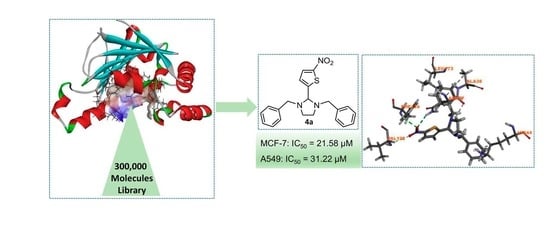
Identification and Structure-Activity Studies of 1,3-Dibenzyl-2-aryl imidazolidines as Novel Hsp90 Inhibitors
Abstract
1. Introduction
2. Results and Discussion
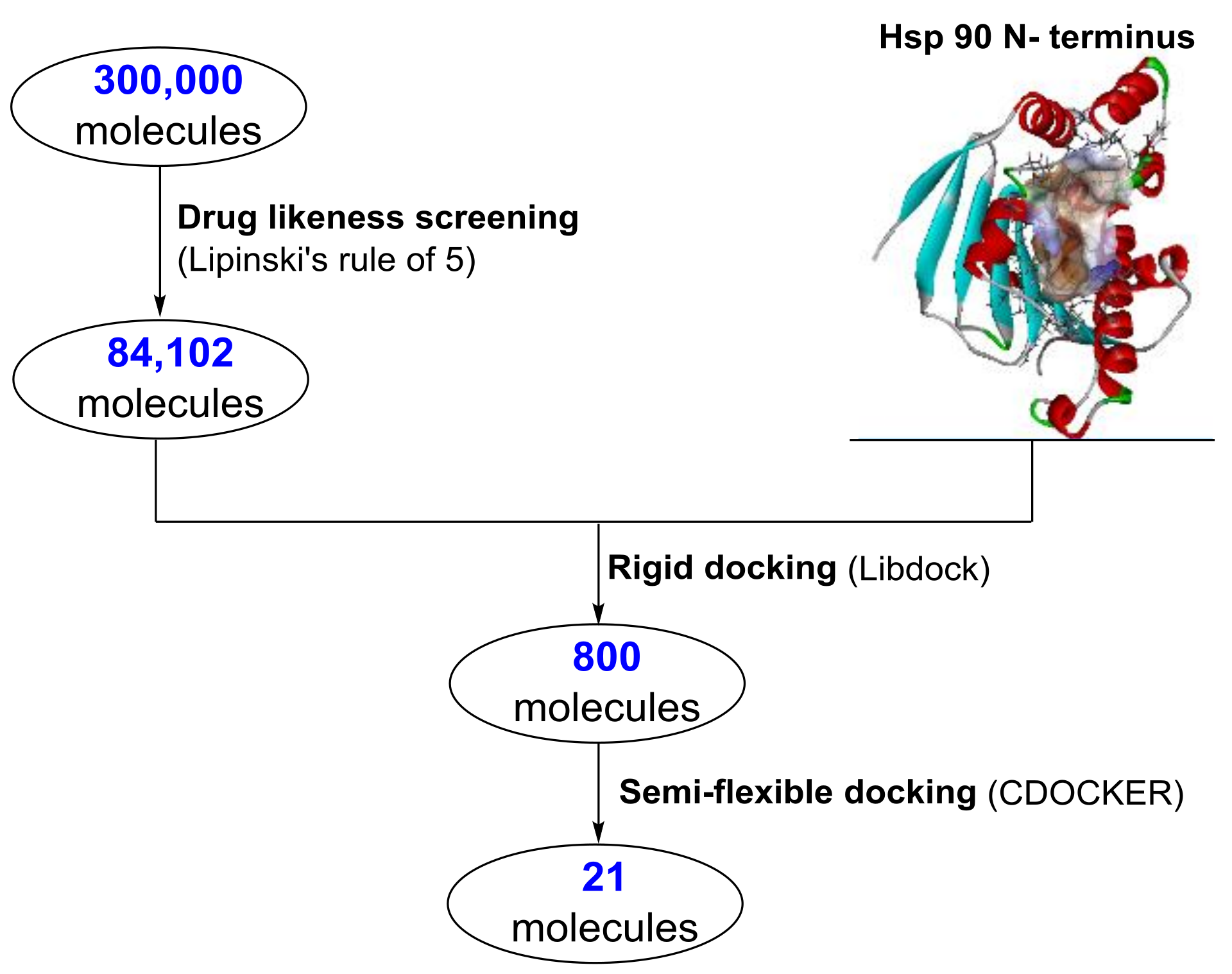
2.1. Structure Based Virtual Screening
2.2. Biological Evaluation of Molecules Identified from Virtual Screening
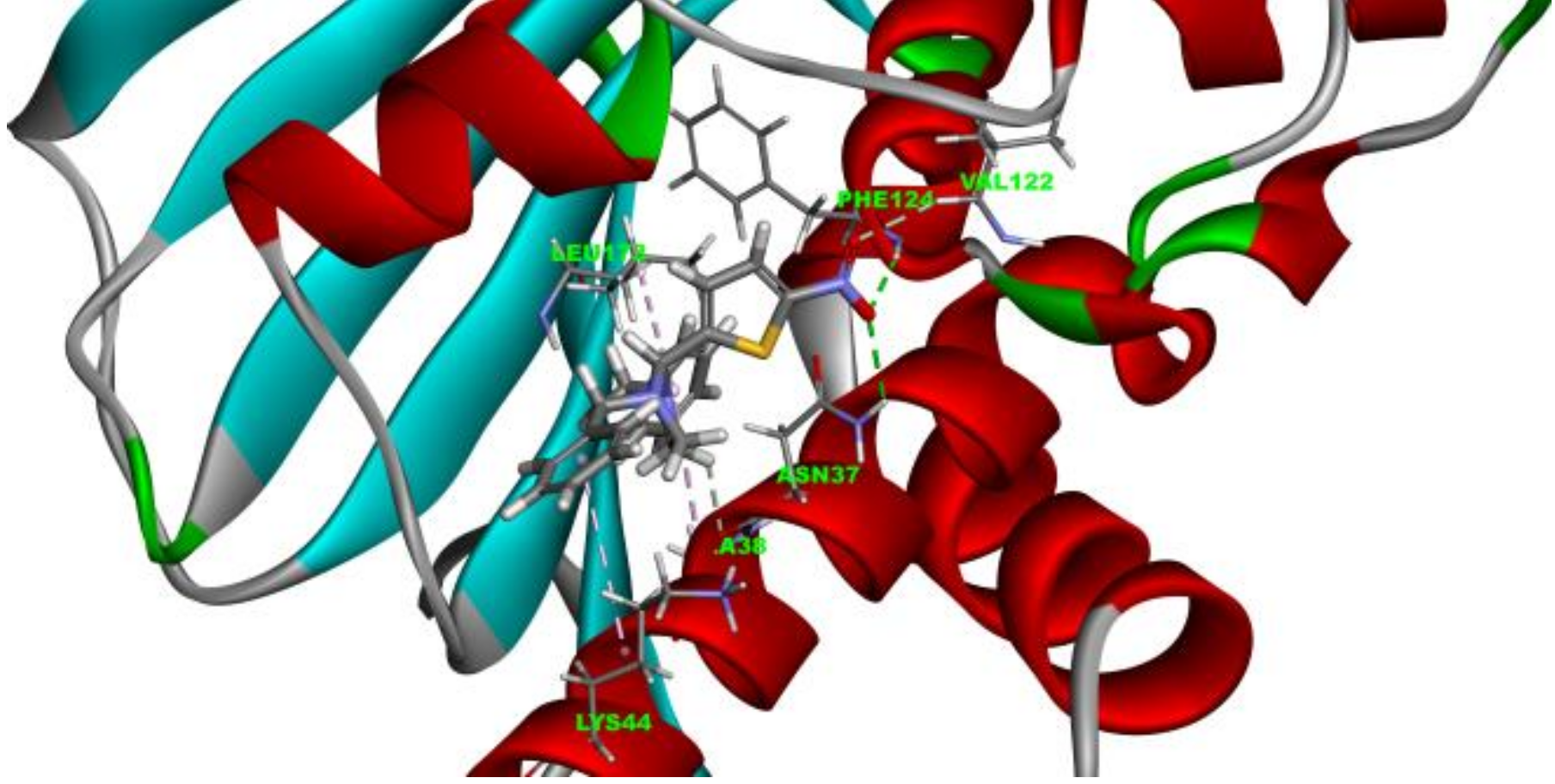
2.3. Molecule Docking Analysis of Hsp90-4a Complex
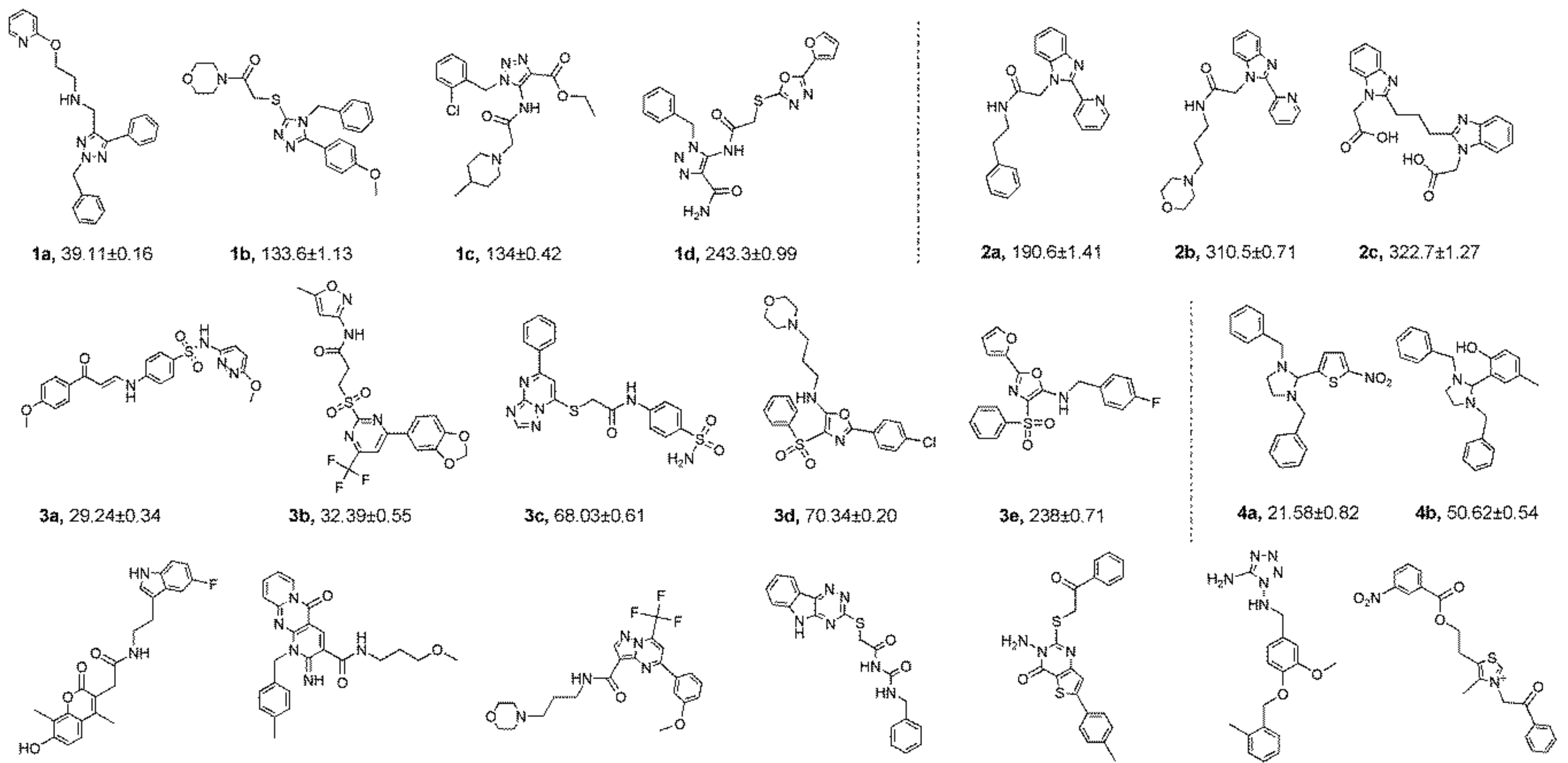
2.4. Structure-Activity Relationship (SAR) Studies
2.5. Binding Affinity Assay
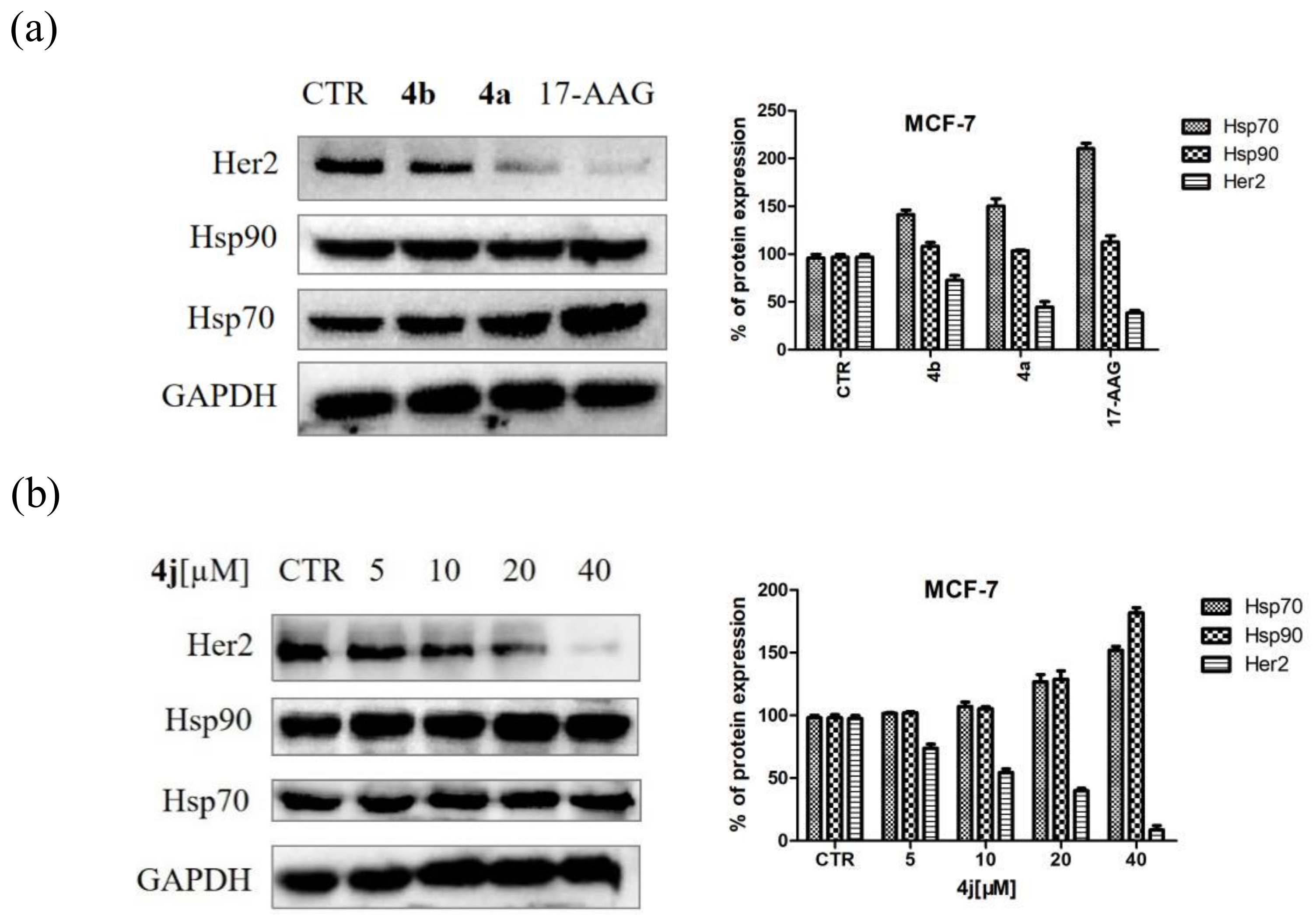
2.6. Western Blotting Assay
3. Materials and Methods
3.1. Virtual Screening
3.2. Evaluation of Anti-proliferative Activity In Vitro
3.3. Fluorescence Polarization (FP) Enzymatic Assay
3.4. Western Immune Blotting Assays
3.5. Chemistry
3.5.1. General Information
3.5.2. General Procedure for the Preparation of 1,3-Dibenzyl-2-aryl Imidazolidine 4c–4r
3.5.3. General Procedure for the Preparation of N,N′-Diphenyl-2-aryl Imidazolidine 6a–6d
3.5.4. General Procedure for Preparation of 1,3-Diethyl-2-aryl Imidazolidine 7a,7b
3.5.5. General Procedure for Preparation of N,N′-Dimethyl-2-aryl Imidazolidine 8a
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sherman, M.; Multhoff, G. Heat shock proteins in cancer. Ann. N. Y. Acad. Sci. 2007, 1113, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761. [Google Scholar] [CrossRef] [PubMed]
- Wandinger, S.; Richter, K.; Buchner, J. The Hsp90 chaperone machinery. J. Biol. Chem. 2008, 283, 18473–18477. [Google Scholar] [CrossRef] [PubMed]
- Pearl, L.; Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 2006, 75, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Graner, M. HSP90 and immune Modulation in cancer. Adv. Cancer Res. 2016, 129, 191–224. [Google Scholar] [PubMed]
- Wong, D.; Jay, D. Emerging roles of extracellular hsp90 in cancer. Adv. Cancer Res. 2016, 129, 141–163. [Google Scholar] [PubMed]
- Miyata, Y.; Nakamoto, H.; Neckers, L. The therapeutic target Hsp90 and cancer hallmarks. Curr. Pharm. Des. 2013, 19, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Maloney, A.; Workman, P. HSP90 as a new therapeutic target for cancer therapy: The story unfolds. Expert Opin. Biol. Ther. 2002, 2, 3–24. [Google Scholar] [CrossRef]
- Moser, C.; Lang, S.; Stoeltzing, O. Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res. 2009, 29, 2031–2042. [Google Scholar]
- Beliakoff, J.; Whitesell, L. Hsp90: An emerging target for breast cancer therapy. Anticancer Drugs 2004, 15, 651–662. [Google Scholar] [CrossRef]
- Mahalingam, D.; Swords, R.; Carew, J.; Nawrocki, S.; Bhalla, K.; Giles, F. Targeting HSP90 for cancer therapy. Br. J. Cancer 2009, 100, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Özgür, A.; Tutar, Y. Heat shock protein 90 inhibition in cancer drug discovery: From chemistry to futural clinical applications. Anticancer Agents Med. Chem. 2016, 16, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Monterrey, I.; Sala, M.; Musella, S.; Campiglia, P. Heat shock protein 90 inhibitors as therapeutic agents. Recent Pat. Anticancer Drug Discov. 2012, 7, 313–336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhat, R.; Tummalapalli, R.; Rotella, D. Progress in the discovery and development of heat shock protein 90 (Hsp90) inhibitors: Miniperspective. J. Med. Chem. 2014, 57, 8718–8728. [Google Scholar] [CrossRef] [PubMed]
- Claudia, C.; Andrea, P.; Celeste, C.; Paola, M.; Antonella, M.; Silvestre, B. Heat shock proteins in alzheimer’s disease: Role and targeting. Int. J. Mol. Sci. 2018, 19, 10. [Google Scholar]
- Lackie, R.; Maciejewski, A.; Ostapchenko, V.; Marques-Lopes, J. The Hsp70/Hsp90 Chaperone machinery in neurodegenerative diseases. Front Neurosci. 2017, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.; Neubert, T.; Liu, M.; Sperry, S.; Zuccola, H. Identification of novel HSP90α/β isoform selective inhibitors using structure-based drug design demonstration of potential utility in treating CNS disorders such as Huntington’s disease. J. Med. Chem. 2014, 57, 3382–3400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, F.; Wang, R.; Li, F.; Wu, Y.; Kitazato, K.; Wang, Y. HSP90: A promising broad-spectrum antiviral drug target. Arch. Virol. 2017, 162, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.; Singh, S.; Kohler, J.; Collins, C.; Zaas, A. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. USA 2009, 106, 2818–2823. [Google Scholar] [CrossRef]
- Devaney, E.; Gillan, V. Hsp90 Inhibitors in parasitic nematodes: Prospects and challenges. Curr. Top Med. Chem. 2016, 16, 2805–2811. [Google Scholar] [CrossRef]
- Wang, H.; Lu, M.; Yao, M.; Zhu, W. Effects of treatment with an Hsp90 inhibitor in tumors based on 15 phase II clinical trials. Mol. Clin. Oncol. 2016, 5, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Yuno, A.; Lee, M.; Lee, S.; Tomita, Y.; Rekhtman, D.; Moore, B.; Trepel, J. Clinical evaluation and biomarker profiling of hsp90 inhibitors. Methods Mol. Boil. 2018, 1709, 423–441. [Google Scholar]
- Jhaveri, K.; Taldone, T.; Modi, S.; Chiosis, G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. BBA Mol. Cell Res. 2012, 1823, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Petrikaite, V.; Matulis, D. Binding of natural and synthetic inhibitors to heat shock protein 90 and their clinical applications. Medicina (Kaunas) 2011, 47, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Xu, J.; Shi, J.; Jiang, L.; Yao, N.; Ye, W. Discovery and development of natural heat shock protein 90 inhibitors in cancer treatment. Acta Pharm. Sin. B 2012, 2, 238–245. [Google Scholar] [CrossRef]
- Roe, S.; Prodromou, C.; O’Brien, R.; Ladbury, J.; Piper, P.; Pearl, L. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 1999, 42, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Sausville, E.; Tomaszewski, J.; Ivy, P. Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr. Cancer Drug Targets 2003, 3, 377–383. [Google Scholar] [CrossRef]
- Schulte, T.; Akinaga, S.; Soga, S.; Sullivan, W.; Stensqard, B.; Toft, D.; Neckers, L. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperone 1998, 3, 100–108. [Google Scholar] [CrossRef]
- Eccles, S.; Massey, A.; Raynaud, F.; Sharp, S.; Box, G.; Valenti, V. NVP-AUY922: A novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008, 68, 2850–2859. [Google Scholar] [CrossRef] [PubMed]
- Canonici, A.; Qadir, Z.; Conlon, N.; Collins, D.; O’Brien, N. The HSP90 inhibitor NVP-AUY922 inhibits growth of HER2 positive and trastuzumab-resistant breast cancer cells. Invest. New Drugs 2018, 36, 581–589. [Google Scholar] [CrossRef]
- Shapiro, G. Hsp90 inhibitors in clinical development: STA-9090 (Ganetespib). Ann. Oncol. 2011, 22, 16–17. [Google Scholar]
- Cavenagh, J.; Oakervee, H.; Baetiong-Caguioa, P.; Davies, F.; Gharibo, M. A phase I/II study of KW-2478, an Hsp90 inhibitor, in combination with bortezomib in patients with relapsed/refractory multiple myeloma. Br. J. Cancer 2017, 117, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.; Cavet, J.; Johnson, P.; Morgan, G.; Williams, C. Phase I study of KW-2478, a novel Hsp90 inhibitor, in patients with B-cell malignancies. Br. J. Cancer 2016, 114, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.; Weiss, G.; Jones, S.; Tibes, R.; Bauer, T. Phase I dose-escalation studies of SNX-5422, an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumours. Euro. J. Cancer 2014, 50, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, R.; Matta, H.; Chaudhary, P. A purine scaffold HSP90 inhibitor BIIB021 has selective activity against KSHV-associated primary effusion lymphoma and blocks vFLIP K13-induced NF-κB. Clin. Cancer Res. 2013, 19, 5016–5026. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Hu, H. BIIB021, an Hsp90 inhibitor: A promising therapeutic strategy for blood malignancies. Oncol. Rep. 2018, 40, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Canella, A.; Welker, A.; Yoo, J.; Xu, J.; Abas, F. Efficacy of onalespib, a long-acting second-generation HSP90 inhibitor, as a single agent and in combination with temozolomide against malignant gliomas. Clin. Cancer Res. 2017, 23, 6215–6226. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4c–4r, 6a–6d, 7a, 7b and 8a are available from the authors. |
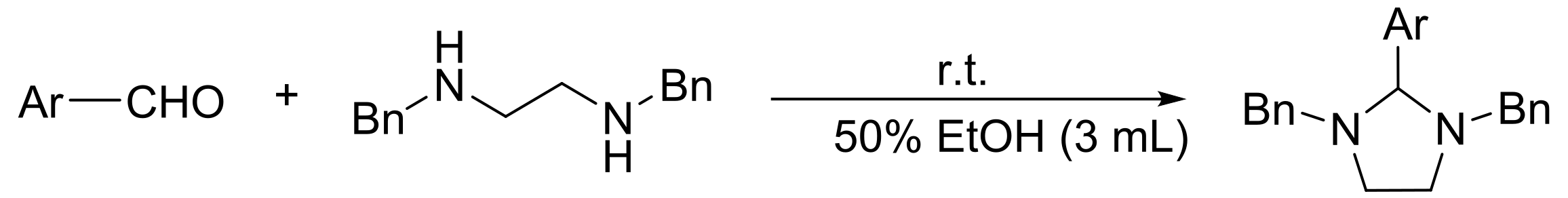
| Entry | Product | Ar | IC50 a (μM) | |
|---|---|---|---|---|
| MCF-7 | A549 | |||
| 1 | 4a | 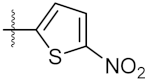 | 21.58 ± 0.57 | 31.22 ± 0.31 |
| 2 | 4b | 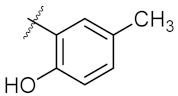 | 50.62 ± 0.26 | 88.58 ± 0.08 |
| 3 | 4c | 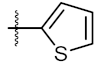 | 80.04 ± 0.63 | 78.18 ± 0.15 |
| 4 | 4d | 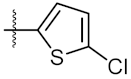 | 68.35 ± 0.44 | 56.30 ± 1.27 |
| 5 | 4e | 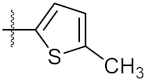 | 119.30 ± 0.42 | 112.80 ± 1.41 |
| 6 | 4f | 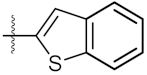 | 219.40 ± 0.57 | 135.80 ± 1.41 |
| 7 | 4g | 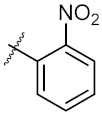 | 40.91 ± 0.05 | 40.64 ± 0.51 |
| 8 | 4h | 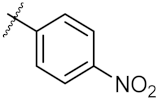 | 117.70 ± 0.01 | 55.60 ± 0.49 |
| 9 | 4i | 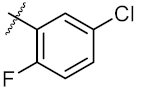 | 27.86 ± 0.01 | 59.27 ± 0.83 |
| 10 | 4j | 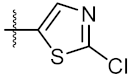 | 33.12 ± 0.23 | 35.30 ± 0.14 |
| 11 | 4k | 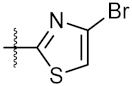 | 77.01 ± 0.01 | 498.80 ± 0.25 |
| 12 | 4l | 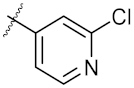 | 31.48 ± 0.60 | 64.66 ± 0.48 |
| 13 | 4m | 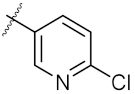 | 76.42 ± 0.31 | 70.42 ± 0.10 |
| 14 | 4n | 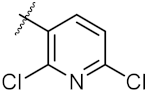 | 40.36 ± 0.51 | 52.11 ± 0.50 |
| 15 | 4o | 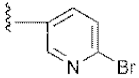 | 53.12 ± 0.77 | 56.92 ± 1.27 |
| 16 | 4p | 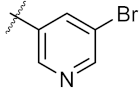 | 45.25 ± 0.63 | 72.87 ± 0.66 |
| 17 | 4q | 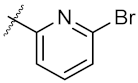 | 46.97 ± 0.04 | 71.36 ± 0.88 |
| 18 | 4r | 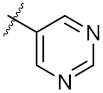 | 51.37 ± 0.54 | 80.78 ± 1.00 |
| 19 | 17-AAG | - | 7.18 ± 0.13 | 3.54 ± 0.08 |
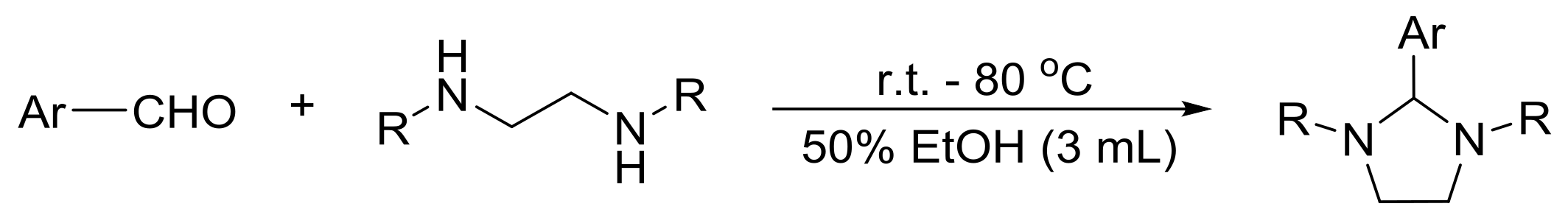
| Entry | Product | R | Ar | IC50 a (μM) | |
|---|---|---|---|---|---|
| MCF-7 | A549 | ||||
| 1 | 6a | Ph | 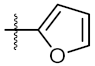 | 40.77 ± 0.39 | 123.40 ± 0.85 |
| 2 | 6b | Ph | 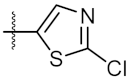 | 161.60 ± 0.57 | 172.00 ± 1.41 |
| 3 | 6c | Ph | 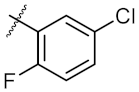 | 43.67 ± 1.03 | 46.14 ± 0.20 |
| 4 | 6d | Ph | 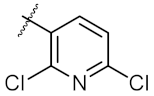 | 323.00 ± 2.28 | 248.60 ± 0.57 |
| 5 | 7a | Et | 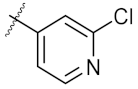 | >500 | 250.10 ± 1.27 |
| 6 | 7b | Et | 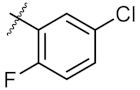 | 158.90 ± 0.14 | 164.00 ± 1.41 |
| 7 | 8a | Me | 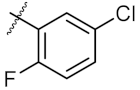 | 250.10 ± 0.27 | 148.70 ± 1.56 |
| Compounds | FP IC50 (μM) a |
|---|---|
| 17-AAG | 0.017 ± 0.003 |
| 4a | 0.012 ± 0.010 |
| 4b | 0.612 ± 0.014 |
| 4d | 0.672 ± 0.010 |
| 4i | 0.610 ± 0.014 |
| 4j | 0.310 ± 0.014 |
| 4n | 0.102 ± 0.010 |
| 6c | 1.930 ± 0.013 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, X.; Li, L.; Dai, R.; Shi, M.; Xue, H.; Liu, Y.; Wang, H. Identification and Structure-Activity Studies of 1,3-Dibenzyl-2-aryl imidazolidines as Novel Hsp90 Inhibitors. Molecules 2019, 24, 2105. https://doi.org/10.3390/molecules24112105
Liu Y, Liu X, Li L, Dai R, Shi M, Xue H, Liu Y, Wang H. Identification and Structure-Activity Studies of 1,3-Dibenzyl-2-aryl imidazolidines as Novel Hsp90 Inhibitors. Molecules. 2019; 24(11):2105. https://doi.org/10.3390/molecules24112105
Chicago/Turabian StyleLiu, Yajun, Xiaoxia Liu, Lihong Li, Rui Dai, Meiyun Shi, Hongyu Xue, Yong Liu, and Hecheng Wang. 2019. "Identification and Structure-Activity Studies of 1,3-Dibenzyl-2-aryl imidazolidines as Novel Hsp90 Inhibitors" Molecules 24, no. 11: 2105. https://doi.org/10.3390/molecules24112105
APA StyleLiu, Y., Liu, X., Li, L., Dai, R., Shi, M., Xue, H., Liu, Y., & Wang, H. (2019). Identification and Structure-Activity Studies of 1,3-Dibenzyl-2-aryl imidazolidines as Novel Hsp90 Inhibitors. Molecules, 24(11), 2105. https://doi.org/10.3390/molecules24112105