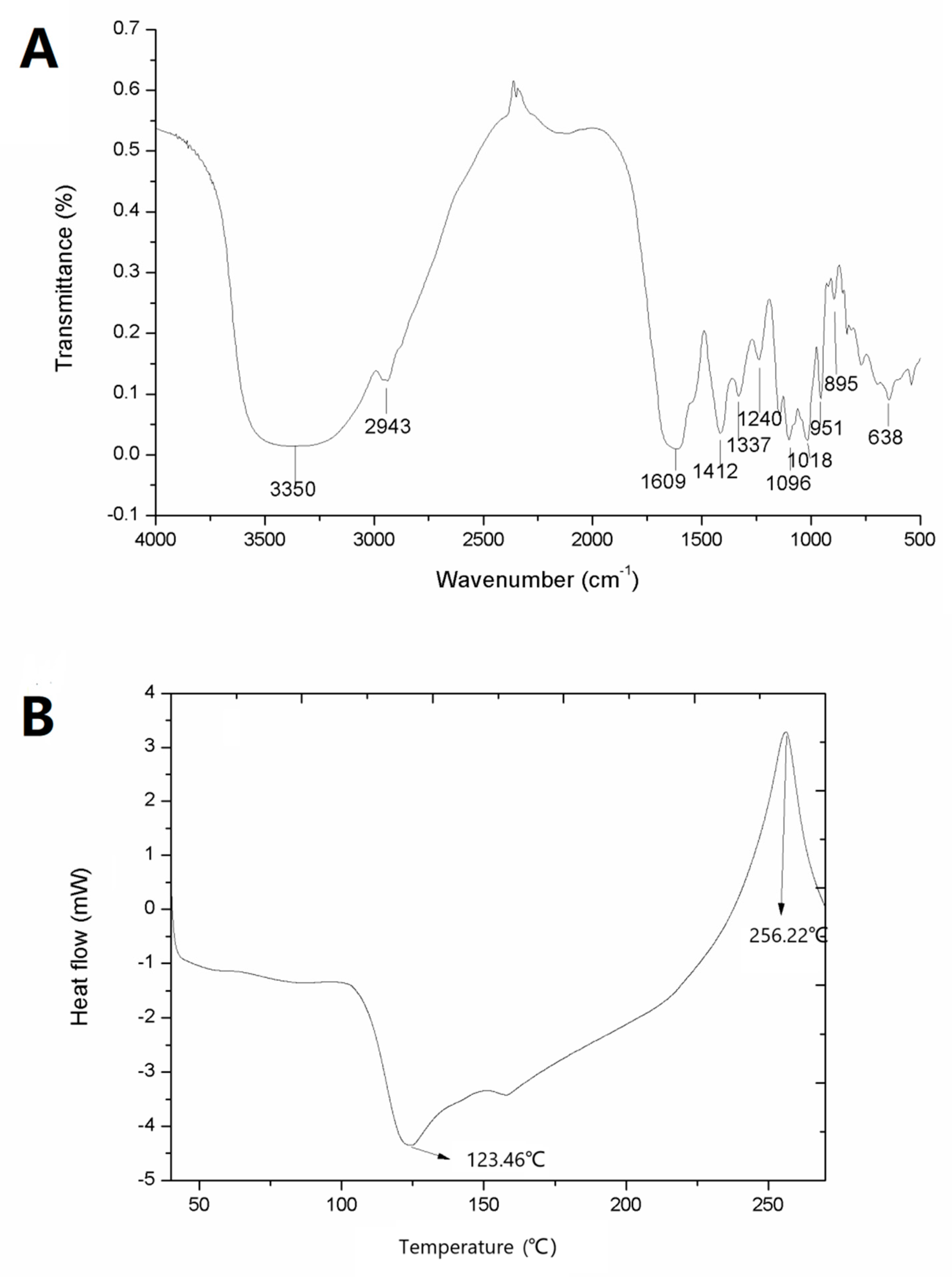
2.4. Physical and Chemical Properties of ZJRP
2.4.1. Chemical Analysis
2.4.2. FT-IR Analysis
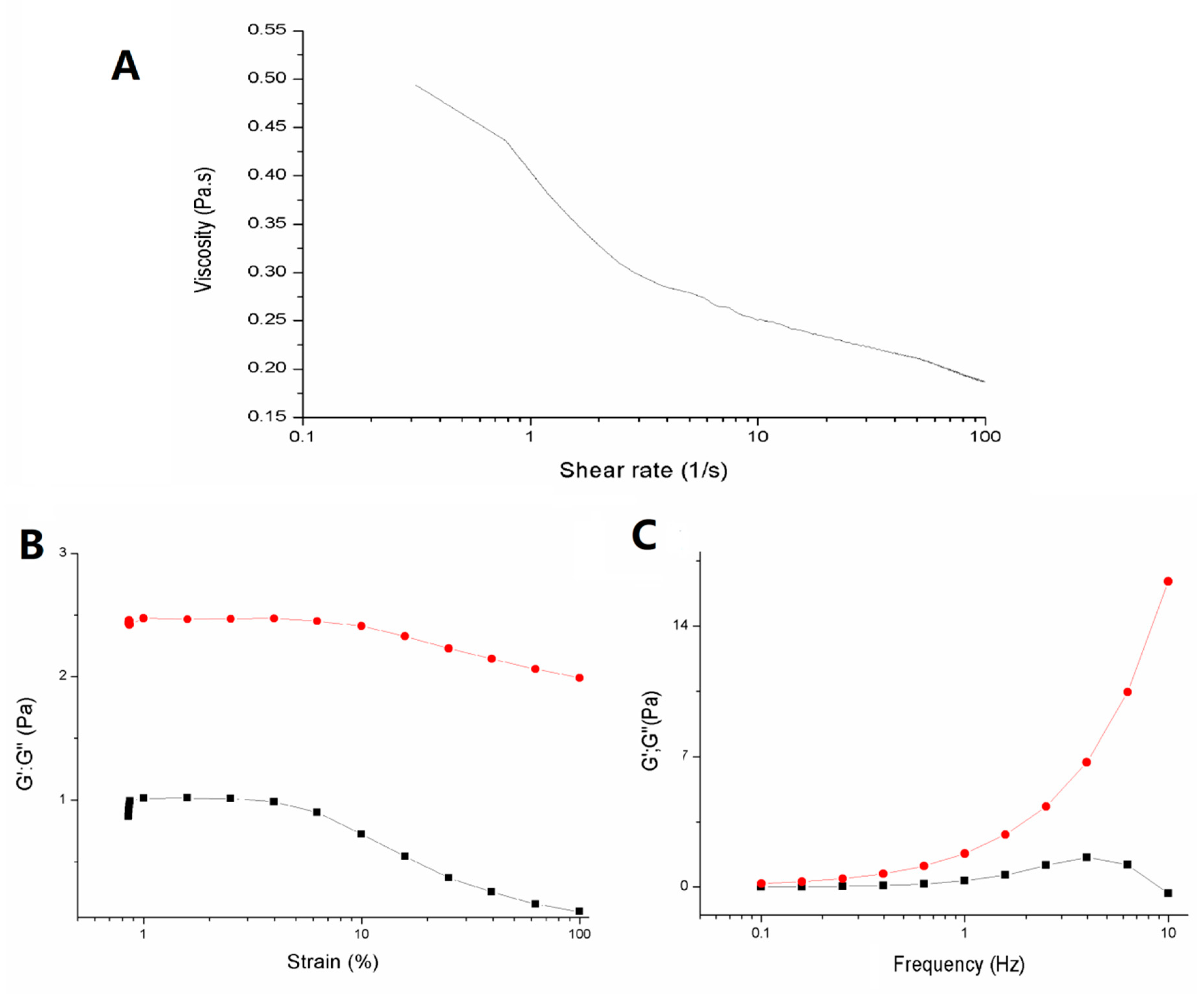
2.4.3. Thermal and Rheological Properties
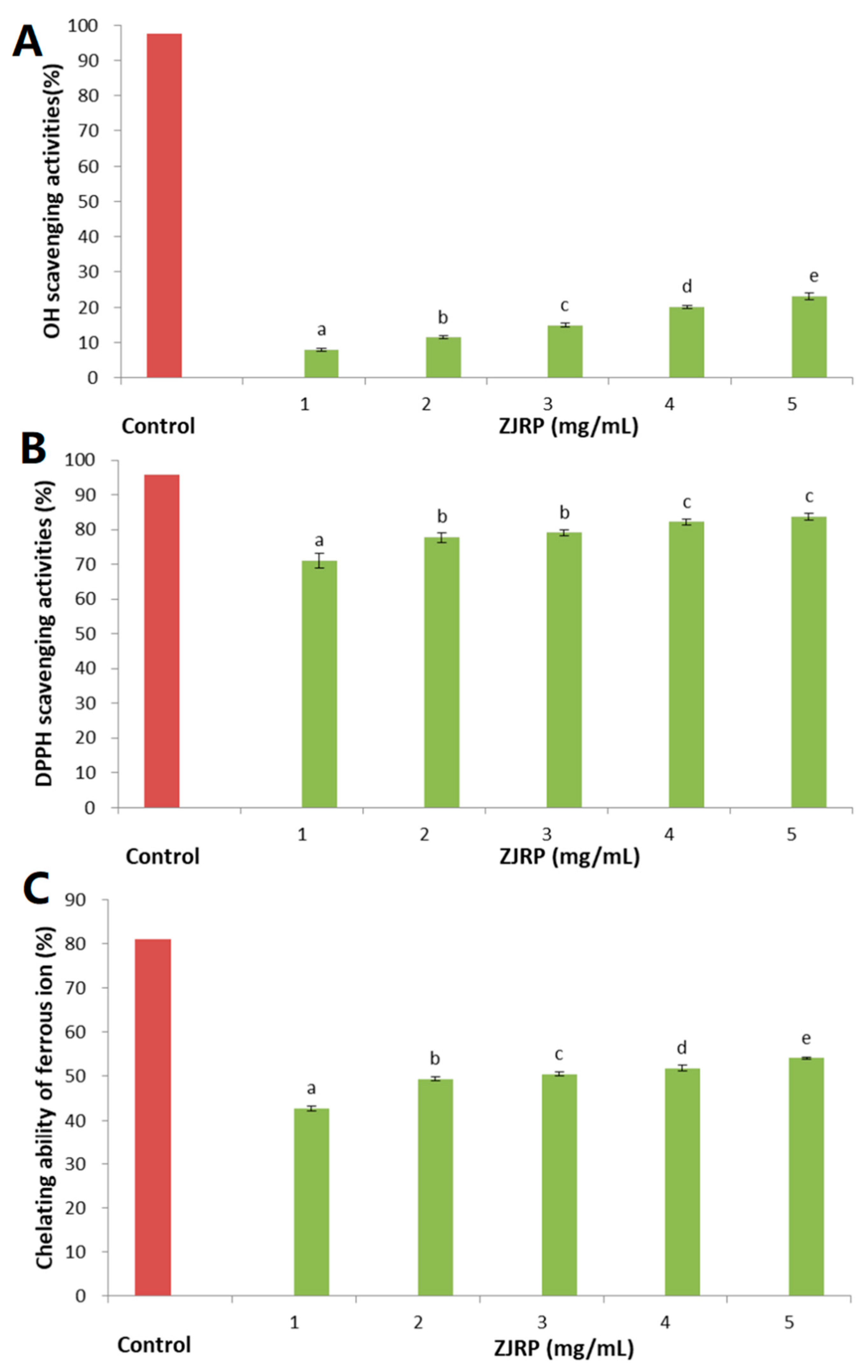
2.5. In Vitro Antioxidant Activity of ZJRP
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Ziziphus jujuba cv Residue
3.3. Extraction of Polysaccharides
3.4. Box–Behnken Design
3.5. Chemical Analysis
3.6. Monosaccharide Analysis
3.7. Analysis of FT-IR Spectra
3.8. Thermal Analysis
3.9. Rheological Analysis
3.10. In Vitro Antioxidant Activity Analysis
3.10.1. DPPH Radical Scavenging Capacity
3.10.2. Hydroxyl Radical Scavenging Activity
3.10.3. Chelating Ability of Ferrous Ion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ZJRP | Ziziphus jujuba cv. Muzao Residue Polysaccharides |
| BBD | Box–Benhnken design |
| EDTA | Ethylenediaminetetraacetic acid |
| TFA | Trifluoroacetic acid |
| RSM | Response surface methodology |
References
- Wang, Y.; Liu, X.; Zhang, J.; Liu, G.; Liu, Y.; Wang, K.; Yang, M.; Cheng, H.; Zhao, Z. Structural characterization and in vitro antitumor activity of polysaccharides from Zizyphus jujuba cv. Muzao. Rsc Adv. 2015, 5, 7860–7867. [Google Scholar] [CrossRef]
- The Jujube (Ziziphus jujuba Mill.) Fruit: A Review of Current Knowledge of Fruit Composition and Health Benefits. Available online: http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_PM23480594 (accessed on 16 April 2019).
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.T.W.; Chan, P.H.; Lee, P.S.C.; Lau, K.M.; Kong, A.Y.Y.; Gong, A.G.W.; Xu, M.L.; Lam, K.Y.C.; Dong, T.T.X.; Lin, H.; et al. Chemical and biological assessment of Jujube (Ziziphus jujube)-containing herbal decoctions: Induction of erythropoietin expression in cultures. J. Chromatogr. B 2016, 1026, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.T.W.; Gong, A.G.W.; Lam, K.Y.C.; Zhang, L.M.; Chen, J.-P.; Dong, T.T.X.; Lin, H.-Q.; Tsim, K.W.K. Jujube-containing herbal decoctions induce neuronal differentiation and the expression of anti-oxidant enzymes in cultured PC12 cells. J. Ethnopharmacol. 2016, 188, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Tang, W.; Jin, M.-L.; Li, J.-E.; Xie, M.-Y. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Xie, J.-H.; Liu, X.; Shen, M.-Y.; Nie, S.-P.; Zhang, H.; Li, C.; Gong, D.-M.; Xie, M.-Y. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef]
- Ji, X.; Peng, Q.; Yuan, Y.; Liu, F.; Wang, M. Extraction and physicochemical properties of polysaccharides from Ziziphus Jujuba cv. Muzao by ultrasound-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018, 108, 541–549. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef]
- Romdhane, M.B.; Haddar, A.; Ghazala, I.; Jeddou, K.B.; Helbert, C.B.; Ellouz-Chaabouni, S. Optimization of polysaccharides extraction from watermelon rinds: Structure, functional and biological activities. Food Chem. 2017, 216, 355–364. [Google Scholar] [CrossRef]
- Optimization of Extraction Process by Response Surface Methodology and Preliminary Characterization of Polysaccharides from Phellinus Igniarius-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0144861709006699 (accessed on 16 April 2019).
- Zou, Y.; Xie, C.; Fan, G.; Gu, Z.; Han, Y. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innov. Food Sci. Emerg. Technol. 2010, 11, 611–615. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Wang, P.; Ma, C.; He, G.; Rahman, M.R.T. Optimization of PEG-based extraction of polysaccharides from Dendrobium nobile Lindl. and bioactivity study. Int. J. Biol. Macromol. 2016, 92, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Zhou, H.; Duan, M.; Li, R.; Wu, H.; Lou, Y. Extraction condition optimization and effects of drying methods on physicochemical properties and antioxidant activities of polysaccharides from comfrey (Symphytum officinale L.) root. Int. J. Biol. Macromol. 2018, 112, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dang, J.; Wang, Q.; Yu, M.; Jiang, L.; Mei, L.; Shao, Y.; Tao, Y. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mauron, J. The Maillard reaction in food; a critical review from the nutritional standpoint. Prog. Food Nutr. Sci. 1981, 5, 5–35. [Google Scholar] [PubMed]
- Assessment of Antiproliferative Activity of Pectic Substances Obtained by Different Extraction Methods from Rapeseed Cake on Cancer Cell Lines-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0144861714010327 (accessed on 16 April 2019).
- Optimisation of Pressurised Water Extraction of Polysaccharides from Blackcurrant and Its Antioxidant Activity-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0308814615012595 (accessed on 16 April 2019).
- Chen, C.; You, L.-J.; Abbasi, A.M.; Fu, X.; Liu, R.H.; Li, C. Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro. Food Funct. 2016, 7, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Vinod, V.T.P.; Sashidhar, R.B.; Suresh, K.I.; Rama Rao, B.; Vijaya Saradhi, U.V.R.; Prabhakar Rao, T. Morphological, physico-chemical and structural characterization of gum kondagogu (Cochlospermum gossypium): A tree gum from India. Food Hydrocoll. 2008, 22, 899–915. [Google Scholar] [CrossRef]
- Iijima, M. Phase transition of pectin with sorbed water. Carbohydr. Polym. 2000, 41, 101–106. [Google Scholar] [CrossRef]
- Lee, S.; Warner, K.; Inglett, G.E. Rheological properties and baking performance of new oat β-Glucan-Rich hydrocolloids. J. Agric. Food Chem. 2005, 53, 9805–9809. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, L.; Zhang, L.; Wang, T.; Zhou, Y.; Ding, C.; Yang, R.; Wang, X.; Yu, L. Optimization of extraction and antioxidant activity of polysaccharides from Salvia miltiorrhiza Bunge residue. Int. J. Biol. Macromol. 2015, 79, 533–541. [Google Scholar] [CrossRef]
- Liu, L.; Cao, J.; Huang, J.; Cai, Y.; Yao, J. Extraction of pectins with different degrees of esterification from mulberry branch bark. Bioresour. Technol. 2010, 101, 3268–3273. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Q.; Lü, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Sagis, L.M.C.; de Vries, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Physicochemical properties of pectins from okra (Abelmoschus esculentus (L.) Moench). Food Hydrocoll. 2010, 24, 35–41. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Bektaşoğlu, B.; Güçlü, K.; Apak, R. Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal. Chim. Acta 2008, 616, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Chemical Characterization and Anti-Inflammatory Activity of Polysaccharides from Zizyphus jujube cv. Muzao. Available online: http://www.doc88.com/p-1866397138638.html (accessed on 16 April 2019).
- Chang, S.C.; Hsu, B.Y.; Chen, B.H. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int. J. Biol. Macromol. 2010, 47, 445–453. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, J.; Shi, L. Antioxidant activity of the derivatives of polysaccharide extracted from a Chinese medical herb (Ramulus mori). Food Sci. Technol. Res. 2008, 14, 160–168. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Bo, R.; Ma, X.; Feng, Y.; Zhu, Q.; Huang, Y.; Liu, Z.; Liu, C.; Gao, Z.; Hu, Y.; Wang, D. Optimization on conditions of Lycium barbarum polysaccharides liposome by RSM and its effects on the peritoneal macrophages function. Carbohydr. Polym. 2015, 117, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- New Method for Quantitative Determination of Uronic Acids. Available online: http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_PM4269305 (accessed on 16 April 2019).
- Wang, X.; Zhang, L.; Wu, J.; Xu, W.; Wang, X.; Lü, X. Improvement of simultaneous determination of neutral monosaccharides and uronic acids by gas chromatography. Food Chem. 2017, 220, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, M.; Besbes, S.; Abbes, F.; Robert, C.; Paquot, M.; Blecker, C.; Attia, H. Pectin extraction from lemon by-product with acidified date juice: Effect of extraction conditions on chemical composition of pectins. Food Bioprocess Technol. 2012, 5, 687–695. [Google Scholar] [CrossRef]
- Sharma, R.; Ahuja, M. Thiolated pectin: Synthesis, characterization and evaluation as a mucoadhesive polymer. Carbohydr. Polym. 2011, 85, 658–663. [Google Scholar] [CrossRef]
- Wang, X.; Lü, X. Characterization of pectic polysaccharides extracted from apple pomace by hot-compressed water. Carbohydr. Polym. 2014, 102, 174–184. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
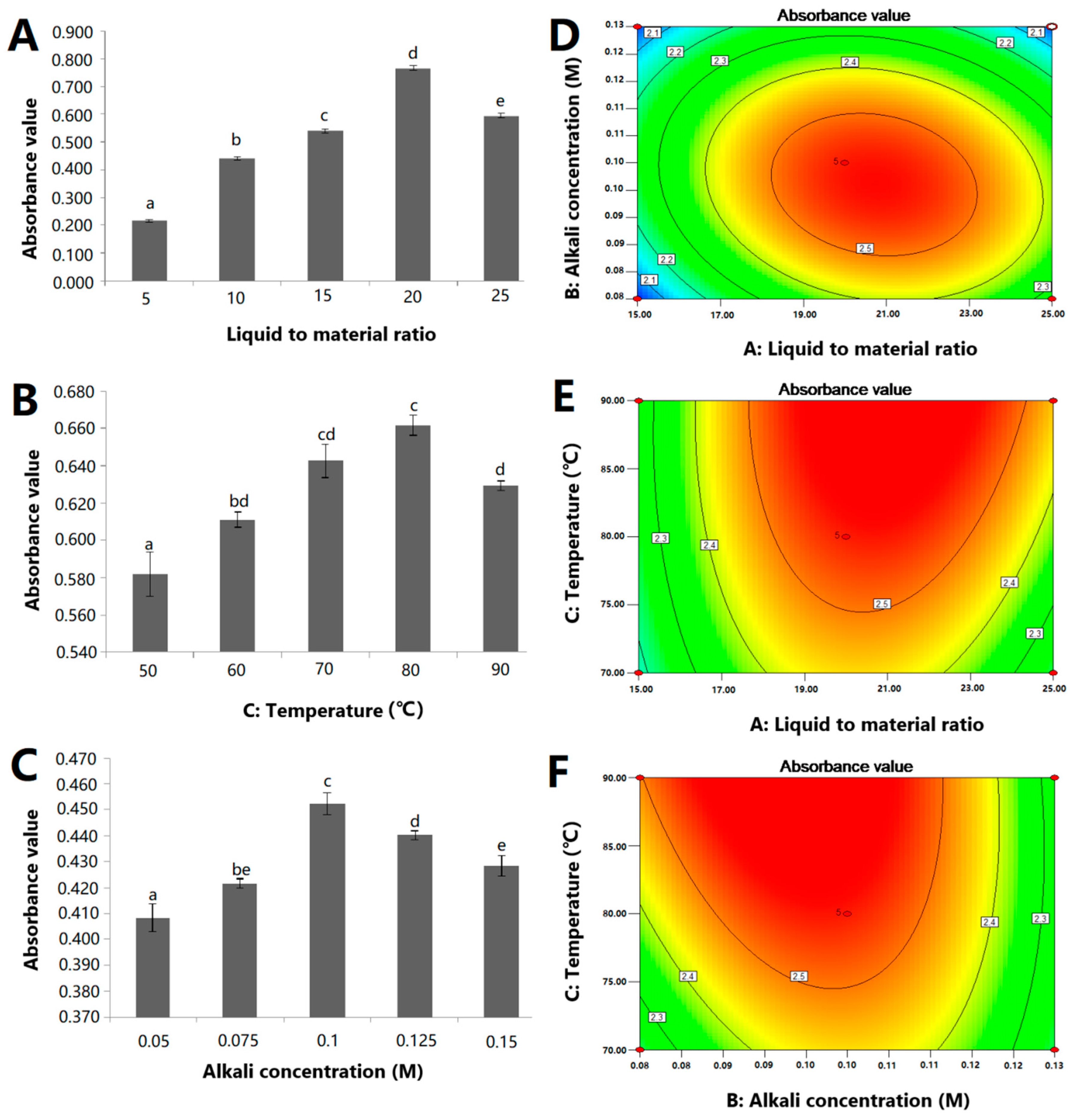
| Run | Independent Variables | Actual Value | Predicted Value | ||
|---|---|---|---|---|---|
| Liquid to Material Ratio | Alkali Concentration (M) | Extraction Temperature (°C) | |||
| 1 | 15.00 | 0.08 | 80.00 | 2.01 | 2.02 |
| 2 | 20.00 | 0.10 | 80.00 | 2.57 | 2.55 |
| 3 | 20.00 | 0.08 | 90.00 | 2.48 | 2.50 |
| 4 | 20.00 | 0.08 | 70.00 | 2.22 | 2.23 |
| 5 | 15.00 | 0.10 | 70.00 | 2.20 | 2.18 |
| 6 | 20.00 | 0.13 | 90.00 | 2.28 | 2.26 |
| 7 | 25.00 | 0.10 | 90.00 | 2.43 | 2.45 |
| 8 | 20.00 | 0.13 | 70.00 | 2.23 | 2.22 |
| 9 | 20.00 | 0.10 | 80.00 | 2.53 | 2.55 |
| 10 | 20.00 | 0.10 | 80.00 | 2.55 | 2.55 |
| 11 | 15.00 | 0.13 | 80.00 | 1.99 | 2.03 |
| 12 | 25.00 | 0.08 | 80.00 | 2.31 | 2.27 |
| 13 | 20.00 | 0.10 | 80.00 | 2.54 | 2.55 |
| 14 | 25.00 | 0.10 | 70.00 | 2.19 | 2.22 |
| 15 | 25.00 | 0.13 | 80.00 | 2.03 | 2.02 |
| 16 | 20.00 | 0.10 | 80.00 | 2.56 | 2.55 |
| 17 | 15.00 | 0.10 | 90.00 | 2.29 | 2.26 |
| Source | Sum of Squares | df | Mean of Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 0.630 | 9 | 0.070 | 62.590 | <0.001 ** |
| X1 | 0.028 | 1 | 0.028 | 24.870 | 0.002 ** |
| X2 | 0.030 | 1 | 0.030 | 26.370 | 0.001 ** |
| X3 | 0.049 | 1 | 0.049 | 43.290 | <0.001 ** |
| X1X2 | 0.016 | 1 | 0.016 | 14.460 | 0.007 ** |
| X1X3 | 0.006 | 1 | 0.006 | 5.140 | 0.058 |
| X2X3 | 0.012 | 1 | 0.012 | 10.760 | 0.013 * |
| X12 | 0.250 | 1 | 0.250 | 223.830 | <0.001 ** |
| X22 | 0.200 | 1 | 0.200 | 181.220 | <0.001 ** |
| X32 | 0.003 | 1 | 0.003 | 2.980 | 0.128 * |
| Residual | 0.008 | 7 | 0.001 | ||
| Lack of Fit | 0.007 | 3 | 0.002 | 11.180 | 0.021 |
| Pure Error | 0.001 | 4 | 0.000 | ||
| Cor Total | 0.640 | 16 | |||
| R2 | 0.988 | ||||
| Adj R2 | 0.972 | ||||
| C.V% | 1.450 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Zhu, Y.; Bao, X.; Zhang, L.; Li, N.; Jiang, G.; Peng, Q. Optimization of Alkali Extraction and Properties of Polysaccharides from Ziziphus jujuba cv. Residue. Molecules 2019, 24, 2221. https://doi.org/10.3390/molecules24122221
He Z, Zhu Y, Bao X, Zhang L, Li N, Jiang G, Peng Q. Optimization of Alkali Extraction and Properties of Polysaccharides from Ziziphus jujuba cv. Residue. Molecules. 2019; 24(12):2221. https://doi.org/10.3390/molecules24122221
Chicago/Turabian StyleHe, Zongxing, Yulian Zhu, Xingyu Bao, Liuxin Zhang, Nan Li, Gonglingxia Jiang, and Qiang Peng. 2019. "Optimization of Alkali Extraction and Properties of Polysaccharides from Ziziphus jujuba cv. Residue" Molecules 24, no. 12: 2221. https://doi.org/10.3390/molecules24122221
APA StyleHe, Z., Zhu, Y., Bao, X., Zhang, L., Li, N., Jiang, G., & Peng, Q. (2019). Optimization of Alkali Extraction and Properties of Polysaccharides from Ziziphus jujuba cv. Residue. Molecules, 24(12), 2221. https://doi.org/10.3390/molecules24122221







