Rapid Identification of Berberine Metabolites in Rat Plasma by UHPLC-Q-TOF-MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Detection Conditions
2.2. Identification of Berberine Metabolites in Rat Plasma
2.3. Proposed Metabolic Pathway of Berberine in Rats
3. Materials and Methods
3.1. Chemicals
3.2. Rat Studies
3.3. Sample Preparation
3.4. Instrumental Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karaosmanoglu, K.; Sayar, N.A.; Kurnaz, I.A.; Akbulut, B.S. Assessment of berberine as a multi-target antimicrobial: A multi-omics study for drug discovery and repositioning. Omics 2014, 18, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Long, Y.; Sun, Y.; Wang, Y.; Li, Q.; Wu, H.; Guo, Z.; Li, Y.; Niu, Y.; Li, C.; et al. Evidence for the complementary and synergistic effects of the three-alkaloid combination regimen containing berberine, hypaconitine and skimmianine on the ulcerative colitis rats induced by trinitrobenzene-sulfonic acid. Eur. J. Pharmacol. 2011, 651, 187–196. [Google Scholar] [CrossRef]
- Lau, C.W.; Yao, X.Q.; Chen, Z.Y.; Ko, W.H.; Huang, Y. Cardiovascular actions of berberine. Cardiovasc Drug Rev. 2001, 19, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Piao, X.S.; Kim, S.W.; Wang, L.; Liu, P. The effects of berberine on the magnitude of the acute inflammatory response induced by Escherichia coli lipopolysaccharide in broiler chickens. Poultry Sci. 2010, 89, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.; Li, L.; Yuen, M.; Feng, Y. Berberine and Coptidis Rhizoma as potential anticancer agents: Recent updates and future perspectives. J. Ethnopharmacol. 2015, 176, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Doggrell, S.A. Berberine - a novel approach to cholesterol lowering. Expert Opin. Investig. Drugs 2005, 14, 683–685. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef]
- Tsai, P.L.; Tsai, T.H. HPLC determination of berberine in medicinal herbs and a related traditional Chinese medicine. Anal. Lett. 2002, 35, 2459–2470. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Cha, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef]
- Ma, J.; Feng, R.; Tan, X.; Ma, C.; Shou, J.; Fu, J.; Huang, M.; He, C.; Chen, S.; Zhao, Z.; et al. Excretion of Berberine and Its Metabolites in Oral Administration in Rats. J. Pharm. Sci. 2013, 102, 4181–4192. [Google Scholar] [CrossRef]
- Pan, J.F.; Yu, C.; Zhu, D.Y.; Zhang, H.; Zeng, J.F.; Jiang, S.H.; Ren, J.Y. Identification of three sulfite-conjugated metabolites of berberine chloride in healthy volunteers’ urine after oral administration. Acta Pharmacol. Sin. 2002, 23, 77–82. [Google Scholar] [PubMed]
- Tsai, P.L.; Tsai, T.H. Hepatobiliary excretion of berberine. Drug Metab. Dispos. 2004, 32, 405–412. [Google Scholar] [CrossRef]
- Tan, X.; Ma, J.; Feng, R.; Ma, C.; Chen, W.; Sun, Y.; Fu, J.; Huang, M.; He, C.; Shou, J.; et al. Tissue Distribution of Berberine and Its Metabolites after Oral Administration in Rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef]
- Li, Y.; Ren, G.; Wang, Y.; Kong, W.; Yang, P.; Wang, Y.; Li, Y.; Yi, H.; Li, Z.; Song, D.; et al. Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes. J. Trans. Med. 2011, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yin, L.; Xu, L.; Wang, X.; Peng, J. Simultaneous determination of ten active components in chinese medicine “Huang-Lian-Shang-Qing” tablets by high-performance liquid chromatography coupled with photodiode array detection. Anal. Lett. 2010, 43, 545–556. [Google Scholar] [CrossRef]
- Qi, M.; Xiong, A.; Li, P.; Yang, Q.; Yang, L.; Wang, Z. Identification of acteoside and its major metabolites in rat urine by ultra-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. B 2013, 940, 77–85. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.; Teng, L.; Cheng, X.; Wang, Z.; Wang, C. Metabolites identification of harmane in vitro/in vivo in rats by ultra-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Pharmaceut. Biomed. 2014, 92, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Zhu, Z.; Kang, N.; Piao, S.; Qin, G.; Yao, X. Isolation and Identification of Urinary Metabolites of Berberine in Rats and Humans. Drug Metab. Dispos. 2008, 36, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab. Dispos. 2006, 34, 2064–2072. [Google Scholar] [CrossRef]
- Spinozzi, S.; Colliva, C.; Camborata, C.; Roberti, M.; Ianni, C.; Neri, F.; Calvarese, C.; Lisotti, A.; Mazzella, G.; Roda, A. Berberine and Its Metabolites: Relationship between Physicochemical Properties and Plasma Levels after Administration to Human Subjects. J. Nat. Prod. 2014, 77, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Porru, E.; Franco, P.; Calabria, D.; Spinozzi, S.; Roberti, M.; Caliceti, C.; Roda, A. Combined analytical approaches to define biodistribution and biological activity of semi-synthetic berberrubine, the active metabolite of natural berberine. Anal. Bioanal. Chem. 2018, 15, 3533–3545. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, S.; Wang, Y.; Xu, P.; Yan, J.; Bin, W.; Qiu, F.; Kang, N. Berberine metabolites could induce low density lipoprotein receptor up-regulation to exert lipid-lowering effects in human hepatoma cells. Fitoterapia 2014, 92, 230–237. [Google Scholar] [CrossRef]
- Abd El-Salam, M.; Mekky, H.; El-Naggar, E.M.B.; Ghareeb, D.; El-Demellawy, M.; El-Fiky, F. Hepatoprotective properties and biotransformation of berberine and berberrubine by cell suspension cultures of Dodonaea viscosa and Ocimum basilicum. S. Afr. J. Bot. 2015, 97, 191–195. [Google Scholar] [CrossRef]
- Cao, S.; Xu, P.; Yan, J.; Liu, H.; Liu, L.; Cheng, L.; Qiu, F.; Kang, N. Berberrubine and its analog, hydroxypropyl-berberrubine, regulate LDLR and PCSK9 expression via the ERK signal pathway to exert cholesterol-lowering effects in human hepatoma HepG2 cells. J. Cell Biochem. 2019, 120, 1340–1349. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Xiao, J.; Gao, H.; Wang, H.; Zhang, X.; Zhang, C.; Ji, X. Berberine Attenuates Vascular Remodeling and Inflammation in a Rat Model of Metabolic Syndrome. Biol. Pharm. Bull. 2015, 38, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhou, Y.; Xu, P.; Wang, Y.; Yan, J.; Bin, W.; Qiu, F.; Kang, N. Berberine metabolites exhibit triglyceride-lowering effects via activation of AMP-activated protein kinase in Hep G2 cells. J. Ethnopharmacol. 2013, 149, 576–582. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Yan, Y.; Qiang, X.; Zhou, C.; Li, R.; Chen, H.; Zhang, Y. Demethyleneberberine Protects against Hepatic Fibrosis in Mice by Modulating NF-κB Signaling. Int. J. Mol. Sci. 2016, 17, 1036. [Google Scholar] [CrossRef]
- Rackova, L.; Majekova, M.; Kost’alova, D.; Stefek, M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorg. Med. Chem. 2004, 12, 4709–4715. [Google Scholar] [CrossRef]
- Luo, T.; Jiang, W.; Kong, Y.; Li, S.; He, F.; Xu, J.; Wang, H.Q. The protective effects of jatrorrhizine on b-amyloid (25-35)-induced neurotoxicity in rat cortical neurons. CNS Neurol Disord. Drug Targets 2016, 11, 1030–1037. [Google Scholar] [CrossRef]
- Liu, R.F.; Cao, Z.F.; Pan, Y.Y.; Zhang, G.C.; Yang, P.; Guo, P.D.; Zhou, Q.S. Jatrorrhizine hydrochloride inhibits the proliferation and neovascularization of C8161 metastatic melanoma cells. Anticancer Drugs 2013, 24, 667–676. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the berberine are available from the authors. |
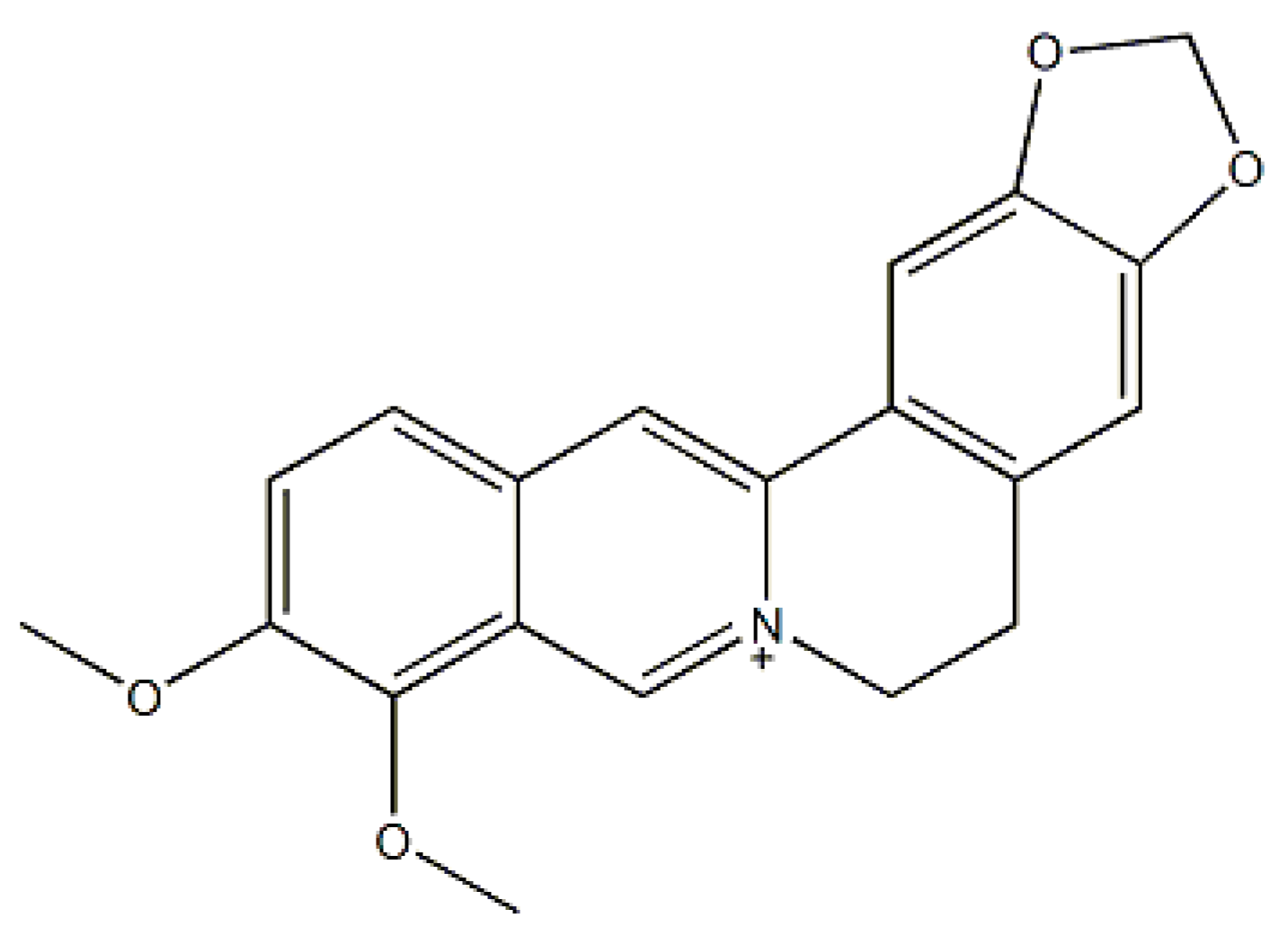
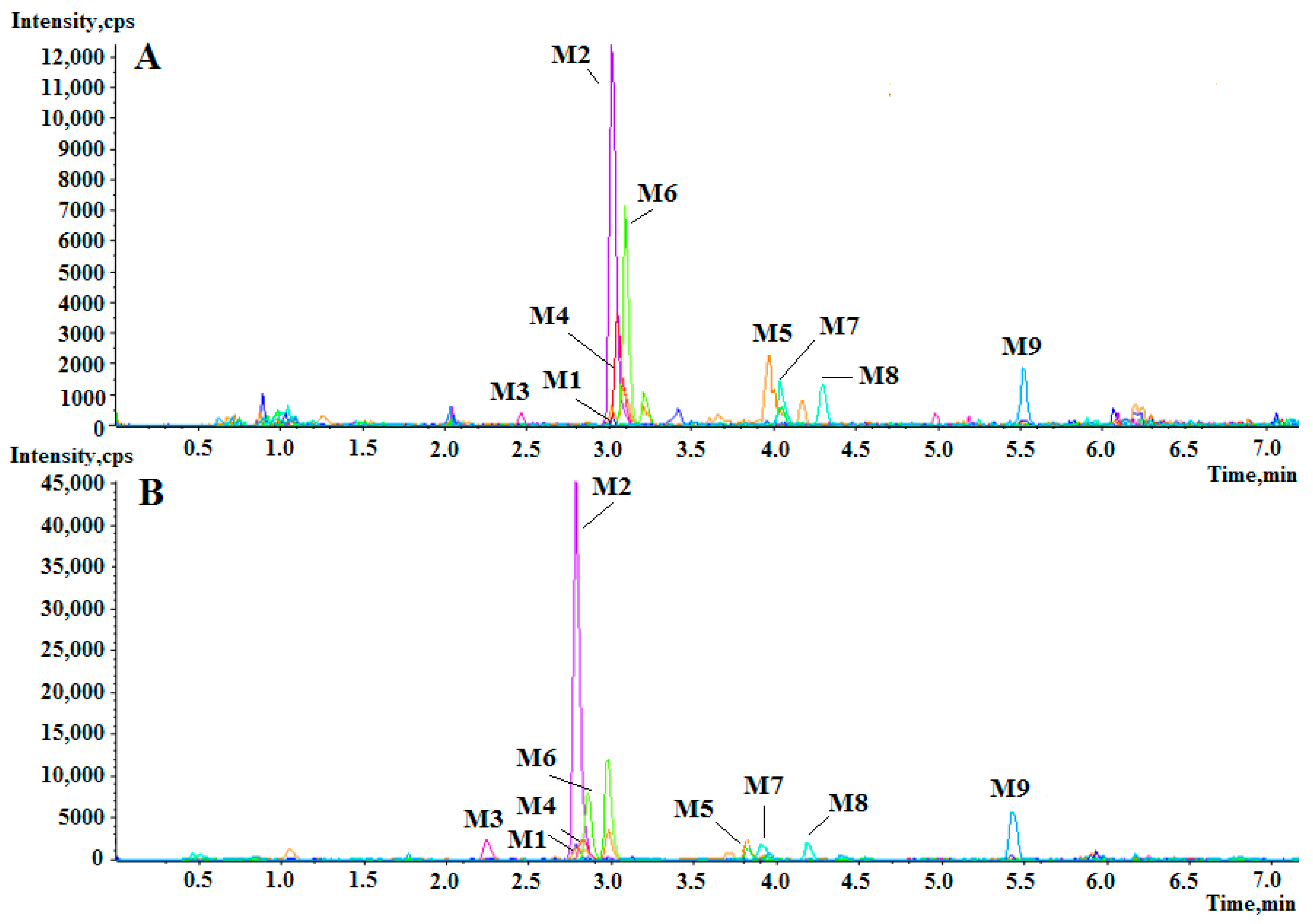

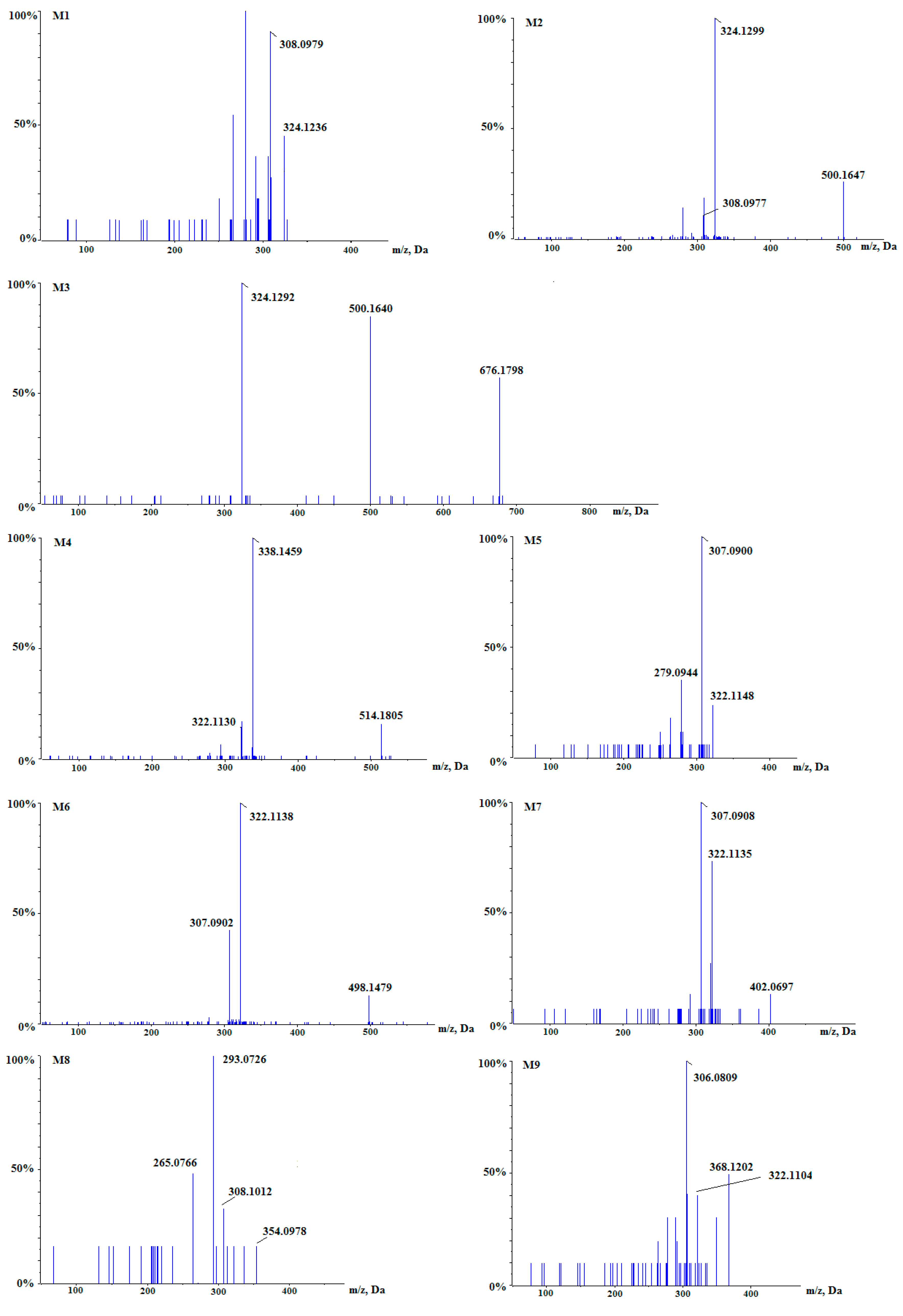
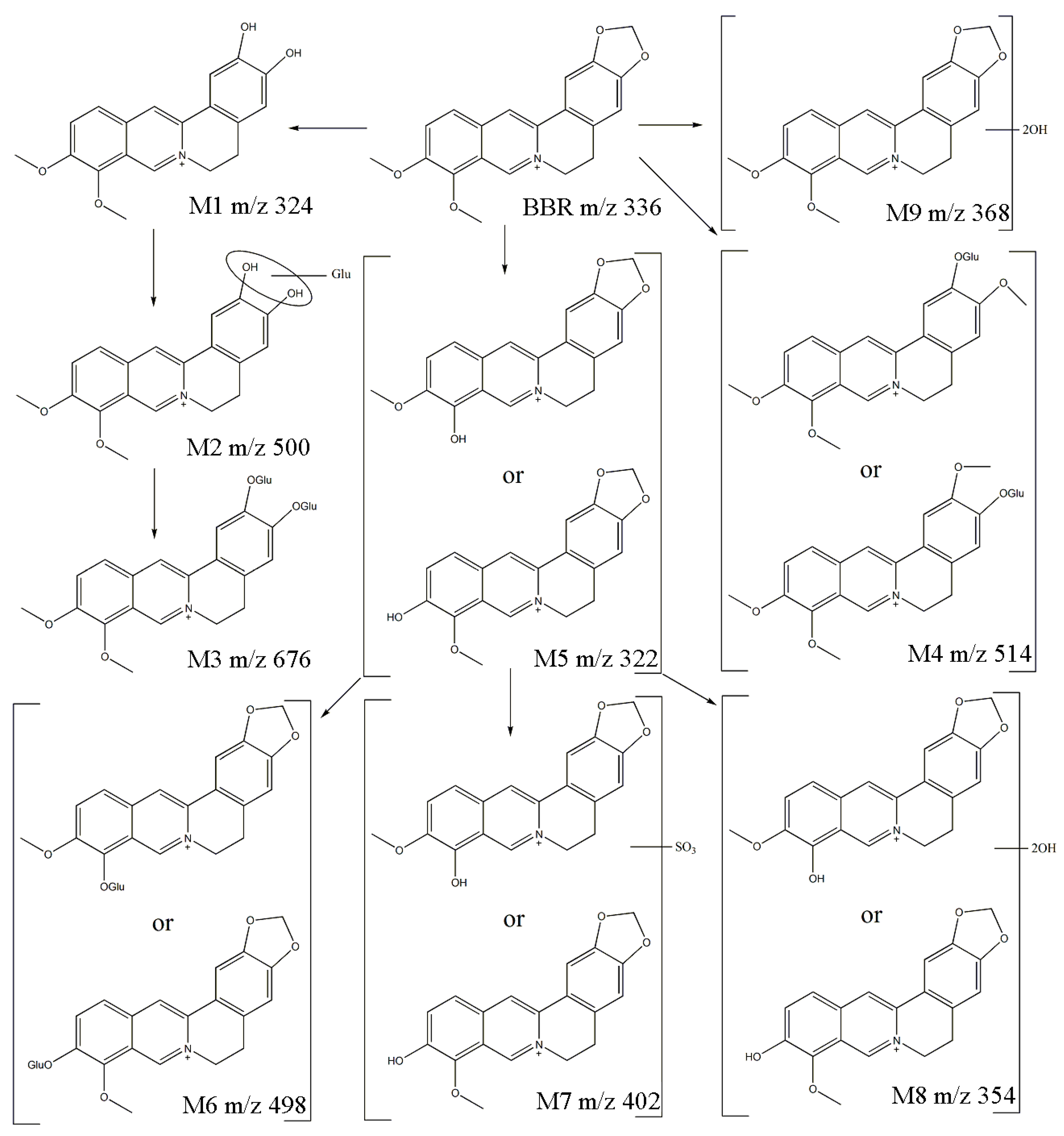
| Meta-Bolites | Formula | RT 1 (min) | m/z (Da) | Error (ppm) | Iso Diff 2 (%) | Fragments (m/z) | Identification |
|---|---|---|---|---|---|---|---|
| M1 | C19H18NO4 | 2.79 | 324.1236 | −1.4 | 0.3 | 308.0979 | Demethylene-berberine |
| M2 | C25H26NO10 | 2.78 | 500.1647 | −0.1 | 0.3 | 324.1299 308.0977 | glucuronic acid-conjugated M1 |
| M3 | C31H34NO16 | 2.23 | 676.1798 | −0.8 | 2.6 | 500.1640 324.1292 | diglucuronide-conjugated M1 |
| M4 | C26H28NO10 | 2.81 | 514.1805 | −0.3 | 2.8 | 338.1459 322.1130 | glucuronic acid-conjugated jatrorrhizine or columbamine |
| M5 | C19H16NO4 | 3.72 | 322.1148 | −1.7 | 4.7 | 307.0900 279.0944 | berberrubine or thalifendine |
| M6 | C25H24NO10 | 2.85 | 498.1479 | −0.6 | 1.4 | 322.1138 307.0902 | glucuronic acid-conjugated M5 |
| M7 | C19H16NO7S | 3.82 | 402.0697 | −0.9 | 2.6 | 322.1135 307.0908 | sulfite-conjugat-ed M5 |
| M8 | C19H16NO6 | 3.90 | 354.0978 | −1.0 | 2.3 | 308.1012 293.0726 | dihydroxy M5 |
| M9 | C20H18NO6 | 5.43 | 368.1202 | −0.8 | 3.2 | 322.1104 306.0809 | Dihydroxy berberine |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Xu, C.; Li, X.; Li, D.; Li, Y.; Jiang, J.; Yang, P.; Duan, G. Rapid Identification of Berberine Metabolites in Rat Plasma by UHPLC-Q-TOF-MS. Molecules 2019, 24, 1994. https://doi.org/10.3390/molecules24101994
Xu P, Xu C, Li X, Li D, Li Y, Jiang J, Yang P, Duan G. Rapid Identification of Berberine Metabolites in Rat Plasma by UHPLC-Q-TOF-MS. Molecules. 2019; 24(10):1994. https://doi.org/10.3390/molecules24101994
Chicago/Turabian StyleXu, Peng, Chen Xu, Xiaoxia Li, Dan Li, Yan Li, Jiebing Jiang, Ping Yang, and Gengli Duan. 2019. "Rapid Identification of Berberine Metabolites in Rat Plasma by UHPLC-Q-TOF-MS" Molecules 24, no. 10: 1994. https://doi.org/10.3390/molecules24101994
APA StyleXu, P., Xu, C., Li, X., Li, D., Li, Y., Jiang, J., Yang, P., & Duan, G. (2019). Rapid Identification of Berberine Metabolites in Rat Plasma by UHPLC-Q-TOF-MS. Molecules, 24(10), 1994. https://doi.org/10.3390/molecules24101994




