Cytotoxic Effects of Newly Synthesized Heterocyclic Candidates Containing Nicotinonitrile and Pyrazole Moieties on Hepatocellular and Cervical Carcinomas
Abstract
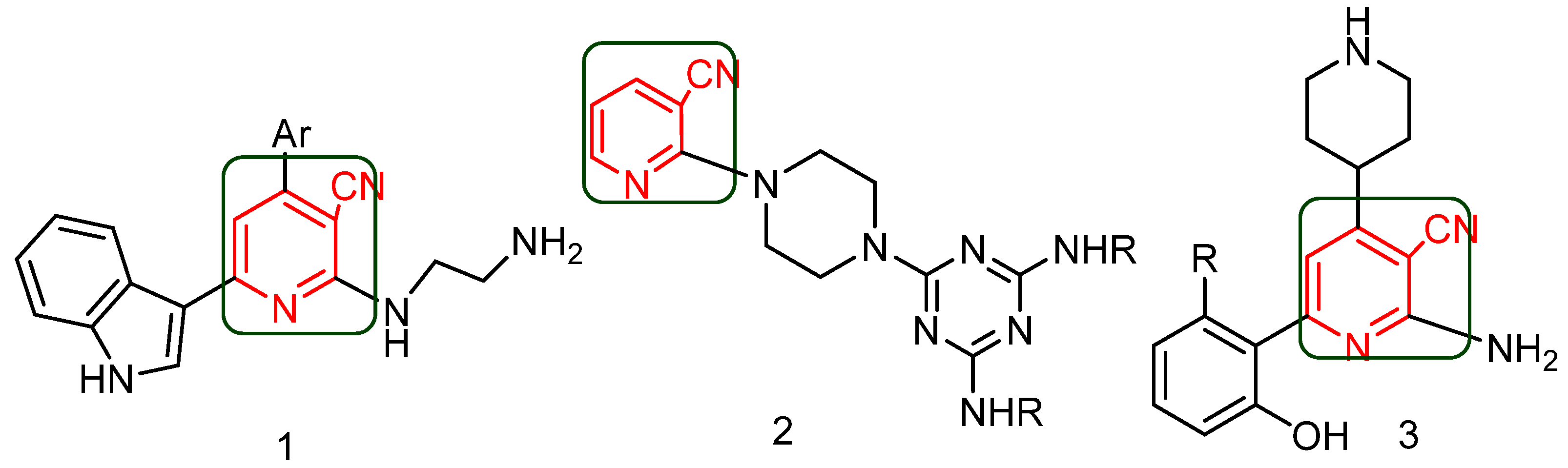
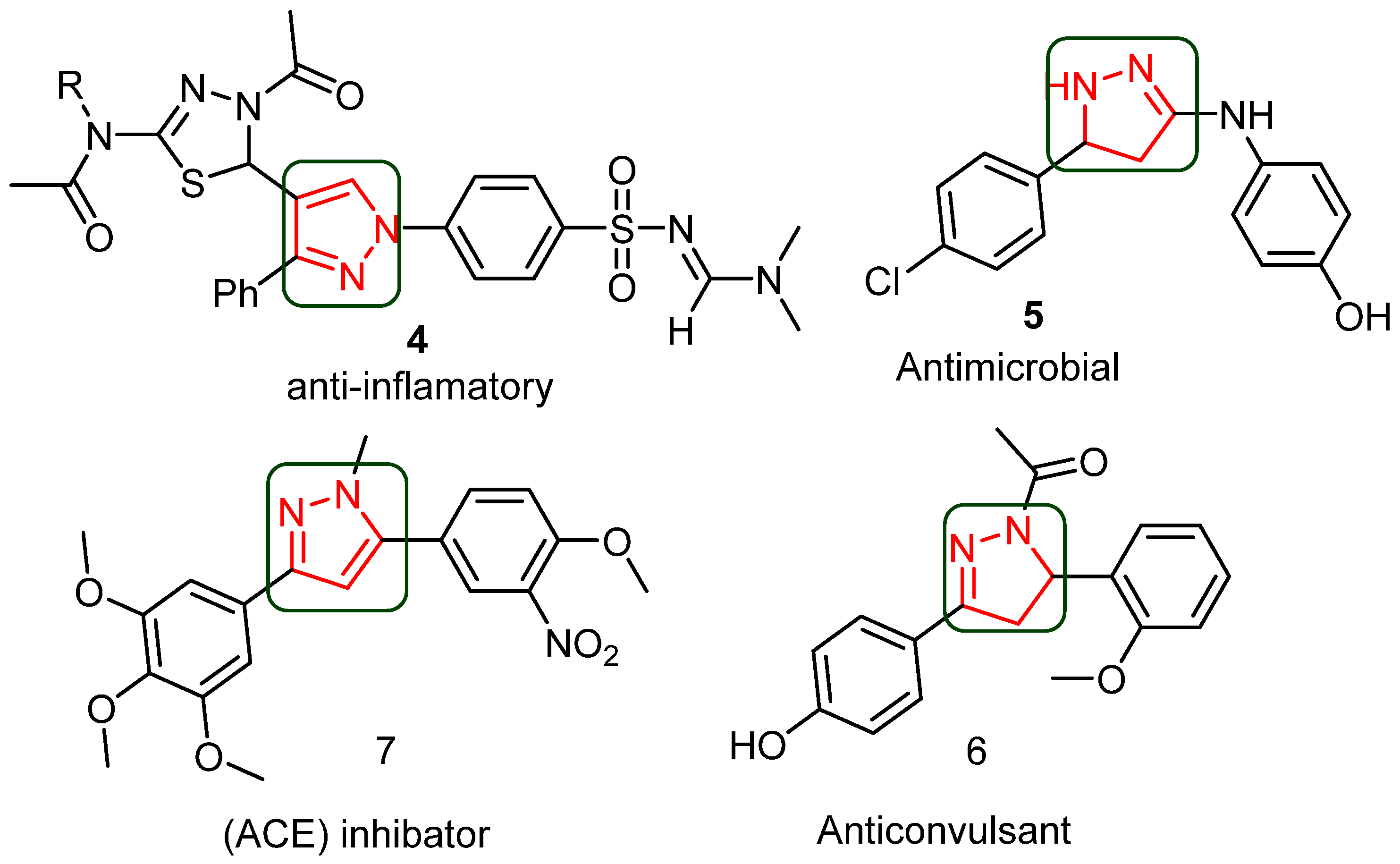
1. Introduction
2. Results
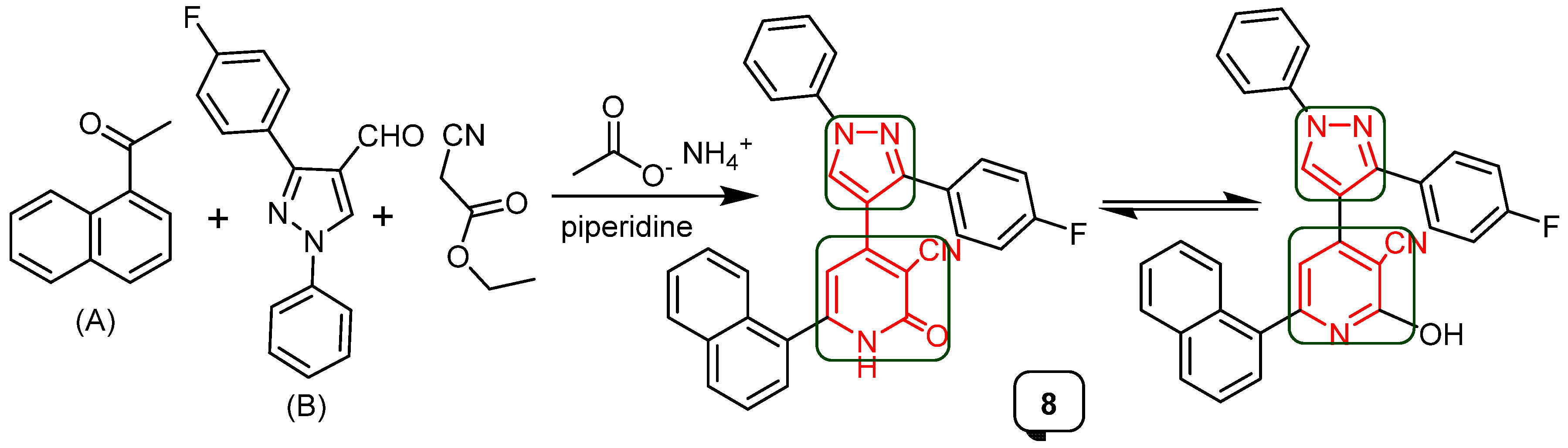
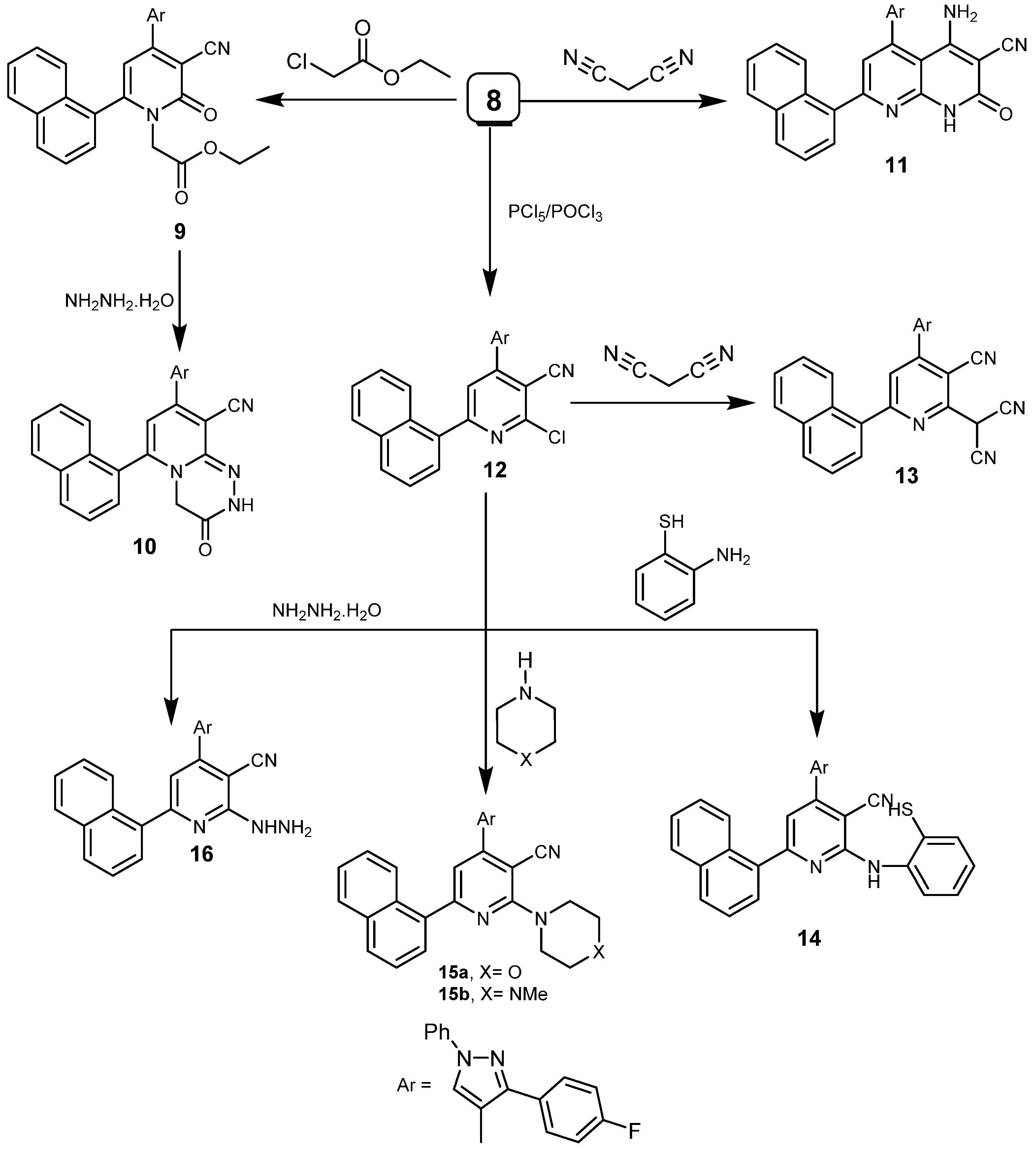
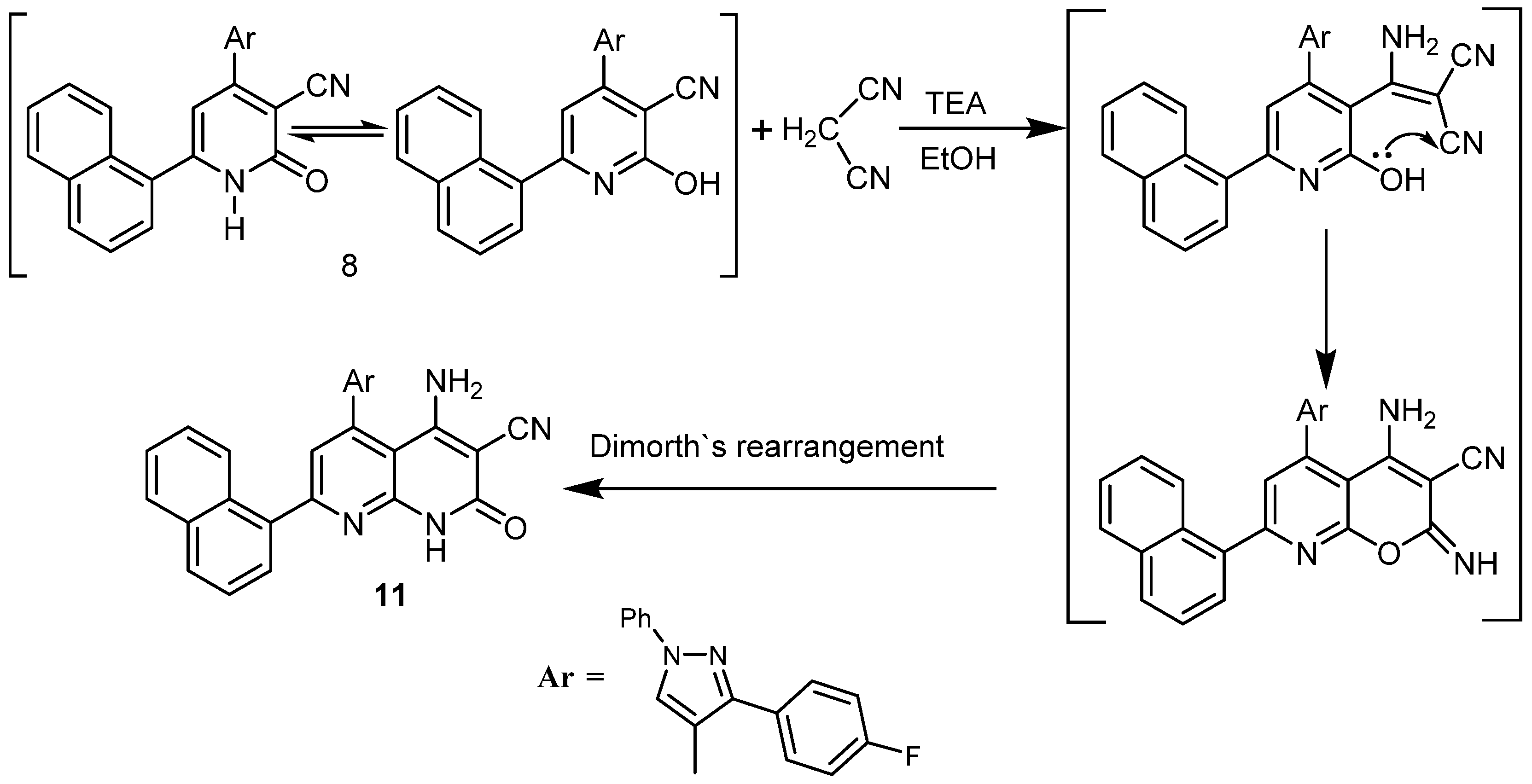
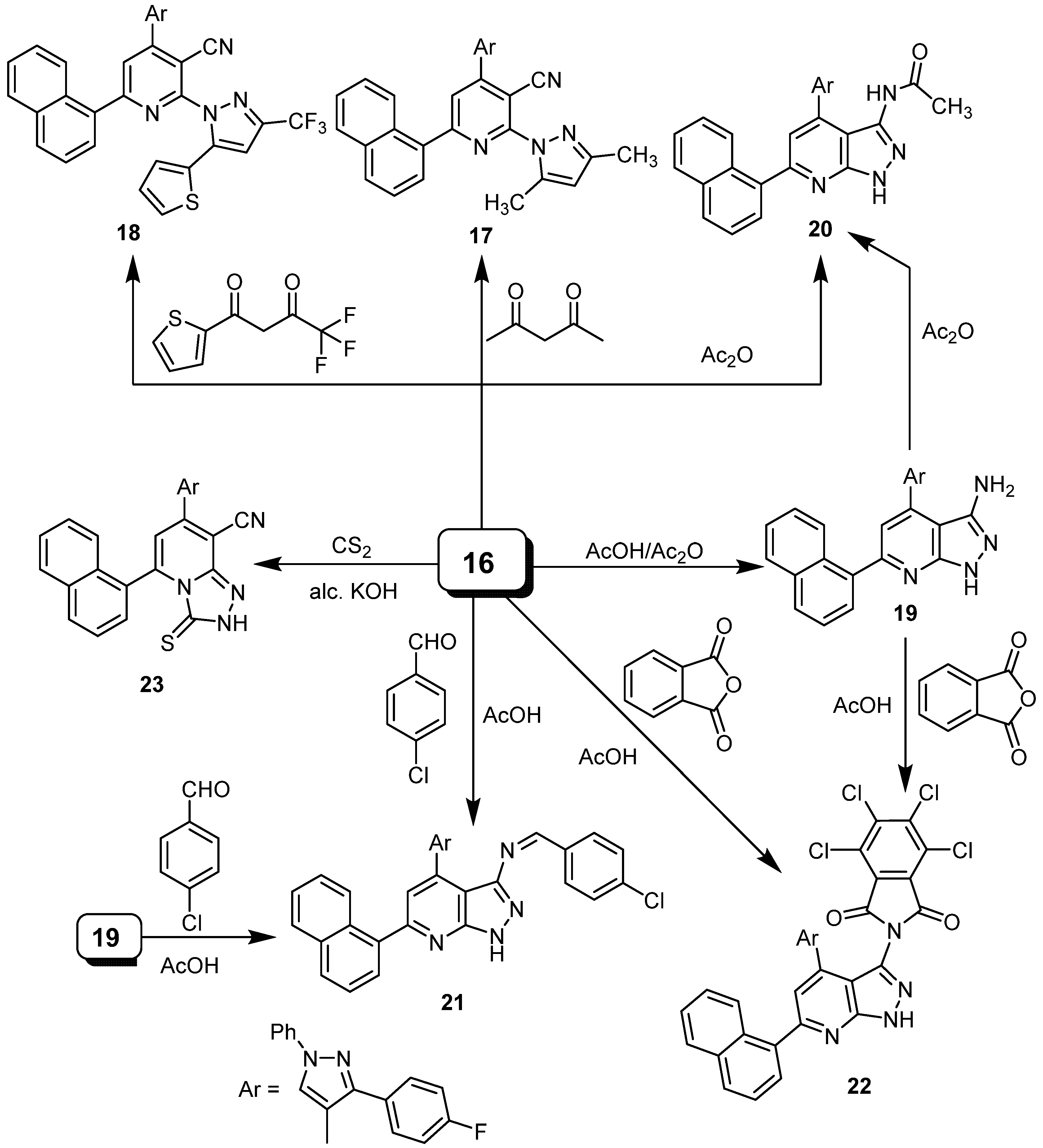
2.1. Chemistry
2.2. Cytotoxic Activity
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Synthesis of 4-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-2-hydroxy-6-(naphthalen-1-yl)-nicotinenitrile (8)
4.1.2. Synthesis of ethyl 2-(3-cyano-4-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-6-(naphthalene-1-yl)-2-oxopyridin-1(2H)-yl)acetate (9)
4.1.3. Synthesis of 8-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-6-(naphthalen-1-yl)-3-oxo-3,4-dihydro-2H-pyrido[2,1-c][1,2,4]triazine-9-carbonitrile (10)
4.1.4. Synthesis of 5-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-7-(naphthalen-1-yl)-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carbonitrile (11)
4.1.5. Synthesis of 2-chloro-4-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-6-(naphthalen-1-yl)-nicotinenitrile (12)
4.1.6. Synthesis of 2-[4-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-6-(naphthalen-1-yl)-3-cyano-pyridinyl]malononitrile (13)
4.1.7. Synthesis of 14 and 15a,b
4.1.8. Synthesis of 4-(3-(4-Fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-2-hydrazinyl-6-(naphthalen-1-yl)nicotinonitrile (16)
4.1.9. Synthesis of 17 and 18
4.1.10. Synthesis of 19 and 20
4.1.11. Synthesis of N-(4-chlorobenzylidene)-4-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-6- (naphthalen-1-yl)-1H-pyrazolo[3,4-b]pyridine-3-amine (21)
4.1.12. Synthesis of 2-(4-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-6-(naphthalen-1-yl)-1H- pyrazolo[3,4-b]-pyridin-3-yl)isoindoline-1,3-dione (22)
4.2.13. Synthesis of 7-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-(naphthalen-1-yl)-3-thioxo- 2,3-dihydro[1,2,4]triazolo[4,3-a]pyridine-8-carbonitrile (23)
4.2. Cytotoxicity Assay
4.2.1. Materials and Cell Lines
4.2.2. MTT Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajeswari, M.; Saluja, P.; Khurana, J.M. A facile and green approach for the synthesis of spiro[naphthalene-2,50-pyrimidine]-4-carbonitrile via a one-pot three-component condensation reaction using DBU as a catalyst. Rsc. Adv. 2016, 6, 1307–1312. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Moustafa, A.H.; Haikal, A.Z.; Abu-El-Halawa, R.; El Ashry, E.H. Synthesis, antitumor and antimicrobial activities of 4-(4-chlorophenyl)-3-cyano-2-(b-o-glycosyloxy)-6-(thien-2-yl)nicotine- nitrile. Eur. J. Med. Chem. 2011, 46, 2948–2954. [Google Scholar] [CrossRef]
- Kotb, E.R.; El-Hashash, M.A.; Salama, M.A.; Kalf, H.S.; Abdel Wahed, N.A.M. Synthesis and reactions of some novel nicotinonitrile derivatives for anticancer and antimicrobial evaluation. Acta Chim. Slov. 2009, 56, 908–919. [Google Scholar]
- Hamdy, N.A.; Anwar, M.M.; Abu-Zied, K.M.; Awad, H.M. Synthesis, tumor inhibitory and antioxidant activity of new polyfunctionally 2-substituted 5,6,7,8-tetrahydronaphthalene derivatives containing pyridine, thioxopyridine and pyrazolopyridine moieties. Acta Polo. Pharm. Drug Res. 2013, 70, 987–1001. [Google Scholar]
- Salem, M.S.; Sakr, S.I.; El-Senousy, W.M.; Madkour, H.M.F.; El-Senousy, W.M. Synthesis, Antibacterial, and Antiviral Evaluation of New Heterocycles Containing the Pyridine Moiety. Arch. der Pharm. 2013, 346, 766–773. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Shirazi, A.N.; El-Meligy, M.G.; El-Ziaty, A.K.; Rowley, D.; Sun, J.; Nagib, Z.A.; Parang, K. Synthesis of 4-aryl-6-indolylpyridine-3-carbonitriles and evaluation of their anti-proliferative activity. Tetra. Lett. 2014, 55, 1154–1158. [Google Scholar] [CrossRef]
- Ruiz, J.F.M.; Kedziora, K.; Keogh, B.; Maguire, J.; Reilly, M.; Windle, H.; Kelleher, D.P.; Gilmer, J.F. A double prodrug system for colon targeting of benzenesulfonamide COX-2 inhibitors. Bioorganic Med. Chem. Lett. 2011, 21, 6636–6640. [Google Scholar] [CrossRef]
- Balsamo, A.; Coletta, I.; Guglielmotti, A.; Landolfi, C.; Mancini, F.; Martinelli, A.; Milanese, C.; Minutolo, F.; Nencetti, S.; Orlandini, E.; et al. Synthesis of heteroaromatic analogues of (2-aryl-1-cyclopentenyl-1-alkylidene)-(arylmethyloxy)amine COX-2 inhibitors: effects on the inhibitory activity of the replacement of the cyclopentene central core with pyrazole, thiophene or isoxazole ring. Eur. J. Med. Chem. 2003, 38, 157–168. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Al-Aizari, F.; Ansar, M.; A Al-Aizari, F.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Ashour, H.M.; Ghany, Y.S.A.; Bekhit, A.E.-D.A.; Baraka, A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 456–463. [Google Scholar] [CrossRef]
- Christodoulou, M.S.; Liekens, S.; Kasiotis, K.M.; Haroutounian, S.A. Novel pyrazole derivatives: Synthesis and evaluation of anti-angiogenic activity. Bioorganic Med. Chem. 2010, 18, 4338–4350. [Google Scholar] [CrossRef]
- Bondock, S.; Fadaly, W.; Metwally, M.A. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur. J. Med. Chem. 2010, 45, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Bolasco, A.; Manna, F.; Secci, D.; Chimenti, P.; Befani, O.; Turini, P.; Giovannini, V.; Mondovi, B.; Cirilli, R.; et al. Synthesis and Selective Inhibitory Activity of 1-Acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole Derivatives against Monoamine Oxidase. J. Med. Chem. 2004, 47, 2071–2074. [Google Scholar] [CrossRef]
- Rashad, A.E.; Hegab, M.I.; Abdel-Megeid, R.E.; Micky, J.A.; Abdel-Megeid, F.M. Synthesis and antiviral evaluation of some new pyrazole and fused pyrazolopyrimidine derivatives. Bioorganic Med. Chem. 2008, 16, 7102–7106. [Google Scholar] [CrossRef]
- Bonesi, M.; Loizzo, M.R.; Statti, G.A.; Michel, S.; Tillequin, F.; Menichini, F. The synthesis and Angiotensin Converting Enzyme (ACE) inhibitory activity of chalcones and their pyrazole derivatives. Bioorganic Med. Chem. Lett. 2010, 20, 1990–1993. [Google Scholar] [CrossRef]
- Mahmoud, M.R.; El-Ziaty, A.K.; Abu El-Azm, F.S.M.; Ismail, M.F.; Shiba, S.A. Utility of Cyano-N-(2-oxo-1,2-dihydroindol-3-ylidene)acetohydrazide in the Synthesis of Novel Heterocycles. J. Chem. 2013, 37, 80–85. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Shirazi, A.N.; El-Meligy, M.G.; El-Ziaty, A.K.; Nagieb, Z.A.; Parang, K.; Tiwari, R.K. Design, synthesis, and evaluation of chitosan conjugated GGRGDSK peptides as a cancer cell-targeting molecular transporter. Int. J. Boil. Macromol. 2016, 87, 611–622. [Google Scholar] [CrossRef]
- El-Ziaty, A.K.; Shiba, S.A. Antibacterial activities of new (E) 2-cyano-3-(3,4-dimethoxyphenyl) -2-propenoylamide derivatives. Synth. Commun. 2007, 37, 4043–4057. [Google Scholar] [CrossRef]
- Mahmoud, M.R.; Shiba, S.A.; El-Ziaty, A.K.; Abu El-Azm, F.S.M.; Ismail, M.F. Synthesis and reactions of novel 2,5-disubistituted 1,3,4-thiadiazoles. Synth. Commun. 2014, 44, 1094–1102. [Google Scholar] [CrossRef]
- El-Shahawi, M.M.; El-Ziaty, A.K. Enaminonitrile as Building Block in Heterocyclic Synthesis: Synthesis of Novel 4H -Furo[2,3-d ][1,3]oxazin-4-one and Furo[2,3- d ]pyrimidin-4 (3H) -one Derivatives. J. Chem. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Mahmoud, M.R.; El-Ziaty, A.K.; Hussein, A.M. Synthesis and Spectral Characterization of Novel Thiazolopyridine and Pyrimidine Derivatives. Synth. Commun. 2013, 43, 961–978. [Google Scholar] [CrossRef]
- Ismail, M.F.; El-Sayed, A.A. Synthesis and in-vitro antioxidant and antitumor evaluation of novel pyrazole-based heterocycles. Iran. Chem. Soc. 2019, 16, 921–937. [Google Scholar] [CrossRef]
- Fahmy, A.F.M.; Rizk, S.A.; Hemdan, M.M.; El-Sayed, A.A.; Hassaballah, A.I. Efficient Green Synthesis and Computational Chemical Study of Some Interesting Heterocyclic Derivatives as Insecticidal Agents. J. Chem. 2018, 55, 2545–2555. [Google Scholar] [CrossRef]
- Rizk, S.A.; El-Sayed, A.A.; Mounier, M.M.; El-Sayed, A.A. Synthesis of Novel Pyrazole Derivatives as Antineoplastic Agent. J. Chem. 2017, 54, 3358–3371. [Google Scholar] [CrossRef]
- Fahmy, A.F.M.; El-Sayed, A.A.; Hemdan, M.M. Multicomponent synthesis of 4-arylidene-2-phenyl-5(4H)-oxazolones (azlactones) using a mechanochemical approach. Chem. Central J. 2016, 10, 59. [Google Scholar] [CrossRef]
- Hemdan, M.M.; El-Sayed, A.A. Use of phthalimidoacetylisothiocyanate as a scaffold in synthesis of target heterocyclic systems with their antimicrobial assessment. Chem. Pharm. Bull. 2016, 64, 483–489. [Google Scholar] [CrossRef]
- Hemdan, M.M.; El-Sayed, A.A. Synthesis of some new heterocycles derived from novel 2-(1,3-dioxisoindolin-2-yl)benzoyl isothiocyanate. J. Heterocycl. Chem. 2016, 53, 487–492. [Google Scholar] [CrossRef]
- Metwally, M.; Gouda, M.; Harmal, A.N.; Khalil, A. Synthesis, antitumor, cytotoxic and antioxidant evaluation of some new pyrazolotriazines attached to antipyrine moiety. Eur. J. Med. Chem. 2012, 56, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Hossan, A.; Abu-Melha, H. Synthesis, mass spectroscopic studies, cytotoxicity evaluation and quantitative structure activity relationship of novel isoindolin-1,3-dione derivatives. Chem. Process Eng. Res. 2014, 21, 60–71. [Google Scholar]
- Eissa, I.H.; El-Naggar, A.M.; El-Hashash, M.A. Design, synthesis, molecular modeling and biological evaluation of novel 1H-pyrazolo[3,4-b]pyridine derivatives as potential anticancer agents. Bioorganic Chem. 2016, 67, 43–56. [Google Scholar] [CrossRef]
- Shaaban, S.; Negm, A.; Sobh, M.A.; Wessjohann, L.A. Organoselenocyanates and symmetrical diselenides redox modulators: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 97, 190–201. [Google Scholar] [CrossRef]
- Elsayed, E.A.; Farooq, M.; Dailin, D.; El-Enshasy, H.A.; Othman, N.Z.; Malek, R.; Danial, E.; Wadaan, M. In vitro and in vivo biological screening of kefiran polysaccharide produced by Lactobacillus kefiranofaciens. Biomed. Res. 2017, 28, 594–600. [Google Scholar]
- Amr, A.E.-G.E.; El-Naggar, M.; Al-Omar, M.A.; Elsayed, E.A.; Abdalla, M.M. In Vitro and In Vivo Anti-Breast Cancer Activities of Some Synthesized Pyrazolinyl-estran-17-one Candidates. Molecules 2018, 23, 1572. [Google Scholar] [CrossRef]
- Amr, A.E.-G.E.; Abo-Ghalia, M.H.; Moustafa, G.O.; Al-Omar, M.A.; Nossier, E.S.; Elsayed, E.A. Design, Synthesis and Docking Studies of Novel Macrocyclic Pentapeptides as Anticancer Multi-Targeted Kinase Inhibitors. Molecules 2018, 23, 2416. [Google Scholar] [CrossRef]
- Dailin, D.J.; Elsayed, E.A.; Othman, N.Z.; Malek, R.; Phin, H.S.; Aziz, R.; Wadaan, M.; El Enshasy, H.A. Bioprocess development for kefiran production by Lactobacillus kefiranofaciens in semi industrial scale bioreactor. Saudi J. Biol. Sci. 2016, 23, 495–502. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
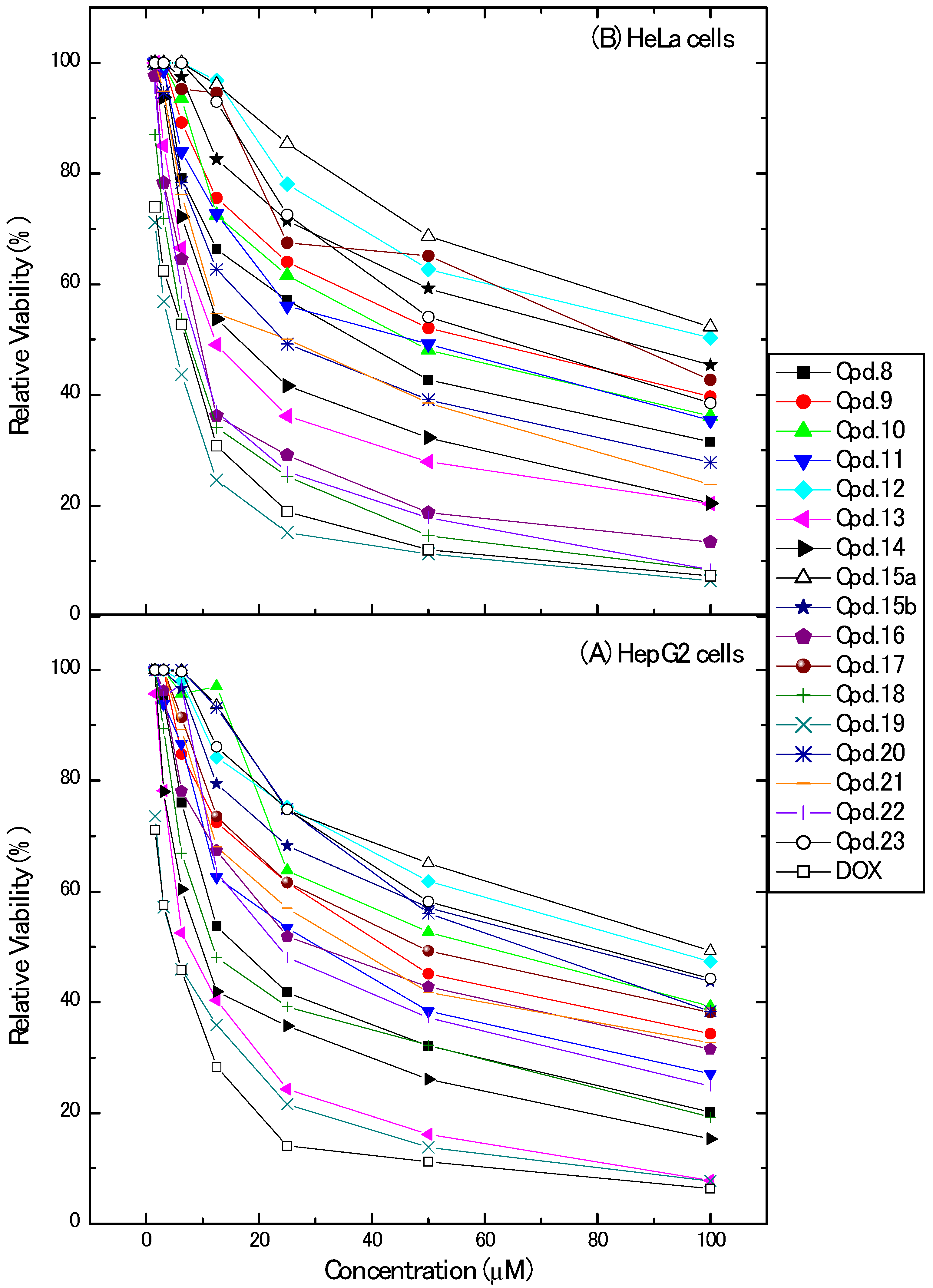
| Compound | IC50 (µM) * | |
|---|---|---|
| HepG2 | HeLa | |
| 8 | 20.00 ± 1.7 | 35.58 ± 2.6 |
| 9 | 42.95 ± 3.2 | 55.00 ± 3.7 |
| 10 | 56.57 ± 3.4 | 47.02 ± 3.4 |
| 11 | 30.22 ± 2.1 | 43.64 ± 3.3 |
| 12 | 83.82 ± 4.5 | 89.72 ± 4.7 |
| 13 | 8.87 ± 0.70 | 15.32 ± 1.2 |
| 14 | 12.20 ± 1.0 | 19.44 ± 1.4 |
| 15a | 90.05 ± 5.1 | >100 |
| 15b | 68.19 ± 3.7 | 75.05 ± 4.5 |
| 16 | 33.45 ± 2.3 | 10.37 ± 0.9 |
| 17 | 49.66 ± 3.2 | 65.91 ± 4.1 |
| 18 | 16.70 ± 1.3 | 7.67 ± 0.60 |
| 19 | 5.16 ± 0.40 | 4.26 ± 0.30 |
| 20 | 64.39 ± 3.6 | 28.15 ± 2.2 |
| 21 | 37.42 ± 2.5 | 24.83 ± 1.8 |
| 22 | 26.64 ± 1.9 | 9.33 ± 0.80 |
| 23 | 73.48 ± 4.0 | 62.07 ± 3.9 |
| Doxorubicin | 4.50 ± 0.20 | 5.57 ± 0.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. El-Sayed, A.; E. Amr, A.E.-G.; K. EL-Ziaty, A.; A. Elsayed, E. Cytotoxic Effects of Newly Synthesized Heterocyclic Candidates Containing Nicotinonitrile and Pyrazole Moieties on Hepatocellular and Cervical Carcinomas. Molecules 2019, 24, 1965. https://doi.org/10.3390/molecules24101965
A. El-Sayed A, E. Amr AE-G, K. EL-Ziaty A, A. Elsayed E. Cytotoxic Effects of Newly Synthesized Heterocyclic Candidates Containing Nicotinonitrile and Pyrazole Moieties on Hepatocellular and Cervical Carcinomas. Molecules. 2019; 24(10):1965. https://doi.org/10.3390/molecules24101965
Chicago/Turabian StyleA. El-Sayed, Amira, Abd El-Galil E. Amr, Ahmed K. EL-Ziaty, and Elsayed A. Elsayed. 2019. "Cytotoxic Effects of Newly Synthesized Heterocyclic Candidates Containing Nicotinonitrile and Pyrazole Moieties on Hepatocellular and Cervical Carcinomas" Molecules 24, no. 10: 1965. https://doi.org/10.3390/molecules24101965
APA StyleA. El-Sayed, A., E. Amr, A. E.-G., K. EL-Ziaty, A., & A. Elsayed, E. (2019). Cytotoxic Effects of Newly Synthesized Heterocyclic Candidates Containing Nicotinonitrile and Pyrazole Moieties on Hepatocellular and Cervical Carcinomas. Molecules, 24(10), 1965. https://doi.org/10.3390/molecules24101965







