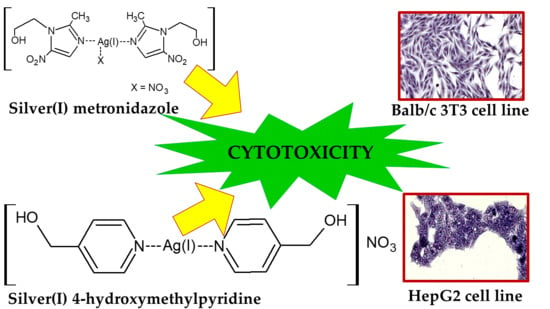
Silver(I) Complexes of the Pharmaceutical Agents Metronidazole and 4-Hydroxymethylpyridine: Comparison of Cytotoxic Profile for Potential Clinical Application
Abstract
1. Introduction
2. Results and Discussion
2.1. General Aspects
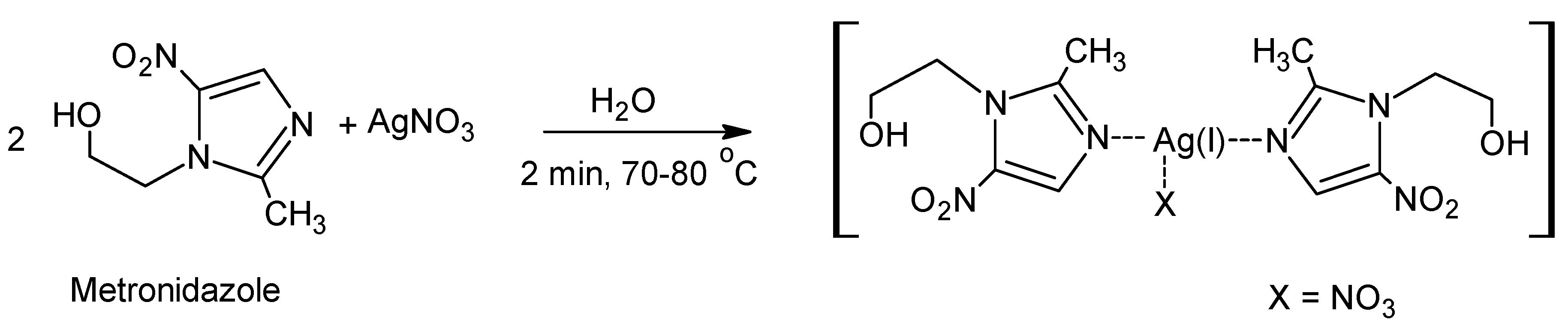
2.2. Synthesis of [(Metronidazole)2AgNO3]
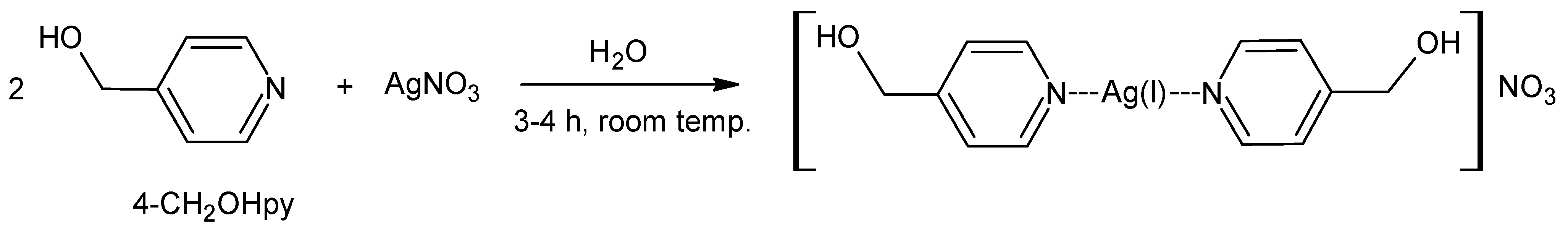
2.3. Synthesis of [(4-hydroxymethylpyridine)2Ag]NO3
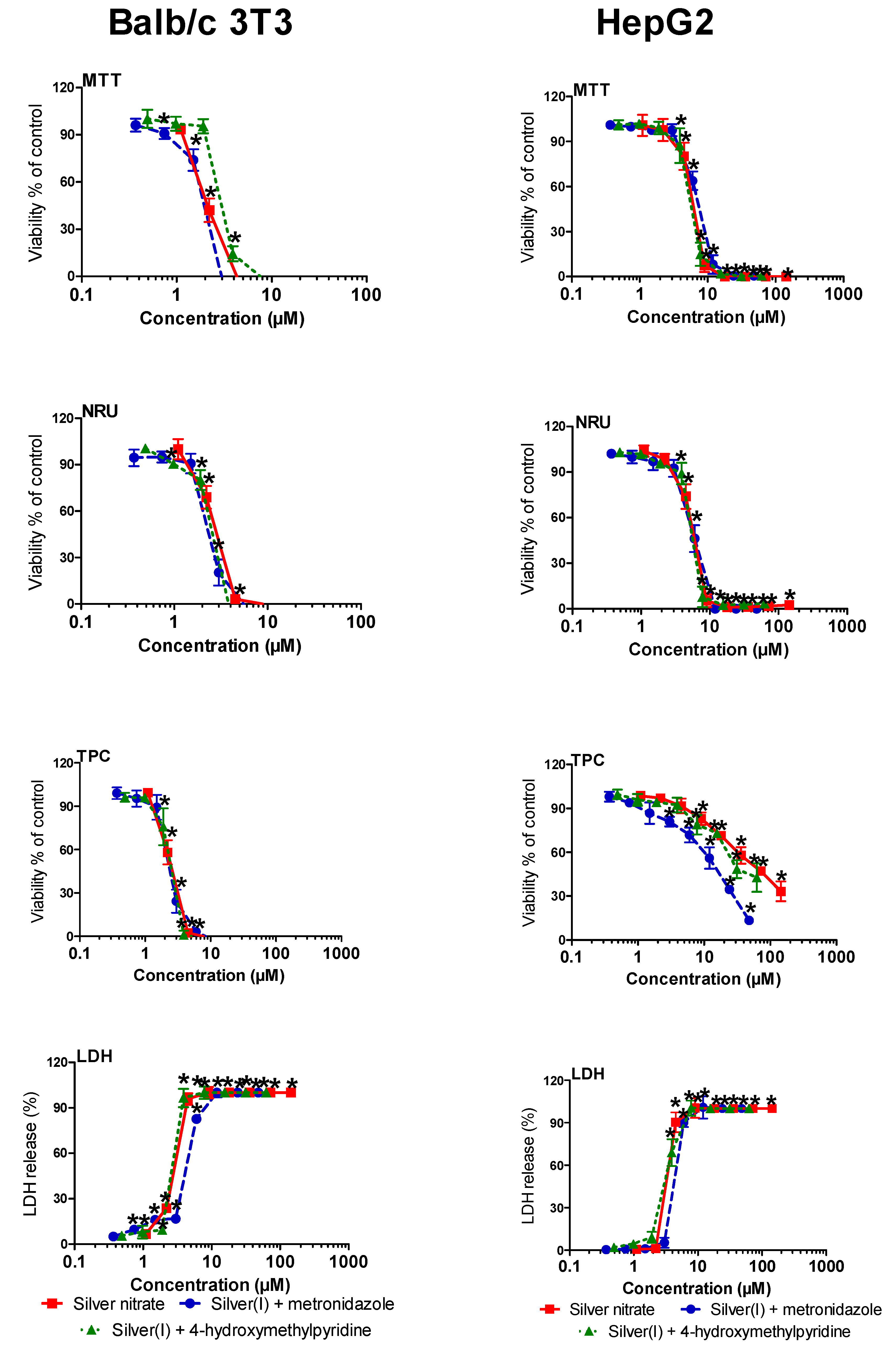
2.4. Cytotoxic Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthetic Procedures
3.2.1. Synthesis of [(Metronidazole)2AgNO3]
3.2.2. Synthesis of [(4-hydroxymethylpyridine)2Ag]NO3
3.3. Cell Line Cultures
3.4. Preparation of Compounds and Exposure
3.5. Cytotoxicity Assessment
3.5.1. MTT Assay
3.5.2. NRU Assay
3.5.3. TPC Assay
3.5.4. Leakage LDH Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Zawadzka, K.; Patyna, E.; Lisowska, K.; Ochocki, J. Synthesis, characterization and antimicrobial activity of water-soluble silver(I) complexes of metronidazole drug and selected counter-ions. Dalton Trans. 2015, 44, 8178–8189. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska-Lis, U.; Szewczyk, E.M.; Chęcińska, L.; Wojciechowski, J.M.; Wolf, W.M.; Ochocki, J. Synthesis, characterization, and antimicrobial activity of silver(I) and copper(II) complexes of phosphate derivatives of pyridine and benzimidazole. Chem. Med. Chem. 2014, 9, 169–176. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Szabłowska-Gadomska, I.; Patyna, E.; Małecki, M.; Lisowska, K.; Ochocki, J. Antibacterial Activity and Cytotoxicity of Silver(I) Complexes of Pyridine and (Benz)Imidazole Derivatives. X-ray Crystal Structure of [Ag(2,6-di(CH2OH)py)2]NO3. Molecules 2016, 28, 87. [Google Scholar] [CrossRef]
- Ochocki, J.; Kalinowska-Lis, U. EP 2848608B1, Silver complex compounds, method for their production and their use. European Patent Specification. Bulletin 2017/51. Available online: https://register.epo.org/application?number=EP14174623 (accessed on 18 May 2019).
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Lisowska, K.; Ochocki, J. Synthesis, characterization and antimicrobial activity of silver(I) complexes of hydroxymethyl derivatives of pyridine and benzimidazole. J. Organomet. Chem. 2014, 749, 394–399. [Google Scholar] [CrossRef]
- Banti, C.N.; Hadjikakou, S.K. Anti-proliferative and anti-tumor activity of silver(I) compounds. Metallomics 2013, 5, 569–596. [Google Scholar] [CrossRef]
- Zhu, H.-L.; Zhang, X.-M.; Liu, X.-Y.; Wang, X.-J.; Liu, G.-F.; Usman, A.; Fun, H.-K. Clear Ag–Ag bonds in three silver(I) carboxylate complexes with high cytotoxicity properties. Inorg. Chem. Commun. 2003, 6, 1113–1116. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhu, H.L. Strong silver-silver interactions in three silver(I) carboxylate complexes with high cytotoxicity properties. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2005, 35, 325–332. [Google Scholar] [CrossRef]
- Lencastre, A.; Lobo, M.; João, A. Argyria—Case report. An. Bras. Dermatol. 2013, 88, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef]
- Kadayat, T.M.; Song, C.; Shin, S.; Magar, T.B.; Bist, G.; Shrestha, A.; Thapa, P.; Na, Y.; Kwon, Y.; Lee, E.S. Synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship study of 2-phenyl- or hydroxylated 2-phenyl-4-aryl-5H-indeno [1,2-b]pyridines. Bioorg. Med. Chem. 2015, 23, 3499–3512. [Google Scholar] [CrossRef]
- Adhami, F.; Safavi, M.; Ehsani, M.; Ardestani, S.K.; Emmerling, F.; Simyari, F. Synthesis, crystal structure, and cytotoxic activity of novel cyclic systems in [1,2,4]thiadiazolo[2,3-a]pyridine benzamide derivatives and their copper(II) complexes. Dalton Trans. 2014, 43, 7945–7957. [Google Scholar] [CrossRef]
- Sun, G.-X.; Yang, M.-Y.; Shi, Y.-X.; Sun, Z.-H.; Liu, X.-H.; Wu, H.-K.; Li, B.-J.; Zhang, Y.-G. Microwaveassistant synthesis, antifungal activity and dft theoretical study of some novel 1,2,4-triazole derivatives containing pyridine moiety. Int. J. Mol. Sci. 2014, 15, 8075. [Google Scholar] [CrossRef]
- Radko, L.; Minta, M. Cytotoxicity of some nitroimidazole derivatives - comparative studies on human and rat hepatoma cell lines. Bull. Vet. Inst. Pulawy. 2012, 56, 579–584. [Google Scholar] [CrossRef]
- Radko, L.; Minta, M.; Stypuła-Trębas, S. Influence of fluoroquinolones on viability of Balb/c 3T3 and HepG2 cells. Bull. Vet. Inst. Pulawy. 2013, 57, 599–606. [Google Scholar] [CrossRef][Green Version]
- Lozynskyi, A.; Zimenkovsky, B.; Radko, L.; Stypula-Trebas, S.; Roman, O.; Gzella, A.K.; Lesyk, R. Synthesis and cytotoxicity of new thiazolo[4,5-b]pyridine-2(3H)-one derivatives based on α,β-unsaturated ketones and α-ketoacids. Chem. Papers 2018, 72, 669–681. [Google Scholar] [CrossRef]
- Eisenbrand, G.; Pool-Zobel, B.; Baker, V.; Balls, M.; Blaauboer, B.J.; Boobis, A.; Carere, A.; Kevekordes, S.; Lhuguenot, J.C.; Pieters, R.; et al. Methods of in vitro toxicology. Food Chem. Toxicol. 2002, 40, 193–236. [Google Scholar] [CrossRef]
- Weyermenn, J.; Lohmann, D.; Zimmer, A. A practical note on the use of cytotoxicity assays. Int. J. Pharm. 2005, 288, 369–376. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assay. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Korzeniewski, C.; Calleawert, D.M. An enzyme-release assay for natural cytotoxicity. J. Immunol. Method. 1983, 64, 313–320. [Google Scholar] [CrossRef]
- Pohjala, L.; Tammela, P.; Samanta, S.K.; Yli-Kauhaluoma, J.; Vuorela, P. Assessing the data quality in predictive toxicology using a panel of cell lines and cytotoxicity assays. Anal. Biochem. 2007, 362, 221–228. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Irwin, W.; Diaz, D.; Howardo-Cofield, E.; Krejsa, C.M.; Slaughter, M.R.; Gao, B.; Kaludercic, N.; Angeline, A.; Bernardi, P.; et al. High concordance of drug-induced human hapatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch. Toxicol. 2006, 80, 580–604. [Google Scholar] [CrossRef]
- Scheers, E.M.; Ekwall, B.; Dierickx, P.J. In vitro long-term cytotoxicity testing of 27 MEIC chemicals on HepG2 cells and comparison with acute human toxicity data. Toxicol. In Vitro 2001, 15, 153–161. [Google Scholar] [CrossRef]
- Schoonen, W.G.E.J.; de Roos, J.A.D.M.; Westerink, W.M.A.; Débiton, E. Cytotoxic effects of 110 reference compounds on HepG2 cells and for 60 compounds on HeLa, ECC-1 and CHO cells.: II Mechanistic assays on NAD(P)H, ATP and DNA contents. Toxicol. In Vitro 2005, 19, 491–503. [Google Scholar] [CrossRef]
- Schoonen, W.G.E.J.; Westerink, W.M.A.; de Roos, J.A.D.M.; Débiton, E. Cytotoxic effects of 100 reference compounds on HepG2 and HeLa cells and of 60 compounds on ECC-1 and CHO cells. I Mechanistic assays on ROS, glutathione depletion and calcein uptake. Toxicol. In Vitro 2005, 19, 505–516. [Google Scholar] [CrossRef]
- Castell, J.V.; Jover, R.; Martínez-Jiménez, C.P.; Gómez-Lechón, M.J. Hepatocyte cell lines: Their use, scope and limitations in drug metabolism studies. Exp. Opin. Drug Metab. Toxicol. 2006, 2, 183–212. [Google Scholar] [CrossRef]
- Qiu, G.-H.; Xie, X.; Xu, F.; Shi, X.; Wang, Y.; Deng, L. Distinctive pharmacological differences between liver cancer cell lines HepG2 and Hep3B. Cytotechnology 2015, 67, 1–12. [Google Scholar] [CrossRef]
- Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Heteroleptic silver(I) complexes with 2,2′:6′,2″-terpyridines and naproxen: DNA interaction, EGFR/VEGFR2 kinase, growth inhibition and cell cycle arrest studies. Mater Sci. Eng. C Mater Biol. 2017, 76, 601–615. [Google Scholar] [CrossRef]
- Medvetz, D.A.; Hindi, K.M.; Panzner, M.J.; Ditto, A.J.; Yun, Y.H.; Youngs, W.J. Anticancer Activity of Ag(I) N-Heterocyclic Carbene Complexes Derived from 4,5-Dichloro-1H-Imidazole. Metal-Based Drugs 2008. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Ju, H.W.; Moon, B.M.; Park, H.J.; Kim, J.H.; Lee, O.J.; Park, C.H. Facile and highly efficient approach for the fabrication of multifunctional silk nanofibers containing hydroxyapatite and silver nanoparticles. J. Biomed. Mater Res. A 2014, 102, 3459–3469. [Google Scholar] [CrossRef]
- Tamayo, L.V.; Santos, A.F.; Ferreira, I.P.; Santos, V.G.; Lopes, M.T.P.; Beraldo, H. Silver(I) complexes with chromone-derived hydrazones: Investigation on the antimicrobial and cytotoxic effects. Biometals 2017, 30, 379–392. [Google Scholar] [CrossRef]
- Kaplan, A.; Akalin Ciftci, G.; Kutlu, H.M. The apoptotic and genomic studies on A549 cell line induced by silver nitrate. Tumour Biol. 2017, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; AkalinCiftci, G.; Kutlu, H.M. Cytotoxic, anti-proliferative and apoptotic effects of silver nitrate against H-ras transformed 5RP7. Cytotechnology 2016, 68, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Shinohara, Y. Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem. Biophys. Res. Commun. 2009, 390, 733–737. [Google Scholar] [CrossRef]
- Ferreira, M.B.; Myiagi, S.; Nogales, C.G.; Campos, M.S.; Lage-Marques, J.L. Time- and concentration-dependent cytotoxicity of antibiotics used in endodontic therapy. J. Appl. Oral Sci. 2010, 18, 259–263. [Google Scholar] [CrossRef]
- Salahuddin, A.; Agarwal, S.M.; Avecilla, F.; Azam, A. Metronidazole thiosalicylate conjugates: Synthesis, crystal structure, docking studies and antiamoebic activity. Bioorg. Med. Chem. Lett. 2012, 17, 5694–5699. [Google Scholar] [CrossRef]
- Hausen, M.A.; Menna-Barreto, R.F.S.; Lira, D.C.; de Carvalho, L.; Barbosa, H.S. Synergic effect of metronidazole and pyrantelpamoate on Giardia lamblia. Parasitol. Int. 2011, 1, 54–58. [Google Scholar] [CrossRef]
- Silva, L.R.; Gurgel, R.Q.; Lima, D.R.; Cuevas, L.E. Current usefulness of Credé’s method of preventing neonatal ophthalmia. Ann. Trop. Paediatr. 2008, 28, 45–48. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Balb/c 3T3 | HepG2 | |||||||
|---|---|---|---|---|---|---|---|---|
| MTT | NRU | TPC | LDH | MTT | NRU | TPC | LDH | |
| Silver(I) + metronidazole | 2.19 ± 0.29 a | 2.17 ± 0.57 a | 2.13 ± 0.51 a | 4.59 ± 0.08 a | 7.61 ± 1.07 a | 5.92 ± 1.13 ac | 15.2 ± 2.4 b | 4.61 ± 0.1 c |
| Silver(I) + 4-hydroxymethylpyridine | 3.37 ± 0.57 a | 3.22 ± 0.46 a | 3.35 ± 0.75 a | 3.07 ± 0.08 a | 6.53 ± 1.19 a | 5.95 ± 0.08 a | 26.5 ± 5.8 b | 3.50 ± 0.08 c |
| Silver nitrate | 2.13 ± 0.88 a | 2.94 ± 1.06 ab | 2.59 ± 1.30 ab | 3.12 ± 0.06 b | 6.48 ± 1.60 a | 6.18 ± 1.41 a | 60.3 ± 8.1 b | 3.47 ± 0.24 c |
| Metronidazole | >146 | >146 | >146 | >146 | >146 | >146 | >146 | >146 |
| 4-hydroxymethylpyridine | >227 | >227 | >227 | >227 | >227 | >227 | >227 | >227 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radko, L.; Stypuła-Trębas, S.; Posyniak, A.; Żyro, D.; Ochocki, J. Silver(I) Complexes of the Pharmaceutical Agents Metronidazole and 4-Hydroxymethylpyridine: Comparison of Cytotoxic Profile for Potential Clinical Application. Molecules 2019, 24, 1949. https://doi.org/10.3390/molecules24101949
Radko L, Stypuła-Trębas S, Posyniak A, Żyro D, Ochocki J. Silver(I) Complexes of the Pharmaceutical Agents Metronidazole and 4-Hydroxymethylpyridine: Comparison of Cytotoxic Profile for Potential Clinical Application. Molecules. 2019; 24(10):1949. https://doi.org/10.3390/molecules24101949
Chicago/Turabian StyleRadko, Lidia, Sylwia Stypuła-Trębas, Andrzej Posyniak, Dominik Żyro, and Justyn Ochocki. 2019. "Silver(I) Complexes of the Pharmaceutical Agents Metronidazole and 4-Hydroxymethylpyridine: Comparison of Cytotoxic Profile for Potential Clinical Application" Molecules 24, no. 10: 1949. https://doi.org/10.3390/molecules24101949
APA StyleRadko, L., Stypuła-Trębas, S., Posyniak, A., Żyro, D., & Ochocki, J. (2019). Silver(I) Complexes of the Pharmaceutical Agents Metronidazole and 4-Hydroxymethylpyridine: Comparison of Cytotoxic Profile for Potential Clinical Application. Molecules, 24(10), 1949. https://doi.org/10.3390/molecules24101949