Norcantharidin Suppresses YD-15 Cell Invasion Through Inhibition of FAK/Paxillin and F-Actin Reorganization
Abstract
1. Introduction
2. Results
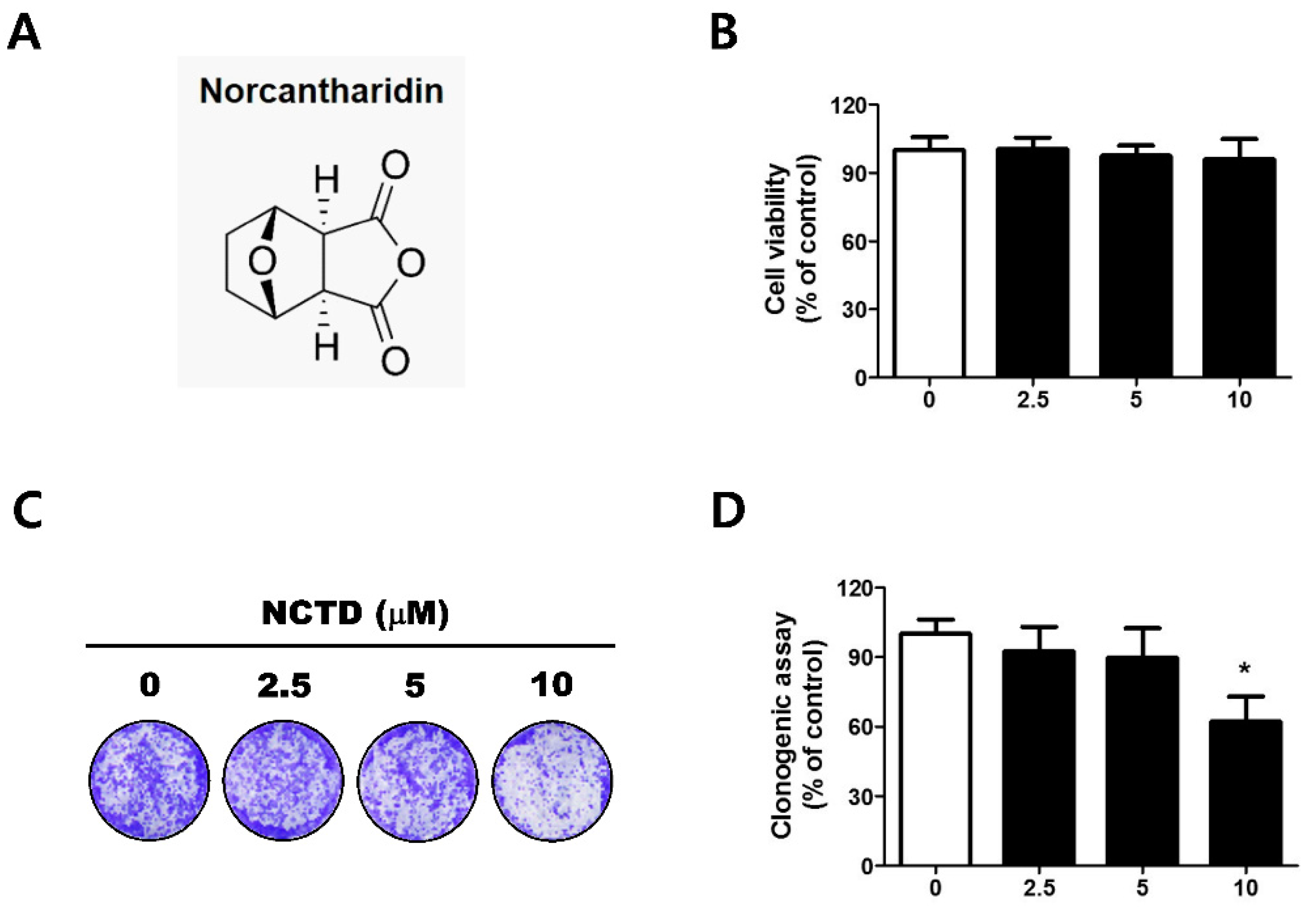
2.1. Low Concentrations of NCTD Affect Survival without Apoptosis in the YD-15 Cell Line
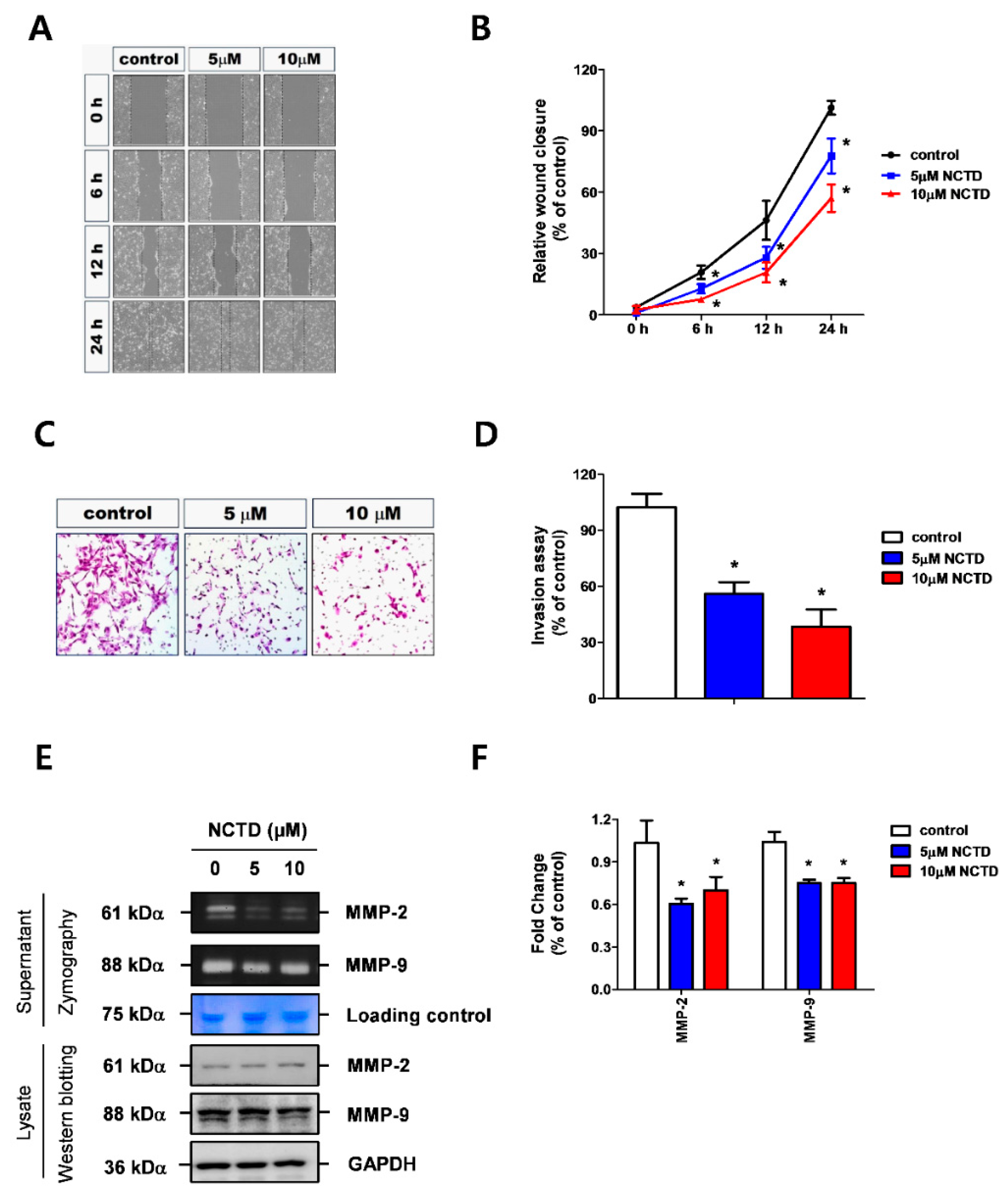
2.2. NCTD Represses YD-15 Cell Migration and Invasion through Down-Regulation of MMP-2 and MMP-9 Activity
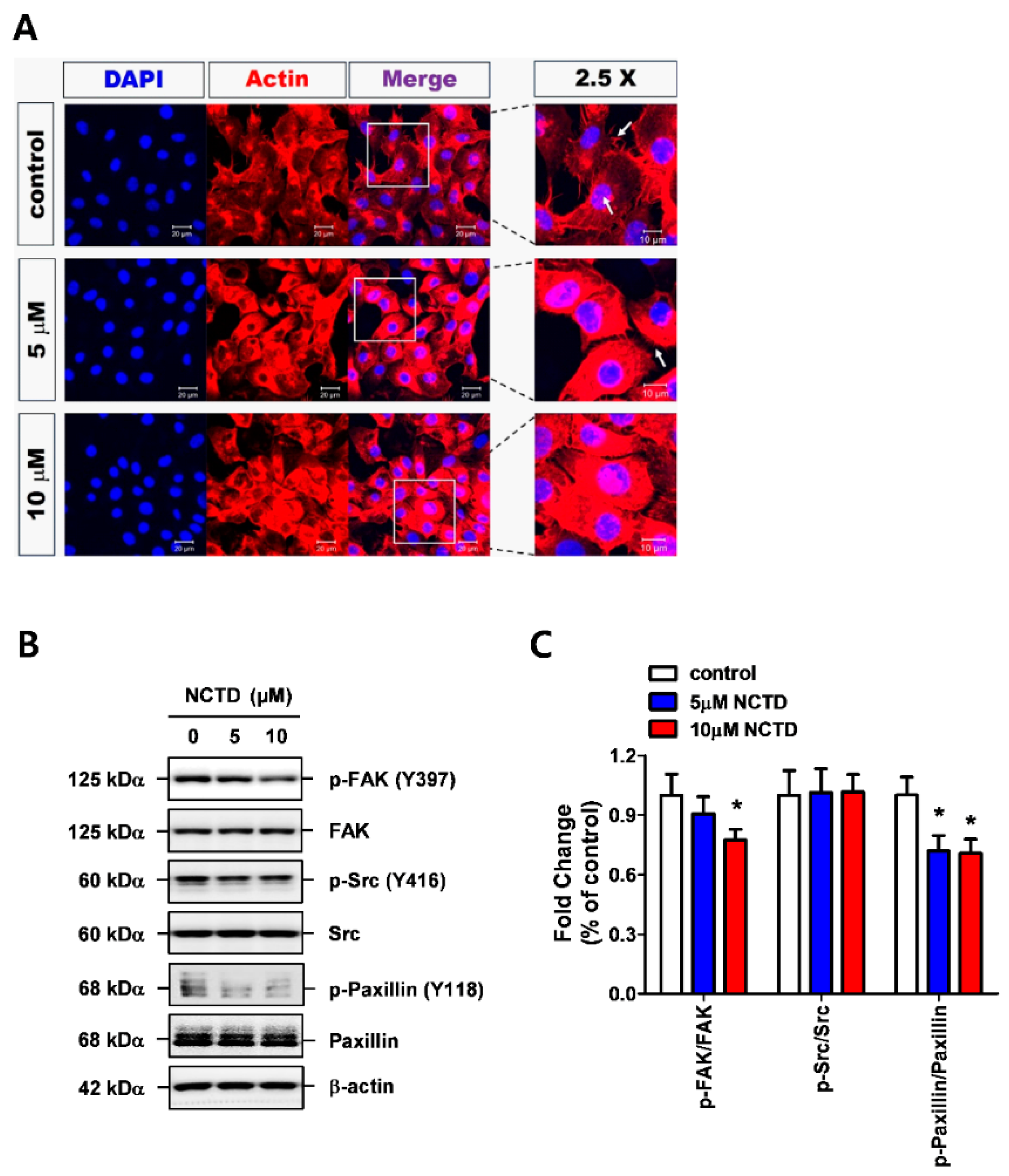
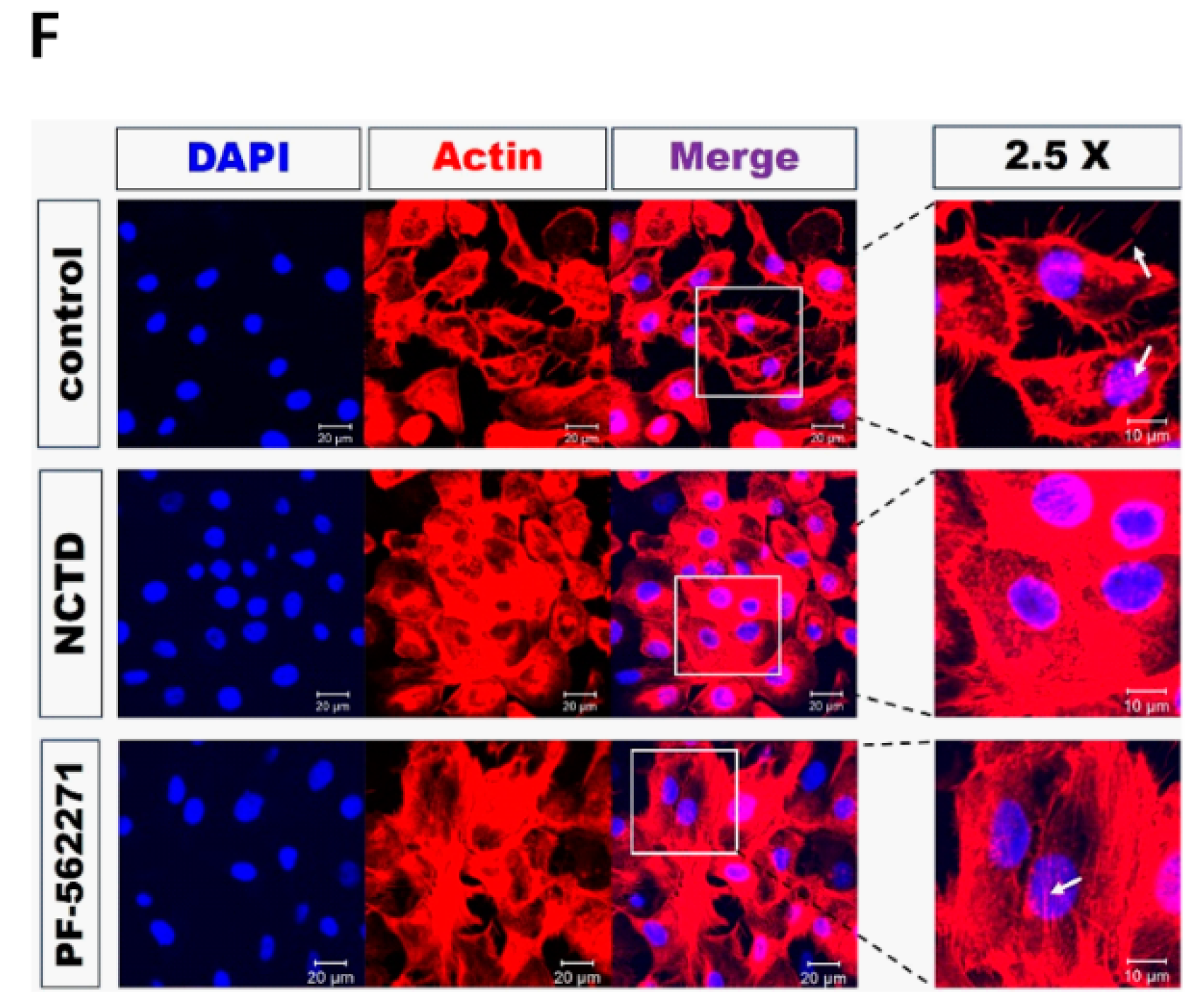
2.3. NCTD Inhibits F-Actin Reorganization of YD-15 Cells through Inactivation of FAK and Paxillin
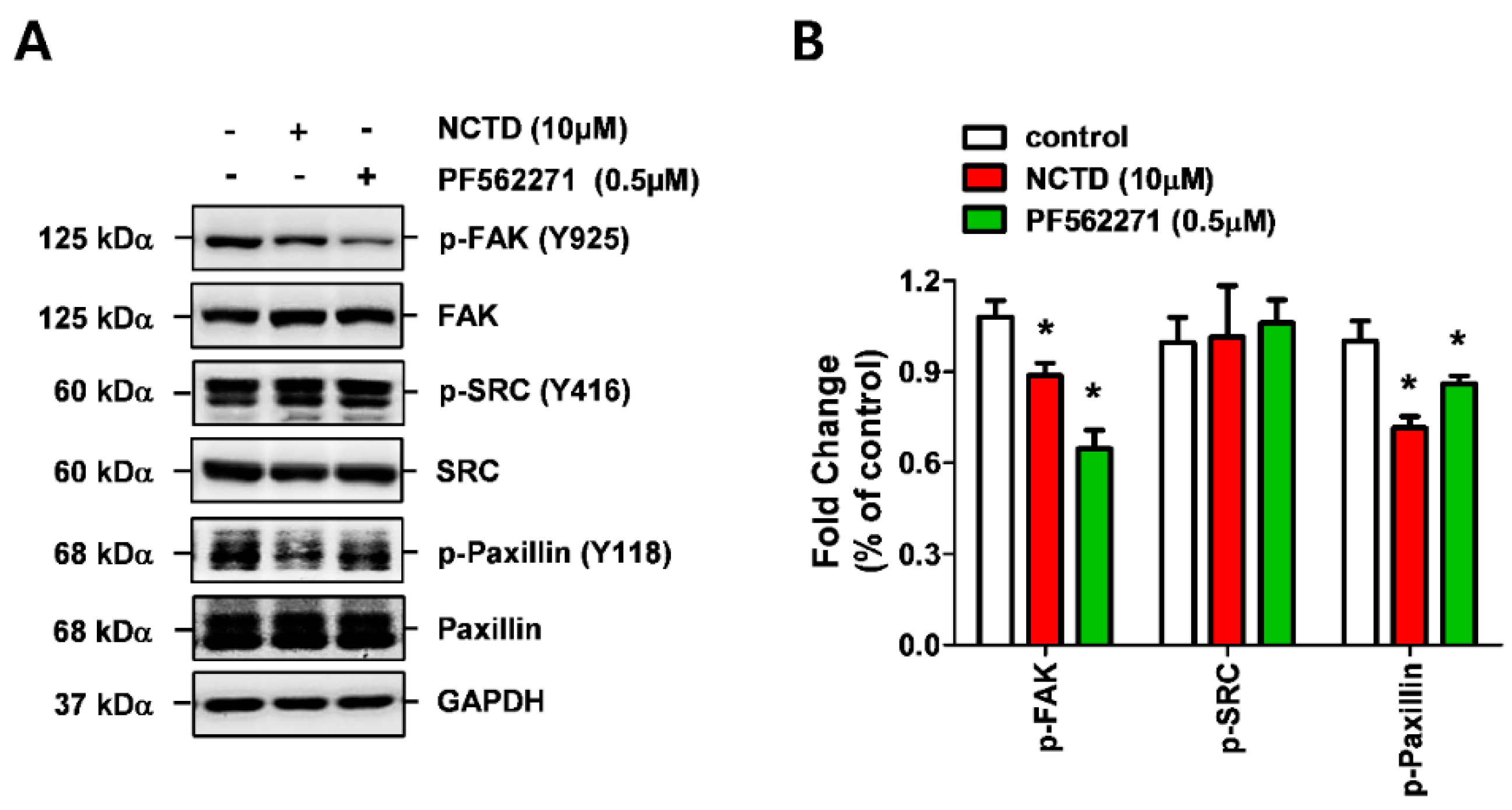
2.4. NCTD Inhibits the Invasiveness of YD-15 Cells More Potently than PF562271
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Chemical Treatment
4.2. Trypan Blue Exclusion Assay
4.3. Clonogenic Formation Assay
4.4. Scratch Wound Healing Assay
4.5. Matrigel Invasion Assay
4.6. Gelatin Zymography
4.7. F-Actin Staining Using TRITC-Conjugated Phalloidin
4.8. Western Blot Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, L.; Eveson, J.; Reichart, P.; Sidransky, D. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Head and Neck; IARC: Lyon, France, 2005. [Google Scholar]
- Bell, D.; El-Naggar, A.K. Molecular heterogeneity in mucoepidermoid carcinoma: Conceptual and practical implications. Head Neck Pathol. 2013, 7, 23–27. [Google Scholar] [CrossRef]
- Spiro, R.H.; Huvos, A.G.; Berk, R.; Strong, E.W. Mucoepidermoid carcinoma of salivary gland origin. A clinicopathologic study of 367 cases. Am. J. Surg. 1978, 136, 461–468. [Google Scholar] [CrossRef]
- Terhaard, C.H.; Lubsen, H.; Rasch, C.R.; Levendag, P.C.; Kaanders, H.H.; Tjho-Heslinga, R.E.; van Den Ende, P.L.; Burlage, F.; Dutch, H.; Neck Oncology Cooperative Group. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 103–111. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Lazebnik, Y. What are the hallmarks of cancer? Nat. Rev. Cancer 2010, 10, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Singer, R.H.; Segall, J.E. The great escape: When cancer cells hijack the genes for chemotaxis and motility. Annu. Rev. Cell Dev. Biol. 2005, 21, 695–718. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 2005, 15, 87–96. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T. Focal adhesion kinase: The first ten years. J. Cell Sci. 2003, 116, 1409–1416. [Google Scholar] [CrossRef]
- Kanteti, R.; Batra, S.K.; Lennon, F.E.; Salgia, R. FAK and paxillin, two potential targets in pancreatic cancer. Oncotarget 2016, 7, 31586–31601. [Google Scholar] [CrossRef] [PubMed]
- Gabarra-Niecko, V.; Schaller, M.D.; Dunty, J.M. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metast. Rev. 2003, 22, 359–374. [Google Scholar] [CrossRef]
- Itoh, S.; Maeda, T.; Shimada, M.; Aishima, S.; Shirabe, K.; Tanaka, S.; Maehara, Y. Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. Clin. Cancer Res. 2004, 10, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, E.; Sankari, L.S.; Malathi, L.; Krupaa, J.R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied Sci. 2015, 7, S181–S183. [Google Scholar] [CrossRef]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chang, W.M.; Liu, Y.W.; Lee, C.Y.; Jang, Y.H.; Kuo, C.D.; Liao, H.F. A small-molecule metastasis inhibitor, norcantharidin, downregulates matrix metalloproteinase-9 expression by inhibiting Sp1 transcriptional activity in colorectal cancer cells. Chem. Biol. Interact 2009, 181, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Liu, K.; Yagasaki, K.; Zhang, G. Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology 2009, 59, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ge, Y.; Liu, F.Y.; Peng, Y.M.; Sun, L.; Li, J.; Chen, Q.; Sun, Y.; Ye, K. Norcantharidin, a protective therapeutic agent in renal tubulointerstitial fibrosis. Mol. Cell Biochem. 2012, 361, 79–83. [Google Scholar] [CrossRef]
- Roberts, W.G.; Ung, E.; Whalen, P.; Cooper, B.; Hulford, C.; Autry, C.; Richter, D.; Emerson, E.; Lin, J.; Kath, J.; et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008, 68, 1935–1944. [Google Scholar] [CrossRef]
- Slack-Davis, J.K.; Hershey, E.D.; Theodorescu, D.; Frierson, H.F.; Parsons, J.T. Differential requirement for focal adhesion kinase signaling in cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol. Cancer Ther. 2009, 8, 2470–2477. [Google Scholar] [CrossRef]
- Irani, S. Distant metastasis from oral cancer: A review and molecular biologic aspects. J. Int. Soc. Prev. Community Dent. 2016, 6, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Brown, P.H. Drug development for cancer chemoprevention: Focus on molecular targets. Semin. Oncol. 2010, 37, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Steele, V.E.; Lubet, R.A. The use of animal models for cancer chemoprevention drug development. Semin. Oncol. 2010, 37, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Liu, Z.P. Natural products as anti-invasive and anti-metastatic agents. Curr. Med. Chem. 2011, 18, 808–829. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Chao, K.S.; Liao, H.F.; Chen, Y.J. Norcantharidin, derivative of cantharidin, for cancer stem cells. Evid. Based Complement. Alternat. Med. 2013, 2013, 838651. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, W.; Zhang, J.T.; Liu, Z.Y.; Li, X.P.; Fan, Y.Z. Norcantharidin enhances TIMP2 antivasculogenic mimicry activity for human gallbladder cancers through downregulating MMP2 and MT1MMP. Int. J. Oncol. 2015, 46, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.B.; Hsieh, M.J.; Hsieh, Y.H.; Chien, M.H.; Chiou, H.L.; Yang, S.F. Antimetastatic effects of norcantharidin on hepatocellular carcinoma by transcriptional inhibition of MMP-9 through modulation of NF-kB activity. PLoS ONE 2012, 7, e31055. [Google Scholar] [CrossRef] [PubMed]
- Mon, N.N.; Ito, S.; Senga, T.; Hamaguchi, M. FAK signaling in neoplastic disorders: A linkage between inflammation and cancer. Ann. N. Y. Acad. Sci. 2006, 1086, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Li, Z.; Niu, Z.; Niu, W.; Xu, Z.; Gao, H.; Niu, W.; Wang, J.; He, Z.; Gao, C.; et al. Norcantharidin Suppresses Colon Cancer Cell Epithelial-Mesenchymal Transition by Inhibiting the alphavbeta6-ERK-Ets1 Signaling Pathway. Sci. Rep. 2016, 6, 20500. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src kinase regulation by phosphorylation and dephosphorylation. Biochem. Biophys. Res. Commun. 2005, 331, 1–14. [Google Scholar] [CrossRef]
- Hsia, C.H.; Lu, W.J.; Lin, K.H.; Chou, D.S.; Geraldine, P.; Jayakuma, T.; Chang, N.C.; Sheu, J.R. Norcantharidin, a clinical used chemotherapeutic agent, acts as a powerful inhibitor by interfering with fibrinogen-integrin alphaIIb beta3 binding in human platelets. J. Cell Mol. Med. 2018, 22, 2142–2152. [Google Scholar] [CrossRef]
- Hu, C.; Chen, X.; Wen, J.; Gong, L.; Liu, Z.; Wang, J.; Liang, J.; Hu, F.; Zhou, Q.; Wei, L.; et al. Antitumor effect of focal adhesion kinase inhibitor PF562271 against human osteosarcoma in vitro and in vivo. Cancer Sci. 2017, 108, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.L.; Mi, P.Y.; Zhao, S.J.; Pan, H.M.; Li, H.J.; Liu, F.; Shao, L.R.; Zhang, H.F.; Zhang, P.; Jiang, S.L. Salinomycin exhibits anti-angiogenic activity against human glioma in vitro and in vivo by suppressing the VEGF-VEGFR2-AKT/FAK signaling axis. Int. J. Mol. Med. 2017, 39, 1255–1261. [Google Scholar] [CrossRef]
- Crompton, B.D.; Carlton, A.L.; Thorner, A.R.; Christie, A.L.; Du, J.; Calicchio, M.L.; Rivera, M.N.; Fleming, M.D.; Kohl, N.E.; Kung, A.L.; et al. High-throughput tyrosine kinase activity profiling identifies FAK as a candidate therapeutic target in Ewing sarcoma. Cancer Res. 2013, 73, 2873–2883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, J.; Han, Y.; Zhang, Y.; Su, L.; Hu, D.; Fu, X. JAM-A knockdown accelerates the proliferation and migration of human keratinocytes and improves wound healing in rats via FAK/Erk signaling. Cell Death Dis. 2018, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, W.H.; Auernheimer, V.; Thievessen, I.; Fabry, B. Vinculin, cell mechanics and tumour cell invasion. Cell Biol. Int. 2013, 37, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, W.H. Role of vinculin in cellular mechanotransduction. Cell Biol. Int. 2016, 40, 241–256. [Google Scholar] [CrossRef]
- Aggarwal, S.; Das, S.N. Garcinol inhibits tumour cell proliferation, angiogenesis, cell cycle progression and induces apoptosis via NF-kappaB inhibition in oral cancer. Tumour Biol. 2016, 37, 7175–7184. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, K.-O.; Ahn, C.-H.; Yang, I.-H.; Han, J.-M.; Shin, J.-A.; Cho, S.-D.; Hong, S.D. Norcantharidin Suppresses YD-15 Cell Invasion Through Inhibition of FAK/Paxillin and F-Actin Reorganization. Molecules 2019, 24, 1928. https://doi.org/10.3390/molecules24101928
Hong K-O, Ahn C-H, Yang I-H, Han J-M, Shin J-A, Cho S-D, Hong SD. Norcantharidin Suppresses YD-15 Cell Invasion Through Inhibition of FAK/Paxillin and F-Actin Reorganization. Molecules. 2019; 24(10):1928. https://doi.org/10.3390/molecules24101928
Chicago/Turabian StyleHong, Kyoung-Ok, Chi-Hyun Ahn, In-Hyoung Yang, Jung-Min Han, Ji-Ae Shin, Sung-Dae Cho, and Seong Doo Hong. 2019. "Norcantharidin Suppresses YD-15 Cell Invasion Through Inhibition of FAK/Paxillin and F-Actin Reorganization" Molecules 24, no. 10: 1928. https://doi.org/10.3390/molecules24101928
APA StyleHong, K.-O., Ahn, C.-H., Yang, I.-H., Han, J.-M., Shin, J.-A., Cho, S.-D., & Hong, S. D. (2019). Norcantharidin Suppresses YD-15 Cell Invasion Through Inhibition of FAK/Paxillin and F-Actin Reorganization. Molecules, 24(10), 1928. https://doi.org/10.3390/molecules24101928




